Abstract
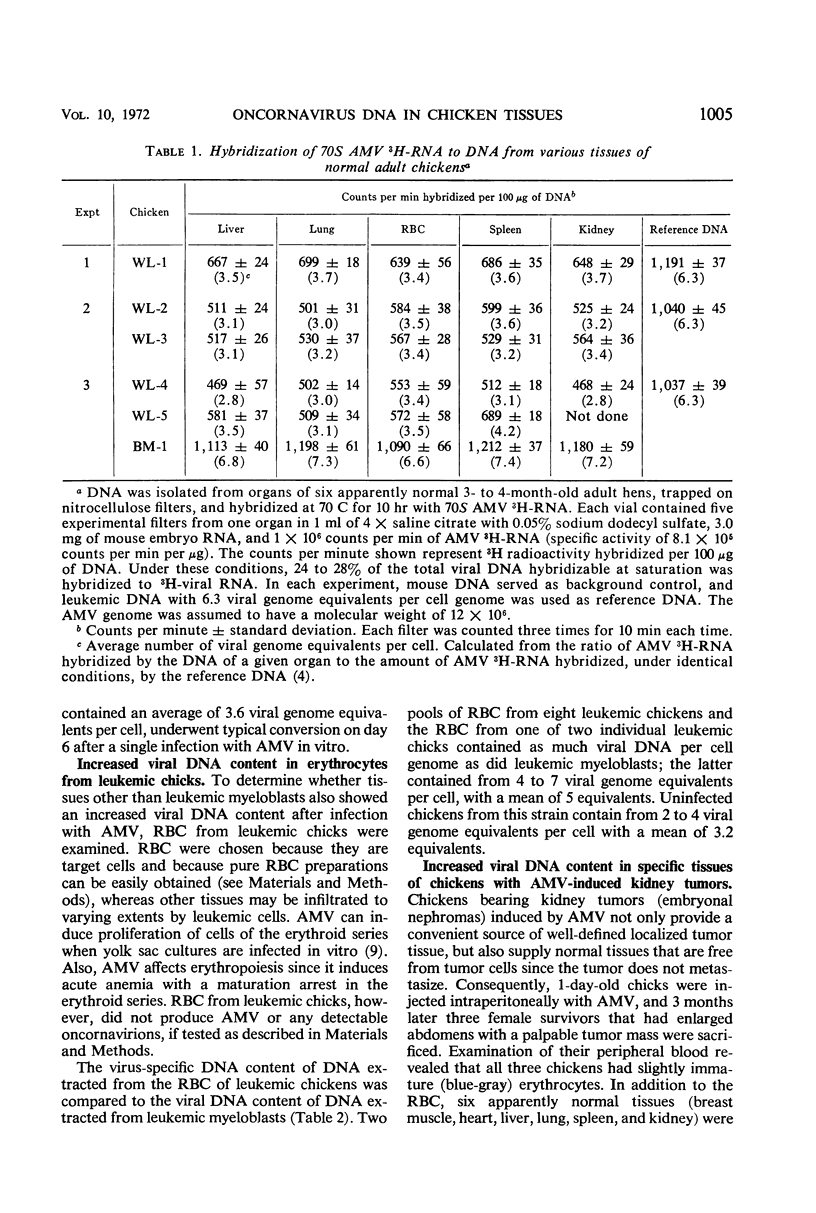
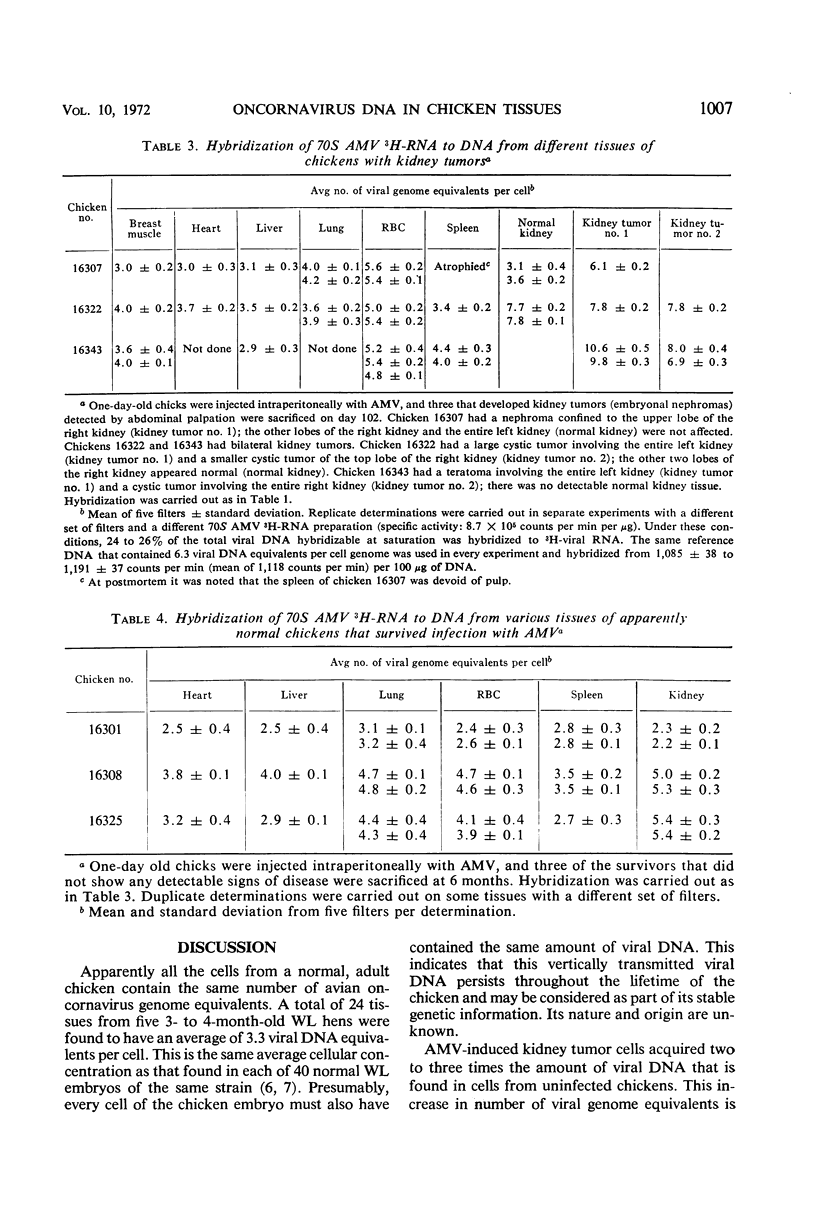
3H-labeled 70S ribonucleic acid (RNA) from purified avian myeloblastosis virus (AMV) was used as a probe in deoxyribonucleic acid (DNA)-RNA hybridization experiments to detect the presence of DNA complementary to the AMV genome in various tissues from noninfected normal chickens and from chickens infected with AMV. There was a remarkable constancy in the average cellular concentration of virus-specific DNA found in every tissue from the same uninfected chicken, and even in different chickens from the same strain. In contrast, different tissues from chickens bearing AMV-induced kidney tumors (embryonal nephromas) revealed an unequal distribution in the average virus-specific DNA content per cell. The increase was limited to tumor cells and to tissues that contain target cells for AMV, i.e., red blood cells, kidney cells, and possibly leukocytes. The red blood cells from AMV-infected chickens suffering from acute myeloblastic leukemia, although producing no virus, contained as many viral genome equivalents per cell as did leukemic myeloblasts known to produce large quantities of AMV. An increased viral DNA content was observed in the target cells of chickens that did not show any sign of tumor formation 6 months after infection with AMV. This study demonstrates that vertically transmitted viral DNA is uniformly and stably distributed among all tissues of the offspring, but that horizontal infection after hatching results in an increase in viral DNA content only in some dividing, target tissues that may or may not give rise to neoplasias.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALUDA M. A., GOETZ I. E. Morphological conversion of cell cultures by avian myeloblastosis virus. Virology. 1961 Oct;15:185–199. doi: 10.1016/0042-6822(61)90234-3. [DOI] [PubMed] [Google Scholar]
- BALUDA M. A., JAMIESON P. P. In vivo infectivity studies with avian mveloblastosis virus. Virology. 1961 May;14:33–45. doi: 10.1016/0042-6822(61)90129-5. [DOI] [PubMed] [Google Scholar]
- BALUDA M. A. Properties of cells infected with avian myeloblastosis virus. Cold Spring Harb Symp Quant Biol. 1962;27:415–425. doi: 10.1101/sqb.1962.027.001.039. [DOI] [PubMed] [Google Scholar]
- BEARD J. W. Etiology of avian leukosis. Ann N Y Acad Sci. 1957 Oct 21;68(2):473–486. doi: 10.1111/j.1749-6632.1957.tb56101.x. [DOI] [PubMed] [Google Scholar]
- BURMESTER B. R., FONTES A. K., WALTER W. G. Pathogenicity of a viral strain (RPL 12) causing avian visceral lymphomatosis and related neoplasms. III. Influence of host age and route of inoculation. J Natl Cancer Inst. 1960 Jun;24:1423–1442. [PubMed] [Google Scholar]
- BURMESTER B. R., WALTER W. G., GROSS M. A., FONTES A. K. The oncogenic spectrum of two pure strains of avian leukosis. J Natl Cancer Inst. 1959 Aug;23:277–291. [PubMed] [Google Scholar]
- Baluda M. A., Markham P. D. Nucleotide composition of RNA hybridized to homologous DNA from cells transformed by avian tumour viruses. Nat New Biol. 1971 May 19;231(20):90–91. doi: 10.1038/newbio231090a0. [DOI] [PubMed] [Google Scholar]
- Baluda M. A., Nayak D. P. DNA complementary to viral RNA in leukemic cells induced by avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1970 Jun;66(2):329–336. doi: 10.1073/pnas.66.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluda M. A., Nayak D. P. Incorporation of precursors into ribonucleic acid, protein, glycoprotein, and lipoprotein of avian myeloblastosis virions. J Virol. 1969 Nov;4(5):554–566. doi: 10.1128/jvi.4.5.554-566.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluda M. A. The role of the bursa-dependent lymphoid tissue in oncogenesis by avian myeloblastosis virus. Virology. 1967 Jul;32(3):428–437. doi: 10.1016/0042-6822(67)90294-2. [DOI] [PubMed] [Google Scholar]
- Baluda M. A. Widespread presence, in chickens, of DNA complementary to the RNA genome of avian leukosis viruses. Proc Natl Acad Sci U S A. 1972 Mar;69(3):576–580. doi: 10.1073/pnas.69.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARR J. G. Renal adenocarcinoma induced by fowl leukaemia virus. Br J Cancer. 1956 Jun;10(2):379–383. doi: 10.1038/bjc.1956.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISTEFANO H. S., DOUGHERTY R. M. VIRUS MULTIPLICATION IN THE OVIDUCT OF HENS INFECTED WITH AN AVIAN LEUKOSIS VIRUS. Virology. 1965 May;26:156–159. doi: 10.1016/0042-6822(65)90040-1. [DOI] [PubMed] [Google Scholar]
- Dougherty R. M., Di Stefano H. S. Lack of relationship between infection with avian leukosis virus and the presence of COFAL antigen in chick embryos. Virology. 1966 Aug;29(4):586–595. doi: 10.1016/0042-6822(66)90282-0. [DOI] [PubMed] [Google Scholar]
- Dougherty R. M., Di Stefano H. S., Roth F. K. Virus particles and viral antigens in chicken tissues free of infectious avian leukosis virus. Proc Natl Acad Sci U S A. 1967 Sep;58(3):808–817. doi: 10.1073/pnas.58.3.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty R. M., Di Stefano H. S. Sites of avian leukosis virus multiplication in congenitally infected chickens. Cancer Res. 1967 Feb;27(2):322–332. [PubMed] [Google Scholar]
- Hill M., Hillova J. Virus recovery in chicken cells tested with Rous sarcoma cell DNA. Nat New Biol. 1972 May 10;237(71):35–39. doi: 10.1038/newbio237035a0. [DOI] [PubMed] [Google Scholar]
- LAGERLOEF B., SUNDELIN P. VARIATIONS IN THE PATHOGENIC EFFECT OF MYELOID FOWL LEUKAEMIA VIRUS. Acta Pathol Microbiol Scand. 1963;59:129–144. doi: 10.1111/j.1699-0463.1963.tb01779.x. [DOI] [PubMed] [Google Scholar]
- RUBIN H., FANSHIER L., CORNELIUS A., HUGHES W. F. Tolerance and immunity in chickens after congenital and contact infection with an avian leukosis virus. Virology. 1962 May;17:143–156. doi: 10.1016/0042-6822(62)90091-0. [DOI] [PubMed] [Google Scholar]
- Rosenthal P. N., Robinson H. L., Robinson W. S., Hanafusa T., Hanafusa H. DNA in uninfected and virus-infected cells complementary to avian tumor virus RNA. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2336–2340. doi: 10.1073/pnas.68.10.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda J., Hlozánek I., Mach O. Detection of chicken sarcoma virus after transfection of chicken fibroblasts with DNA isolated from mammalian cells transformed with Rous Virus. Folia Biol (Praha) 1972;18(2):149–153. [PubMed] [Google Scholar]
- Temin H. M. Mechanism of cell transformation by RNA tumor viruses. Annu Rev Microbiol. 1971;25:609–648. doi: 10.1146/annurev.mi.25.100171.003141. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Weiss R. A., Friis R. R., Levinson W., Bishop J. M. Detection of avian tumor virus-specific nucleotide sequences in avian cell DNAs (reassociation kinetics-RNA tumor viruses-gas antigen-Rous sarcoma virus, chick cells). Proc Natl Acad Sci U S A. 1972 Jan;69(1):20–24. doi: 10.1073/pnas.69.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. A., Friis R. R., Katz E., Vogt P. K. Induction of avian tumor viruses in normal cells by physical and chemical carcinogens. Virology. 1971 Dec;46(3):920–938. doi: 10.1016/0042-6822(71)90091-2. [DOI] [PubMed] [Google Scholar]



