Abstract
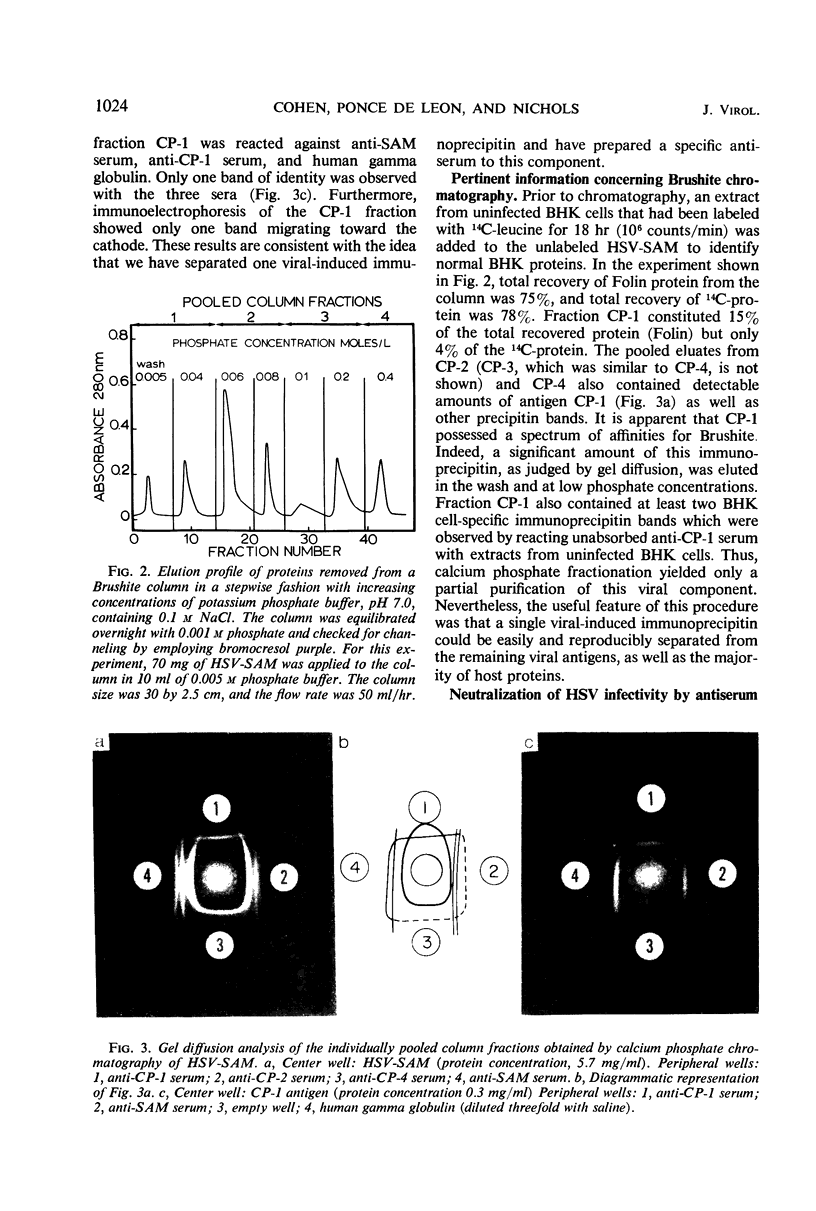
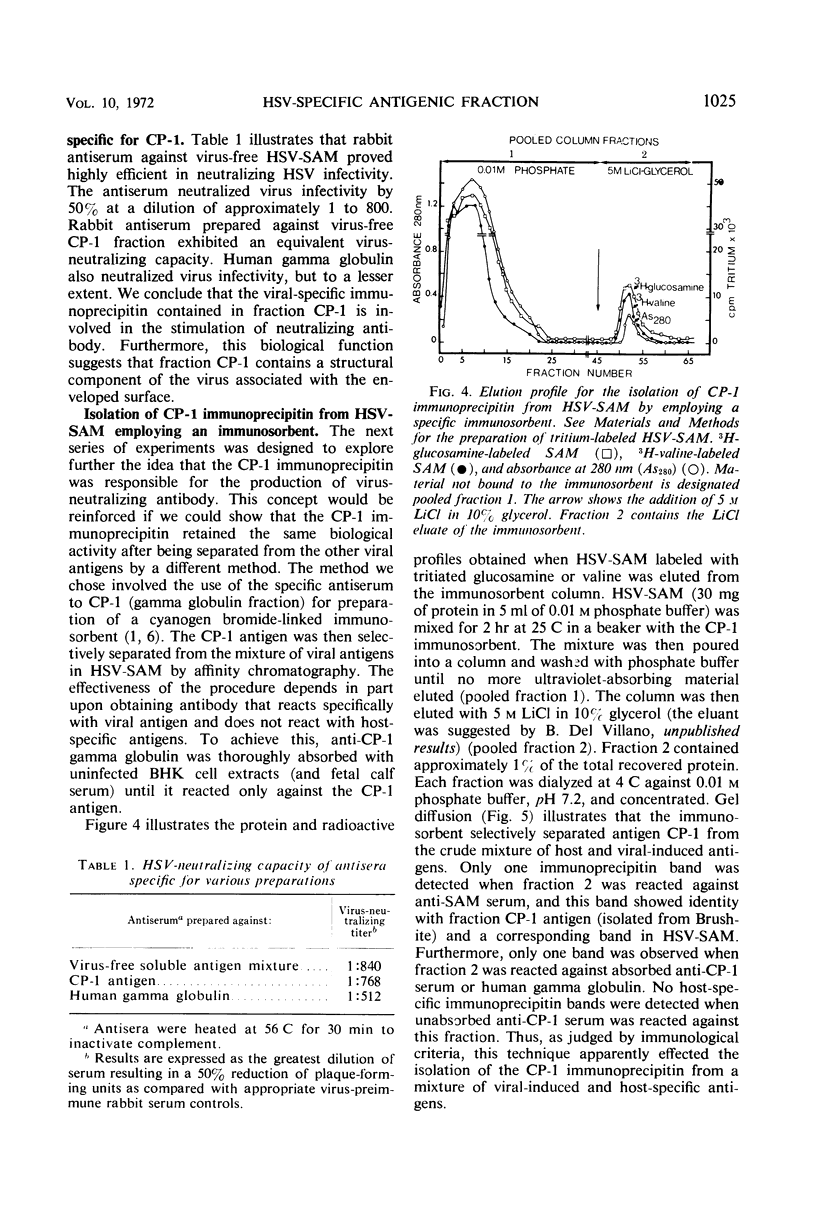
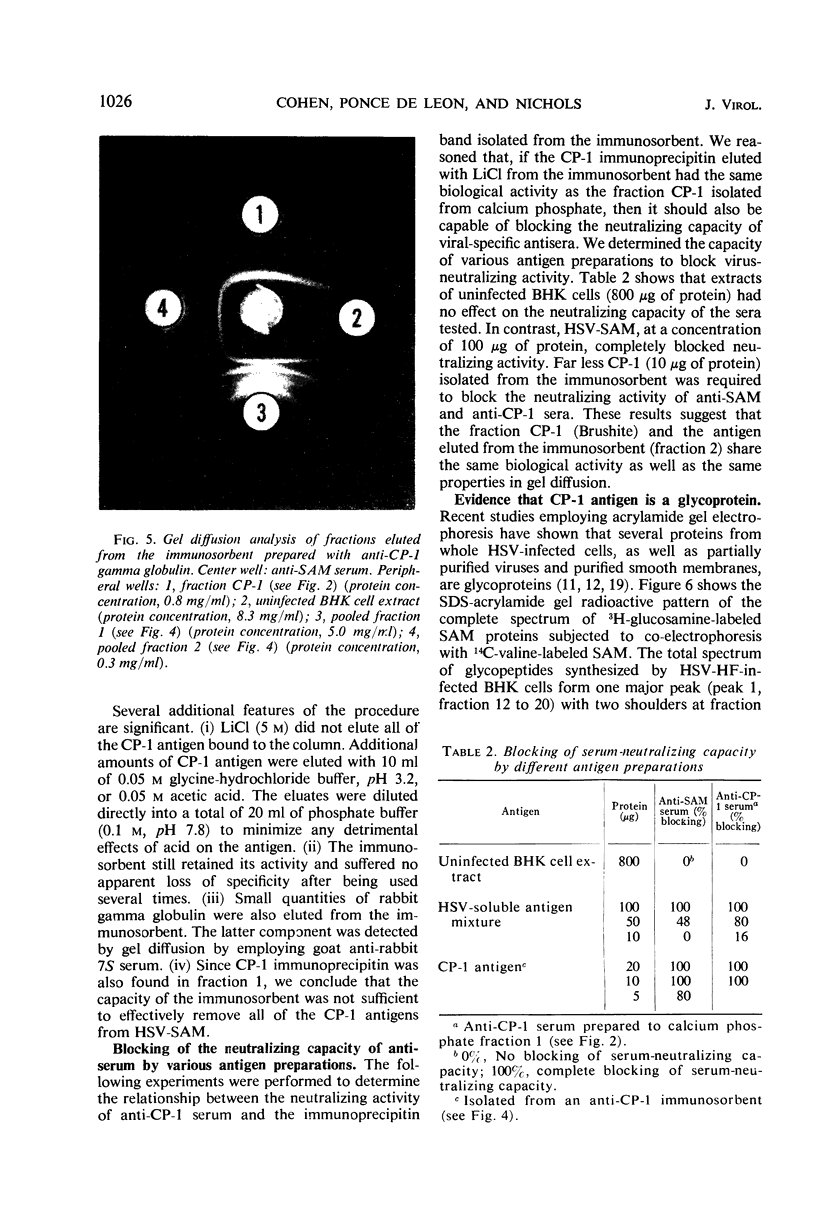
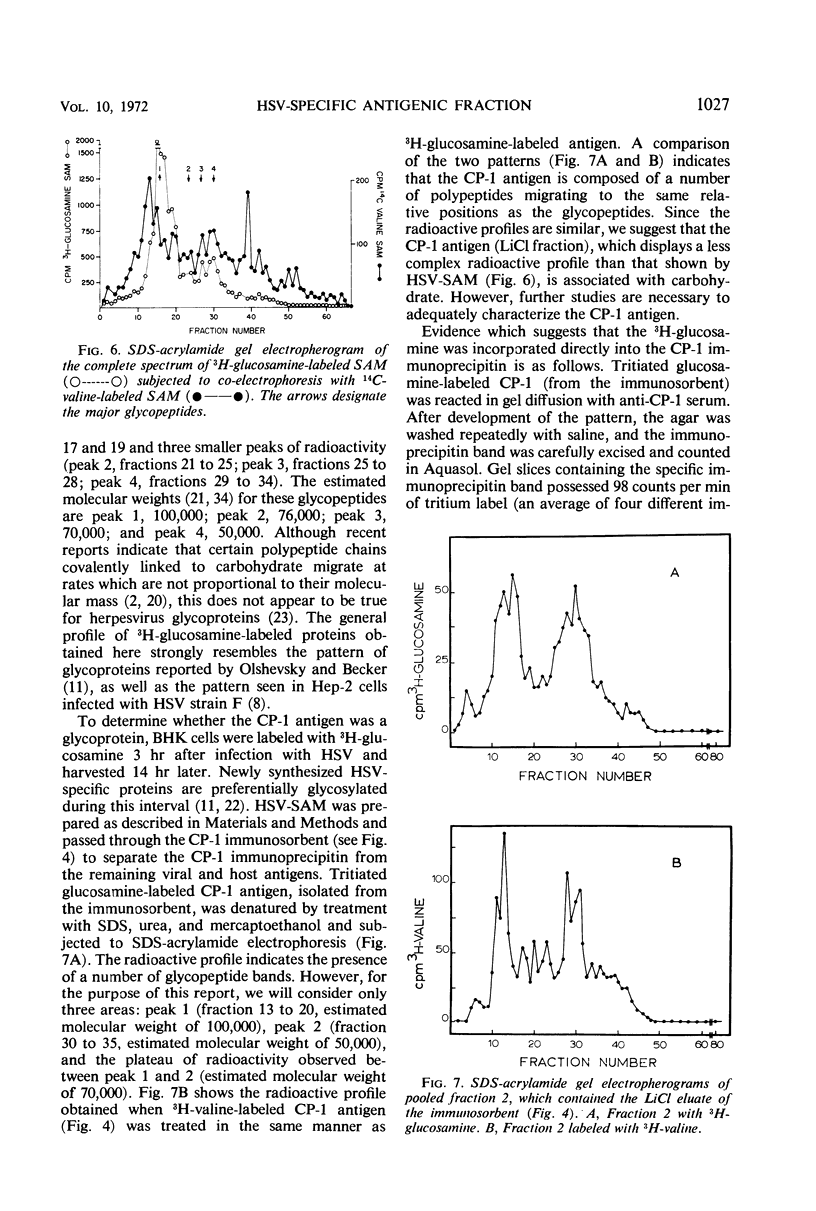
Infection of mammalian cells with herpes simplex virus (HSV) results in the production of a number of virus-induced soluble antigens. Immunodiffusion analyses of the soluble antigen mixture (SAM) obtained from HSV-infected KB or BHK cells revealed at least six well-defined immunoprecipitin bands. Calcium phosphate chromatography (Brushite) was employed to separate one immunoprecipitin (designated CP-1) from the remaining viral and host antigens. We conclude that CP-1 is a viral-specific antigen because (i) specific antiserum, which had been repeatedly absorbed with uninfected cell extracts or serum components, still retained the capacity to react in gel diffusion with CP-1 antigen; (ii) anti-CP-1 serum reacted in gel diffusion with SAM, yielding one precipitin band in identity with the band formed against human gamma globulin; (iii) the CP-1 fraction stimulated the production of HSV-neutralizing antibody of high capacity. The last observation suggests that fraction CP-1 contains a biologically active structural component of the virus which is associated with the envelope. The CP-1 immunoprecipitin was separated from SAM by an alternative method by using a cyanogen bromide-linked immunosorbent prepared from anti-CP-1 gamma globulin. The observation that the CP-1 antigen isolated from the immunosorbent effectively blocked serum-neutralizing activity provided further evidence that neutralizing antibody was directed against CP-1. Acrylamide gel electrophoresis and immunological experiments suggest that the CP-1 antigen is in part a glycoprotein. The finding that CP-1 contains only one antigenic component of the virus will permit future biological studies to be made with a monoprecipitin antiserum. In addition, the techniques described in this paper represent initial steps in the purification of HSV antigens.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPLEYARD G., ZWARTOUW H. T., WESTWOOD J. C. A PROTECTIVE ANTIGEN FROM THE POX-VIRUSES. I. REACTION WITH NEUTRALIZING ANTIBODY. Br J Exp Pathol. 1964 Apr;45:150–161. [PMC free article] [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Major human erythrocyte glycoprotein spans the cell membrane. Nat New Biol. 1971 Jun 23;231(25):229–232. doi: 10.1038/newbio231229a0. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Vaughan R. K., Lawrence W. C. Deoxyribonucleic acid synthesis in synchronized mammalian KB cells infected with herpes simplex virus. J Virol. 1971 Jun;7(6):783–791. doi: 10.1128/jvi.7.6.783-791.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Wilcox W. C. Soluble antigens of vaccinia-infected mammalian cells. I. Separation of virus-induced soluble antigens into two classes on the basis of physical characteristics. J Bacteriol. 1966 Sep;92(3):676–686. doi: 10.1128/jb.92.3.676-686.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968 Oct;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HJERTEN S., LEVIN O., TISELIUS A. Protein chromatography on calcium phosphate columns. Arch Biochem Biophys. 1956 Nov;65(1):132–155. doi: 10.1016/0003-9861(56)90183-7. [DOI] [PubMed] [Google Scholar]
- Hampar B., Miyamoto K., Martos L. M. Serologic classification of Herpes simplex viruses. J Immunol. 1971 Feb;106(2):580–582. [PubMed] [Google Scholar]
- Keller J. M., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. 3. Viruses differing in their effects on the social behavior of infected cells specify different membrane glycoproteins. Proc Natl Acad Sci U S A. 1970 Apr;65(4):865–871. doi: 10.1073/pnas.65.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Montgomery P. C., Dorrington K. J., Rockey J. H. Equine antihapten antibody. The molecular weights of the subunits of equine immunoglobulins. Biochemistry. 1969 Mar;8(3):1247–1258. doi: 10.1021/bi00831a060. [DOI] [PubMed] [Google Scholar]
- Olshevsky U., Becker Y. Herpes simplex virus structural proteins. Virology. 1970 Apr;40(4):948–960. doi: 10.1016/0042-6822(70)90141-8. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B., ROANE P. R., Jr Studies of the determinant antigens of viable cells. I. A method, and its application in tissue culture studies, for enumeration of killed cells, based on the failure of virus multiplication following injury by cytotoxic antibody and complement. J Immunol. 1961 Dec;87:714–727. [PubMed] [Google Scholar]
- Rosenberg G. L., Farber P. A., Notkins A. L. In vitro stimulation of sensitized lymphocytes by herpes simplex virus and vaccinia virus. Proc Natl Acad Sci U S A. 1972 Mar;69(3):756–760. doi: 10.1073/pnas.69.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands D. J., Sangar D. V., Brown F. Relationship of the antigenic structure of foot-and-mouth disease virus to the process of infection. J Gen Virol. 1971 Oct;13(1):85–93. doi: 10.1099/0022-1317-13-1-85. [DOI] [PubMed] [Google Scholar]
- Schaffer P. A., Courtney R. J., McCombs R. M., Benyesh-Melnick M. A temperature-sensitive mutant of herpes simplex virus defective in glycoprotein synthesis. Virology. 1971 Nov;46(2):356–368. doi: 10.1016/0042-6822(71)90037-7. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Andrews E. P., Marchesi V. T. Human erythrocyte membrane glycoprotein: a re-evaluation of the molecular weight as determined by SDS polyacrylamide gel electrophoresis. Biochem Biophys Res Commun. 1971 Jul 16;44(2):390–395. doi: 10.1016/0006-291x(71)90612-7. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAVERNE J., MARSHALL J. H., FULTON F. The purification and concentration of viruses and virus soluble antigens on calcium phosphate. J Gen Microbiol. 1958 Dec;19(3):451–461. doi: 10.1099/00221287-19-3-451. [DOI] [PubMed] [Google Scholar]
- TOKUMARU T. STUDIES OF HERPES SIMPLEX VIRUS BY THE GEL DIFFUSION TECHNIQUE. I. DISTRIBUTION OF PRECIPITATING ANTIBODIES AMONG HUMAN SERA. J Immunol. 1965 Jul;95:181–188. [PubMed] [Google Scholar]
- TOKUMARU T. STUDIES OF HERPES SIMPLEX VIRUS BY THE GEL DIFFUSION TECHNIQUE. II. THE CHARACTERIZATION OF VIRAL AND SOLUBLE PRECIPITATING ANTIGENS. J Immunol. 1965 Jul;95:189–195. [PubMed] [Google Scholar]
- Tokumaru T. Further analysis of physiochemical and immunologic properties of herpes simplex vus precipitating antigens. Arch Gesamte Virusforsch. 1970;29(4):295–306. doi: 10.1007/BF01249884. [DOI] [PubMed] [Google Scholar]
- WATSON D. H., WILDY P. SOME SEROLOGICAL PROPERTIES OF HERPES VIRUS PARTICLES STUDIED WITH THE ELECTRON MICROSCOPE. Virology. 1963 Sep;21:100–111. doi: 10.1016/0042-6822(63)90308-8. [DOI] [PubMed] [Google Scholar]
- WILCOX W. C., GINSBERG H. S. Purification and immunological characterization of types 4 and 5 adenovirus-soluble antigens. Proc Natl Acad Sci U S A. 1961 Apr 15;47:512–526. doi: 10.1073/pnas.47.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins J. F. The relationship of the herpes simplex haemadsorption phenomenon to the virus growth cycle. Virology. 1965 Aug;26(4):746–753. [PubMed] [Google Scholar]
- Watson D. H., Shedden W. I., Elliot A., Tetsuka T., Wildy P., Bourgaux-Ramoisy D., Gold E. Virus specific antigens in mammalian cells infected with herpes simplex virus. Immunology. 1966 Oct;11(4):399–408. [PMC free article] [PubMed] [Google Scholar]
- Watson D. H. The separation of herpes virus-specific antigens by polyacrylamide gel electrophoresis. J Gen Virol. 1969 Mar;4(2):151–161. doi: 10.1099/0022-1317-4-2-151. [DOI] [PubMed] [Google Scholar]
- Watson D. H., Wildy P. The preparation of 'monoprecipitin' antisera to herpes virus specific antigens. J Gen Virol. 1969 Mar;4(2):163–168. doi: 10.1099/0022-1317-4-2-163. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wilcox W. C., Cohen G. H. The poxvirus antigens. Curr Top Microbiol Immunol. 1969;47:1–19. doi: 10.1007/978-3-642-46160-6_1. [DOI] [PubMed] [Google Scholar]






