Abstract
The distribution of health conditions is characterized by extreme inequality. These disparities have been alternately attributed to disease ecology and the economics of poverty. Here, we provide a novel framework that integrates epidemiological and economic growth theory on an individual-based hierarchically structured network. Our model indicates that, under certain parameter regimes, feedbacks between disease ecology and economics create clusters of low income and high disease that can stably persist in populations that become otherwise predominantly rich and free of disease. Surprisingly, unlike traditional poverty trap models, these localized disease-driven poverty traps can arise despite homogeneity of parameters and evenly distributed initial economic conditions.
Keywords: poverty traps, transmission networks, social epidemiology
1. Introduction
The distribution of health and economic conditions across the globe is characterized by extreme inequality, both between and within countries [1,2]. Because prevention and treatment of diseases require economic resources, the social science literature often attributes disparities in disease burdens to disparities in economics [3,4]. The most common explanations for such heterogeneity in the economics literature can be broadly grouped into two schools of thought: (i) variation in parameters (i.e. in aggregate or household ‘production functions’) [5,6]; or (ii) variation in state variables (i.e. ‘initial conditions’) [7]. The literature on poverty traps often focuses on the latter concept that different initial conditions can result in different economic trajectories owing to feedbacks between income and capital accumulation, often defined broadly to include savings, education or health [8–10]. In each case, the role of initial conditions on long-term outcomes has been used as a theoretical justification for external policy interventions, such as healthcare or education.
While the role of disease on poverty traps has long been recognized by economists [11], formal models have tended to focus on individual-level processes (e.g. malnutrition) and have been generally independent of ecological theory. The central importance of an ecological perspective is that population-level processes have a distinctly different character than individual processes. This is especially relevant for infectious diseases for which transmission between hosts is a basic life-history requirement.
Although it is broadly taken for granted in the social sciences that heterogeneity in outcomes is due to heterogeneity in underlying determinants, infectious disease models, rooted in dynamical systems theory and based on a substantial body of evidence, suggest many potential causes of heterogeneity. A single infectious disease system with a homogeneous population can exhibit complex behaviour across space and time, from stationary states to travelling waves, to periodic and chaotic dynamics [12,13]. These disease processes comprise the real-world underlying structure of the dynamics of human capital of the poor, and point to a potentially new family of models for formalizing the structure of health-related economic ‘externalities’.
In the past couple of years, a new body of theory has been put forth on disease-driven poverty traps that is based on explicit epidemiological models [14–16]. This has expanded our theoretical understanding of the potential role of infectious diseases on poverty traps, but also has fundamental limitations of its own. This literature is rooted in ecological theory and explicitly captures population-level processes. However, it is not integrated with economic growth theory, which tends to emphasize the role of the systematic accumulation of capital (or wealth), which may offset the destabilizing effects of fluctuations in income. In addition, these models suppress individual-level effects and the potential complex outcomes that may arise within real-world populations. Our goal here is to develop a theoretical framework that integrates both ecological and economic theory, and accounts for interactions between population-level processes and individual conditions.
To understand how the inherent structure of disease dynamics may feed back on economics at a local level, we turn to network theory [17–20]. Because the basic reproductive number, R0, of an infectious disease depends on the variance of the distribution of number of contacts [21], the topology of contact networks determines critical epidemiological parameters. For example, contact networks with degree distributions with infinite variance (such as the scale-free networks that characterize human sexual contacts) do not have epidemic thresholds [22]. However, scale-free networks with additional structure do have finite epidemic thresholds, underlining the importance of the community structure on the qualitative behaviour of disease dynamics [23].
Here, we provide a novel framework that integrates a hierarchically structured network model of an infectious disease with a formal economic growth model. We find that feedbacks between disease ecology and economics can create clusters of low income and high disease that can stably persist in populations that become, otherwise, predominantly rich and free of disease. These divergent outcomes generate inequality and can arise despite homogeneity of parameters and evenly distributed initial economic conditions.
2. Material and methods
Our general framework expands from previous studies on disease-driven poverty traps [15,16], and is structured in accordance with two canonical theoretical frameworks in respective epidemiology and economics literatures: (i) the susceptible–infected–susceptible (SIS) framework, which has been applied to a wide range of disease systems that serially reinfect their hosts and that are known to be both causes and consequences of poverty, such as malaria and enteric pathogens, and (ii) the neoclassical economic growth model with human capital as a function of health status [24]. The system is coupled via the following three empirically based assumptions: (i) income is determined by the acquisition of human and physical capital; (ii) human capital accumulation is a function of health status; and (iii) disease transmission and recovery rates are determined by income. The first assumption is a universal property of neoclassical economic growth models [25]. The second assumption is based on a recently growing literature in economics and epidemiology that health status influences cognitive development, and schooling with long-term impacts on economic productivity [26–28]. The final assumption is based on the fact that prevention and treatment of infectious diseases requires economic resources, which is why poverty is a social determinant of disease.
We simulate the coupled epidemiological and economic processes on an individual-based network model. We generate random transmission networks with a given degree of community structure, ranging from 0 to 1. The nodes in the network represent individuals, and the vertices (links) represent disease transmission. As in the classic compartmentalized SIS systems, transmission occurs between pairs of susceptible and infected individuals at a rate β, which is determined by the income m of the susceptible individual:
| 2.1 |
This transmission function indicates that poorer individuals are more susceptible to disease. Similarly, the rate of recovery γ also depends on individual incomes such that poorer individuals take longer to recover (e.g. owing to undernutrition or lack of medical treatment):
| 2.2 |
These transmission and recovery functions are drawn from previous studies [15,16].
For each individual, we track human capital hi, physical capital ki and total income mi, which are updated according to the following equations:
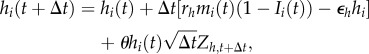 |
2.3 |
| 2.4 |
| 2.5 |
Equation (2.5) represents a classic production function where income is determined by a combination of physical capital k and human capital h, which exhibit diminishing returns on investment (i.e. 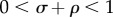 ). Individuals invest a proportion of their current income in human and physical capital at savings rates rh and rk, respectively. Human and physical capital depreciate at rate
). Individuals invest a proportion of their current income in human and physical capital at savings rates rh and rk, respectively. Human and physical capital depreciate at rate 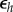 and
and 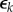 . Importantly, when individuals are sick, they cease to acquire human capital and thus steadily lose income.
. Importantly, when individuals are sick, they cease to acquire human capital and thus steadily lose income.
To analyse the system, we use the Gillespie algorithm to run the simulations until equilibrium, and then calculate summary statistics based on the distribution of long-run average income and the proportion of time spent infected. The simulations are started with half of the population (randomly) infected, and all individuals with half of the maximum possible income. This is the closest approximation to truly uniformly distributed initial conditions; the randomness of the network structure inevitably results in initial heterogeneities in contact structure and disease state.
3. Results
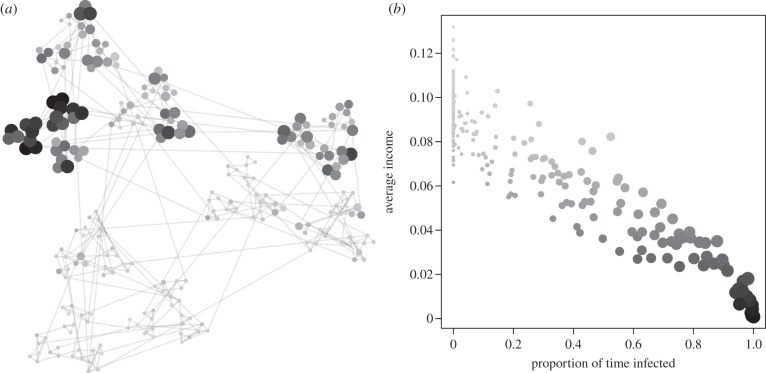
Feedbacks between disease and income generate clusters of poverty and disease in our simulated networks. Figure 1 depicts the result of one simulation, showing the long-run average income and proportion of time spent infected for each individual. There is an obvious cluster of poor and sick individuals, representing a neighbourhood that has fallen into a localized disease-driven poverty trap. Moreover, these divergent outcomes are independent of initial conditions (electronic supplementary material, figure S1). As expected, coupling income and health together results in a correlation between long-term income and time spent infected (figure 1b).
Figure 1.
A sample run of the model. Each point is an individual. Darker points represent lower income, and larger points represent greater time spent infected. (a) Equilibrium distribution of health and income in the network. (b) Average long-term income versus proportion of time spent infected.
To test whether both forces (income-to-disease and disease-to-income) are necessary to generate the observed clusters of poverty, we turn off the feedbacks individually and measure the resulting distribution of income inequality (Theil index; see electronic supplementary material for details). The results indicate that both feedbacks are necessary to generate the localized poverty traps (figure 2c). To explore the role of the infectious disease process in the creation of income disparities, we run 200 simulations for each value of the transmission parameter, β1, and record the observed inequality as well as the mean prevalence (figure 2). Below a certain threshold, transmission cannot be sustained, and thus the only variance in income is due to the stochasticity in the income growth process. However, when the disease is able to stably persist, the observed income inequality rises significantly, evidence that the disease process is allowing for stable disparities in income. The income inequality peaks when disease transmission is quite low (stable prevalence of around 20%) and then starts declining. As the force of infection increases and the likelihood of neighbourhoods persistently escaping from disease decreases, the population approaches a uniformly sick and poor state.
Figure 2.
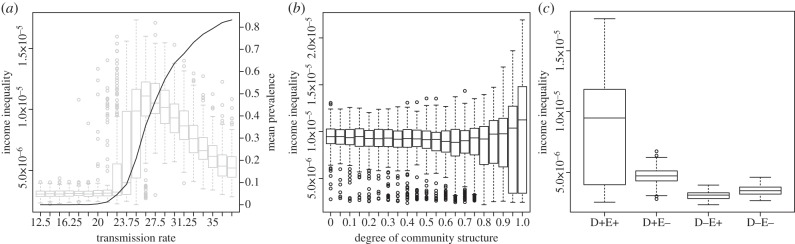
(a) Income inequality as measured by the Theil index (box plot) and average prevalence of the infection (thick line) for different values of the transmission rate parameter β1, with community structure fixed at μ = 0.9. (b) Income inequality versus degree of community structure in the network, ranging from random graphs with no community structure (μ = 0) to networks with full community structure (μ = 1), with the transmission rate parameter fixed at β1 = 25. (c) Income inequality is generated from two-way feedbacks. The D feedback represents the assumption that disease state influences economic growth (the 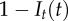 term in equation (2.3)). The D– state removes this term from the equation. The E feedback represents the assumption that the transmission rate β and recovery rate γ depend on income (equations (2.1) and (2.2)). In the E– state, β and γ are held constant, corresponding to values of equations (2.1) and (2.2) at half of maximal income. Mean income inequality is greater for the D+E+ state than all other configurations (pairwise t-tests, all p-values < 10−15).
term in equation (2.3)). The D– state removes this term from the equation. The E feedback represents the assumption that the transmission rate β and recovery rate γ depend on income (equations (2.1) and (2.2)). In the E– state, β and γ are held constant, corresponding to values of equations (2.1) and (2.2) at half of maximal income. Mean income inequality is greater for the D+E+ state than all other configurations (pairwise t-tests, all p-values < 10−15).
We then run the simulations 200 times for different values of μ, which represents the strength of community structure in the network. Figure 2b shows the distribution of the Theil index for income as a function of the degree of community structure. As we vary the degree of community structure, the average observed income inequality falls slightly before rising, but the maximal observed income inequality rises significantly (p-value < 0.001). Moreover, the shape of the distribution of observed income inequalities varies significantly. As the community structure increases, the epidemiology of disease spread changes predictably—there are more stochastic die-outs and there is a wider variance in the distribution of long-term disease prevalence. As a result of this, the distribution of possible income inequality outcomes changes accordingly, with a wider variance and a bimodal distribution (electronic supplementary material, figure S2). Health inequality also shows the same dependence on community structure as income inequality (electronic supplementary material).
4. Discussion
The existing microeconomic literature on poverty traps often focuses on feedbacks between income and human capital at the level of the individual or household, and emphasizes the effects of different initial economic conditions. In our model, clusters of poverty and disease result from two kinds of feedbacks: (i) feedbacks between health and economic conditions at the individual level and (ii) feedback between individual- and population-level health conditions (i.e. from disease transmission). Thus, through the inherent structure of disease dynamics, individual economic conditions are connected to their broader communities. These feedbacks can thus lead to high levels of income and health disparities in otherwise economically homogeneous populations.
Community structure, as well as network structure in general, is becoming appreciated for its importance in determining the observed properties of disease dynamics [29], whether it be high rates of HIV and other STDs in sexual networks with high concurrency [30], or in the role of community structure in explaining the observed power-law distribution in epidemic size and duration [31]. Our results suggest that community structure may have more far-reaching consequences as a factor in the formation and maintenance of pockets of persistent poverty and long-term stable health inequalities.
Social epidemiology, a field dedicated to studying health disparities in various social, economic and ethnic groups, has often focused on the concept of neighbourhood effects [32]. From inner-city neighbourhoods in the developed world, where much of the social epidemiology research has been carried out, to urban slums in the developing world [33], it is increasingly clear that the human environment directly affects the health of communities. Our model is meant to serve as an important framework for exploring such concepts based on formal ecological and economic theory. Our results show that persistent neighbourhood-level differences in health outcomes can arise from individual-level risk factors as long as one accounts for the web of human connections. While our study is based on a simple SIS model, we conjecture that the basic drivers of the system—economic and epidemiological feedback—may be relevant to a wide range of infectious and parasitic disease systems. Indeed, with the increasing evidence that even chronic diseases can have infectious components [34], in addition to the economic dimensions of prevention and treatment, our work should motivate further study into the causative role of transmission networks in the formation and maintenance of general health and economic disparities.
Acknowledgements
This project was catalysed by a workshop on Economic Epidemiology, supported by the DIMACS/MBI US-African Biomathematics Initiative, at Makerere University, Uganda. M.M.P. is financially supported by an NSF Graduate Research Fellowship, C.N.N. is financially supported by a Postdoctoral Fellowship from NIMBioS (NSF award no. ER-0832858), W.M.G. is financially supported by NIH grant no. GM083863, BSF grant no. 2008255 and the Rosalinde and Arthur Gilbert Foundation, and M.H.B. is financially supported by an FIC/NIH International Research Scientist Career Development Award (no. K01TW008773). The authors thank Julia Walsh, Megan Murray, Chris Barrett and two anonymous reviewers for helpful comments and suggestions.
References
- 1.Sala-i-Martin X, Mohapatra S. 2003 Poverty, inequality and the distribution of income in the Group of 20, p. 65. Economic Round-up, Australia. See http://www.g20.utoronto.ca/biblio/salaimartin-mohapatra.pdf . [Google Scholar]
- 2.Bloom DE, Canning D. 2008. Mortality traps and the dynamics of health transitions. Nature 453, 98–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawachi I, Kennedy BP. 1997. Socioeconomic determinants of health: health and social cohesion: why care about income inequality. Br. Med. J. 314, 215–227 10.1136/bmj.314.7086.1037 (doi:10.1136/bmj.314.7086.1037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farmer P. 1999. Infections and inequalities. Berkeley, CA: University of California Press [Google Scholar]
- 5.Gallup JL, Sachs JD, Mellinger AD. 1999. Geography and economic development. Int. Reg. Sci. Rev. 22, 179–232 10.1177/016001799761012334 (doi:10.1177/016001799761012334) [DOI] [Google Scholar]
- 6.Banerjee AV, Duflo E. 2005. Growth theory through the lens of development economics. In Handbook of economic growth, vol. 1 (eds. Durlauf S, Aghion P.), pp. 473–552 Amsterdam, the Netherlands: Elsevier Science [Google Scholar]
- 7.Azariadis C, Drazen A. 1990. Threshold externalities in economic development. Q. J. Econ. 105, 521–526 10.2307/2937797 (doi:10.2307/2937797) [DOI] [Google Scholar]
- 8.Dasgupta P. 1992. An Inquiry into well-being and destitution. Oxford, UK: Oxford University Press [Google Scholar]
- 9.Bowles S, Durlauf SN, Hoff K. 2007. Poverty traps. Princeton, NJ: Princeton University Press [Google Scholar]
- 10.Sommarat C, Barrett CB. 2006. Social network capital, economic mobility and poverty traps. J. Econ. Inequal. 10, 299–342 10.1007/s10888-011-9164-5 (doi:10.1007/s10888-011-9164-5) [DOI] [Google Scholar]
- 11.Barrett CB, Swallow BM. 2006. Fractal poverty traps. World Dev. 34, 1–15 10.1016/j.worlddev.2005.06.008 (doi:10.1016/j.worlddev.2005.06.008) [DOI] [Google Scholar]
- 12.Grenfell BT, Bjornstad ON, Kappey J. 2001. Travelling waves and spatial hierarchies in measles epidemics. Nature 414, 716–723 10.1038/414716a (doi:10.1038/414716a) [DOI] [PubMed] [Google Scholar]
- 13.Keeling MJ, Rohani P. 2007. Modelling infectious diseases. Princeton, NJ: Princeton University Press [Google Scholar]
- 14.Bonds MH, Rohani P. 2010. Herd Immunity caused indirectly by interactions between the ecology of infectious diseases, demography, and economics. J. R. Soc. Interface 7, 541–547 10.1098/rsif.2009.0281 (doi:10.1098/rsif.2009.0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonds MH, Keenan DC, Rohani P, Sachs JD. 2010. Poverty trap formed by the ecology of infectious diseases. Proc. R. Soc. B 277, 1185–1685 10.1098/rspb.2009.1778 (doi:10.1098/rspb.2009.1778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plucinski MM, Ngonghala CN, Bonds MH. 2011. Health safety nets can break cycles of poverty and disease: a stochastic ecological model. J. R. Soc. Interface. 8, 1796–1803 10.1098/rsif.2011.0153 (doi:10.1098/rsif.2011.0153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klovdahl AS. 1985. Social networks and the spread of infectious dieases: the AIDS example. Soc. Sci. Med. 21, 1203–1216 10.1016/0277-9536(85)90269-2 (doi:10.1016/0277-9536(85)90269-2) [DOI] [PubMed] [Google Scholar]
- 18.Watts DJ, Strogatz SH. 1997. Collective dynamics of ‘small-world’ networks. Nature 393, 440–442 10.1038/30918 (doi:10.1038/30918) [DOI] [PubMed] [Google Scholar]
- 19.Liljeros F, Edling CR, Amaral LAN, Stanley HE, Åberg Y. 2001. The web of human sexual contacts. Nature 411, 907–908 10.1038/35082140 (doi:10.1038/35082140) [DOI] [PubMed] [Google Scholar]
- 20.Keeling MJ, Eames KTD. 2005. Networks and epidemic models. J. R. Soc. Interface 2, 295–307 10.1098/rsif.2005.0051 (doi:10.1098/rsif.2005.0051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.May RM, Anderson RM, Irwin M. 1988. The transmission dynamics of human immunodeficiency virus (HIV)(and discussion). Phil. Trans. R. Soc. Lond. B 321, 565–607 10.1098/rstb.1988.0108 (doi:10.1098/rstb.1988.0108) [DOI] [PubMed] [Google Scholar]
- 22.Pastor-Satorras R, Vespignani A. 2001. Epidemic spreading in scale-free networks. Phys. Rev. Lett. 86, 3200–3203 10.1103/PhysRevLett.86.3200 (doi:10.1103/PhysRevLett.86.3200) [DOI] [PubMed] [Google Scholar]
- 23.Eguiluz VM, Klemm K. 2002. Epidemic threshold in structured scale-free networks. Phys. Rev. Lett. 89, 108701. 10.1103/PhysRevLett.89.108701 (doi:10.1103/PhysRevLett.89.108701) [DOI] [PubMed] [Google Scholar]
- 24.Mankiw NG, Romer D, Weil DN. 1992. A contribution to the empirics of economic growth. Q. J. Econ. 107, 407–437 10.2307/2118477 (doi:10.2307/2118477) [DOI] [Google Scholar]
- 25.Barro RJ, Sala-i Martin X. 2003. Economic growth. Cambridge, MA: MIT Press [Google Scholar]
- 26.Nokes C, Grantham-McGregor SM, Sawyer AW, Cooper ES, Bundy DAP. 1992. Parasitic Helminth infection and cognitive function in school children. Proc. R. Soc. Lond. B 247, 77–81 10.1098/rspb.1992.0011 (doi:10.1098/rspb.1992.0011) [DOI] [PubMed] [Google Scholar]
- 27.Miguel E, Kremer M. 2004. Worms: identifying impacts on education and health in the presence of treatment externalities. Econometrica 72, 159–217 10.1111/j.1468-0262.2004.00481.x (doi:10.1111/j.1468-0262.2004.00481.x) [DOI] [Google Scholar]
- 28.Fernando D, De Silva D, Carter R, Mendis KN, Wickremasinghe R. 2006. A randomized, double-blind, placebo-controlled, clinical trial of the impact of malaria prevention on the educational attainment of school children. Am. J. Trop. Med. Hyg. 74, 386–393 [PubMed] [Google Scholar]
- 29.Salathé M, Jones JH. 2010. Dynamics and control of diseases in networks with community structure. PLoS Comput. Biol. 6, e1000736. 10.1371/journal.pcbi.1000736 (doi:10.1371/journal.pcbi.1000736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris M, Kretzschmar M. 1997. Concurrent partnerships and the spread of HIV. AIDS 11, 641. 10.1097/00002030-199705000-00012 (doi:10.1097/00002030-199705000-00012) [DOI] [PubMed] [Google Scholar]
- 31.Watts DJ, Muhamad R, Medina DC, Dodds PS. 2005. Multiscale, resurgent epidemics in a hierarchical metapopulation model. Proc. Natl Acad. Sci. USA 102, 11157–11162 10.1073/pnas.0501226102 (doi:10.1073/pnas.0501226102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yen IH, Syme SL. 1999. The social environment and health: a discussion of the epidemiologic literature. Annu. Rev. Public Health 20, 287–308 10.1146/annurev.publhealth.20.1.287 (doi:10.1146/annurev.publhealth.20.1.287) [DOI] [PubMed] [Google Scholar]
- 33.Riley LW, Ko AI, Unger A, Reis MG. 2007. Slum health: diseases of neglected populations. BMC Int. Health Hum. Rights 7, 2. 10.1186/1472-698X-7-2 (doi:10.1186/1472-698X-7-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finch CE, Crimmins EM. 2004. Inflammatory exposure and historical changes in human life-spans. Science 305, 1736–1739 10.1126/science.1092556 (doi:10.1126/science.1092556) [DOI] [PubMed] [Google Scholar]