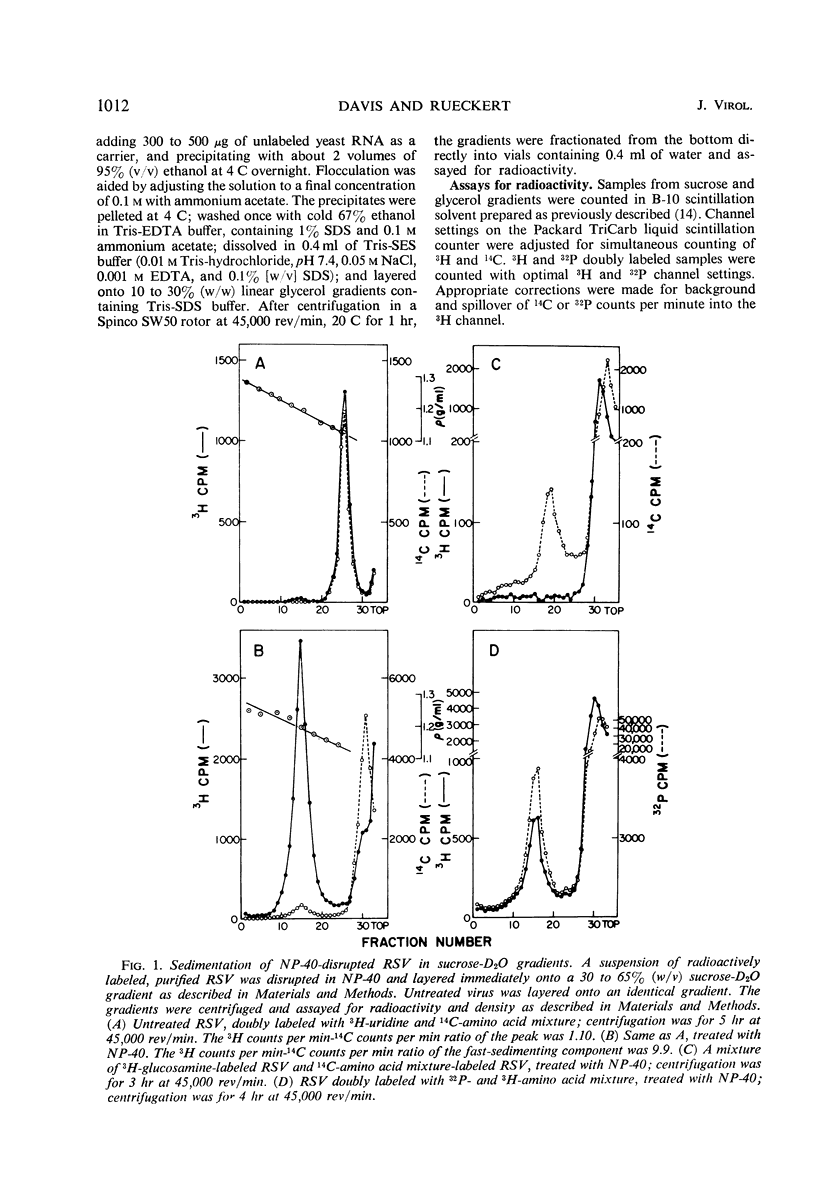
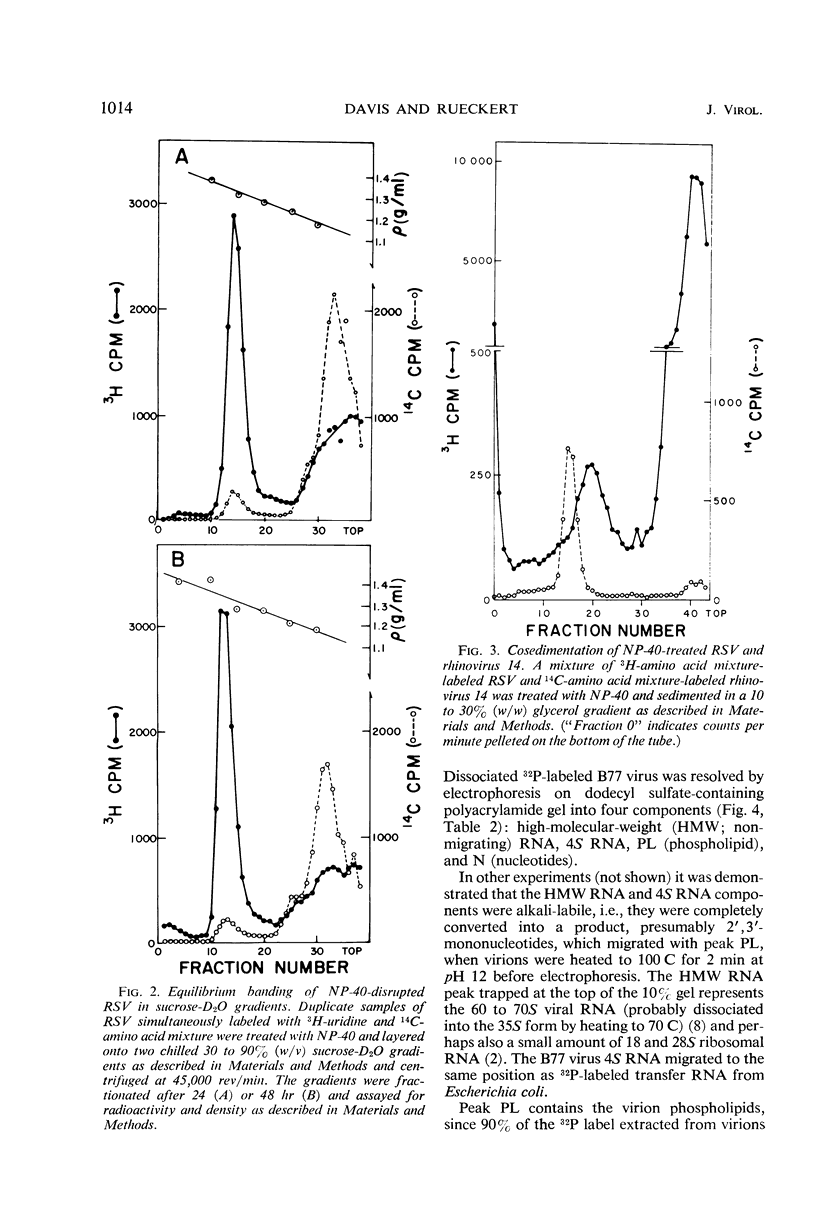
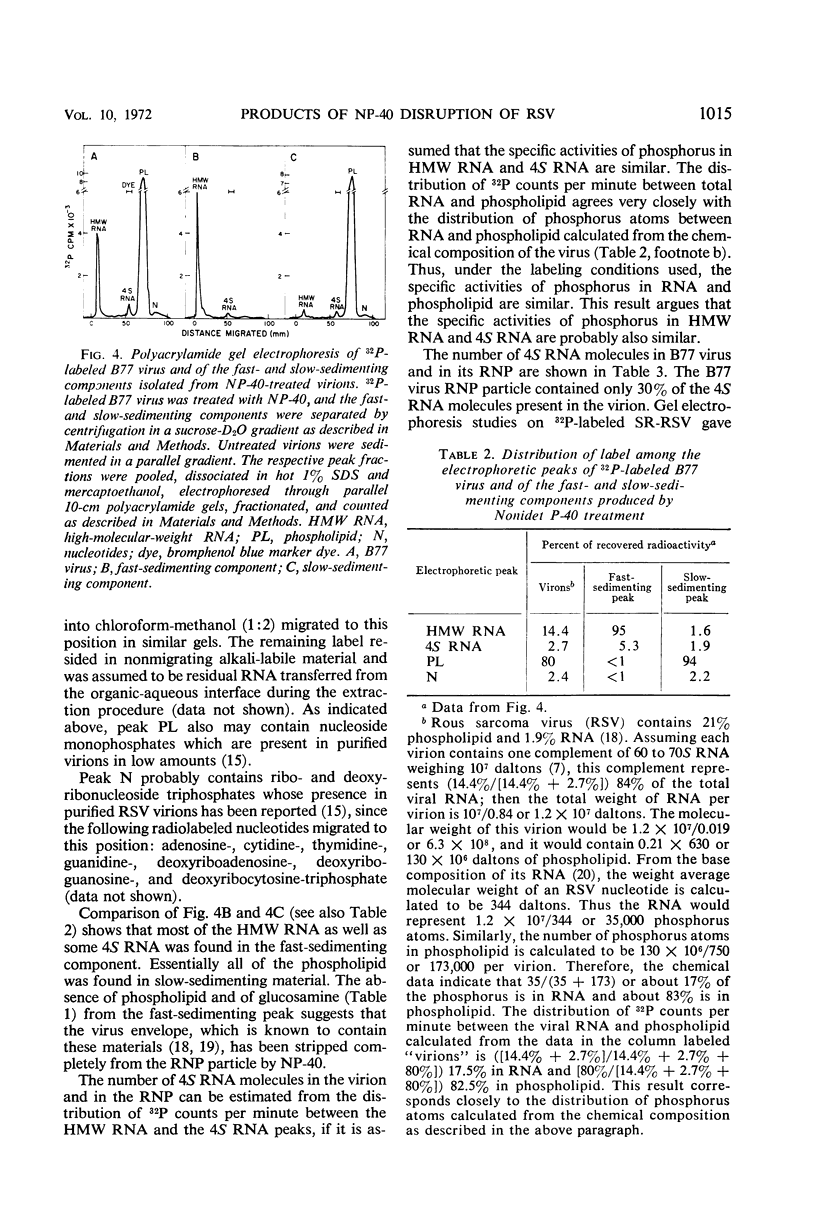
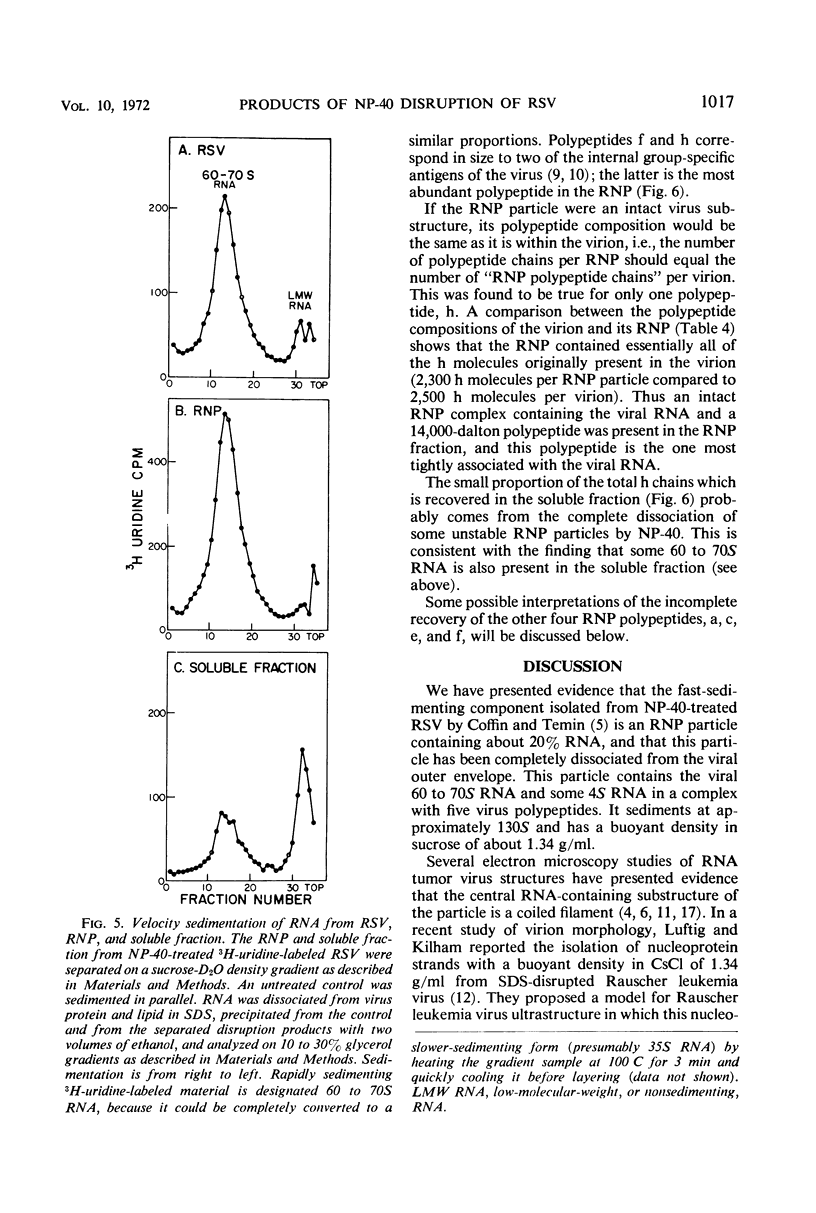
Abstract
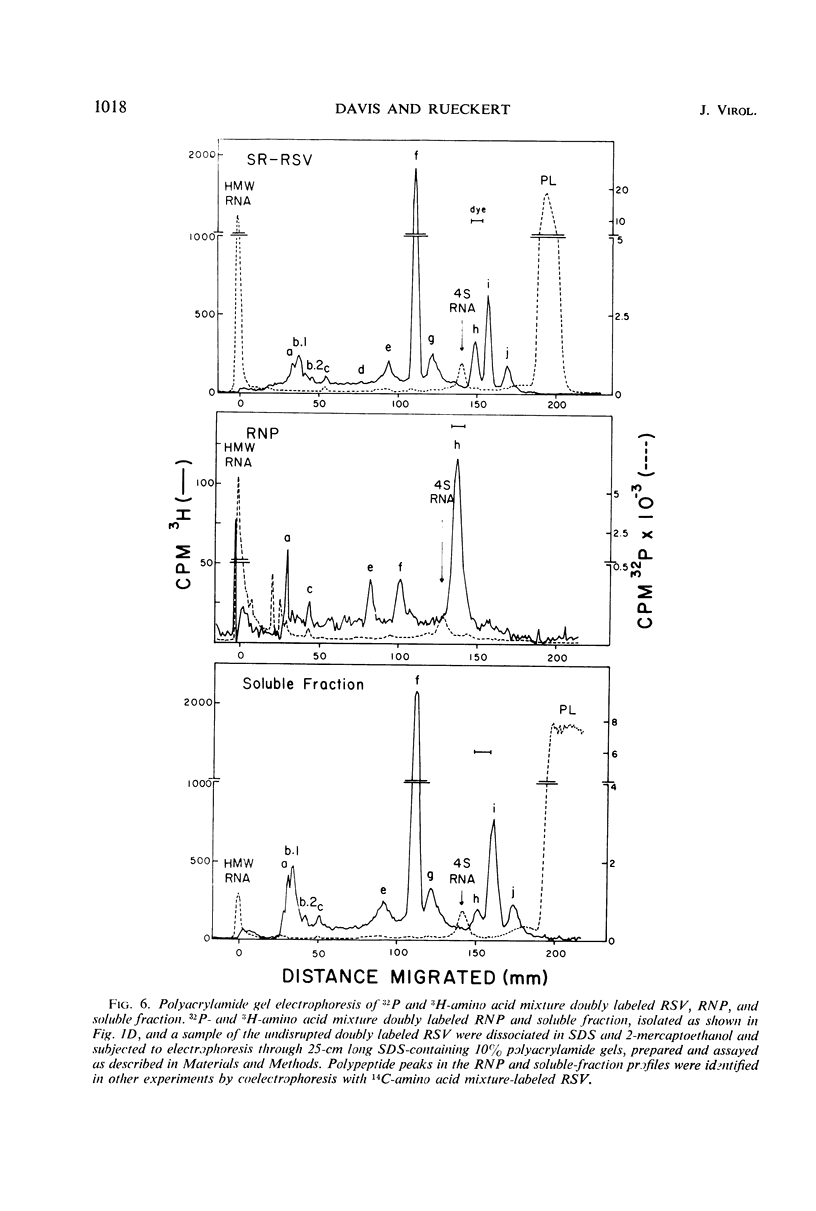
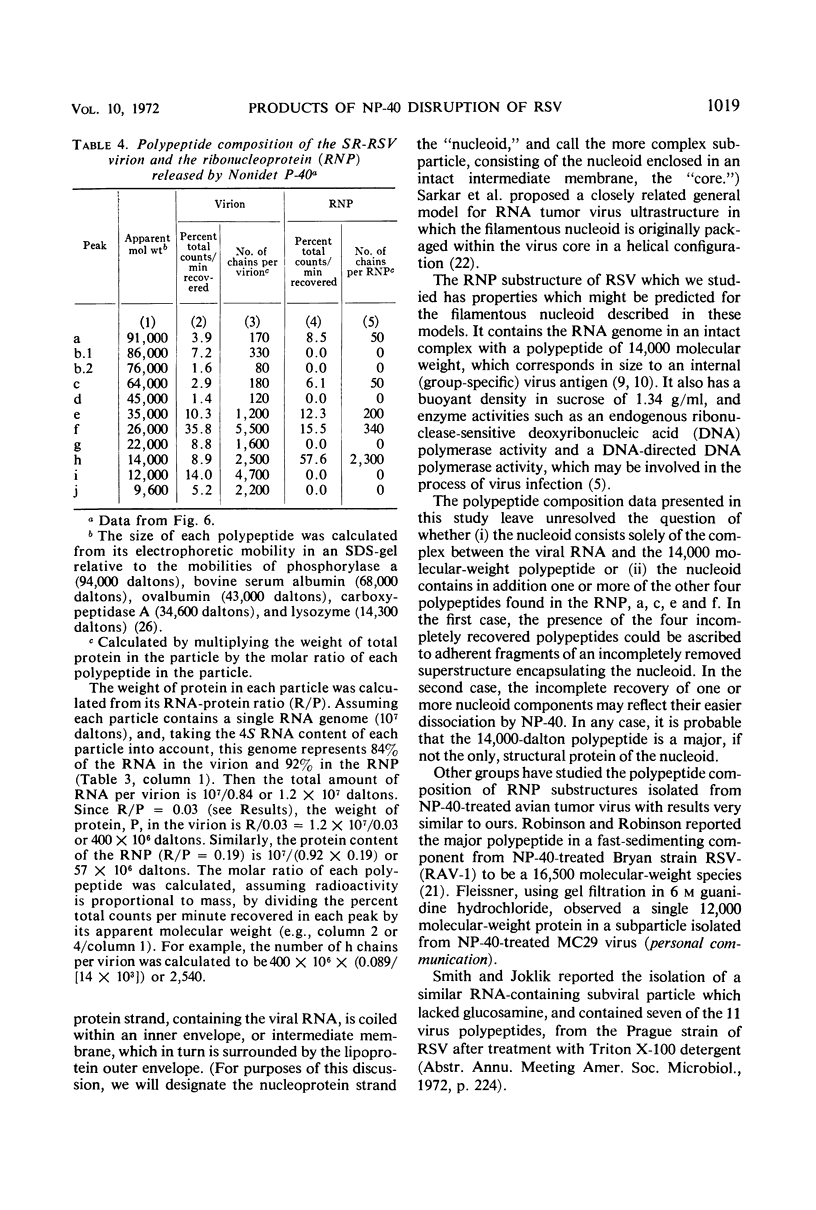
A ribonucleoprotein particle containing about 20% ribonucleic acid (RNA), and containing little if any phospholipid or glucosamine, was recovered in high yield after treatment of Schmidt-Ruppin strain of Rous sarcoma virus and B77 virus with the nonionic detergent Nonidet P-40. This structure, which probably derives from the internal ribonucleoprotein filament described in electron microscopy studies, contained 80 to 90% of the viral 60 to 70S RNA and only about 10% of the protein present in intact virions. It sedimented in glycerol density gradients at approximately 130S and had a buoyant density in sucrose of about 1.34 g/ml. Studies with 32P-labeled virus indicated that the ribonucleoprotein particle contained approximately 30 4S RNA molecules per 107 daltons of high-molecular-weight viral RNA. Intact virions contained about 70 4S RNA molecules per 107 daltons of high-molecular-weight RNA. Electrophoretic studies in dodecyl sulfate-containing polyacrylamide gels showed that the ribonucleoprotein particle contained only 5 of the 11 polypeptides found in the virion; of these the major component was a polypeptide weighing 14,000 daltons.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader J. P., Brown N. R., Bader A. V. Characteristics of cores of avian leuko-sarcoma viruses. Virology. 1970 Aug;41(4):718–728. doi: 10.1016/0042-6822(70)90436-8. [DOI] [PubMed] [Google Scholar]
- Bishop J. M., Levinson W. E., Quintrell N., Sullivan D., Fanshier L., Jackson J. The low molecular weight RNAs of Rous sarcoma virus. I. The 4 S RNA. Virology. 1970 Sep;42(1):182–195. doi: 10.1016/0042-6822(70)90251-5. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Gelderblom H., Bauer H., Mölling K., Hüper G. Polypeptides of avian RNA tumor viruses. V. Analysis of the virus core. Virology. 1972 Mar;47(3):567–578. doi: 10.1016/0042-6822(72)90546-6. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Temin H. M. Comparison of Rous sarcoma virus-specific deoxyribonucleic acid polymerases in virions of Rous sarcoma virus and in Rous sarcoma virus-infected chicken cells. J Virol. 1971 May;7(5):625–634. doi: 10.1128/jvi.7.5.625-634.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Thé G., O'Connor T. E. Structure of a murine leukemia virus after disruption with tween-ether and comparison with two myxoviruses. Virology. 1966 Apr;28(4):713–728. doi: 10.1016/0042-6822(66)90256-x. [DOI] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Association of 4S ribonucleic acid with oncornavirus ribonucleic acids. J Virol. 1971 Aug;8(2):254–256. doi: 10.1128/jvi.8.2.254-256.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleissner E. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. I. Avian leukemia-sarcoma viruses. J Virol. 1971 Nov;8(5):778–785. doi: 10.1128/jvi.8.5.778-785.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung P. P., Robinson H. L., Robinson W. S. Isolation and characterization of proteins from Rous sarcoma virus. Virology. 1971 Jan;43(1):251–266. doi: 10.1016/0042-6822(71)90243-1. [DOI] [PubMed] [Google Scholar]
- Lacour F., Fourcade A., Verger C., Delain E. Coiled structure of the nucleocapsid of avian myeloblastosis virus. J Gen Virol. 1970 Oct;9(1):89–92. doi: 10.1099/0022-1317-9-1-89. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Kilham S. S. An electron microscope study of Rauscher leukemia virus. Virology. 1971 Nov;46(2):277–297. doi: 10.1016/0042-6822(71)90030-4. [DOI] [PubMed] [Google Scholar]
- McGregor S., Mayor H. D. Biophysical and biochemical studies on rhinovirus and poliovirus. II. Chemical and hydrodynamic analysis of the rhinovirion. J Virol. 1971 Jan;7(1):41–46. doi: 10.1128/jvi.7.1.41-46.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medappa K. C., McLean C., Rueckert R. R. On the structure of rhinovirus 1A. Virology. 1971 May;44(2):259–270. doi: 10.1016/0042-6822(71)90258-3. [DOI] [PubMed] [Google Scholar]
- Mizutani S., Temin H. M. Enzymes and nucleotides in virions of Rous sarcoma virus. J Virol. 1971 Oct;8(4):409–416. doi: 10.1128/jvi.8.4.409-416.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll H. Characterization of macromolecules by constant velocity sedimentation. Nature. 1967 Jul 22;215(5099):360–363. doi: 10.1038/215360a0. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Old L. J., Sarkar N. H., Moore D. H. Common properties of the oncogenic RNA viruses (oncornaviruses). Virology. 1970 Dec;42(4):1152–1157. doi: 10.1016/0042-6822(70)90367-3. [DOI] [PubMed] [Google Scholar]
- Quigley J. P., Rifkin D. B., Reich E. Phospholipid composition of Rous sarcoma virus, host cell membranes and other enveloped RNA viruses. Virology. 1971 Oct;46(1):106–116. doi: 10.1016/0042-6822(71)90010-9. [DOI] [PubMed] [Google Scholar]
- Rifkin D., Compans R. W. Identification of the spike proteins of Rous sarcoma virus. Virology. 1971 Nov;46(2):485–489. doi: 10.1016/0042-6822(71)90049-3. [DOI] [PubMed] [Google Scholar]
- Robinson W. S., Pitkanen A., Rubin H. The nucleic acid of the Bryan strain of Rous sarcoma virus: purification of the virus and isolation of the nucleic acid. Proc Natl Acad Sci U S A. 1965 Jul;54(1):137–144. doi: 10.1073/pnas.54.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. S., Robinson H. L. DNA polymerase in defective Rous sarcoma virus. Virology. 1971 May;44(2):457–462. doi: 10.1016/0042-6822(71)90278-9. [DOI] [PubMed] [Google Scholar]
- Sarkar N. H., Nowinski R. C., Moore D. H. Helical nucleocapsid structure of the oncogenic ribonucleic acid viruses (oncornaviruses). J Virol. 1971 Oct;8(4):564–572. doi: 10.1128/jvi.8.4.564-572.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S., Burge B. W. Identification of a second glycoprotein in Sindbis virus. Virology. 1972 Feb;47(2):539–541. doi: 10.1016/0042-6822(72)90298-x. [DOI] [PubMed] [Google Scholar]
- Skehel J. J. The characterization of subviral particles derived from influenza virus. Virology. 1971 May;44(2):409–417. doi: 10.1016/0042-6822(71)90271-6. [DOI] [PubMed] [Google Scholar]
- Wagner R. R., Schnaitman T. C., Snyder R. M., Schnaitman C. A. Protein composition of the structural components of vesicular stomatitis virus. J Virol. 1969 Jun;3(6):611–618. doi: 10.1128/jvi.3.6.611-618.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zachau H. G. Transfer ribonucleic acids. Angew Chem Int Ed Engl. 1969 Oct;8(10):711–727. doi: 10.1002/anie.196907111. [DOI] [PubMed] [Google Scholar]



