Abstract
A dysregulated immune system influencing pathways for cytokine regulation and growth factor expression is implicated in the pathophysiology of several neuropsychiatric disorders. Here, we analyzed cerebrospinal fluid (CSF) cytokines and growth factors with an ultra-sensitive immunoassay system in 43 medication-free suicide attempters and 20 healthy male volunteers. CSF vascular endothelial growth factor (VEGF) and CSF interleukin-8 (IL-8) levels were significantly lower in suicide attempters compared with healthy controls. Further, CSF VEGF showed a significant negative correlation with depression severity. CSF IL-6 levels did not differ between suicide attempters and healthy controls. Low CSF levels of VEGF may represent a lack of trophic support to neurons and downregulation of neurogenesis in the hippocampus reflecting more severe depressive states. IL-8 has also been reported as important in neuroprotection as well as having chemokine activity in the innate immune response. The results support a role for an impaired innate immunity and dysregulation of neuroprotection in the pathophysiology of depression and suicidal behavior.
Keywords: cytokines, depression, interleukin-6, interleukin-8, suicide attempt, VEGF
Introduction
The neurotrophic and neurogenesis hypothesis postulates that growth factor disturbances are important in the pathogenesis of neuropsychiatric disorders such as depression. A dysregulated immune system influencing cytokine regulation and growth factor expression as well as metabolic pathways controlling monoamine regulation is implicated in the pathophysiology of several neuropsychiatric disorders.1, 2 There are several studies reporting differentiated levels of cytokines and growth factors in regards to depression and suicide-related behavior, even though results are conflicting, reflecting the complexity and probable heterogeneity of the disorders.3, 4, 5, 6, 7 Although there are several long-term follow-up studies of dysregulation of hypothalamic–pituitary–adrenal axis and suicide risk, very few studies have focused on growth factors and proinflammatory cytokines in the cerebrospinal fluid (CSF).8 There is an urgent need for reliable biomarkers in relation to suicide risk both for more accurate prediction as well as for new therapeutic opportunities.
In this study, we analyzed CSF cytokines and vascular endothelial growth factor-A (VEGF) with a sensitive immunoassay system in medication-free suicide attempters and healthy volunteers. We hypothesized that growth factor levels, as well as proinflammatory cytokines would differentiate in the CSF of patients with a recent suicide attempt, as compared with healthy controls.
Materials and methods
Subjects
A total of 43 suicide attempters (15 men, mean age 45 years, s.d.=12.8, range 22–69 years and 28 women, mean age 36 years, s.d.=12.7, range 18–68 years) admitted to the psychiatric wards at the Karolinska University Hospital were recruited to the present study. Inclusion criteria were a recent suicide attempt (within 1 month), fair capacity to communicate verbally and in writing in the Swedish language and the age of 18 years or older. Exclusion criteria were schizophrenia spectrum psychosis, dementia, mental retardation and intravenous drug abuse.
Suicide attempt was defined as any nonfatal, self-injurious behavior with intent to die. Patients did not receive any antidepressant or antipsychotic medication during a washout period of mean 21 days (s.d.=13.6) after the suicide attempt. Seven of patients (16%) had been treated with antidepressants before the suicide attempt; the mean washout time for these patients was 34 days (s.d.=14.1; range 26–62 days).
A trained psychiatrist interviewed the participants, using the Structured Clinical Interview for DSM (Diagnostic and Statistical Manual of Mental Disorders) Axis I disorders (SCID I) research version interview to establish diagnosis according to DSM–III. Axis II diagnoses were established with SCID II interview. In all, 95% of participants had at least one current Axis I psychiatric diagnosis; 79% of patients fulfilled criteria for mood disorders (unipolar, major depressive disorder, single episode or recurrent, bipolar disorder, depressed or dysthymic disorder), 7% for adjustment disorder and 5% for anxiety disorders, 1 patient had a substance-related disorder and 1 an unspecified psychiatric disorder (not psychotic). Overall, 20% of the patients had a co-morbid substance-related disorder (mostly alcohol dependence). Among Axis II diagnoses, 37% of the patients fulfilled criteria for a personality disorder. Concerning somatic diagnoses, two patients had cardiovascular disease, three patients diabetes, one patient Mb Crohn, one patient celiac disease and four patients suffered from chronic pain not otherwise specified.
Depression severity was rated using the Montgomery Åsberg Depression Rating Scale (MADRS).9
A total of 20 healthy male volunteers (mean age 29 years, s.d.=5.0, range 22–41) were recruited and screened with a SCID interview performed by a trained psychiatrist to exclude previous or current psychiatric problems or medical conditions. Healthy volunteers were screened for absence of psychiatric illness in the first-degree relatives. The volunteers had to be medication-free (had never taken psychiatric medication or even anti-inflammatory medication during the last month) and free from any form of substance abuse.
The study was approved by the Research Ethical Committees at the Karolinska Institutet, Sweden.
Lumbar puncture procedure
Lumbar punctures were performed at the end of the washout period in a standardized manner between 0800 and 0900 hours after fasting in bed since midnight. A volume of 12 ml of CSF was withdrawn with the patient in the sitting position, the needle being inserted between lumbar vertebrae IV and V. The CSF was immediately centrifuged and stored at −80 °C until analyzed. The aliquoted CSF samples had never been thawed before cytokine analysis in November 2011.
Blood sampling procedures
Blood samples were collected in conjunction to the suicide attempt, between 0730 and 0800 hours after a night of fasting and bed rest. Blood samples were collected from all seasons throughout the year between 1993 and 1998. The same conditions applied to all the samples. The blood was centrifuged within 5 min in room temperature (1000 g for 10 min). Plasma was collected and stored at −80 °C until cytokine measurements. Samples had never been thawed before the cytokine analysis in 2010.
Cytokine analysis in CSF
Vascular endothelial growth factor
Samples were run on two MSD Human VEGF 96-well plates (K151BMC-1; Gaithersburg, MD, USA) and the results were pooled. Assay calibrator was diluted according to the manufacturer's recommendation with exception for the standard curve starting at 1000 pg μl−1. Each well was blocked with 150 μl Blocker C (provided) for 2 h in room temperature on an orbital shaker (400 r.p.m.). The plates were washed three times with 150 μl per well phosphate-buffered saline+0.05% Tween-20 (PBS-T). Diluent 7 (provided) was added at 25 μl per well. Calibrator, samples and controls were added at 25 μl per well. The plate was sealed and incubated overnight at 4 °C on an orbital shaker (400 r.p.m.). At the end of the incubation, the wells were washed three times with PBS-T. After the last wash, detection antibody was added at 25 μl per well. The plate was sealed and incubated in dark for 2 h at room temperature on an orbital shaker (400 r.p.m.). At the end of the incubation, the plate was washed three times as before. A volume of 150 μl of the MSD 1 × Read Buffer T was added to each well and the plate was measured immediately on the MSD Sector Imager 2400 plate reader.
Interleukin-6 (IL-6) and Interleukin-8 (IL-8)
Samples were run on two MSD Human Pro-inflammatory-4 II Ultra-Sensitive 96-well plates (K15025C-1). The samples were run according to the manufacturer's recommendation with the following exceptions: standard points starting at 1000 pg μl−1. Calibrator, samples and controls were incubated overnight at 4 °C.
Cytokine analysis in plasma
VEGF, IL-6 and IL-8 were analyzed with a high-throughput automated biochip immunoassay system, EvidenceH, Randox Laboratories (Crumlin, UK), see previous study for methods.10
Statistical analysis
One patient was identified as being clearly both a univariate outlier (3.5 s.d. above the mean of the patients) and a multivariate outlier calculated using Mahalanobis distance.11 Although not affecting the statistical significance of the results, the patient was excluded in all statistical analyses. Both CSF VEGF and IL-8 levels were normally distributed, whereas CSF IL-6 was not normally distributed. The potential effect of the confounding factors was tested in linear regression models. The model consisted of age, gender, body mass index, a co-morbid diagnosis of personality disorder and abuse. Only age was a significant predictor of CSF VEGF levels in the model (t=2.14, P=0.04), other P-values ranged between 0.25–0.82. The group comparisons were adjusted for age using linear regression.
Concerning plasma levels of VEGF and IL-8, one patient was identified as being both a univariate outlier (6 s.d. above the mean of the patients) and a multivariate outlier calculated using Mahalanobis distance. This patient was excluded from the correlation analysis between CSF and plasma levels of VEGF and IL-8. Tests of parametric correlations were performed using Pearson's r. Tests of nonparametric correlations were performed using Spearman's rho.
Alpha was set at 0.05. The Statistical Package JMP VI software, SAS Institute, Cary, NC, USA was used for all statistical analyses.
Results
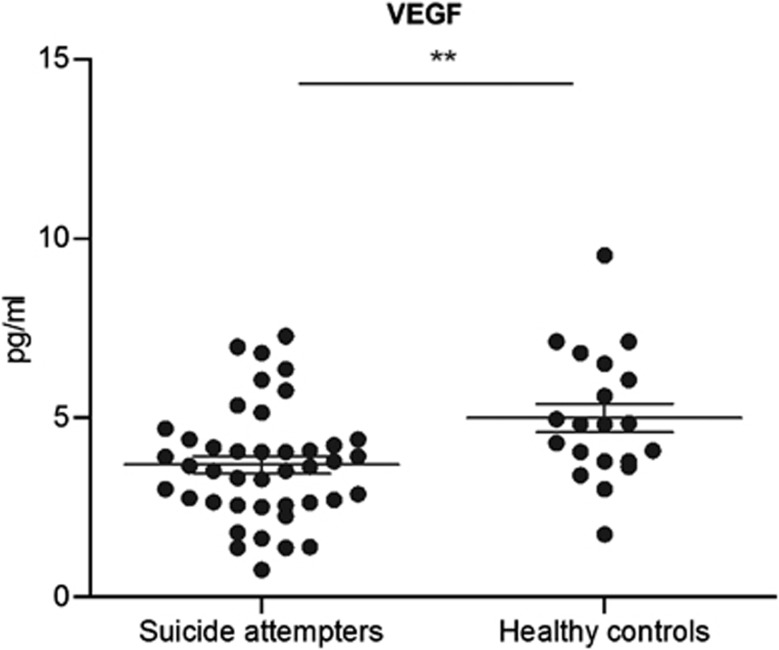
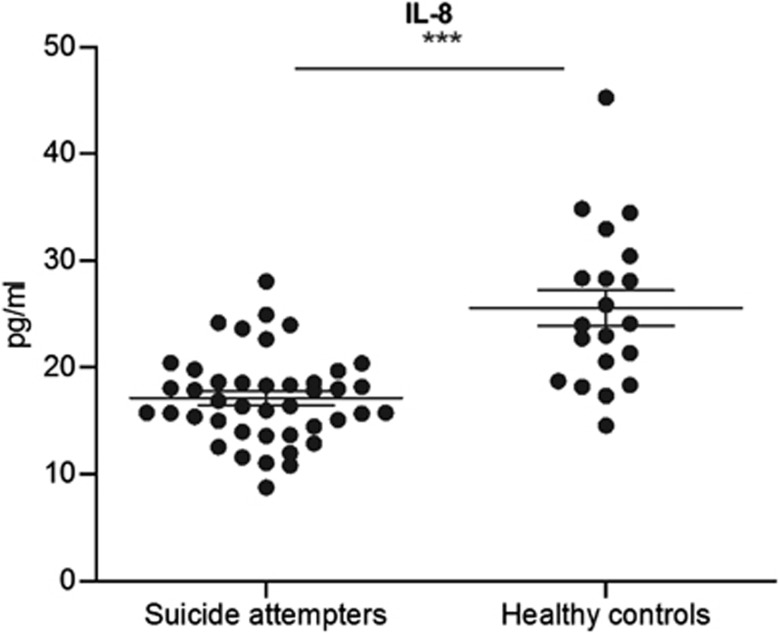
CSF VEGF and IL-8 levels were significantly lower in suicide attempters (VEGF mean 3.7 pg ml−1, IL−8 mean 17.1 pg ml−1) compared with controls (VEGF mean 5.0 pg ml−1, IL-8 mean 25.6 pg ml−1) (t-ratio=−3.76, P=0.0004; t-ratio=−5.82, P<0.0001, both adjusted for age) (Figure 1, 2). CSF VEGF levels showed a significant negative correlation with depression severity as measured with the MADRS (r=−0.31; P=0.049). Correlation analysis between CSF IL-8 levels and MADRS was not significant (r=−0.13, P=0.40).
Figure 1.
VEGF concentrations in CSF of suicide attempters vs. healthy controls, measured with an MSD immunoassay. Horizontal lines indicate mean concentrations and standard error of mean (SEM). VEGF levels are significantly decreased compared to healthy controls. (**p<0.01).
Figure 2.
IL-8 concentrations in CSF of suicide attempters vs. healthy controls, measured with an MSD immunoassay. Horizontal lines indicate mean concentrations and standard error of mean (SEM). IL-8 levels are significantly decreased compared to healthy controls. (***p<0.001).
In suicide attempters VEGF showed a trend for positive correlation with IL-8 levels (r=0.29, P=0.066). Interestingly, the levels of CSF VEGF and IL-8 did not correlate with plasma levels of VEGF and IL-8 in suicide attempters (Spearman's rho=0.04, P=0.80; Spearman's rho=0.06, P=0.70). CSF IL-6 levels did not differ in suicide attempters (mean 1.1 pg ml−1) compared with controls (0.9 pg ml−1) (t-ratio=0.91, P=0.37, adjusted for age).
Discussion
To the best of our knowledge, this is the first study to report lower CSF VEGF and IL-8 levels in suicide attempters. The suicide attempters with the highest depression scores as measured on MADRS, had the lowest levels of VEGF. Our finding suggests that downregulated VEGF and IL-8 levels could be involved in the pathophysiology underlying suicidal behavior and depression. In a previous study, we have reported that low plasma levels of VEGF were associated with suicide risk in suicide attempters.10
VEGF has been increasingly appreciated as a neurotrophic factor. It has pleiotropic properties, and apart from being a vital angiogenic factor, it is having a role in regulating neurogenesis and synaptogenesis, but also regulating glia, in the adult brain.12, 13 Dysregulated VEGF levels have previously been implicated in several neurodegenerative disorders, such as Alzheimer, amyotrophic lateral sclerosis and multiple sclerosis.14
There are several animal studies suggesting that VEGF mediates the biological effect of common antidepressants, such as fluoxetine and lamotrigine.15, 16, 17 In addition, there are animal studies showing that VEGF could mediate the effect of electroconvulsive seizures.15, 18, 19 These effects are believed to be mediated via the main brain receptor for VEGF action, the flk-1 receptor. VEGF is also believed to be having a part in the antidepressive effect that is seen as an effect of regular physical exercise.20 There are reports that have shown a downregulation of VEGF in response to stress or corticosteroids, putatively putting VEGF in context of the diathesis–stress model for depression.21, 22
There are several clinical studies on the role of VEGF in depressive patients. Most studies have been small, with mixed patient populations and the results have been inconsistent or negative.23, 24, 25, 26 There are several known functional single-nucleotide polymorphisms for the VEGF gene, as well as known promoter regions, such as the hypoxia inducible factor (HIF-1).27 In a recent study, VEGF has been suggested as a biomarker for more severe depressive states, and possibly for treatment resistance, as shown in a genetic association study to the VEGF gene.28
Interestingly, the levels of CSF VEGF and IL-8 did not correlate with plasma levels of VEGF and IL-8 in suicide attempters. VEGF is known to regulate blood–brain barrier (BBB) permeability in certain acute conditions.12, 29, 30 However, we are not aware of any experimental data suggesting that VEGF can tune permeability across the largely intact BBB, such as in depression. Lack of correlation between blood and CSF levels may therefore reflect differences in local production of VEGF rather than a dysfunctional BBB. However, it is still possible that this may have more subtle effects on BBB function in relevant brain regions.
IL-8 is important in neuroprotection and innate immunity.31 A few reports have linked IL-8 to neuropsychiatric disorders.31, 32 In a recent study, CSF IL-8 levels did not differ between suicide attempters and controls, whereas CSF IL-6 levels were higher in suicide attempters.5 In our study, a higher proportion of suicide attempters fulfilled criteria for depression, which may partly explain these differences. The CSF level of IL-8 was higher than that of IL-6 with less within-group variation, which likely affects the possibility to detect significant between group differences of the latter. However, our study also lacked a female control group, which is a limitation, and must be taken into account when comparing these two studies. One study has reported lower serum levels of IL-8 in a long-term follow-up study of depressive patients compared with controls.31
Some limitations should also be pointed out, particularly the fact that the groups were not age and gender matched. However, even though not optimal, when we used both age and gender as covariates in the regression model, patients had significantly lower CSF VEGF levels. As VEGF is difficult to measure in the CSF and the levels found here were close to the detection limit of the assay, our findings should be interpreted with caution until replicated.
To summarize, low CSF levels of VEGF may represent a lack of trophic support to neurons and downregulation of neurogenesis in important regions such as the hippocampus. Low CSF VEGF and IL-8 levels in suicide attempters may be associated with an impaired innate immunity and as a consequence dysregulation of neuroprotection adding to the hypothesis of depression being an inflammatory disorder.33, 34
Acknowledgments
Funding for this study was provided by the Swedish Research Council (Project numbers: 5454; K2009-61P-21304-04-4; K2009-61X-21305-01-1) and Thuring foundation.
The authors declare no conflict of interest.
References
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-K, Lee S-W, Kim S-H, Shim S-H, Han S-W, Choi S-H, et al. Differences in cytokines between non-suicidal patients and suicidal patients in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:356–361. doi: 10.1016/j.pnpbp.2007.08.041. [DOI] [PubMed] [Google Scholar]
- Tonelli LH, Stiller J, Rujescu D, Giegling I, Schneider B, Maurer K, et al. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr Scand. 2008;117:198–206. doi: 10.1111/j.1600-0447.2007.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, et al. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 2009;66:287–292. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelidze S, Mattei D, Westrin Å, Träskman-Bendz L, Brundin L. Cytokine levels in the blood may distinguish suicide attempters from depressed patients. Brain Behav Immun. 2011;25:335–339. doi: 10.1016/j.bbi.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Jokinen J, Carlborg A, Mårtensson B, Forslund K, Nordström A-L, Nordström P. DST non-suppression predicts suicide after attempted suicide. Psychiatry Res. 2007;150:297–303. doi: 10.1016/j.psychres.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Isung J, Mobarrez F, Nordström P, Asberg M, Jokinen J. Low plasma vascular endothelial growth factor (VEGF) associated with completed suicide. World J Biol Psychiatry. 2012;13:468–473. doi: 10.3109/15622975.2011.624549. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS.Using Multivariate Statistics Pearson Allyn & Bacon, Boston, Mass; London: Pearson Education; 2007. p980 [Google Scholar]
- Ruiz de Almodovar C, Lambrechts D, Mazzone M, Carmeliet P. Role and therapeutic potential of VEGF in the nervous system. Physiol Rev. 2009;89:607–648. doi: 10.1152/physrev.00031.2008. [DOI] [PubMed] [Google Scholar]
- Nowacka MM, Obuchowicz E. Vascular endothelial growth factor (VEGF) and its role in the central nervous system: a new element in the neurotrophic hypothesis of antidepressant drug action. Neuropeptides. 2012;46:1–10. doi: 10.1016/j.npep.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Iacobaeus E, Amoudruz P, Ström M, Khademi M, Brundin L, Hillert J, et al. The expression of VEGF-A is down regulated in peripheral blood mononuclear cells of patients with secondary progressive multiple sclerosis. PLoS ONE. 2011;6:e19138. doi: 10.1371/journal.pone.0019138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. VEGF as a potential target for therapeutic intervention in depression. Curr Opin Pharmacol. 2008;8:14–19. doi: 10.1016/j.coph.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene J, Banasr M, Lee B, Warner-Schmidt J, Duman RS. Vascular endothelial growth factor signaling is required for the behavioral actions of antidepressant treatment: pharmacological and cellular characterization. Neuropsychopharmacology. 2009;34:2459–2468. doi: 10.1038/npp.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R, Li N, Li T. VEGF regulates antidepressant effects of lamotrigine. Eur Neuropsychopharmacol. 22:424–430. doi: 10.1016/j.euroneuro.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Segi-Nishida E. Exploration of new molecular mechanisms for antidepressant actions of electroconvulsive seizure. Biol Pharm Bull. 2011;34:939–944. doi: 10.1248/bpb.34.939. [DOI] [PubMed] [Google Scholar]
- Elfving B, Wegener G. Electroconvulsive seizures stimulate the vegf pathway via mTORC1. Synapse. 2012;66:340–345. doi: 10.1002/syn.21518. [DOI] [PubMed] [Google Scholar]
- Kiuchi T, Lee H, Mikami T. Regular exercise cures depression-like behavior via VEGF-Flk-1 signaling in chronically stressed mice. Neuroscience. 5:208–217. doi: 10.1016/j.neuroscience.2012.01.023. [DOI] [PubMed] [Google Scholar]
- Heine VM, Zareno J, Maslam S, Joëls M, Lucassen PJ. Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur J Neurosci. 2005;21:1304–1314. doi: 10.1111/j.1460-9568.2005.03951.x. [DOI] [PubMed] [Google Scholar]
- Howell KR, Kutiyanawalla A, Pillai A. Long-term continuous corticosterone treatment decreases VEGF receptor-2 expression in frontal cortex. PLoS ONE. 2011;6:e20198. doi: 10.1371/journal.pone.0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iga J-I, Ueno S-I, Yamauchi K, Numata S, Tayoshi-Shibuya S, Kinouchi S, et al. Gene expression and association analysis of vascular endothelial growth factor in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:658–663. doi: 10.1016/j.pnpbp.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Kahl KG, Bens S, Ziegler K, Rudolf S, Kordon A, Dibbelt L, et al. Angiogenic factors in patients with current major depressive disorder comorbid with borderline personality disorder. Psychoneuroendocrinology. 2009;34:353–357. doi: 10.1016/j.psyneuen.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Takebayashi M, Hashimoto R, Hisaoka K, Tsuchioka M, Kunugi H. Plasma levels of vascular endothelial growth factor and fibroblast growth factor 2 in patients with major depressive disorders. J Neural Transm. 2010;117:1119–1122. doi: 10.1007/s00702-010-0452-1. [DOI] [PubMed] [Google Scholar]
- Lee B-H, Kim Y-K. Increased plasma VEGF levels in major depressive or manic episodes in patients with mood disorders. J Affect Disord. 2012;136:181–184. doi: 10.1016/j.jad.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Mazure NM, Chen EY, Laderoute KR, Giaccia AJ. Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood. 1997;90:3322–3331. [PubMed] [Google Scholar]
- Viikki M, Anttila S, Kampman O, Illi A, Huuhka M, Setälä-Soikkeli E, et al. Vascular endothelial growth factor (VEGF) polymorphism is associated with treatment resistant depression. Neurosci Lett. 2010;477:105–108. doi: 10.1016/j.neulet.2010.04.039. [DOI] [PubMed] [Google Scholar]
- Amburgey OA, Chapman AC, May V, Bernstein IM, Cipolla MJ. Plasma from preeclamptic women increases blood-brain barrier permeability: role of vascular endothelial growth factor signaling. Hypertension. 2010;56:1003–1008. doi: 10.1161/HYPERTENSIONAHA.110.158931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argaw AT, Asp L, Zhang J, Navrazhina K, Pham T, Mariani JN, et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest. 2012;122:2454–2468. doi: 10.1172/JCI60842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto SM, Niskanen L, Herzig K-H, Tolmunen T, Huotari A, Viinamäki H, et al. Serum chemokine levels in major depressive disorder. Psychoneuroendocrinology. 2010;35:226–232. doi: 10.1016/j.psyneuen.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Uher T, Bob P. Cerebrospinal fluid IL-8 levels reflect symptoms of alexithymia in patients with non-inflammatory neurological disorders. Psychoneuroendocrinology. 2011;36:1148–1153. doi: 10.1016/j.psyneuen.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. Is depression an inflammatory disorder. Curr Psychiatry Rep. 2011;13:467–475. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH.The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D) Mol Psychiatrye-pub ahead of print 31 January 2012; doi: 10.1038/mp.2012.2 [DOI] [PMC free article] [PubMed]