Abstract
The improvement of therapeutic efficacy for cancer agents has been a big challenge which includes the increase of tumor selectivity and the reduction of adverse effects at non-tumor sites. In order to achieve those goals, prodrug approaches have been extensively investigated. In this report, the potential activation enzymes for 5′-amino acid/dipeptide monoester floxuridine prodrugs in pancreatic cancer cells were selected and the feasibility of enzyme specific activation of prodrugs was evaluated. All prodrugs exhibited the range of 3.0–105.7 min of half life in Capan-2 cell homogenate with the presence and the absence of selective enzyme inhibitors. 5′-O-L--Phenylalanyl-L-tyrosyl-floxuridine exhibited longer half life only with the presence of pepstatin A. Human cathepsin B and D selectively hydrolized 5′-O-L-phenylalanyl-L-tyrosylfloxuridine and 5′-O-L-phenylalanyl-L-glycylfloxuridine compared to the other tested prodrugs. The wide range of growth inhibitory effect by floxuridine prodrugs in Capan-2 cells was observed due to the different affinities of prodrug promoieties to enyzmes. In conclusion, it is feasible to design prodrugs which are activated by specific enzymes. Cathepsin D might be a good candidate as a target enzyme for prodrug activation and 5′-O-L-phenylalanyl-L-tyrosylfloxuridine may be the best candidate among the tested floxuridine prodrugs.
Keywords: prodrugs, Capan-2 cell, cathepsin, enzymatic activation, cell proliferation assay
1. Introduction
Prodrug applications have been utilized in cancer treatment for improving drug delivery, minimizing toxicity at non-target sites, maximizing therapeutic effect and increasing oral bioavailability [1,2,3]. Amino acid monoester prodrugs of antiviral agents like acyclovir and ganciclovir have been reported to improve their oral bioavailability by facilitating carrier-mediated transport [4,5,6,7]. Amino acid monoester prodrugs of the anti-cancer drug gemcitabine have also exhibited enhanced affinity to the oligopeptide transporter (PEPT1) [8]. The PEPT1 transporter has been extensively examined as a delivery site to improve oral drug absorption and the pro-moieties targeting this transporter have been investigated to improve their binding affinity [9,10,11].
The anticancer agent 5-fluoro-2′-deoxyuridine (floxuridine) has been clinically used in the treatment of colon carcinoma and colorectal cancer metastases to the liver. The mechanical action of 5-fluorouracil (5-FU) and floxuridine is well understood [12]. Floxuridine has a specific activity in DNA and has less cytotoxicity in RNA and, thus, this specificity of floxuridine minimizes adverse effects unlike 5-FU [13,14]. It has been reported that the potency and the growth inhibition of floxuridine is 10- to 100-fold better than those of 5-FU [15,16]. Hence, the protection of the glycosidic bond in floxuridine will produce better clinical outputs. Floxuridine, however, is rapidly converted to 5-FU by thymidine phosphorylase, which is ubiquitously observed in many tissues, including the liver [17]. Therefore, the protection of glycosidic bond of floxuridine leads to improve therapeutic efficiency. A variety of enzymes have been tested as the possible target enzyme to activate prodrugs because those prodrugs have to be activated in order to reveal their therapeutic effects. Irinotecan (CPT-11) is an anti-cancer prodrug of active metabolite SN-38 and has been approved for the treatment of colon cancer. CPT-11 has been investigated for specific activation of irinotecan at tumor sites by carboxylesterase to minimize toxicity in non-tumor sites [3,18,19,20,21]. The progression of technology can make it possible to examine the crystal structure of carboxylesterase and to design more efficient prodrugs activated by specific enzymes [18,22,23]. Highly expressed enzymes have been studied as possible enzyme targets in certain disease stages. The enzyme targeting for prodrug activation in various diseases has also been carried out. It has been reported that endopeptidase cathepsins have been upregulated in tumor cells and are expressed more than those in non-cancer cells [24,25,26]. Furthermore, the signaling pathways related to upregulation of cathepsins have been gradually recognized [27,28]. It is well documented that cathepsins B, D, and L are the most abundant proteases and the increased expression of those cathepsins plays a part in the invasion and metastasis in the tumor growth [29,30,31,32,33,34]. Therefore, cathepsin B, D, and L have a potential to be good targets for prodrug activation. Doxorubicin and daunorubicin prodrugs conjugated with peptides and amino acids show the less toxicity than their parent drugs, doxorubicin and daunorubicin, and are activated by hydrolytic enzymes like the cathepsins [35,36,37,38,39,40]. Capecitabine is a prodrug of 5-FU like floxuridine and is used for the treatment of breast and colorectal cancers. This prodrug, which requires a few activation steps by enzymes to show its therapeutic effect, has been investigated in its activation, distribution, and pharmacokinetics [41,42,43]. However, the avoidance of adverse effects in CPT-11 and capecitabine still has not been fully successful. The understanding of prodrug activation in tumor cells is necessary and leads to the design and production of more advanced prodrugs. Yet, the activation of prodrugs neither has been examined nor has been given adequate attention even though this is one of the most important steps in prodrug approaches.
In this report, the feasibility of cathepsin D targeting for the activation of amino acid/dipeptide monoester prodrugs of floxuridine and the capability of inhibiting cancer cell growth for those activated prodrugs are described. Also, those 5′-dipeptide monoester prodrugs of floxuridine are compared with the 5′-mono amino acid monoester prodrugs of floxuridine. The enzymatic activation of these prodrugs was evaluated to determine the effects of amino acid/dipeptide promoieties and esterification site for enzyme-mediated activation. The activation of translocated prodrugs in tumor cells, the enzymes involved in the prodrug activation, and the ability of tumor growth inhibition for tested prodrugs were discussed.
2. Results and Discussion
Prodrug approaches have been investigated to improve oral absorption and therapeutic effects of poorly permeant drugs [44]. Amino acid/dipeptide monoester prodrugs of antiviral and anticancer drugs such as gemcitabine, acyclovir, 2-bromo-5,6-dichloro-1-(β-D-ribofuranosyl)benzimidazole (BDCRB) and floxuridine have been synthesized and characterized in our previous reports [8,45,46,47,48,49,50,51]. The synthesis methods and characterization of those floxuridine prodrugs have been reported [49,50].
Lysosomal proteases called cathepsins may be attractive target enzymes because of high expression in tumor cells. It has been reported that lysosomal cathepsins, especially cathepsin B and D, are upregulated in tumors and redistributed to the outside of lysosomes such as cytosol and plasma membrane [28,52,53,54,55,56,57,58]. Thus, cathepsin B and D in tumors might be good target enzymes even though they are lysosomal enzymes. It has been reported that cathepsin D is present in all tissues but the expression levels significantly vary in the type of tissues and their physiological state [59]. The location and expression level of enzymes are important decisive factors to select possible target enzymes for prodrug activation. For this purpose, enzymes which are located in the plasma membrane and cytosol, as well as highly expressed enzymes in lysosomes, were selected as candidates of prodrug-activation enzymes.
The identification and selection of the specific enzymes for prodrug activation along with designing prodrugs have been investigated in order to minimize adverse effects and improve therapeutic index [60,61,62,63,64]. One of the most common tools for performing high-throughput expression measurements would be a microarray analysis to select target candidates. The Affymetrix GeneChip (GeneChip Human Genome U133 Plus 2.0) was used to obtain the gene expression profile of top 50 enzymes in specific locations, cytosol and plasma membrane as well as highly expressed lysosomal enzymes, of Capan-2 cell. Cathepsin D, puromycin-sensitive aminopeptidase, TPP2, and DPP3 were chosen as candidates of target enzymes due to their locations and high expression levels of gene in Capan-2 cell (Table 1). The experiments concerning identifying activation enzymes for floxuridine prodrugs were performed at 37 °C in Capan-2 cell homogenates in pH 7.4 phosphate buffer with the presence and the absence of selective enzyme inhibitors.
Table 1.
Expression levels of top 50 enzyme genes in plasma membrane and cytosol with cathepsins in pancreatic cancer cell line, capan-2 and AsPC-1, with Affymetrix GeneChip Human Genome U133 Plus 2.0.
| Enzyme | Capan-2 | AsPC-1 |
|---|---|---|
| Cathepsin B | 1526.6 | 38 |
| Zinc Metalloproteinase | 1057.9 | 48 |
| Cathepsin H | 913.4 | 58.6 |
| Cathepsin D | 891.2 | 3.7 |
| gamma-Glutamyl Hydrolase | 840.6 | 20.3 |
| Calpastatin (CAST) | 746.2 | 28.3 |
| Aminopeptidase B | 726.2 | 14.9 |
| Leucine Aminopeptidase | 700.7 | 15.1 |
| Cathepsin C | 593.4 | 17.6 |
| Puromycin Sensitive Aminopeptidase | 530.2 | 4.9 |
| Prolylcarboxypeptidase | 516.3 | 16.8 |
| Aminoacylase 1 | 511.9 | 16.2 |
| Cytochrome P450 | 432.4 | 13.5 |
| N-Acylaminoacyl-Peptide Hydrolase | 335.5 | 9.7 |
| Tripeptidyl peptidase II (TPP2) | 296.7 | 36.4 |
| Cathepsin U | 288.4 | 7.4 |
| Aminopeptidase P | 264 | 328.2 |
| Dipeptidylpeptidase III (DPP3) | 257.5 | 4.2 |
| Leucine Aminopeptidase | 238 | 20.7 |
| Mitochondrial Intermediate Peptidase (MIPEP) | 206.8 | 35 |
| Aminopeptidase PILS (APPILS) | 195.1 | 2.4 |
| UDP-N-Acetylglucosamine-2-Epimerase (GNE) | 180.4 | 42.3 |
| Caspase 6 | 179 | 0.8 |
| Sentrin-Specific Protease (SENP2) | 176 | 116.5 |
| Glycosylasparaginase | 176 | 130.1 |
| Carboxypeptidase D | 168.2 | 57.3 |
| Transmembrane Protease(TMPRSS4) | 167.8 | 3.9 |
| Cysteine Protease | 167.1 | 17.1 |
| Aspartyl Aminopeptidase (DNPEP) | 163.6 | 24.7 |
| Peptidase D (PEPD) | 163.2 | 3.3 |
| Heparan Sulfate (glucosamine) 3-O-Sulfotransferase 1 (HS3ST1) | 157.1 | 5.3 |
| Aspartylglucosaminidase | 121.5 | 28.6 |
| GPI Transamidase | 119.6 | 112.6 |
| SentrinSUMO-specific protease 3 (SENP3) | 117.8 | 111.9 |
| Caspase 3 | 114.8 | 5.1 |
| Carboxy-Terminal Hydrolase | 111.7 | 56 |
| Caspase 8 | 108.8 | 49.8 |
| Phosphatidylcholine 2-acylhydrolase (cPLA2) | 108.3 | 3.3 |
| Polypeptide 5 (RPS6KA5) | 103.3 | 32.1 |
| beta-Site APP-Cleaving Enzyme | 100.2 | 178.3 |
| Protease 5 (isopeptidase T) (USP5) | 99.9 | 84 |
| Arginyl Aminopeptidase (Aminopeptidase B)-Like 1 (RNPEPL1) | 99.3 | 16.1 |
| SentrinSUMO-Specific Protease (SENP1) | 98.8 | 48.3 |
| Carboxypeptidase M | 97.4 | 7.8 |
| Carboxypeptidase D | 96.9 | 4.9 |
| 3-Phosphoinositide Dependent Protein Kinase-1 (PDPK1) | 94.7 | 53.3 |
| Putative Metalloglycoprotease | 94 | 55.4 |
| Aspartylglucosaminidase (AGA) | 93.2 | 12.5 |
| Carboxypeptidase 1 | 92.8 | 15 |
| Matrix Metalloproteinase 14 (MMP14) | 88.5 | 10.3 |
BOLD: selected for enzyme inhibition studies.
Table 2 displays the estimated half-lives (t1/2) obtained from the initial rates of hydrolysis by plotting the logarithm of remaining prodrugs as a function of time. All prodrugs showed shorter half-lives in cell homogenates than in pH 7.4 phosphate buffer suggesting enzyme catalyzed hydrolysis. No degradation of prodrugs was confirmed in lower than pH 6.0 phosphate buffer (data not shown). Amino acid monoester prodrugs of floxuridine and their parent drug were observed from 5′-O-L-valyl-L-phenylalanylfloxuridine, 5′-O-L-phenylalanyl-L-tyrosylfloxuridine and 5′-O-L-glycyl-L-leucyl-floxuridine. However, only floxuridine was observed from the rest of tested prodrugs. The stabilities of 5′-O-L-leucyl-L-glycylfloxuridine and 5′-O-L-valyl-L-phenylalanylfloxuridine were 1.4- to 8.3-fold and 0.8- to 1.7- fold better with the presence of enzyme inhibitors, respectively. However, significant differences among them could not be observed. Only 5′-O-L-glycyl-L-leucylfloxuridine exhibited no change in its stability in all conditions. This result is indicating that there are many enzymes to activate 5′-O-L-glycyl-L-leucylfloxuridine or/and selected enzyme inhibitors did not effectively inhibit enzymatic activation of this prodrug. 5′-O-L-phenylalanyl-L-glycylfloxuridine and 5′-O-L-phenyl-alanylfloxuridine exhibited 2.4- to 7.0-fold and 5.1- to 20.2-fold longer half lives with the presence of enzyme inhibitors. This suggests that those targeted enzymes associate to those prodrug activations. However, activation enzymes for those two prodrugs could not be specified. The half life of 5′-O-L-phenylalanyl-L-tyrosylfloxuridine in Capan-2 cell homogenate with the presence of pepstatin A was significantly prolonged, while the half lives of this prodrug with other enzyme inhibitors were not improved. Thus, the activation enzyme of 5′-O-L-phenylalanyl-L-tyrosylfloxuridine would be specific and cathepsin D might be the one of major activation enzymes for this prodrug activation.
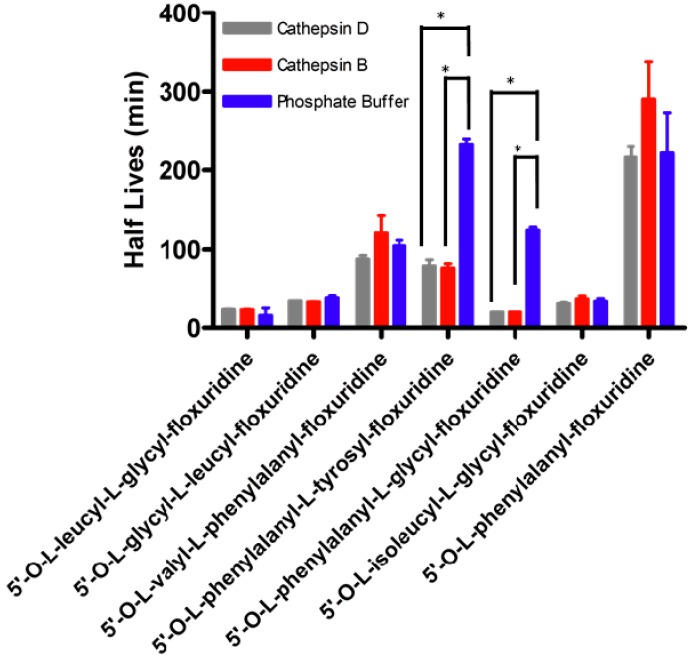
The high expression levels of cathepsins in tumors have been reported [65,66,67,68]. It has been investigated if the protein levels of cathepsin B and D would associate with pathological parameters in order to assess the difference in protein levels of cathepsins as possible tumor biomarkers [34,69,70,71]. Cathepsin B, one of the highest gene expressions in enzymes of Capan-2 cell, is a cysteine endopeptidase and reportedly has similar activity to carboxypeptidase and peptidyl-dipeptidase [72,73,74]. On the other hand, cathepsin D has been reported that it preferentially hydrolyzes the peptide bond of bulky hydrophobic amino acids and the peptide bond between Phe-Phe of Bz-Arg-Gly-Phe-Phe-Pro-4MbNA [75,76,77]. The metabolic stability of floxuridine prodrugs was assessed using human cathepsin B and D in pH 7.4 phosphate buffer. Figure 1 shows the stability of floxuridine prodrugs against cathepsin B and D compared with the absence of those endopeptidases, which representing chemical stability of prodrugs. All tested floxuridine prodrugs with exception of 5′-O-L-phenylalanyl-L-glycyl-floxuridine and 5′-O-L-phenylalanyl-L-tyrosylfloxuridine did not exhibit differences in their half lives. Only 5′-O-L-phenylalanyl-L-glycylfloxuridine and 5′-O-L-phenylalanyl-L-tyrosylfloxuridine showed significant degradation by cathepsin B and D. The production of amino acid mono ester prodrug of floxuridine was clearly observed from 5′-O-L-phenylalanyl-L-tyrosyl-floxuridine. However, the enzyme specificity between cathepsin B and D in prodrug activation for those two prodrugs was not observed. As Offermann et al. have reported, aromatic amino acid incorporated prodrugs would be possible substrates for cathepsin B and D but the result showed that 5′-O-L-valyl-L-phenylalanyl-floxuridine was not a good substrate for cathepsins despite the incorporation of phenylalanine in the dipeptide promoiety [76]. The differences in the half lives of amino acid monoester prodrug of floxuridine, 5′-O-L-phenylalanylfloxuridine, was not observed with cathepsin enzymes. Thus, this result indicates that not only N-terminus aromatic amino acid but also dipeptide are required to be a substrate for cathepsins.
Table 2.
Prodrug stability in capan-2 cell homogenate with the presence and the absence of inhibitors.
| Half Life (min) | ||||||
|---|---|---|---|---|---|---|
| Prodrug | Phosphate Buffer pH 7.4 | Capan-2 Cell Homogenate | Cathepsin D Inhibitor | Aminopeptidase Inhibitor | TPP 2 Inhibitor | DPP 3 Inhibitor |
| 5'-O-L-leucyl-L-glycylfloxuridine | 23.1 ± 4.1 § | 3.9 ± 1.1 | 7.9 ± 0.7 | 14.0 ± 4.3 | 5.6 ± 0.6 | 32.2 ± 9.3 |
| 5'-O-L-glycyl-L-leucylfloxuridine | 35.7 ± 0.9 § | 29.2 ± 0.7 | 32.2 ± 0.1 | 33.0 ± 0.4 | 21.0 ± 0.9 | 26.5 ± 17.5 |
| 5'-O-L-valyl-L-phenylalanyl-floxuridine | 104.7 ± 7.0 § | 56.2 ±12.8 | 76.4 ±6.1 | 93.6 ± 29.3 | 46.1 ± 1.9 | 67.7 ± 15.1 |
| 5'-O-L-phenylalanyl-L-tyrosyl-floxuridine | 233.9 ± 6.6 § | 42.8 ± 0.0 | 105.7 ± 12.8 * | 54.7 ± 2.8 | 42.2 ± 1.3 | 47.2 ± 3.6 |
| 5'-O-L-phenylalanyl-L-glycyl-floxuridine | 132.1 ± 10.2 § | 4.3 ± 0.9 | 10.2 ± 3.0 | 25.7 ± 0.4 * | 19.6 ± 0.4 * | 30.1 ± 10.0 |
| 5'-O-L-phenylalanylfloxuridine | 187.0 ± 19.0 § | 3.0 ± 0.1 | 60.6 ± 4.6* | 48.7 ± 0.37 * | 15.3 ± 0.6 * | 28.9 ± 10.7 |
* <0.01; cathepsin D inhibitor–pepstatin A, aminopeptidase inhibitor–puromycin, TPP2 (tripeptidylpepptidase 2) inhibitor-H-Ala-Ala-Phe-CMK CF3CO2H, DPP3 (dipeptidylpeptidase 3)–1,10-phenanthroline. Asterisks indicate significant difference in half lives in capan-2 cell homogenate with an inhibitor to ones without inhibitors. Values presented are the mean ± s.d. (*p < 0.01; t-test). § from Reference [50].
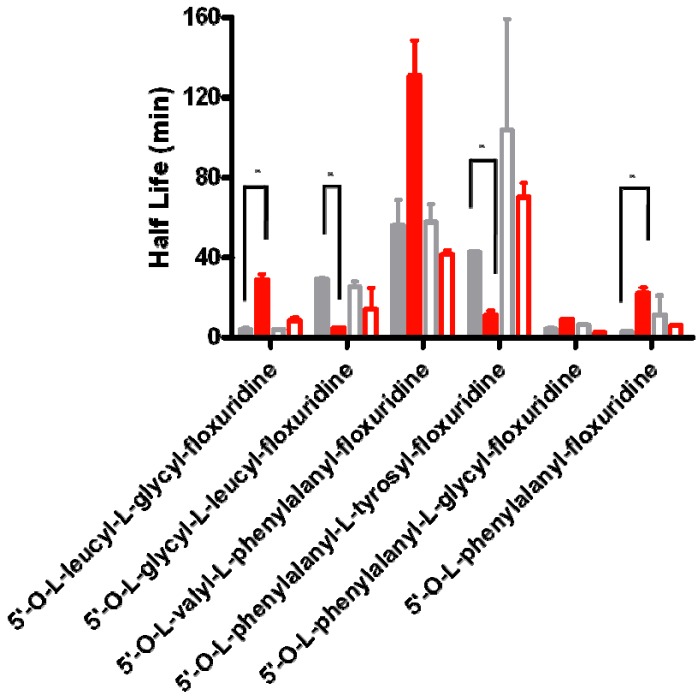
In stability studies of test compounds in cell homogenates, the location of enzymes in living cells is not considered and the inaccessible enzymes in vivo condition may be able to degrade those analytes in this in vitro setting. Therefore, the result of prodrug stabilities in cell homogenate may be different to one in vivo, where all enzymes are arranged in all specific locations. The experiments of prodrug activation concerning the location of enzymes in cells were performed at 37 °C in Capan-2 cell homogenates and in cytosolic enzyme extracts. For the comparison purpose, the same experiments were performed at 37 °C in Caco-2 cell homogenates and in cytosolic enzyme extracts. All tested prodrugs except 5′-O-L-phenylalanyl-L-tyrosylfloxuridine and 5′-O-L-glycyl-L-leucylfloxuridine showed 2.0- to 7.4-fold longer half lives in cytosolic extract than ones in Capan-2 cell homogenate in pH 7.4 phosphate buffer (Figure 2). On the other hand, all tested prodrugs except 5′-O-L-leucyl-L-glycylfloxuridine exhibited shorter half lives in cytosolic extract than ones in Caco-2 cell homogenate in pH 7.4 phosphate buffer. All tested prodrugs in Caco-2 cells did not exhibit significant difference between in whole cell homogenate and in the cytosolic extract. The difference between them was minimal (1.4- to 2.5 fold). Floxuridine and amino acid mono ester prodrugs of floxuridine were observed from 5′-O-L-valyl-L-phenylalanylfloxuridine, 5′-O-L-phenylalanyl-L-tyrosylfloxuridine and 5′-O-L-glycyl-L-leucylfloxuridine but only floxuridine was detected from 5′-O-L-leucyl-L-glycyl-floxuridine, 5′-O-L-isoleucyl-L-glycylfloxuridine, 5′-O-L-phenylalanyl-L-glycylfloxuridine, and 5′-O-L-phenylalanylfloxuridine. In Capan-2 cells, the stabilities of 5′-O-L-phenylalanyl-L-glycylfloxuridine and 5′-O-L-phenylalanylfloxuridine were improved 7.4- and 7.3-fold, while the ones of 5′-O-L- phenylalanyl-L-tyrosylfloxuridine and 5′-O-L-glycyl-L-leucylfloxuridine were declined 3.9- and 6.0-fold, respectively. The latter result suggests: (1) there is the same amount of protein but there is difference in the population of enzymes between cell homogenate and cytosolic fraction; (2) there are more enzymes in cytosol extract to activate 5′-O-L-phenylalanyl-L-tyrosylfloxuridine and 5′-O-L-glycyl-L-leucylfloxuridine than ones to activate other prodrugs; and (3) the redistribution/trafficking of upregulated lysosomal enzymes to outside of lysosomal compartments [53,54,55,78]. Those results indicate that the prodrug stabilities in vitro might mislead prodrug stabilities in vivo due to the difference in cellular enzymatic population and location.
Figure 1.
Prodrug stability in human cathepsin B and D. Values presented are the mean ± s.d., n = 4, (*p < 0.01; t-test).
Figure 2.
The difference of prodrug stability in the cytosolic fraction of cell homogenate and in whole cell homogenate of Capan-2 and Caco-2 cells. Gray bars represent the prodrug stability in whole cell homogenate and red bars represent the prodrug stability in the cytosolic fraction of cell homogenate. Closed bars represent the prodrug stability in Capan-2 cells and open bars represent the prodrug stability in Caco-2 cells. Values presented are the mean ± s.d., n = 4, (* p < 0.01; t-test).
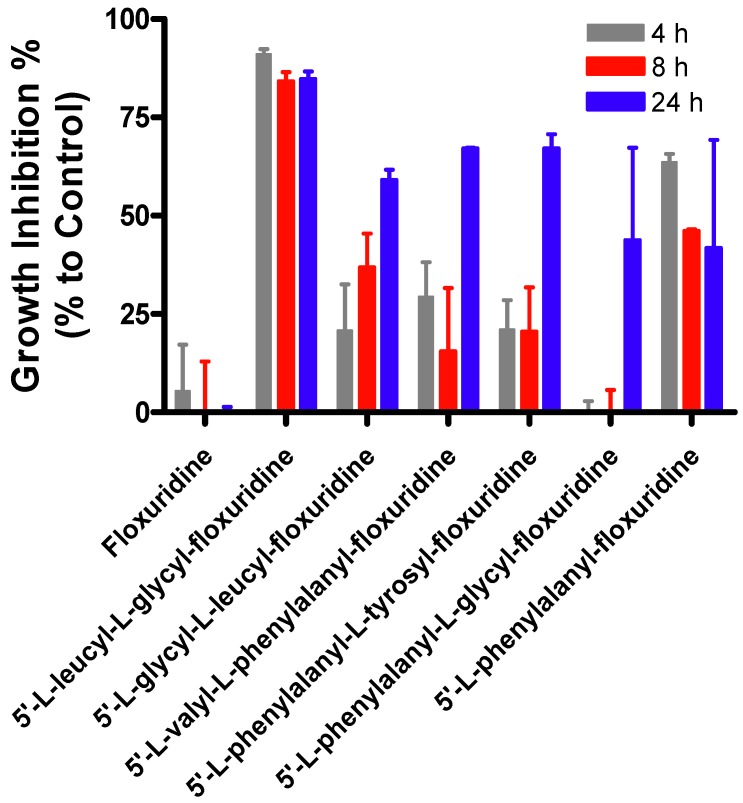
The percentage of growth inhibition for amino acid/dipeptide monoester prodrugs of floxuridine in Capan-2 cell was evaluated via 2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilideinner salt (XTT) assays (Figure 3). The growth inhibitory effect of tested floxuridine prodrugs after 4 h drug treatment range from 0.0–91.1% at 4 h, 0.0–84.2% at 8 h, and 41.8–84.8% at 24 h in Capan-2 cell lines. 5′-O-L-leucyl-L-glycylfloxuridine and 5′-O-L-phenylalanylfloxuridine immediately inhibited the growth of tumor cells while the rest of floxuridine prodrugs needed a certain time to be activated for their inhibitory effects. While specific activation enzymes for 5′-O-L-leucyl-L-glycylfloxuridine and 5′-O-L-phenylalanylfloxuridine have not been identified, those enzymes may be highly localized at the plasma membrane and cytosol. Thus, the activation of those prodrugs took place immediately after translocation of prodrugs into the cells and started inhibiting tumor growth. Leucine and phenylalanine are reportedly good substrates for aminopeptidases and carboxylesterase, respectively.
Thus, 5′-O-L-leucyl-L-glycylfloxuridine and 5′-O-L-phenylalanylfloxuridine might be activated by aminopeptidases and carboxylesterases, which are highly expressed at the plasma membrane and cytosol, to inhibit the growth of cancer cells [79,80]. 5′-O-L-phenylalanyl-L-glycyl-floxuridine, which exhibited a shorter half life in cell homogenate, did not exhibit the growth inhibition of cancer cells at 4 h and 8 h, suggesting that 5′-O-L-phenylalanyl-L-glycylfloxuridine might be more stable in vivo cell condition than one in cell homogenate. Those results imply that floxuridine prodrugs have to be activated in order to inhibit tumor growth. The prodrug activation would be initiated at different time periods due to the affinity between promoieties and enzymes and the location and population of prodrug-activation-enzymes.
Figure 3.
The growth inhibitory effects (%) of floxuridine prodrugs with varied post exposure time (4, 8, and 24 h). Values presented are the mean ± s.d. (n = 4).
The sites of prodrug activation in cells, the duration for activating prodrugs, the enzyme population, and the activation enzymes based on promoieties are all different. Therefore, in vitro experiments have to be carefully designed and prodrug approaches should be monitored by not only the amount of delivered prodrug but also prodrug activation and their therapeutic effect.
3. Experimental
3.1. Materials
Floxuridine was obtained from Lancaster (Windham, NH, USA). The tert-butyloxycarbonyl (Boc) protected amino acids Boc-L-phenylalanine, Boc-L-glycyl-leucine, Boc-L-phenylalanyl-glycine, Boc-L-leucyl-glycine, Boc-L-isolucylglycine, Boc-L-valyl-phenylalanine, and Boc-L-phenylalanyl-tyrosine were obtained from Chem-Impex (Wood Dale, IL, USA). Pepstatin A, cathepsin B (human liver), and cathepsin D (human liver) were obtained from Sigma Chemical Co (St. Louis, MO, USA). Enzyme inhibitors were purchased from EMD Chemicals (Gibbstown, NJ, USA). High-performance liquid chromatography (HPLC) grade acetonitrile was obtained from Fisher Scientific (St. Louis, MO, USA). N,N-dicyclohexylcarbodiimide, N,N-dimethylaminopyridine, trifluoroacetic acid (TFA), and all other reagents and solvents were purchased from Aldrich Chemical Co. (Milwaukee, WI, USA). Cell culture reagents were obtained from Invitrogen (Carlsbad, CA, USA) and cell culture supplies were obtained from Corning (Corning, NY, USA) and Falcon (Lincoln Park, NJ, USA). The Affymetrix GeneChip Human Genome U133 Plus 2.0 was purchased from Affymetrix Inc. (Santa Clara, CA, USA). All chemicals were either analytic or HPLC grade.
3.2. Floxuridine Prodrug Synthesis
The synthesis and characterization of 5′-monoamino acid and dipeptide ester prodrugs of floxuridine have been reported previously [46,49,50]. Briefly, Boc-protected amino acid and dipeptide (1,1 mmol), N,N-dicyclohexylcarbodiimide (1,1 mmol), and N,N-dimethylaminopyridine (0.1 mmol) were allowed to react with floxuridine (1 mmol) in dry DMF (7 mL) for 24 h. The reaction progress was monitored by TLC (ethyl acetate as eluent). The reaction mixture was filtered and DMF was removed under vacuum at 40 °C. The residue was extracted with ethyl acetate (30 mL) and washed with water (2 × 20 mL), and saturated NaCl (20 mL). The organic layer was dried over MgSO4 and concentrated under vacuum. The reaction yielded a mixture of 3′-monoester, 5′-monoester, and 3′,5′-diester floxuridine prodrugs. The three spots observed on TLC were separated and purified using column chromatography (dichloromethane/methanol, 20:1). Fractions from each spot were concentrated under vacuum separately. The Boc group was cleaved by treating the residues with TFA-dichloromethane (1:1, 5 mL). After 4 h, the solvent was removed and the residues werereconstituted with water and lyophilized. The TFA salts of amino acid/dipeptide monoester prodrugs of floxuridine were obtained as white fluffy solids. The combined yield of floxuridine prodrugs was ~60%. HPLC was used to evaluate the prodrug purity. Prodrugs were between 90% and 99% pure. These prodrugs were easily separated from parent drug by HPLC. Electrospray ionization mass spectra(ESI-MS) were obtained on a Thermoquest LCQ ESI-MS. The observed molecular weights of all prodrugs were found to be consistent with that required by their structure. The structural identity of the prodrugs was then confirmed using proton nuclear magnetic resonance spectra (1H-NMR). 1H-NMR spectra were obtained on a 300 MHz Bruker DPX-300 NMR spectrometer.
3.3. Cell Culture
Capan-2 cells (passages 50–58) from American Type Culture Collection (Rockville, MD) were routinely maintained in RPMI-1640 containing 10% fetal bovine serum. Caco-2 cells (passages 30–33) from American Type Culture Collection (Rockville, MD, USA) were routinely maintained in DMEM containing 10% fetal bovine serum, 1% nonessential amino acids, 1 mmol/L sodium pyruvate, and 1% L-glutamine. Cells were grown in an atmosphere of 5% CO2 and 90% relative humidity at 37 °C.
3.4. Affymetrix Oligonucleotide Array
Capan-2 cells were cultured at 37 °C, 5% CO2 in RPMI-1640 supplemented with 10% fetal bovine serum. Our group previously described the preparation of Capan-2 total RNA samples [81,82]. The gene expression profile of Capan-2 cell was obtained using Affymetrix GeneChip Human Genome U133 Plus 2.0.
Hydrolysis StudiesPreparation of Capan-2 cell homogenates. Preparation of cell homogenates has been reported previously [49]. Confluent Capan-2 cells (2 plates; 150 mm × 25 mm plate) were rinsed with saline twice. The cells were washed off with 5 mL of pH 7.4 phosphate buffer (10 mmol/L) and the lysed cells by ultrasonication (Micro ultrasonic cell disrupter Model KT40, Kontes, Vineland, NJ, USA) were spun down by centrifugation.
Preparation of Cytosolic Enzyme Extract. Confluent Caco-2 cells and Capan-2 cells (2 plates; 150 mm × 25 mm plate) were rinsed with saline twice. The cells were washed off with 1 mL of pH 7.4 phosphate buffer (10 mmol/L) and the enzymes were extracted through 30 gauge needle 10 times in ice-cold bath. Cell suspension was spun at 10,000 × g for 2 h at 4 °C and the supernatant was spun at 39,000 × g for 2 h at 4 °C. Protein amount was quantified with the Bio-Rad (Hercules, CA, USA) DC Protein Assay using bovine serum albumin as a standard. The protein amount was adjusted to 500 µg/mL and the hydrolysis reactions were carried out in 96-well plates (Corning). Caco-2 cells and Capan-2 cell suspensions (250 µL) were placed in triplicate wells either presence or absence of various inhibitors, the reactions were started with the addition of substrate (200 µM), and wells were incubated at 37 °C for 120 min (time points; 0, 5, 10, 30, 60, and 120 min). At each time point, aliquot sample (35 µL) was removed and added to 150 µL of acetonitrile (ACN) with 0.1% TFA to prevent the further degradation. The mixtures were filtered with a 0.45 µm filters at 1,000 × g for 10 min at 4 °C. The filtrate wasthen analyzed via reverse-phaseHPLC.
Prodrug stability with Cathepsin B and D. Lyophilized enzyme powder was dissolved and adjusted to 2.5 mg/mL of pure enzyme, human cathepsin B and D. The hydrolysis reactions were immediately started by adding substrates (200 µM) in 96-well plates (Corning). The reactions were carried out at 37 °C for 120 min (time points; 0, 5, 10, 30, 60, and 120 min). At each time point, aliquot sample (35 µL) was removed and added to 150 µL of acetonitrile (ACN) with 0.1% TFA. The mixtures were filtered with a 0.45 µm filters at 1,000 × g for 10 min at 4 °C. The filtrate was then analyzed via reverse-phase HPLC.
3.5. Data Analysis
The initial rates of hydrolysis were used to obtain the apparent first-order rate constants and estimate the half-lives. The apparent first-order degradation rate constants of various floxuridine prodrugs at 37 °C were determined by plotting the logarithm of prodrug remaining as a function of time. The slopes of these plots are related to the rate constant, k, and given by:
k = 2.303 × slope (log C vs. time) (1)
The degradation half-lives were then estimated by the equation:
t1/2 = 0.693/k (2)
Statistical significance was evaluated with GraphPad Prism version 3.0 by performing one-way analysis of variance with post-hoc Tukey’s test to compare means. A p value of <0.01 was considered significant.
3.6. HPLC Analysis
The concentrations of prodrugs and their metabolites were determined on a Waters HPLC system (Waters, Inc., Milford, MA, USA). The HPLC system consisted of two Waters pumps (model 515), a Waters autosampler (WISP model 712), and a Waters UV detector (996 photodiode array detector). The system was controlled by Waters Millennium 32 software (version 3.0.1). Samples were injected onto a Waters Xterra C18 reverse-phase column (5 µm, 4.6 × 250 mm) equipped with a guard column. The compounds were eluted using gradient method. All prodrugs were run with the solvent B gradient changing 0% to 56% at a rate of 2%/min during a 28-min run. Standard curves generated for each prodrug and their parent drugs were used for quantitation of integrated area under peaks.
3.7. Cell Proliferation Assays
Cell proliferation studies were conducted with Capan-2 cell lines. The cells were seeded into 96-well plates at 125,000 cells per well and allowed to attach/grow for 24 h before drug solutions were added. The culture medium (RPMI-1640 + 10% fetal bovine serum) was removed and the cells were gently washed once with sterile pH 6.0 uptake buffer (145 mM NaCl, 0.5 mM MgCl2, 1 mM NaH2PO4, 1 mM CaCl2, 3 mM KCl, 5 mM glucose, and 5 mM MES). Floxuridine prodrugs and floxuridine were diluted in pH 6.0 uptake buffer to 4 mmol/L using no drug as a 100% viability control. The wash buffer was removed and 25 µL drug solution per well were added and incubated at 37 °C for 4 h in the cell incubator. After this time, the drug solutions were removed and the cells were again gently washed twice with sterile uptake buffer. RPMI-1640 was then added to each well after washing. The cells were allowed to recover for 4 h, 8 h, or 24 h before evaluating cell viability via 2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide inner salt (XTT) assays. A mixture (30 µL) containing 2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide inner salt in sterile RPMI-1640 without phenol red (1 mg/mL) and phenazine methosulfate (N-methyldibenzopyrazine methyl sulfate) (0.383 mg/mL) reagents were added to the cells and incubated at 37 °C for 1 h for the color to develop. Absorbance readings at 450 nm were recorded.
4. Conclusions
In summary, it is feasible to design prodrugs which are activated by specific enzymes. The prodrug stabilities in cell homogenate do not necessary reflect their stabilities in vivo. Cathepsin D might be a good candidate as a target enzyme for prodrug activation because of its upregulation and redistribution to other cellular compartments in tumor cells and its substrate specificity. The results of stability studies with the presence of enzyme inhibitors indicate that there are particular enzymes activating 5′-O-L-phenylalanyl-L-tyrosyl-floxuridine and 5′-O-L-phenylalanyl-L-glycylfloxuridine. Cathepsin B and D significantly activated 5′-O-L-phenylalanyl-L-tyrosyfloxuridine and 5′-O-L-phenylalanyl-L-glycyl-floxuridine to produce floxuridine. Our studies demonstrate that cathepsin D can significantly contribute to the activation of 5′-O-L-phenylalanyl-L-tyrosylfloxuridine. For tumors that express large amounts of cathepsins, it is likely that a substantial proportion of 5′-O-L-phenylalanyl-L-tyrosyl-floxuridine is hydrolyzed by cathepsin D. Taken together, our experimental results demonstrate that cathepsin D has the significant ability to activate dipeptide monoester prodrug of floxuridine, 5′-O-L-phenylalanyl-L-tyrosylfloxuridine, and, therefore, has the potential to be a target enzyme for prodrug activation in tumors. 5′-O-L-phenylalanyl-L-tyrosylfloxuridine has the capability of being a cathepsin D-targeted prodrug for enzymatic activation and exhibits the respectable growth inhibition of cancer cells. In conclusion, with the consideration of chemical/enzymatic stability of those prodrugs, 5′-O-L-phenylalanyl-L-tyrosylfloxuridine may be the best candidate for in vivo tumor reduction studies because this prodrug would be stable enough to stay longer in the systemic circulation to reach target sites for the activation by the specific enzymes. With careful evaluations, the prodrug approaches designed to ameliorate the toxicity of anti-cancer drugs and to maximize prodrug activation based on enzyme specificity are feasible.
Acknowledgements
This work was supported by NIH Grant NIGMD-2R01GM037188.
Footnotes
Sample Availability: Not available.
References and Notes
- 1.Escalona-Benz E., Jockovich M.E., Murray T.G., Hayden B., Hernandez E., Feuer W., Windle J.J. Combretastatin A-4 prodrug in the treatment of a murine model of retinoblastoma. Invest. Ophthalmol. Vis. Sci. 2005;46:8–11. doi: 10.1167/iovs.04-0751. [DOI] [PubMed] [Google Scholar]
- 2.Moody T.W., Mantey S.A., Pradhan T.K., Schumann M., Nakagawa T., Martinez A., Fuselier J., Coy D.H., Jensen R.T. Development of high affinity camptothecin-bombesin conjugates that have targeted cytotoxicity for bombesin receptor-containing tumor cells. J. Biol. Chem. 2004;279:23580–23589. doi: 10.1074/jbc.M401938200. [DOI] [PubMed] [Google Scholar]
- 3.Senter P.D., Beam K.S., Mixan B., Wahl A.F. Identification and activities of human carboxylesterases for the activation of CPT-11, a clinically approved anticancer drug. Bioconjug. Chem. 2001;12:1074–1080. doi: 10.1021/bc0155420. [DOI] [PubMed] [Google Scholar]
- 4.Bras A.P., Sitar D.S., Aoki F.Y. Comparative bioavailability of acyclovir from oral valacyclovir and acyclovir in patients treated for recurrent genital herpes simplex virus infection. Can. J. Clin. Pharmacol. 2001;8:207–211. [PubMed] [Google Scholar]
- 5.Curran M., Noble S. Valganciclovi. Drugs. 2001;61:1145–1150. doi: 10.2165/00003495-200161080-00013. discussion 1151-1152. [DOI] [PubMed] [Google Scholar]
- 6.Linden K., Zhou X.X., Stable L. Validation of microdialysis sampling for oral availability studies by means of a new ganciclovir prodrug. Pharmacol. Toxicol. 2002;90:297–302. doi: 10.1034/j.1600-0773.2002.900602.x. [DOI] [PubMed] [Google Scholar]
- 7.Tolle-Sander S., Lentz K.A., Maeda D.Y., Coop A., Polli J.E. Increased acyclovir oral bioavailability via a bile acid conjugate. Mol. Pharm. 2004;1:40–48. doi: 10.1021/mp034010t. [DOI] [PubMed] [Google Scholar]
- 8.Song X., Lorenzi P.L., Landowski C.P., Vig B.S., Hilfinger J.M., Amidon G.L. Amino acid ester prodrugs of the anticancer agent gemcitabine: Synthesis, bioconversion, metabolic bioevasion, and hPEPT1-mediated transport. Mol. Pharm. 2005;2:157–167. doi: 10.1021/mp049888e. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen C.U., Andersen R., Brodin B., Frokjaer S., Taub M.E., Steffansen B. Dipeptide model prodrugs for the intestinal oligopeptide transporter. Affinity for and transport via hPepT1 in the human intestinal Caco-2 cell line. J. Control. Release. 2001;76:129–138. doi: 10.1016/S0168-3659(01)00427-8. [DOI] [PubMed] [Google Scholar]
- 10.Thomsen A.E., Friedrichsen G.M., Sorensen A.H., Andersen R., Nielsen C.U., Brodin B., Begtrup M., Frokjaer S., Steffansen B. Prodrugs of purine and pyrimidine analogues for the intestinal di/tri-peptide transporter PepT1: affinity for hPepT1 in Caco-2 cells, drug release in aqueous media and in vitro metabolism. J. Control. Release. 2003;86:279–292. doi: 10.1016/s0168-3659(02)00413-3. [DOI] [PubMed] [Google Scholar]
- 11.Vabeno J., Nielsen C.U., Ingebrigtsen T., Lejon T., Steffansen B., Luthman K. Dipeptidomimetic ketomethylene isosteres as pro-moieties for drug transport via the human intestinal di-/tripeptide transporter hPEPT1: Design, synthesis, stability, and biological investigations. J. Med. Chem. 2004;47:4755–4765. doi: 10.1021/jm040780c. [DOI] [PubMed] [Google Scholar]
- 12.Grem J.L. 5-Fluorouracil: Forty-plus and still ticking. A review of its preclinical and clinical development. Invest. New Drugs. 2000;18:299–313. doi: 10.1023/A:1006416410198. [DOI] [PubMed] [Google Scholar]
- 13.Parker W.B., Cheng Y.C. Metabolism and mechanism of action of 5-fluorouracil. Pharmacol. Ther. 1990;48:381–395. doi: 10.1016/0163-7258(90)90056-8. [DOI] [PubMed] [Google Scholar]
- 14.Willmore E., Durkacz B.W. Cytotoxic mechanisms of 5-fluoropyrimidines. Relationships with poly(ADP-ribose) polymerase activity, DNA strand breakage and incorporation into nucleic acids. Biochem. Pharmacol. 1993;46:205–211. doi: 10.1016/0006-2952(93)90405-l. [DOI] [PubMed] [Google Scholar]
- 15.Laskin J.D., Evans R.M., Slocum H.K., Burke D., Hakala M.T. Basis for natural variation in sensitivity to 5-fluorouracil in mouse and human cells in culture. Cancer Res. 979;39:383–390. [PubMed] [Google Scholar]
- 16.Yamada M., Nakagawa H., Fukushima M., Shimizu K., Hayakawa T., Ikenaka K. In vitro study on intrathecal use of 5-fluoro-2'-deoxyuridine (FdUrd) for meningeal dissemination of malignant brain tumors. J. Neurooncol. 1998;37:115–121. doi: 10.1023/a:1005869226496. [DOI] [PubMed] [Google Scholar]
- 17.Birnie G.D., Heidelberger C. In vitro synthesis of acidsoluble thymine compounds by human neoplastic tissues. Cancer Res. 1963;23:420–430. [PubMed] [Google Scholar]
- 18.Bencharit S., Morton C.L., Howard-Williams E.L., Danks M.K., Potter P.M., Redinbo M.R. Structural insights into CPT-11 activation by mammalian carboxylesterases. Nat. Struct. Biol. 2002;9:337–342. doi: 10.1038/nsb790. [DOI] [PubMed] [Google Scholar]
- 19.Oosterhoff D., Pinedo H.M., van der Meulen I.H., de Graaf M., Sone T., Kruyt F.A., van Beusechem V.W., Haisma H.J., Gerritsen W.R. Secreted and tumour targeted human carboxylesterase for activation of irinotecan. Br. J. Cancer. 2002;87:659–664. doi: 10.1038/sj.bjc.6600519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu M.H., Yan B., Humerickhouse R., Dolan M.E. Irinotecan activation by human carboxylesterases in colorectal adenocarcinoma cells. Clin. Cancer Res. 2002;8:2696–2700. [PubMed] [Google Scholar]
- 21.Xu G., Zhang W., Ma M.K., McLeod H.L. Human carboxylesterase 2 is commonly expressed in tumor tissue and is correlated with activation of irinotecan. Clin. Cancer Res. 2002;8:2605–2611. [PubMed] [Google Scholar]
- 22.Harel M., Hyatt J.L., Brumshtein B., Morton C.L., Wadkins R.M., Silman I., Sussman J.L., Potter P.M. The 3D structure of the anticancer prodrug CPT-11 with Torpedo californica acetylcholinesterase rationalizes its inhibitory action on AChE and its hydrolysis by butyrylcholinesterase and carboxylesterase. Chem. Biol. Interact. 2005;157–158:153–157. doi: 10.1016/j.cbi.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Harel M., Hyatt J.L., Brumshtein B., Morton C.L., Yoon K.J., Wadkins R.M., Silman I., Sussman J.L., Potter P.M. The crystal structure of the complex of the anticancer prodrug 7-ethyl-10-[4-(1-piperidino)-1-piperidino]-carbonyloxycamptothecin (CPT-11) with Torpedo californica acetylcholinesterase provides a molecular explanation for its cholinergic action. Mol. Pharmacol. 2005;67:1874–1881. doi: 10.1124/mol.104.009944. [DOI] [PubMed] [Google Scholar]
- 24.Kondoh K., Tsuji N., Kamagata C., Sasaki M., Kobayashi D., Yagihashi A., Watanabe N. A novel aspartic protease gene, ALP56, is up-regulated in human breast cancer independently from the cathepsin D gene. Breast Cancer Res. Treat. 2003;78:37–44. doi: 10.1023/a:1022149226430. [DOI] [PubMed] [Google Scholar]
- 25.Steinfeld S., Maho A., Chaboteaux C., Daelemans P., Pochet R., Appelboom T., Kiss R. Prolactin up-regulates cathepsin B and D expression in minor salivary glands of patients with Sjogren’s syndrome. Lab. Invest. 2000;80:1711–1720. doi: 10.1038/labinvest.3780181. [DOI] [PubMed] [Google Scholar]
- 26.Skrzydlewska E., Sulkowska M., Wincewicz A., Koda M., Sulkowski S. Evaluation of serum cathepsin B and D in relation to clinicopathological staging of colorectal cancer. World J. Gastroenterol. 2005;11:4225–4229. doi: 10.3748/wjg.v11.i27.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumartin L., Whiteman H.J., Weeks M.E., Hariharan D., Dmitrovic B., Iacobuzio-Donahue C.A., Brentnall T.A., Bronner M.P., Feakins R.M., Timms J.F., et al. AGR2 is a novel surface antigen that promotes the dissemination of pancreatic cancer cells through regulation of cathepsins B and D. Cancer Res. 2011;71:7091–7102. doi: 10.1158/0008-5472.CAN-11-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whiteman H.J., Weeks M.E., Dowen S.E., Barry S., Timms J.F., Lemoine N.R., Crnogorac-Jurcevic T. The role of S100P in the invasion of pancreatic cancer cells is mediated through cytoskeletal changes and regulation of cathepsin D. Cancer Res. 2007;67:8633–8642. doi: 10.1158/0008-5472.CAN-07-0545. [DOI] [PubMed] [Google Scholar]
- 29.Chauhan S.S., Goldstein L.J., Gottesman M.M. Expression of cathepsin L in human tumors. Cancer Res. 1991;51:1478–1481. [PubMed] [Google Scholar]
- 30.Koblinski J.E., Ahram M., Sloane B.F. Unraveling the role of proteases in cancer. Clin. Chim. Acta. 2000;291:113–135. doi: 10.1016/s0009-8981(99)00224-7. [DOI] [PubMed] [Google Scholar]
- 31.Rochefort H., Liaudet-Coopman E. Cathepsin D in cancer metastasis: A protease and a ligand. Apmis. 1999;107:86–95. doi: 10.1111/j.1699-0463.1999.tb01530.x. [DOI] [PubMed] [Google Scholar]
- 32.Turk B., Stoka V., Rozman-Pungercar J., Cirman T., Droga-Mazovec G., Oresic K., Turk V. Apoptotic pathways: involvement of lysosomal proteases. Biol. Chem. 2002;383:1035–1044. doi: 10.1515/BC.2002.112. [DOI] [PubMed] [Google Scholar]
- 33.Shen J., Person M.D., Zhu J., Abbruzzese J.L., Li D. Protein expression profiles in pancreatic adenocarcinoma compared with normal pancreatic tissue and tissue affected by pancreatitis as detected by two-dimensional gel electrophoresis and mass spectrometry. Cancer Res. 2004;64:9018–9026. doi: 10.1158/0008-5472.CAN-04-3262. [DOI] [PubMed] [Google Scholar]
- 34.Abbott D.E., Margaryan N.V., Jeruss J.S., Khan S., Kaklamani V., Winchester D.J., Hansen N., Rademaker A., Khalkhali-Ellis Z., Hendrix M.J. Reevaluating cathepsin D as a biomarker for breast cancer: serum activity levels versus histopathology. Cancer Biol. Ther. 2010;9:23–30. doi: 10.4161/cbt.9.1.10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baurain R., Masquelier M., Deprez-De Campeneere D., Trouet A. Amino acid and dipeptide derivatives of daunorubicin. 2. Cellular pharmacology and antitumor activity on L1210 leukemic cells in vitro and in vivo. J. Med. Chem. 1980;23:1171–1174. doi: 10.1021/jm00185a004. [DOI] [PubMed] [Google Scholar]
- 36.Briozzo P., Morisset M., Capony F., Rougeot C., Rochefort H. In vitro degradation of extracellular matrix with Mr 52,000 cathepsin D secreted by breast cancer cells. Cancer Res. 1988;48:3688–3692. [PubMed] [Google Scholar]
- 37.Keppler D., Fondaneche M.C., Dalet-Fumeron V., Pagano M., Burtin P. Immunohistochemical and biochemical study of a cathepsin B-like proteinase in human colonic cancers. Cancer Res. 1988;48:6855–6862. [PubMed] [Google Scholar]
- 38.Maciewicz R.A., Wardale R.J., Etherington D.J., Paraskeva C. Immunodetection of cathepsins B and L present in and secreted from human pre-malignant and malignant colorectal tumour cell lines. Int. J. Cancer. 1989;43:478–486. doi: 10.1002/ijc.2910430323. [DOI] [PubMed] [Google Scholar]
- 39.Masquelier M., Baurain R., Trouet A. Amino acid and dipeptide derivatives of daunorubicin. 1. Synthesis, physicochemical properties, and lysosomal digestion. J. Med. Chem. 1980;23:1166–1170. doi: 10.1021/jm00185a003. [DOI] [PubMed] [Google Scholar]
- 40.Breistol K., Hendriks H.R., Berger D.P., Langdon S.P., Fiebig H.H., Fodstad O. The antitumour activity of the prodrug N-L--leucyl-doxorubicin and its parent compound doxorubicin in human tumour xenografts. Eur. J. Cancer. 1998;34:1602–1606. doi: 10.1016/s0959-8049(98)00152-x. [DOI] [PubMed] [Google Scholar]
- 41.Quinney S.K., Sanghani S.P., Davis W.I., Hurley T.D., Sun Z., Murry D.J., Bosron W.F. Hydrolysis of capecitabine to 5'-deoxy-5-fluorocytidine by human carboxylesterases and inhibition by loperamide. J. Pharmacol. Exp. Ther. 2005;313:1011–1016. doi: 10.1124/jpet.104.081265. [DOI] [PubMed] [Google Scholar]
- 42.Schuller J., Cassidy J., Dumont E., Roos B., Durston S., Banken L., Utoh M., Mori K., Weidekamm E., Reigner B. Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother. Pharmacol. 2000;45:291–297. doi: 10.1007/s002800050043. [DOI] [PubMed] [Google Scholar]
- 43.Tsukamoto Y., Kato Y., Ura M., Horii I., Ishikawa T., Ishitsuka H., Sugiyama Y. Investigation of 5-FU disposition after oral administration of capecitabine, a triple-prodrug of 5-FU, using a physiologically based pharmacokinetic model in a human cancer xenograft model: Comparison of the simulated 5-FU exposures in the tumour tissue between human and xenograft model. Biopharm. Drug Dispos. 2001;22:1–14. doi: 10.1002/bdd.250. [DOI] [PubMed] [Google Scholar]
- 44.Hu M., Subramanian P., Mosberg H.I., Amidon G.L. Use of the peptide carrier system to improve the intestinal absorption of L-alpha-methyldopa: Carrier kinetics, intestinal permeabilities,and in vitro hydrolysis of dipeptidyl derivatives of L--alpha-methyldopa. Pharm. Res. 1989;6:66–70. doi: 10.1023/a:1015855820488. [DOI] [PubMed] [Google Scholar]
- 45.Han H., de Vrueh R.L., Rhie J.K., Covitz K.M., Smith P.L., Lee C.P., Oh D.M., Sadee W., Amidon G.L. 5'-Amino acid esters of antiviral nucleosides, acyclovir, and AZT are absorbed by the intestinal PEPT1 peptide transporter. Pharm. Res. 1998;15:1154–1159. doi: 10.1023/a:1011919319810. [DOI] [PubMed] [Google Scholar]
- 46.Landowski C.P., Song X., Lorenzi P.L., Hilfinger J.M., Amidon G.L. Floxuridine amino acid ester prodrugs: enhancing Caco-2 permeability and resistance to glycosidic bond metabolism. Pharm. Res. 2005;22:1510–1518. doi: 10.1007/s11095-005-6156-9. [DOI] [PubMed] [Google Scholar]
- 47.Landowski C.P., Vig B.S., Song X., Amidon G.L. Targeted delivery to PEPT1-overexpressing cells: Acidic, basic, and secondary floxuridine amino acid ester prodrugs. Mol. Cancer Ther. 2005;4:659–667. doi: 10.1158/1535-7163.MCT-04-0290. [DOI] [PubMed] [Google Scholar]
- 48.Lorenzi P.L., Landowski C.P., Song X., Borysko K.Z., Breitenbach J.M., Kim J.S., Hilfinger J.M., Townsend L.B., Drach J.C., Amidon G.L. Amino acid ester prodrugs of 2-bromo-5,6-dichloro-1-(beta-D-ribofuranosyl)benzimidazole enhance metabolic stability in vitro and in vivo. J. Pharmacol. Exp. Ther. 2005;314:883–890. doi: 10.1124/jpet.104.082412. [DOI] [PubMed] [Google Scholar]
- 49.Tsume Y., Hilfinger J.M., Amidon G.L. Enhanced cancer cell growth inhibition by dipeptide prodrugs of floxuridine: Increased transporter affinity and metabolic stability. Mol. Pharm. 2008;5:717–727. doi: 10.1021/mp800008c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsume Y., Vig B.S., Sun J., Landowski C.P., Hilfinger J.M., Ramachandran C., Amidon G.L. Enhanced absorption and growth inhibition with amino acid monoester prodrugs of floxuridine by targeting hPEPT1 transporters. Molecules. 2008;13:1441–1454. doi: 10.3390/molecules13071441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vig B.S., Lorenzi P.J., Mittal S., Landowski C.P., Shin H.C., Mosberg H.I., Hilfinger J.M., Amidon G.L. Amino acid ester prodrugs of floxuridine: Synthesis and effects of structure, stereochemistry, and site of esterification on the rate of hydrolysis. Pharm. Res. 2003;20:1381–1388. doi: 10.1023/a:1025745824632. [DOI] [PubMed] [Google Scholar]
- 52.Berg T., Gjoen T., Bakke O. Physiological functions of endosomal proteolysis. Biochem. J. 1995;307:313–326. doi: 10.1042/bj3070313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Claus V., Jahraus A., Tjelle T., Berg T., Kirschke H., Faulstich H., Griffiths G. Lysosomal enzyme trafficking between phagosomes, endosomes, and lysosomes in J774 macrophages. Enrichment of cathepsin H in early endosomes. J. Biol. Chem. 1998;273:9842–9851. doi: 10.1074/jbc.273.16.9842. [DOI] [PubMed] [Google Scholar]
- 54.Kageshita T., Yoshii A., Kimura T., Maruo K., Ono T., Himeno M., Nishimura Y. Biochemical and immunohistochemical analysis of cathepsins B, H, L and D in human melanocytic tumour. Arch. Dermatol. Res. 1995;287:266–272. doi: 10.1007/BF01105077. [DOI] [PubMed] [Google Scholar]
- 55.Li W., Yuan X.M. Increased expression and translocation of lysosomal cathepsins contribute to macrophage apoptosis in atherogenesis. Ann. NY Acad. Sci. 2004;1030:427–433. doi: 10.1196/annals.1329.053. [DOI] [PubMed] [Google Scholar]
- 56.Roberg K., Ollinger K. Oxidative stress causes relocation of the lysosomal enzyme cathepsin D with ensuing apoptosis in neonatal rat cardiomyocytes. Am. J. Pathol. 1998;152:1151–1156. [PMC free article] [PubMed] [Google Scholar]
- 57.Sameni M., Elliott E., Ziegler G., Fortgens P.H., Dennison C., Sloane B.F. Cathepsin B and D are localized at the surface of human breast cancer cells. Pathol. Oncol. Res. 1995;1:43–53. doi: 10.1007/BF02893583. [DOI] [PubMed] [Google Scholar]
- 58.Gronborg M., Kristiansen T.Z., Iwahori A., Chang R., Reddy R., Sato N., Molina H., Jensen O.N., Hruban R.H., Goggins M.G., et al. Biomarker discovery from pancreatic cancer secretome using a differential proteomic approach. Mol. Cell. Proteomics. 2006;5:157–171. doi: 10.1074/mcp.M500178-MCP200. [DOI] [PubMed] [Google Scholar]
- 59.Reid W.A., Valler M.J., Kay J. Immunolocalization of cathepsin D in normal and neoplastic human tissues. J. Clin. Pathol. 1986;39:1323–1330. doi: 10.1136/jcp.39.12.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deville-Bonne D., El Amri C., Meyer P., Chen Y., Agrofoglio L.A., Janin J. Human and viral nucleoside/nucleotide kinases involved in antiviral drug activation: Structural and catalytic properties. Antivir. Res. 2010;86:101–120. doi: 10.1016/j.antiviral.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 61.Kohchi Y., Hattori K., Oikawa N., Mizuguchi E., Isshiki Y., Aso K., Yoshinari K., Shirai H., Miwa M., Inagaki Y., et al. Design and synthesis of novel prodrugs of 2'-deoxy-2'-methylidenecytidine activated by membrane dipeptidase overexpressed in tumor tissues. Bioorg. Med. Chem. Lett. 2007;17:2241–2245. doi: 10.1016/j.bmcl.2007.01.066. [DOI] [PubMed] [Google Scholar]
- 62.Major Jourden J.L., Cohen S.M. Enzymatic activation of a matrix metalloproteinase inhibitor. Chem. Commun. (Camb) 2010;46:1241–1243. doi: 10.1039/b923302d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Major Jourden J.L., Cohen S.M. Hydrogen peroxide activated matrix metalloproteinase inhibitors: A prodrug approach. Angew. Chem. Int. Ed. Engl. 2010;49:6795–6797. doi: 10.1002/anie.201003819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tabata T., Katoh M., Tokudome S., Nakajima M., Yokoi T. Identification of the cytosolic carboxylesterase catalyzing the 5'-deoxy-5-fluorocytidine formation from capecitabine in human liver. Drug Metab. Dispos. 2004;32:1103–1110. doi: 10.1124/dmd.104.000554. [DOI] [PubMed] [Google Scholar]
- 65.Alevizos I., Mahadevappa M., Zhang X., Ohyama H., Kohno Y., Posner M., Gallagher G.T., Varvares M., Cohen D., Kim D., et al. Oral cancer in vivo gene expression profiling assisted by laser capture microdissection and microarray analysis. Oncogene. 2001;20:6196–6204. doi: 10.1038/sj.onc.1204685. [DOI] [PubMed] [Google Scholar]
- 66.Elie B.T., Gocheva V., Shree T., Dalrymple S.A., Holsinger L.J., Joyce J.A. Identification and pre-clinical testing of a reversible cathepsin protease inhibitor reveals anti-tumor efficacy in a pancreatic cancer model. Biochimie. 2010;92:1618–1624. doi: 10.1016/j.biochi.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nagler D.K., Kruger S., Kellner A., Ziomek E., Menard R., Buhtz P., Krams M., Roessner A., Kellner U. Up-regulation of cathepsin X in prostate cancer and prostatic intraepithelial neoplasia. Prostate. 2004;60:109–119. doi: 10.1002/pros.20046. [DOI] [PubMed] [Google Scholar]
- 68.Turk V., Turk B., Guncar G., Turk D., Kos J. Lysosomal cathepsins: structure, role in antigen processing and presentation, and cancer. Adv. Enzyme Regul. 2002;42:285–303. doi: 10.1016/s0065-2571(01)00034-6. [DOI] [PubMed] [Google Scholar]
- 69.Devetzi M., Scorilas A., Tsiambas E., Sameni M., Fotiou S., Sloane B.F., Talieri M. Cathepsin B protein levels in endometrial cancer: Potential value as a tumour biomarker. Gynecol. Oncol. 2009;112:531–536. doi: 10.1016/j.ygyno.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 70.Lah T.T., Nanni I., Trinkaus M., Metellus P., Dussert C., De Ridder L., Rajcevic U., Blejec A., Martin P.M. Toward understanding recurrent meningioma: the potential role of lysosomal cysteine proteases and their inhibitors. J. Neurosurg. 2010;112:940–950. doi: 10.3171/2009.7.JNS081729. [DOI] [PubMed] [Google Scholar]
- 71.Szumilo J., Burdan F., Zinkiewicz K., Dudka J., Klepacz R., Dabrowski A., Korobowicz E. Expression of syndecan-1 and cathepsins D and K in advanced esophageal squamous cell carcinoma. Folia Histochem. Cytobiol. 2009;47:571–578. doi: 10.2478/v10042-008-0012-8. [DOI] [PubMed] [Google Scholar]
- 72.Linebaugh B.E., Sameni M., Day N.A., Sloane B.F., Keppler D. Exocytosis of active cathepsin B enzyme activity at pH 7.0, inhibition and molecular mass. Eur. J. Biochem. 1999;264:100–109. doi: 10.1046/j.1432-1327.1999.00582.x. [DOI] [PubMed] [Google Scholar]
- 73.Pohl J., Davinic S., Blaha I., Strop P., Kostka V. Chromophoric and fluorophoric peptide substrates cleaved through the dipeptidyl carboxypeptidase activity of cathepsin B. Anal. Biochem. 1987;165:96–101. doi: 10.1016/0003-2697(87)90205-3. [DOI] [PubMed] [Google Scholar]
- 74.Polgar L., Csoma C. Dissociation of ionizing groups in the binding cleft inversely controls the endo- and exopeptidase activities of cathepsin B. J. Biol. Chem. 1987;262:14448–14453. [PubMed] [Google Scholar]
- 75.Ju B.G., Kim W.S. Upregulation of cathepsin D expression in the dedifferentiating salamander limb regenerates and enhancement of its expression by retinoic acid. Wound Repair Regen. 1998;6:349–357. doi: 10.1046/j.1524-475x.1998.60410.x. [DOI] [PubMed] [Google Scholar]
- 76.Offermann M.K., Chlebowski J.F., Bond J.S. Action of cathepsin D on fructose-1,6-bisphosphate aldolase. Biochem. J. 1983;211:529–534. doi: 10.1042/bj2110529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hulkower K.I., Butler C.C., Linebaugh B.E., Klaus J.L., Keppler D., Giranda V.L., Sloane B.F. Fluorescent microplate assay for cancer cell-associated cathepsin B. Eur. J. Biochem. 2000;267:4165–4170. doi: 10.1046/j.1432-1327.2000.01458.x. [DOI] [PubMed] [Google Scholar]
- 78.Carvelli L.F., Bannoud N., Aguilera C.A., Morales C.R., Sosa M.A. Castration induces changes in the cation-dependent mannose-6-phosphate receptor in rat epididymis: Possible implications in secretion of lysosomal enzymes. J. Cell. Biochem. 2010;110:1101–1110. doi: 10.1002/jcb.22622. [DOI] [PubMed] [Google Scholar]
- 79.Landowski C.P., Lorenzi P.L., Song X., Amidon G.L. Nucleoside ester prodrug substrate specificity of liver carboxylesterase. J. Pharmacol. Exp. Ther. 2006;316:572–580. doi: 10.1124/jpet.105.092726. [DOI] [PubMed] [Google Scholar]
- 80.Murray H., Turner A.J., Kenny A.J. The aminopeptidase activity in the human T-cell lymphoma line (Jurkat) is not at the cell surface and is not aminopeptidase N (CD-13) Biochem. J. 1994;298:353–360. doi: 10.1042/bj2980353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Landowski C.P., Anderle P., Sun D., Sadee W., Amidon G.L. Transporter and ion channel gene expression after Caco-2 cell differentiation using 2 different microarray technologies. AAPS J. 2004;6:e21. doi: 10.1208/aapsj060321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun D., Lennernas H., Welage L.S., Barnett J.L., Landowski C.P., Foster D., Fleisher D., Lee K.D., Amidon G.L. Comparison of human duodenum and Caco-2 gene expression profiles for 12,000 gene sequences tags and correlation with permeability of 26 drugs. Pharm. Res. 2002;19:1400–1416. doi: 10.1023/a:1020483911355. [DOI] [PubMed] [Google Scholar]