Abstract
The wear of teeth is a major factor limiting mammalian lifespans in the wild. One method of describing worn surfaces, dental microwear texture analysis, has proved powerful for reconstructing the diets of extinct vertebrates, but has yielded unexpected results in early hominins. In particular, although australopiths exhibit derived craniodental features interpreted as adaptations for eating hard foods, most do not exhibit microwear signals indicative of this diet. However, no experiments have yet demonstrated the fundamental mechanisms and causes of this wear. Here, we report nanowear experiments where individual dust particles, phytoliths and enamel chips were slid across a flat enamel surface. Microwear features produced were influenced strongly by interacting mechanical properties and particle geometry. Quartz dust was a rigid abrasive, capable of fracturing and removing enamel pieces. By contrast, phytoliths and enamel chips deformed during sliding, forming U-shaped grooves or flat troughs in enamel, without tissue loss. Other plant tissues seem too soft to mark enamel, acting as particle transporters. We conclude that dust has overwhelming importance as a wear agent and that dietary signals preserved in dental microwear are indirect. Nanowear studies should resolve controversies over adaptive trends in mammals like enamel thickening or hypsodonty that delay functional dental loss.
Keywords: teeth, dental enamel, nanowear, material properties, hominins
1. Introduction
Dental wear, the loss of tooth tissue, threatens the survival of individual mammals in the wild by jeopardizing their rate of food acquisition and processing [1–3]. Studied intensively for 60 years [4], wear patterns reflect the diet of living mammals and are capable of predicting diet in extinct forms [5–8]. This has been studied at various scales of measurement [9]. Macroscopic wear is ultimately what affects masticatory ability to reduce food particle sizes. The visible facets that form on molar teeth can be used to track changes in dental function and jaw movement [10], and are stable enough in position across species to trace the relationships between groups of early mammals [11]. However, the actual marks produced by wear mechanisms are microscopic. One analytical technique, dental microwear analysis, concentrates on describing the microscopic surface damage sustained by teeth when they collide with each other or with extraneous particles during feeding [12]. Species that consume leaves and other non-reproductive parts of plants seem to accumulate finely scratched tooth enamel surfaces with aligned features, while ‘hard object’ feeders tend to exhibit irregular or pitted wear patterns [12,13]. Hard object feeders are hypothesized to fracture food items between tooth facets that are moving directly towards each other, leading to pit formation, while scratches are suspected to form as food is trapped between tooth surfaces sliding past each other [12,13].
Much significance has been placed on microwear analysis for dietary reconstruction in fossil hominins. However, a disparity between functional morphology and microwear has become apparent. For example, the microwear of the East African robust australopith Paranthropus boisei differs from that of South African Paranthropus robustus despite craniodental similarities. Both species share large low-cusped thick-enamelled post-canine teeth rooted in thick mandibles moved by large masticatory muscles. All this suggests a diet of hard food items for both these australopiths [14–17]. However, despite suffering heavy macroscopic wear, the teeth of P. boisei appear finely scratched, apparently suggesting a diet including tough, compliant foods that isotopic evidence indicates might have included tropical grasses and sedges [18]. Similarly, it has been argued on biomechanical grounds that the gracile australopith Australopithecus africanus was also adapted to eat hard foods, and yet the microwear of this species appears not to preserve evidence that such foods were consumed regularly [13,19–21]. However, interpretations are complicated by the fact that the mechanical basis of microwear formation has yet to be established experimentally. Importantly, the hypothesis of feature formation described above does not consider the mechanisms involved, particularly as influenced by mechanical properties and contact geometry, although these factors are known to be of utmost importance [22,23].
The literature suggests that three types of particles warrant special consideration for microwear because of their supposed hardness: phytoliths, quartz dust and enamel chips. Some authors contend that plant phytoliths, made of amorphous, hydrated and porous silica, are important wear agents [24–27]. However, although portrayed long ago as harder than enamel [24], phytoliths are actually softer, possibly rendering them ineffectual [28]. Despite this, experimental evidence shows that phytolith formation is induced in plants by mammalian herbivory and that this deters further feeding [29,30]. Extraneous dust ingested with food is often composed of quartz, but the efficacy of this crystalline form of silica as a wear agent has been based on microhardness measurement. This scale of indentation must be inaccurate for small particulate matter because indentations need to be much smaller than particle dimensions not to result in erroneous hardness estimates [31,32]. Finally, although there are few published comments on the mechanisms by which teeth wear each other, it is known that enamel surfaces do so, producing smooth facets [33] that perforate into dentine. It is then entirely possible that dental contacts are the main culprit and that teeth often simply wear themselves out.
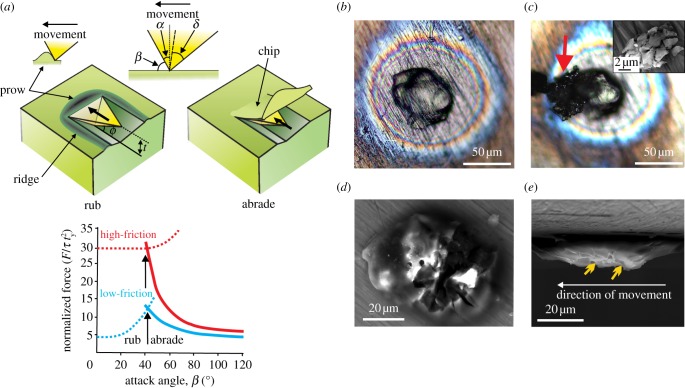
In order to investigate the major mechanism operating in dental wear and characterize the damage caused, we have developed a method of mounting individual particles of known hardness, morphology and elemental composition on a customized nanoindenter tip (figures 1a, 2b and 4a). We combine this with a novel model of the wear process, which distinguishes between two actions: that of rubbing and abrasion. When a rigid particle damages enamel, the latter can either be abraded by elastic/plastic chipping or displaced by a ‘standing wave’ (prow) moving ahead of the particle. Abrasion results in wear (material loss), while a prow simply rearranges the surface, creating ridges alongside an indentation (figure 2a). The latter is a ‘rubbing’ action without material loss. These alternatives depend on particle geometry, friction, the shear yield stress (represented in our study by indentation hardness, of which it is a simple multiple) and fracture toughness (see appendix A). Both rubbing and abrasion lead to depressions in a surface. In the following account, we refer to a linear depression as a ‘groove’ if it is narrow or a ‘trough’ if it is wide, reserving the term ‘scratch’ for when there is evidence of material removal (i.e. of wear).
Figure 1.
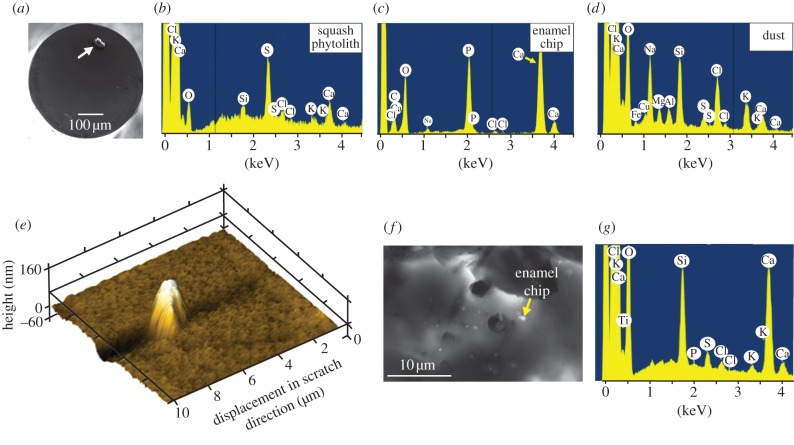
(a) Scanning electron microscopy (SEM) image of a squash phytolith (arrowed) mounted on the flat-ended titanium nanoindenter tip. (b–d) Pre-test screening of chemical identity of each mounted particle by energy dispersive spectroscopy (EDS). No sputter-coating was necessary. Dust and phytoliths have distinct elemental compositions, while Ca and P peaks for enamel reflect hydroxyapatite. (e) A squash phytolith has rubbed an enamel surface, a broken fragment of which remains embedded in the enamel. Note the U-shaped trough lying to the left of the fragment. (f) Part of the surface of a quartz dust particle, post-test, littered with small enamel chips, one of which is arrowed. (g) The joint identity of quartz particle and an enamel chip was confirmed by spot EDS analysis, with the Ca and P peaks reflecting enamel, as in (c), with the other peaks mirroring those in (d).
Figure 2.
A rigid particle sliding on a surface rubs or abrades depending on the attack angle β. (a) Abrasion removes material by chipping, while rubbing produces plastic displacement with a prow in front of the particle displacing enamel into lateral ridges. Assuming a triangular pyramid moving facet-first on enamel (angles as shown), a sliding force F, normalized to enamel shear yield stress τy and the square of indentation depth t (= 0.5 µm to conform with experiment), abrades when β > 40°, but rubs when β < 40°. (b–e) Features of a quartz dust particle that removed an enamel chip at a fixed vertical force of 1800 µN. (b) Stereo light microscopic views show quartz attached to the titanium tip (cyanoacrylate glue produces the halo effect) prior to the scratching test. (c) The same quartz dust particle post-test, showing a clump of enamel chips (arrowed) retained on the particle after having been fractured away from the enamel surface. Inset for (c) shows a clump of these enamel chips (SEM). (d,e) Top and side views of same particle post-test, but the enamel chips having been removed (SEM). Particle has peaks, arrowed in (e), with β > 40° that removed the enamel in (c).
2. Material and methods
Phytoliths were obtained via low-temperature acid extraction [34] from Cucurbita moschata Duchesne ex Poir. fruit (squash) rind and from Ampelodesmos mauritanicus (Poir.) T. Durand & Schinz (grass) leaves. Such extraction should not affect phytolith properties [35]. Dust was obtained from a Kuwaiti landscape, washed, dried and then sieved to obtain a sub-70 µm fraction. Samples of these particles were set in resin prior to nanoindentation. We chipped enamel from the molar of an orangutan (isolated molar of Bornean Pongo pygmaeus, from the Raffles Museum, National University of Singapore) by holding it against the edge of a low-speed diamond saw to obtain particles for the nanoindentation experiments. This molar was set in resin and sectioned longitudinally with that saw. Enamel located mid-way between the enamel–dentine junction and the tooth surface was used in all tests. All surfaces for nanoindentation were polished down to 20 nm r.m.s. surface roughness using colloidal silica. Indentation hardness was obtained by nanoindentation with a Hysitron Ubi1 (Minneapolis, MN, USA) with a Berkovich diamond tip. The diamond tip was calibrated against fused quartz samples. Depths of indentation were 150–400 nm with forces of 2–4 mN. Dust, phytoliths and orangutan enamel chips were attached individually to a customized flat-ended 500 µm diameter titanium tip, dipping the tip first into a drop of cyanoacrylate glue, then inverting it onto the particle (figure 1a). Attachment was confirmed by optical reflectance microscopy and scanning electron microscopy (SEM, Jeol 7001F, Tokyo, Japan). Prior to nanotesting, the elemental composition of each particle was confirmed by energy dispersive spectroscopy (EDS, Oxford, Abdingdon, UK) attached to the SEM (figure 1b–d). Specimens were not sputter-coated for this purpose because this would affect the nanowear tests. Some charging of the specimens resulted, but this did not interfere with elemental analysis. The titanium tip with particle was then placed in the nanoindenter. Tests involved particle–enamel contacts with lateral displacements of a maximum 10–15 µm at fixed vertical forces of between 600 and 1800 µN. Lateral forces could be monitored. Results were examined by scanning in an atomic force microscope set in tapping mode (Agilent 5500 AFM, Santa Clara, CA, USA). All the above nanowear tests were conducted in an air-dry, but not desiccated, state.
3. Results
Nanohardness results (table 1) showed that quartz dust was approximately 2.5 times harder than enamel. Both types of phytolith were substantially softer than enamel, but those from squash rinds were softer than those from grasses (table 1). Ranges for the hardness of squash phytoliths, quartz dust and enamel did not overlap. Thus, these phytoliths, enamel chips (derived from this same enamel surface) and quartz dust were used in further tests (EDS spectra shown in figure 1b–d). Each of these particle types could mark enamel at vertical forces ranging between 600 and 1800 µN. Averaged lateral forces varied between 30 per cent and 80 per cent of the vertical load. The quartz particle shown in figure 2b was responsible for detaching enamel chips (figure 2c) and thus scratching it since it possessed surface features with the right attack angles (figure 2d,e). Squash phytoliths marked enamel, but made rubbing contacts with prow formation (figure 3). The grooves that phytoliths formed resembled U-shaped valleys (figures 2e and 3). Enamel chips (figure 4a) could make multiple marks on their parental surface depending on their form, but these appeared as flat troughs (figure 4b) indicating rubbing due to mutual deformation.
Table 1.
Means (s.d.) of nanoindentation hardness values for potential wear agents. n is the number of samples, mean the s.d. All tests used a Berkovich diamond tip.
| property | phytoliths |
quartz dust (n = 117) | enamel (n = 100) | |
|---|---|---|---|---|
| squash (n = 14) | grass (n = 17) | |||
| indentation hardness (GPa) | 0.89 (0.48) | 2.56 (0.81) | 12.8 (1.07) | 5.0 (0.28) |
| data range | 0.43–1.74 | 1.35–4.24 | 10.1–14.1 | 4.08–5.72 |
Figure 3.
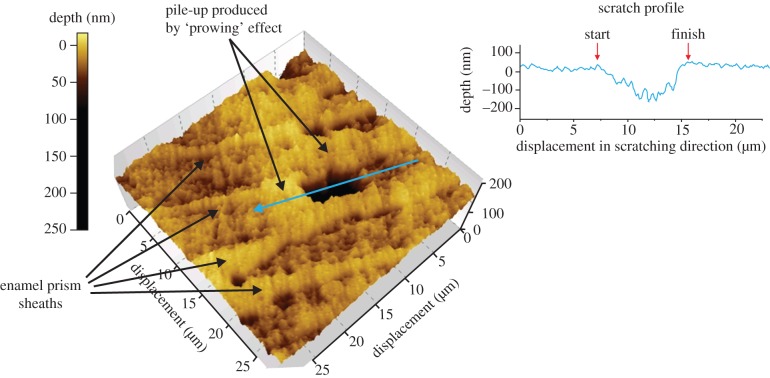
Rubbing action of a phytolith. Topography of a nanogroove produced by squash phytolith on enamel (vertical force of 600 µN, atomic force microscope (AFM) image). The groove is oriented along an enamel prism. The depth profile shows the prow at the end of the groove.
Figure 4.
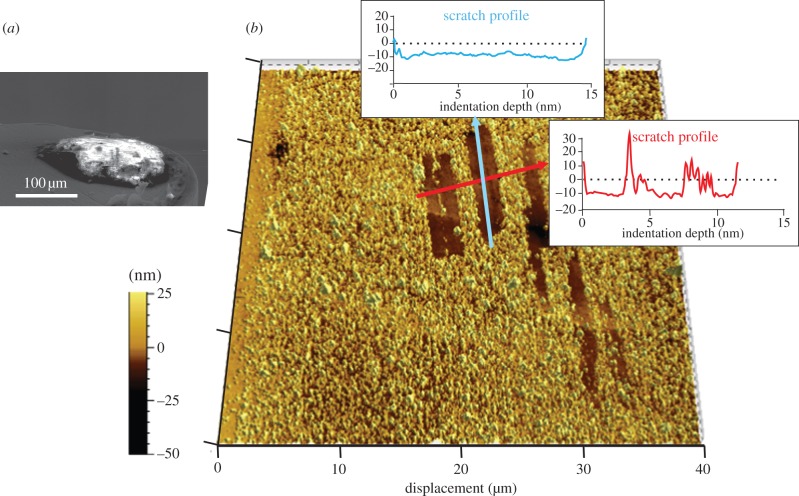
Flat channel-like troughs produced by an enamel chip. (a) Chip on the nanoindenter tip prior to test. (b) Topography of multiple troughs produced by this chip on parental enamel surface (vertical force 1600 µN, AFM image). Depth profiles (graphs) show these scratches that are shallow troughs approximately 10 nm deep.
4. Discussion
Our wear theory (see appendix A) suggests that enamel can only be abraded by particles hard enough to make rigid-plastic contacts and which possess a sufficiently high attack angle β (figure 2a). Enamel is protected from abrasion by its high hardness, which reduces the types of particle that can contact it without mutually deforming, and also by its toughness, which controls the minimum value of β. From this perspective, many potentially dangerous particles would lack the attack geometry to inflict wear on enamel. Contacts with these and other particles would, at most, merely rearrange the enamel surface via rubbing. While such rubbing may eventually lead to wear, many further contacts are required before cracks detach the tissue [23]. Neither the theory given here nor our experiments, enlightens about such long-term damage, but the immediate response of enamel to contact with individual particles could be investigated.
We have shown that quartz dust abrades enamel under the right circumstances (figure 2b–e), resulting in immediate loss of enamel volume. By contrast, contacts between enamel and a squash phytolith, as also with an enamel chip on its parental surface, appear to involve substantial mutual deformation [36]. The latter rub enamel with a ‘prowing’ action, without producing tissue loss (figures 3 and 4). These results are as predicted, given the relative hardness of each of these particles (table 1).
In these experiments, abrasion by quartz particles produced rough-edged microwear pits reflecting the fracture and separation of micron-sized enamel chips. Enamel is surprisingly a damage-tolerant tissue [37], partly because its constitutive crystallites can jostle to some extent to rest in new locations, so accommodating an indentation without fracturing [38]. However, the fracture and removal of small enamel chips were clear in our experiments (figures 1f and 2c inset). In this respect, enamel may resemble modern ‘tough’ ceramics that permit micro-cracking in a confined region under an indenter because, somewhat ironically, this inhibits the possibility of any catastrophic fracture [39]. Cone cracks, typical of scratching damage on homogeneous fine-grained ceramics [40], have been suggested as explanations for such fractures in enamel [41]. However, we saw no evidence of them and they appear to be suppressed by enamel structure [39]. Instead, abrasion is likely to be the result of median cracks that turn towards the enamel surface, as is also seen in larger-scale chipping events [42].
The release of enamel chips via abrasion may rub the parental surface. If so, then our results suggest that this would leave smooth flat-bottomed troughs (figure 4). Production of these troughs via enamel chips or larger-scale tooth–tooth contacts is consistent with in vivo observations of the relatively featureless enamel surfaces that form on guinea pig molars via jaw movements in utero [33]. Variations in enamel properties could complicate this picture. Hardness increases gradually from inside to out [43,44], but the gradient is generally too shallow to affect predictions from our study. Enamel toughness is affected strongly by the decussation of enamel prisms, but this effect is much more pronounced in larger cracks than those encountered during indentation [45]. Although the shape of crystallites and discontinuities across prism boundaries must have an effect on the shape of the chips shown in figure 2c, it may be for the above material property reasons that enamel microstructure tends to be ignored in microwear reports.
Finally, phytoliths produce U-shaped grooves with ridges beside them. Remnants of a prow at one end of the groove indicate its termination, and thus give directional information. No previous study seems to have found this. Most in vitro experiments have employed mass contacts of enamel with ‘sand’ (probably quartz) [46,47] or very hard silicon carbide [48] particles, and have thus studied rigid-plastic contacts. Ryan [46,47] focused on the inconstant width of grooves along their length, but varying patterns have been reported by others [33,49], any of which would be predicted simply to reflect a variable vertical force during sliding.
It would appear then that the patterns found in our experiments could be useful in deciphering in vivo microwear patterns via the identification of individual features on AFM scans. Current trends in microwear analysis have moved away from individual feature recognition on worn surfaces [6,50], concentrating instead on descriptions of its texture [8,13,51–53]. However, although this is helpful for comparisons, this will not indicate how the surface was worn. From the perspective of materials science, neither the troughs nor grooves produced by enamel chips and phytoliths, respectively, are true scratches because neither removes enamel from the surface directly. When individual features in microwear analyses are described, it would be helpful in terms of mechanisms and causes to distinguish them.
Enamel markings produced in these experiments probably indicate the level of force involved in their formation in the wild. Dust particles can be smaller [54] and chips very much larger [17,42], but our model nevertheless predicts that the forces involved in micro-feature formation are tiny compared with the bite force maxima of mammals. Orangutans, whose enamel was used in these experiments, can produce bite forces of 2000 N [55], giving capacity for thousands of scratches, grooves, troughs or pits from just one chew were particles to be present in large quantity. The saving grace is that quartz is probably only ingested in small quantities by mammals. It would be important to establish this by distinguishing phytoliths from quartz, for example in the silica intake of herbivores [56]. Despite this lack of knowledge, our experiments strongly support the view that anatomical adaptations such as hypsodonty evolved to combat quartz abrasion by prolonging dental function [57].
The above viewpoint suggests an ironic interpretation of dental microwear: although a little dust causes greater enamel wear than many phytoliths, the latter may dominate a microwear image. For example, East African P. boisei had heavily worn molars despite their lightly scratched appearance. It is as yet unclear whether or not these marks are true scratches or rubbing features. These hominins seem to have inhabited a relatively wet refugium, suggesting a low-dust environment. However, even small amounts of dust, particularly as brought in early in the year on winter plumes [58,59], could explain this heavy wear. This could be masked by light rubbing from regular phytolith ingestion later on during the wet season. Much depends on how far hominins were protected from aridity by the microhabitat that they occupied, since evidence for some modern groups in arid locations shows that heavy tooth wear is possible [60]. However, either possibility indicates some independence of microwear and food type. Alternatively, occasional consumption of plant underground storage organs [61] might have periodically introduced quartz into the mouth, their abrasive effect erased by subsequent rubbing against phytoliths from more frequent above-ground foods. Phytoliths in themselves do not seem to provide a clear dietary guide to plant parts, insofar as many leaves and seeds contain them [34]. Moreover, it is unlikely that non-siliceous plant components like seed shells could abrade enamel [62], given that they have only 10 per cent of the hardness of enamel at most [55]. Their consumption would likely be hidden from microwear analysis. At best, seed shells would rub enamel, although this still has to be established experimentally.
This work provides a first step towards defining mechanisms that determine wear rates in enamel, but the effect of enamel structure and salivary factors need to be established. Here, we used the highest values for enamel toughness in the formulae, adopting those for long cross-prismatic paths. Wear in other directions may be easier. The effect of saliva is more complicated in that many proteins adsorb onto the enamel surface, possibly protecting it to some degree [63]. Physiological mechanisms to avoid wear in mammals include the stimulation of saliva in response to dust ingestion [64]. Phytoliths are easily large enough for a mammal to detect between its teeth, and their presence does seem to interfere with food particle reduction in the mouth [65]. Some existing evidence suggests that the saliva itself does little to affect conditions, as judged from scratching experiments on enamel with diamond tips [66], but may help clear particles quickly from tooth surfaces.
Yet, as developed in this paper, this work appears already to have profound implications for dietary reconstruction in hominins. Heavy pitting on enamel may not be evidence of a ‘hard’ food diet, but rather quartz dust. Microgrooves with remnants of a prow may document the presence of phytoliths, but not necessarily reveal any information about food material properties. We suggest that hypotheses about hominin diets be reconsidered in the light of our experimental evidence. The absence of pitting on early hominin teeth should not be interpreted to indicate an absence of ‘hard’ foods in the diet; neither should the absence of scratches/grooves be interpreted to indicate the absence of leaves or other structural plant parts. Examination of the anthropoid comparative microwear database [13] shows that some species exhibit microwear patterns consistent with their contrasting diets. However, others have patterns that are broadly similar despite profound dietary differences [21]. The latter supports our experimental results: it is likely that diet is not the most important influence on microwear patterns. Rather, the interpretation of microwear patterns is likely to require an understanding of the relative abundance of quartz dust and phytoliths being consumed by primates within particular habitats.
5. Conclusions
The disparity between the wear potential of crystalline quartz dust and amorphous, variably hydrated and porous, plant silica on enamel seems much greater than previously envisaged. Longstanding microhardness estimates of 7 GPa for quartz and 5 GPa for phytoliths [24] have clearly been misleading, almost certainly owing to the use of overly large indentations in obtaining them. The actual value for quartz is nearly twice this estimate, which is supported by measurements on bulk specimens of the mineral [67]. Phytoliths are far softer [28]. However, particle hardness is not enough to predict what happens. Although engineers and biologists may think of wear and hardness as linked, doubts about a causal interrelationship are not new. Mohs scale, the classic estimate of scratchability, was long ago found ‘… utterly unreliable, as a softer body was found to be able to scratch a harder one, provided a certain angle of the scratching surface were presented to the surface to be scratched …’ [68, p. 215]. Fracture toughness is the missing factor controlling this angulation and is the property that resists wear [22,23]. Much needs to be done. On the theoretical side, variation of the critical attack angle β with indenter geometry requires investigation. Further research is needed to extend the theory to include conditions of mutual wear. On the practical side, the work needs to be extended to dentine. Phytoliths would form rigid-plastic contacts with this tissue because dentinal hardness is generally 650 MPa or less [69]. This would influence the enamel–dentine wear ratio in teeth, where both tissues are exposed, in a manner not previously contemplated.
Acknowledgements
We thank A. Shekeban and J. R. Mathew for technical assistance, B. W. Darvell for advice, and J. F. Prinz, J. W. Osborn, M. B. Bush, J. Green, K. Zink and N. J. Dominy for comments on the manuscript. It was improved by comments from four anonymous reviewers. We acknowledge support from Kuwait University General Facilities Project GE01/07 for access to nanoindentation, SEM and AFM, and a grant from the National Science Foundation HOMINID program (NSF BCS 0725126).
Appendix A
Our mechanical model assumes some translation between opposing surfaces forced into contact because this has been demonstrated to be a more important wear mechanism than static indentation. A groove formed by a rigid pointed indenter sliding on a surface displaces material in one of two ways, either (i) by pushing a ‘standing wave’ (prow) ahead of the indenter through which material is displaced upwards into ridges alongside the groove or (ii) by cutting away a ribbon or chips of material [22,23]. When prows and ridges are formed, material is merely moved around on the surface. The first mechanism is called ‘rubbing’ or ‘ploughing’ in tribology. However, the use of the term ‘ploughing’ is now argued against because soil is fractured by an agricultural plough, which is not what happens in the current context. The term ‘prowing’ has been suggested as an alternative and is used in this paper. The second mechanism, often called ‘cutting’, is referred to as ‘abrasion’ in this paper.
The mechanism of prow/ridge formation described in the paper involves plasticity and friction between the faces of the indenter and the material; that for abrasion additionally involves work of formation of new surfaces (fracture toughness). Rigid-plastic upper-bound solutions for the two modes are restricted to the case of a pyramidal indenter sliding face-first at variable attack angle β (figure 2a; also shown in figs. 4a and 5a of Atkins [23]). The normalized forces for ‘prowing’ are obtained by minimizing the total work done, given by ΣτyV*s, where τy is the shear yield strength and V* the velocity discontinuity along a slip band of length s in the chosen kinematically admissible velocity field. There is no closed form solution for the field and the problem has to be solved numerically [23]. Calculations show that the normalized forces for prowing, Fprow/τyt2, with t being indentation depth, are least when β is small. Then they increase gradually with β until rapid increase as β → 90°. These forces are larger, the broader the semi-apical angle δ of the indenter (fig. 4b of [23]). By contrast, forces for abrasion are least at very large β, increasing as β reduces. As with prowing, the magnitude of the forces increases with friction, as would be expected, but more significant is the parameter, Z = R/τyt, where R is the fracture toughness (in terms of the energy required to detach unit area of tissue) of the surface in which the groove is formed (fig. 5b of Atkins [23]). The solution for abrasion is given in closed form as
where δ is the semi-apical angle of the leading face of the indenter, α the rake angle of the leading face and ϕ the angle of the primary shear plane that depends upon Z. Q, the frictional factor, is given by
where λ is the friction angle, i.e. tan λ = μ, the coefficient of friction [22,23]. On the assumption that the mode of deformation that occurs is that requiring least work (least force over the same displacement parallel to the surface), the transition from prow formation to cutting as the attack angle increases, and vice versa, will occur when Fprow/τyt2 = Fabrade/τyt2. Thus, in an abrasive event, forces rise with friction, but are greatest at small β. On the assumption that the process requiring least work is what will be observed, a critical value of β demarcates the rub-abrade transition (the curve intersection in figure 2a). Taking t = 0.5 µm, R = 50 J m−2 (for the toughest fracture path in enamel at this length-scale) and τy = 1 GPa, we calculate the critical angle for enamel as approximately 40°, almost independent of friction (figure 2a). Thus, only a rigid particle that is sufficiently angulated can abrade enamel. Geometry of contact will have some influence on these results. When a pyramid is slid edge-first, the transition to abrading occurs at lower β. However, other shapes of indenter will have similar transitions, but there is no theoretical solution yet for these geometries. Existing experimental evidence with diamond tips on enamel already supports the general validity of our model and this geometrical influence: cube corners moving face-first (β = 55.7°) can abrade an enamel surface, while a Berkovich indenter (β = 24.7°), sphere or blunt cone cannot [22,70]. The biological issue though is not what diamond does to teeth, but what natural particles achieve. Particles may not be rigid, in which case the above argument does not apply, because both surface and particle will mutually deform plastically. This is known for materials in static indentation, where the hardness of one is less than approximately 2.5 times that of the other [36]. The explanation for this is that most materials possess a hardness of about 2.8 times their tensile yield stress, σy, with significant yielding beginning at 1.1 σy [70]. Thus, if one material does not reach stresses greater than 2.8/1.1 = 2.5σy prior to the other exceeding 110 per cent of its own tensile yield stress, then both interacting solids will plastically deform [71]. In such circumstances of mutual deformation, the edges of particles quickly flatten, regardless of their original shape, leading to rubbing marks. So a major problem in establishing the cause of tooth wear lies in distinguishing rubbing from abrasion. This requires nanoscale study to observe what individual wear candidate particles do when slid against a flat enamel surface at known load. While the use of ‘real’ particles loses control of particle shape, they establish wear potential while still allowing estimations of β via inspection of their morphology. Although literature descriptions of dentinal wear patterns are rare, the low hardness of dentine (approx. 0.6 GPa) means that grass phytoliths and enamel chips would be rigid against it. However, abrasion conditions will differ: the critical attack angle β for dentine, assuming R = 550 J m−2, is estimated much higher at approximately 70°.
References
- 1.Logan M, Sanson GD. 2002. The effects of tooth wear on the feeding behaviour of free-ranging koalas (Phascolarctos cinereus Goldfuss). J. Zool. 256, 63–69 10.1017/S0952836902000080 (doi:10.1017/S0952836902000080) [DOI] [Google Scholar]
- 2.King SJ, Arrigo-Nelson SJ, Pochron ST, Semprebon GM, Godfrey LR, Wright PC, Jernvall J. 2005. Dental senescence in a long-lived primate links infant survival to rainfall. Proc. Natl Acad. Sci. USA 102, 16 579–16 583 10.1073/pnas.0508377102 (doi:10.1073/pnas.0508377102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuozzo FP, Sauther ML. 2006. Severe wear and tooth loss in wild ring-tailed lemurs (Lemur catta): a function of feeding ecology, dental structure, and individual life history. J. Hum. Evol. 51, 490–505 10.1016/j.jhevol.2006.07.001 (doi:10.1016/j.jhevol.2006.07.001) [DOI] [PubMed] [Google Scholar]
- 4.Butler PM. 1952. The milk-molars of Perissodactyla, with remarks on molar occlusion. Proc. Zool. Soc. Lond. 121, 777–817 10.1111/j.1096-3642.1952.tb00784.x (doi:10.1111/j.1096-3642.1952.tb00784.x) [DOI] [Google Scholar]
- 5.Walker A, Hoeck H, Perez L. 1978. Microwear of mammalian teeth as an indicator of diet. Science 201, 908–910 10.1126/science.684415 (doi:10.1126/science.684415) [DOI] [PubMed] [Google Scholar]
- 6.Teaford MF. 1994. Dental microwear and dental function. Evol. Anthropol. 3, 17–30 10.1002/evan.1360030107 (doi:10.1002/evan.1360030107) [DOI] [Google Scholar]
- 7.Fortelius M, Solounias N. 2000. Functional characterization of ungulate molars using the abrasion–attrition wear gradient: a new method for reconstructing paleodiets. Am. Mus. Novitates 3301, 1–36 (doi:10.1206/0003-0082(2000)301<0001:FCOUMU>2.0.CO;2) [DOI] [Google Scholar]
- 8.Scott RS, Ungar PS, Bergstrom TS, Brown CA, Grine FE, Teaford MF, Walker A. 2005. Dental microwear texture analysis shows within-species diet variability in fossil hominins. Nature 436, 693–695 10.1038/nature03822 (doi:10.1038/nature03822) [DOI] [PubMed] [Google Scholar]
- 9.Lucas PW, Omar R. 2012. New perspectives on tooth wear. Int. J. Dent. 2012, 287–573 10.1155/2012/287573 (doi:10.1155/2012/287573). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benazzi S, Kullmer O, Grosse IR, Weber GW. 2011. Using occlusal wear information and finite element analysis to investigate stress distributions in human molars. J. Anat. 219, 259–272 10.1111/j.1469-7580.2011.01396.x (doi:10.1111/j.1469-7580.2011.01396.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crompton AW. 1971. The origin of the tribosphenic molar. In Early mammals (eds Kermack DM, Kermack KA.), pp. 65–97 London, UK: Academic Press [Google Scholar]
- 12.Ungar PS, Sponheimer M. 2011. The diets of early hominins. Science 334, 190–193 10.1126/science.1207701 (doi:10.1126/science.1207701) [DOI] [PubMed] [Google Scholar]
- 13.Scott RS, Teaford MF, Ungar PS. 2012. Dental microwear texture and anthropoid diets. Am. J. Phys. Anthropol. 147, 551–579 10.1002/ajpa.22007 (doi:10.1002/ajpa.22007) [DOI] [PubMed] [Google Scholar]
- 14.Jolly CJ. 1970. Seed-eaters: new model of hominid differentiation based on a baboon analogy. Man 5, 5–26 10.2307/2798801 (doi:10.2307/2798801) [DOI] [Google Scholar]
- 15.Peters CR. 1979. Towards an ecological model of African Plio-Pleistocene hominid adaptations. Am. Anthropol. 81, 261–278 10.1525/aa.1979.81.2.02a00010 (doi:10.1525/aa.1979.81.2.02a00010) [DOI] [Google Scholar]
- 16.Lucas PW, Corlett RT, Luke DA. 1985. Plio-Pleistocene hominids: an approach combining masticatory and ecological analysis. J. Hum. Evol. 14, 187–202 10.1016/S0047-2484(85)80006-3 (doi:10.1016/S0047-2484(85)80006-3) [DOI] [Google Scholar]
- 17.Lee JJ-W, Constantino P, Lucas PW, Lawn BR. 2011. Fracture in teeth: a diagnostic for inferring tooth function and diet. Biol. Rev. 86, 959–974 10.1111/j.1469-185X.2011.00181.x (doi:10.1111/j.1469-185X.2011.00181.x) [DOI] [PubMed] [Google Scholar]
- 18.Cerling TE, Mbua E, Kirera FM, Kyalo Manthi F, Grine FE, Leakey MG, Sponheimer M, Uno KT. 2011. Diet of Paranthropus boisei in the early Pleistocene of East Africa. Proc. Natl Acad. Sci. USA 108, 9337–9341 10.1073/pnas.1104627108 (doi:10.1073/pnas.1104627108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ungar PS, Scott RS, Grine FE, Teaford MF. 2010. Molar microwear textures and the diets of Australopithecus anamensis and Australopithecus afarensis. Phil. Trans. R. Soc. B 365, 3345–3354 10.1098/rstb.2010.0033 (doi:10.1098/rstb.2010.0033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strait DS, et al. 2009. The feeding biomechanics and dietary ecology of Australopithecus africanus. Proc. Natl Acad. Sci. USA 106, 2124–2129 10.1073/pnas.0808730106 (doi:10.1073/pnas.0808730106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strait DS, et al. 2012. Microwear, mechanics and the feeding adaptations of Australopithecus africanus. J. Hum. Evol. 62, 165–168 10.1016/j.jhevol.2011.10.006 (doi:10.1016/j.jhevol.2011.10.006) [DOI] [PubMed] [Google Scholar]
- 22.Atkins AG, Liu JH. 2007. Toughness and the transition between cutting and rubbing in abrasive contacts. Wear 262, 146–159 10.1016/j.wear.2006.04.002 (doi:10.1016/j.wear.2006.04.002) [DOI] [Google Scholar]
- 23.Atkins AG. 2009. The science and engineering of cutting. Oxford, UK: Elsevier Press [Google Scholar]
- 24.Baker G, Jones LHP, Wardrop ID. 1959. Cause of wear in sheeps’ teeth. Nature 184, 1583–1584 10.1038/1841583b0 (doi:10.1038/1841583b0) [DOI] [PubMed] [Google Scholar]
- 25.Ciochon RL, Piperno DR, Thompson RG. 1990. Opal phytoliths found on the teeth of the extinct ape, Gigantopithecus blacki: implications for paleodietary studies. Proc. Natl Acad. Sci. USA 87, 8120–8124 10.1073/pnas.87.20.8120 (doi:10.1073/pnas.87.20.8120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gügel IL, Grupe G, Kunzelmann K-H. 2001. Simulation of dental microwear: characteristic traces by opal phytoliths give clues to ancient human dietary behavior. Am. J. Phys. Anthropol. 114, 124–138 (doi:10.1002/1096-8644(200102)114:2<124::AID-AJPA1012>3.0.CO;2-S) [DOI] [PubMed] [Google Scholar]
- 27.Rabenold D, Pearson OM. 2011. Abrasive, silica phytoliths and the evolution of thick molar enamel in primates, with implications for the diet of Paranthropus boisei. PLoS ONE 6, e28379. 10.1371/journal.pone.0028379 (doi:10.1371/journal.pone.0028379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanson GD, Kerr SA, Gross KA. 2007. Do silica phytoliths really wear mammalian teeth? J. Archaeol. Sci. 34, 526–531 10.1016/j.jas.2006.06.009 (doi:10.1016/j.jas.2006.06.009) [DOI] [Google Scholar]
- 29.Massey FP, Ennos R, Hartley SE. 2007. Herbivore specific induction of silica-based plant defences. Oecologia 152, 677–683 10.1007/s00442-007-0703-5 (doi:10.1007/s00442-007-0703-5) [DOI] [PubMed] [Google Scholar]
- 30.Massey FP, Massey K, Ennos R, Hartley SE. 2009. Impacts of silica-based defences in grasses on the feeding preferences of sheep. Basic Appl. Ecol. 10, 622–630 10.1016/j.baae.2009.04.004 (doi:10.1016/j.baae.2009.04.004) [DOI] [Google Scholar]
- 31.Hill R. 1950. LXVII. A theoretical investigation of the effect of specimen size in the measurement of hardness. Philos. Mag. 41, 745–753 10.1080/14786445008561007 (doi:10.1080/14786445008561007) [DOI] [Google Scholar]
- 32.Samuels LE, Mulhearn TO. 1957. An experimental investigation of the deformed zone associated with indentation hardness impressions. J. Mech. Phys. Solids 5, 125–134 10.1016/0022-5096(57)90056-X (doi:10.1016/0022-5096(57)90056-X) [DOI] [Google Scholar]
- 33.Teaford MF, Walker A. 1983. Dental microwear in adult and stillborn guinea pigs (Cavia porcellus). Arch. Oral Biol. 28, 1077–1081 10.1016/0003-9969(83)90067-5 (doi:10.1016/0003-9969(83)90067-5) [DOI] [PubMed] [Google Scholar]
- 34.Piperno DR. 2006. Phytoliths: a comprehensive guide for archaeologists and paleoecologists. Lanham, MD: Altamira Press [Google Scholar]
- 35.Jones LHP, Milne AA. 1966. Studies of silica in the oat plant I. Chemical and physical properties of the silica. Plant Soil 18, 207–220 10.1007/BF01347875 (doi:10.1007/BF01347875) [DOI] [Google Scholar]
- 36.Atkins AG, Felbeck DK. 1974. Applying mutual indentation hardness phenomena to service failures. Met. Eng. Q. 14, 55–61 [Google Scholar]
- 37.Chai H, Lee JJ-W, Constantino P, Lucas PW, Lawn BR. 2009. Remarkable resilience of teeth. Proc. Natl Acad. Sci. USA 106, 7289–7293 10.1073/pnas.0902466106 (doi:10.1073/pnas.0902466106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He LH, Swain MV. 2007. Contact induced deformation of enamel. Appl. Phys. Lett. 90, 171916. 10.1063/1.2450649 (doi:10.1063/1.2450649) [DOI] [Google Scholar]
- 39.Lawn BR, Lee JJ, Constantino PJ, Lucas PW. 2009. Predicting failure in mammalian enamel. J. Mech. Behav. Biomed. Mater. 2, 33–42 10.1016/j.jmbbm.2008.05.007 (doi:10.1016/j.jmbbm.2008.05.007) [DOI] [PubMed] [Google Scholar]
- 40.Lawn BR. 1967. Partial cone crack formation in a brittle material loaded with a sliding spherical indenter. Proc. R. Soc. Lond. A 299, 307–316 10.1098/rspa.1967.0138 (doi:10.1098/rspa.1967.0138) [DOI] [Google Scholar]
- 41.Gordon KR. 1984. Microfracture patterns of abrasive wear striations on teeth indicate directionality. Am. J. Phys. Anthropol. 63, 315–322 10.1002/ajpa.1330630308 (doi:10.1002/ajpa.1330630308) [DOI] [PubMed] [Google Scholar]
- 42.Constantino P, Lee JJ-W, Chai H, Zipfel B, Ziscovici C, Lawn BR, Lucas PW. 2010. Tooth chipping can reveal the diet and bite forces of fossil hominins. Biol. Lett. 6, 719–722 10.1098/rsbl.2010.0304 (doi:10.1098/rsbl.2010.0304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cuy JL, Mann AB, Livi KJ, Teaford MF, Weihs TP. 2002. Nanoindentation mapping of the mechanical properties of human molar tooth enamel. Arch. Oral Biol. 7, 281–291 10.1016/S0003-9969(02)00006-7 (doi:10.1016/S0003-9969(02)00006-7) [DOI] [PubMed] [Google Scholar]
- 44.Lee JJ-W, Morris D, Constantino P, Chai H, Lucas PW, Smith TM, Lawn BR. 2010. Properties of tooth enamel in great apes. Acta Biomater. 12, 4560–4565 10.1016/j.actbio.2010.07.023 (doi:10.1016/j.actbio.2010.07.023) [DOI] [PubMed] [Google Scholar]
- 45.Bajaj D, Park S, Quinn GD, Arola D. 2010. Fracture processes and mechanisms of crack growth resistance in human enamel. J. Mater. 62, 76–82 10.1007/s11837-010-0113-8 (doi:10.1007/s11837-010-0113-8) [DOI] [Google Scholar]
- 46.Ryan AS. 1979. A preliminary scanning electron microscope examination of wear striation direction on primate teeth. J. Dent. Res. 58, 525–530 10.1177/00220345790580011401 (doi:10.1177/00220345790580011401) [DOI] [PubMed] [Google Scholar]
- 47.Ryan AS. 1979. Wear striation direction on primate teeth: a scanning electron microscope examination. Am. J. Phys. Anthropol. 50, 155–168 10.1002/ajpa.1330500204 (doi:10.1002/ajpa.1330500204) [DOI] [PubMed] [Google Scholar]
- 48.Maas MC. 1994. A scanning electron-microscopic study of in vitro abrasion of mammalian tooth enamel under compressive loads. Arch. Oral Biol. 39, 1–11 10.1016/0003-9969(94)90028-0 (doi:10.1016/0003-9969(94)90028-0) [DOI] [PubMed] [Google Scholar]
- 49.Covert HH, Kay RF. 1981. Dental microwear and diet: implications for determining the feeding behaviors of extinct primates, with a comment on the dietary pattern of Sivapithecus. Am. J. Phys. Anthropol. 55, 331–336 10.1002/ajpa.1330550307 (doi:10.1002/ajpa.1330550307) [DOI] [PubMed] [Google Scholar]
- 50.Teaford MF. 1988. A review of dental microwear and diet in modern mammals. Scanning Microsc. 2, 1149–1166 [PubMed] [Google Scholar]
- 51.Kaiser TM, Brinkman G. 2006. Measuring dental wear equilibriums: the use of industrial surface texture parameters to infer the diets of fossil mammals. Palaeogeogr. Palaeoclimatol. Palaeoecol. 239, 221–240 [Google Scholar]
- 52.Purnell M, Seehausen O, Frietson G. 2012. Quantitative three-dimensional microtextural analyses of tooth wear as a tool for dietary discrimination in fishes. J. R. Soc. Interface 9, 2225–2233 10.1098/rsif.2012.0140 (doi:10.1098/rsif.2012.0140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scott JR. 2012. Dental microwear texture analysis of extant African Bovidae. Mammalia 76, 157–174 [Google Scholar]
- 54.Ungar PS, Teaford MF, Glander KE, Pastor RF. 1995. Dust accumulation in the canopy: a potential cause of dental microwear in primates. Am. J. Phys. Anthropol. 97, 93–99 10.1002/ajpa.1330970202 (doi:10.1002/ajpa.1330970202) [DOI] [PubMed] [Google Scholar]
- 55.Lucas PW, Gaskins JT, Lowrey TK, Harrison ME, Morrogh-Bernard H, Cheyne SE, Begley MR. 2012. Evolutionary optimization of material properties of a tropical seed. J. R. Soc. Interface 9, 34–42 10.1098/rsif.2011.0188 (doi:10.1098/rsif.2011.0188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hummel J, Findeisen E, Sudekum K-H, Ruf I, Kaiser TM, Bucher M, Clauss M, Codron D. 2011. Another one bites the dust: faecal silica levels in large herbivores correlate with high-crowned teeth. Proc. R. Soc. B 278, 1742–1747 10.1098/rspb.2010.1939 (doi:10.1098/rspb.2010.1939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Damuth J, Janis CM. 2011. On the relationship between hypsodonty and feeding ecology in ungulate animals, and its utility in palaeoecology. Biol. Rev. 86, 733–758 10.1111/j.1469-185X.2011.00176.x (doi:10.1111/j.1469-185X.2011.00176.x) [DOI] [PubMed] [Google Scholar]
- 58.deMenocal PB. 2004. African climate change and faunal evolution during the Pliocene–Pleistocene. Earth Planet. Sci. Lett. 220, 3–24 10.1016/S0012-821X(04)00003-2 (doi:10.1016/S0012-821X(04)00003-2) [DOI] [Google Scholar]
- 59.Joordens JCA, Vonhof HB, Feibel CS, Lourens LJ, Dupont-Nivet G, van der Lubbe JHLJ, Sier MJ, Davies GR, Kroon D. 2011. An astronomically-tuned climate framework for hominins in the Turkana basin. Earth Planet. Sci. Lett. 307, 1–8 10.1016/j.epsl.2011.05.005 (doi:10.1016/j.epsl.2011.05.005) [DOI] [Google Scholar]
- 60.Johansson A, Fareed K, Omar R. 1991. Analysis of possible factors influencing the occurrence of occlusal tooth wear in a young Saudi population. Acta Odontol. Scand. 49, 139–145 10.3109/00016359109005898 (doi:10.3109/00016359109005898) [DOI] [PubMed] [Google Scholar]
- 61.Dominy NJ, Vogel ER, Yeakel JD, Constantino P, Lucas PW. 2008. Mechanical properties of plant underground storage organs and implications for dietary models of early hominins. Evol. Biol. 35, 159–175 10.1007/s11692-008-9026-7 (doi:10.1007/s11692-008-9026-7) [DOI] [Google Scholar]
- 62.Peters CR. 1982. Electron-optical microscopic study of incipient dental microdamage from experimental seed and bone crushing. Am. J. Phys. Anthropol. 57, 283–301 10.1002/ajpa.1330570306 (doi:10.1002/ajpa.1330570306) [DOI] [PubMed] [Google Scholar]
- 63.Joiner A, Schwarz A, Philpotts CJ, Cox TF, Huber K, Hannig M. 2008. The protective nature of pellicle towards toothpaste abrasion on enamel and dentine. J. Dent. 36, 360–368 10.1016/j.jdent.2008.01.010 (doi:10.1016/j.jdent.2008.01.010) [DOI] [PubMed] [Google Scholar]
- 64.Prinz JF. 2004. Abrasives in foods and their effect on intra-oral processing: a two-colour chewing gum study. J. Oral Rehabil. 31, 968–974 10.1111/j.1365-2842.2004.01328.x (doi:10.1111/j.1365-2842.2004.01328.x) [DOI] [PubMed] [Google Scholar]
- 65.Hunt JW, Dean AP, Webster RE, Johnson GN, Ennos AR. 2008. A novel mechanism by which silica defends against herbivory. Ann. Bot. 102, 653–656 10.1093/aob/mcn130 (doi:10.1093/aob/mcn130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guidoni GM, Swain MV, Jäger I. 2010. Nano-scale sliding contact deformation behaviour of enamel under wet and dry conditions. J. Mater. Sci.: Mater. Med. 21, 1195–1203 10.1007/s10856-010-3988-6 (doi:10.1007/s10856-010-3988-6) [DOI] [PubMed] [Google Scholar]
- 67.Whitney DL, Broz M, Cook RF. 2008. Hardness, toughness and modulus of some common metamorphic minerals. Am. Mineral. 92, 281–288 10.2138/am.2007.2212 (doi:10.2138/am.2007.2212) [DOI] [Google Scholar]
- 68.Bottone S. 1873. On a relation existing between atomic weights, specific gravities and hardness of the metallic elements. Chemistry News, 2 May 1873, pp. 215.–
- 69.Lucas PW. 2004. Dental functional morphology. Cambridge, UK: Cambridge University Press [Google Scholar]
- 70.Guidoni GM, Swain MV, Jäger I. 2009. Wear behaviour of dental enamel at the nanoscale with a sharp and blunt indenter tip. Wear 266, 60–68 10.1016/j.wear.2008.05.007 (doi:10.1016/j.wear.2008.05.007) [DOI] [Google Scholar]
- 71.Tabor D. 1951. The hardness of metals. Oxford, UK: Oxford University Press [Google Scholar]