Abstract
Many fishes make frequent ascents to surface waters and often show prolonged surface swimming following descents to deep water. This affinity for the surface is thought to be related to the recovery of body heat lost at depth. We tested this hypothesis using data from time–depth recorders deployed on four whale sharks (Rhincodon typus). We summarized vertical movements into bouts of dives and classified these into three main types, using cluster analysis. In addition to day and night ‘bounce’ dives where sharks rapidly descended and ascended, we found a third type: single deep (mean: 340 m), long (mean: 169 min) dives, occurring in daytime with extremely long post-dive surface durations (mean: 146 min). Only sharks that were not constrained by shallow bathymetry performed these dives. We found a negative relationship between the mean surface duration of dives in the bout and the mean minimum temperature of dives in the bout that is consistent with the hypothesis that thermoregulation was a major factor driving use of the surface. The relationship broke down when sharks were diving in mean minimum temperatures around 25°C, suggesting that warmer waters did not incur a large metabolic cost for diving and that other factors may also influence surface use.
Keywords: whale shark, thermal recovery, Rhincodon typus, vertical migration, diving behaviour
1. Introduction
Movement ecology is the quantitative study of animal movement and investigates the relationship between an organism's internal state, motion, navigation capabilities and the external factors affecting its movement [1]. Understanding the causes and mechanisms of animal movement is central to developing effective conservation and management strategies, for predicting responses to environmental change and revealing the factors driving the evolution of behaviour [2].
Horizontal movements are often the main focus of movement research; however, for many aquatic animals, vertical movements play an equally important role in their ecology and are critical to behaviours such as foraging and predator avoidance. Many species display repetitive vertical oscillations through the water column that begin and end at or near the surface. In the case of air-breathers, returns to surface waters are driven by the need to respire [3]; however, curiously, they are also typical of many gill-breathers [4–7], some of which spend considerable time at the surface [7]. The hypothesis of thermal recovery is one of the most widely cited to explain this phenomenon [7]. It suggests that surface intervals after diving are required to return the body temperature to levels necessary to regulate physiological processes after time spent in cooler, deeper waters and is one of the few hypotheses explaining differential use of the surface and deep habitats with empirical support [4,8]. Central to this idea is the observation that for ectothermic fishes, body temperature and thus the rate of physiological processes are dependent on water temperature [9]. Other hypotheses suggest that vertical oscillations of fishes are driven by the negative buoyancy of many pelagic species, which means that they must move continuously to counter their tendency to sink [10,11]. However, this hypothesis does not explain the prolonged surface swimming following ascents seen in many elasmobranchs.
Whale sharks (Rhincodon typus), the world's largest fish, are ideal candidates for examining the relationship between movement behaviour and the thermal environment in wild fishes. They are a deep diving (over 1000 m) [12] ectotherm, repeatedly descending throughout the day and night [5,13,14] and spending considerable amounts of time (49 ± 19%; mean for 12 sharks) at the surface (< 2 m) between dives [10]. Here, we construct a suite of models to test the prediction that the time at the surface is correlated with the time spent in cooler, deep waters, using time–depth records collected by archival tags deployed on free-swimming whale sharks. We are thus able to test whether shark movement is consistent with the behavioural thermoregulation hypothesis and provide an insight into how the movement decisions of free-ranging fishes are shaped by the thermal environment.
2. Material and methods
We used data from remote-sensing devices that were attached to four male whale sharks (4.4 ± 0.5 m total length) and subsequently recovered after deployment so that the fine-scale sampled data could be downloaded. Three of the whale sharks were instrumented with Mk-10 PAT tags (Wildlife Computers, Redmond, WA, USA) at Ningaloo Reef, Western Australia (22°45′ S, 113°37′ E) during April–May 2008 and one shark at Christmas Island (10°29′ S, 105°57′ E) during January 2008 with a SPLASH tag (Wildlife Computers). The SPLASH tag was attached to the shark's dorsal fin using a 1 m tether and fin clasp. PAT tags were connected with a tether to a titanium dart that was embedded in the sub-dermal layer at the base of the dorsal fin. Tags sampled depth (± 0.5 m), light and temperature (± 0.05°C) every 10 s (PAT) or 60 s (SPLASH) for the duration of the deployment and the SPLASH tag also collected location data via the ARGOS satellites. Tag deployment durations were 88 days for the SPLASH tag, and 6, 29 and 30 days respectively for the PAT tags.
The time series of dive depths was analysed in R [15], using the library diveMove [16]. The first step in implementing diveMove was to correct for the shift in the pressure transducers. We examined each record separately to determine the offset required, which were −1, 4, 4.5 and 6 m, respectively. In gill-breathers, the reference depth for calibration is not necessarily zero (as it is for air-breathers); however, whale shark vertical movement resembles that of air-breathers, with series of ascents and descents starting and ending near the surface [10].
The remaining steps involved: (i) identifying all dives in the records according to a minimum depth threshold that we set at 10 m; (ii) identifying dive phases (descent, bottom and ascent) and calculating dive statistics, e.g. duration, maximum depth, etc; and (iii) identifying bouts of diving behaviour (logical groupings of dives). Here, bout identification was based on a maximum-likelihood estimation procedure to model the distribution of sequential differences in post-dive surface interval duration [17,18]. A bout-ending criterion was calculated, which determined whether two successive dives should be grouped in the same bout, based on the difference in surface interval duration (see the electronic supplementary material for more details on the implementation of diveMove).
The offset correction and the minimum depth threshold used resulted in some near surface (just below the 10 m cut-off) behaviour being called a dive. These ‘dives’ constituted only a small proportion of the data and were typically short with a bottom time of zero. We isolated these dives and added their duration to the post-dive surface interval of the previous dive.
To identify differences in diving bouts, we applied a hierarchical cluster analysis to a range of bout summary statistics (table 1). We then used principal component analysis (PCA) on these data to determine whether diving behaviour differed among the groups identified by cluster analysis.
Table 1.
Mean and s.d. (for all sharks pooled) for each variable for each bout type. Light refers to light intensity in arbitrary units. Columns labelled as median refer to the median being calculated across the dives in each bout for each shark and the values in the table refer to the means of these values for all sharks.
| bout type | median min. dive temp. (°C) | median max. light per dive | median range in dive temp. (°C) | median max. dive depth (m) | median post-dive surface duration (min) | median dive duration (min) | median dive bottom time (min) | median dive descent rate (m s−1) | median dive ascent rate (m s−1) | bout duration (min) | no. dives per bout | post-bout surface interval (min) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 26.91 ± 1.45 | 181.55 ± 22.10 | 2.08 ± 3.51 | 54.81 ± 38.34 | 38.08 ± 56.52 | 13.93 ± 17.91 | 11.44 ± 18.74 | 0.56 ± 0.67 | −0.43 ± 0.43 | 134.91 ± 193.90 | 4.69 ± 8.94 | 52.60 ± 70.20 |
| 2 | 25.80 ± 3.41 | 57.55 ± 23.31 | 3.68 ± 5.42 | 73.98 ± 75.58 | 23.36 ± 37.22 | 24.04 ± 39.64 | 20.09 ± 35.60 | 0.49 ± 0.82 | −0.41 ± 0.51 | 244.09 ± 318.80 | 9.88 ± 15.51 | 32.05 ± 43.96 |
| 3 | 13.74 ± 4.35 | 197.55 ± 23.62 | 17.78 ± 5.66 | 340.29 ± 120.33 | 141.21 ± 166.51 | 168.54 ± 147.97 | 144.54 ± 146.81 | 0.44 ± 0.22 | −0.27 ± 0.13 | 369.91 ± 283.25 | 1.42 ± 1.65 | 145.73 ± 174.08 |
We determined the ocean depth corresponding to the ARGOS location data from the SPLASH tag using the ETOPO2 v. 2 dataset [19].
We constructed a suite of linear mixed-effect models using the nlme library in R. The log of mean surface duration (during the dive bout) was the response variable, mean minimum temperature, mean bottom time and bout type were predictor variables, and shark identity was a random variable. We compared and ranked models using weights of Akaike's information criterion corrected for small sample size (wAICc) [20]. We tested for autocorrelation in the data using the acf function in R, but none was found.
3. Results
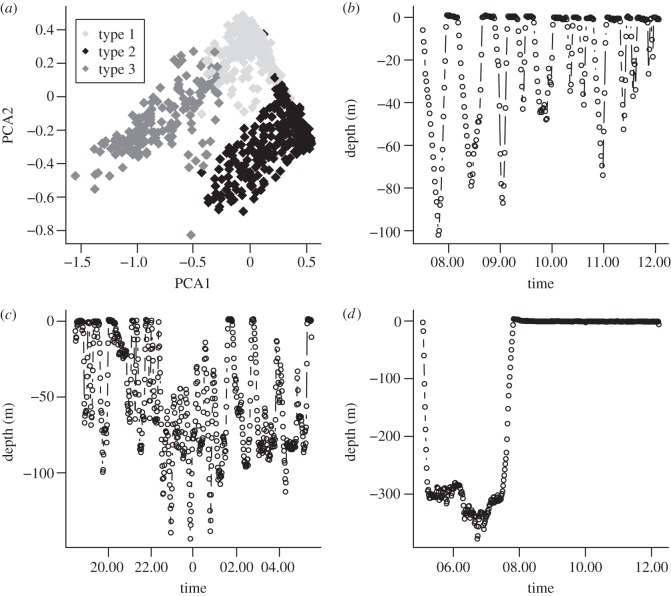
Three main groups of dives were found by cluster analysis and the characteristics of each are shown in table 1. The PCA indicated that 81 per cent of the variation in diving behaviour could be explained by the first two components (figure 1a). Variables associated with median minimum temperature of dives in the bout, median maximum light of dives in the bout (indicating whether day or night) and range in temperature of dives in the bout from the deepest to the shallowest point in the dive had the greatest contribution. Representative plots of the three bout types are shown in figure 1. Type 1 bouts occurred predominantly from 04.00 to 16.00 h, type 2 from 18. 00 to 06.00 h and type 3 from 03.00 to 18.00 h, with peaks at 05.00 and 12.00 h. Overall, type 1 and type 2 bouts were most common, constituting 44 per cent and 40 per cent of all bouts respectively, while type 3 bouts constituted 16 per cent. However, the percentage of type 3 bouts was highly variable. For the shark with the longest deployment (Christmas Island shark, 88 days), 47 per cent of its record was type 3 bouts, whereas the other three sharks had 0 per cent, 7 per cent and 9 per cent of these bouts, respectively (see the electronic supplementary material, table S1).
Figure 1.
(a) The two principal component scores are plotted for the three bout types identified by cluster analysis. Examples of (b) type 1, (c) type 2 and (d) type 3 bout types.
The whale shark tagged at Christmas Island travelled to Indonesia. For the first 17 days, the mean water depth for this shark was 154 ± 139 m. On day 18, the shark entered deeper water (1570 m) and thereafter its movements were largely in deep water (3482 ± 1867 m). This move coincided with an increase in the mean daily dive depth (see the electronic supplementary material, figure S1, shark 4). In comparison, the daily mean dive depth for the other sharks was relatively shallow (see the electronic supplementary material, figure S1).
The model including mean minimum temperature and bout type had majority support (0.70; table 2). There was a negative relationship between the mean minimum temperature of the bout and the mean surface duration (figure 2) for all bout types. Type 3 bouts had the longest mean surface durations (figure 2 and table 1). This relationship broke down in warm mean minimum temperatures (approx. 25°C; figure 2).
Table 2.
The two top-ranked models and the intercept-only model. Full results are shown in the electronic supplementary material, table S1. All models have random effects: individual shark (ID). Also shown are the number of estimable model parameters (k), maximum log-likelihood (LL), Akaike's information criterion corrected for small samples (AICc), the difference in AICc for each model from the top-ranked model (ΔAICc) and the model weight (wAICc).
| model | k | LL | AICc | ΔAICc | wAICc |
|---|---|---|---|---|---|
| ∼ min. temp + bout type + (1|ID) | 6 | −1692.21 | 3396.48 | 0 | 0.70 |
| ∼ min. temp × bout type + (1|ID) | 8 | −1691.06 | 3398.22 | 1.74 | 0.30 |
| ∼ 1 (NULL model) + (1|ID) | 3 | −1792.17 | 3590.34 | 193.86 | <0.01 |
Figure 2.
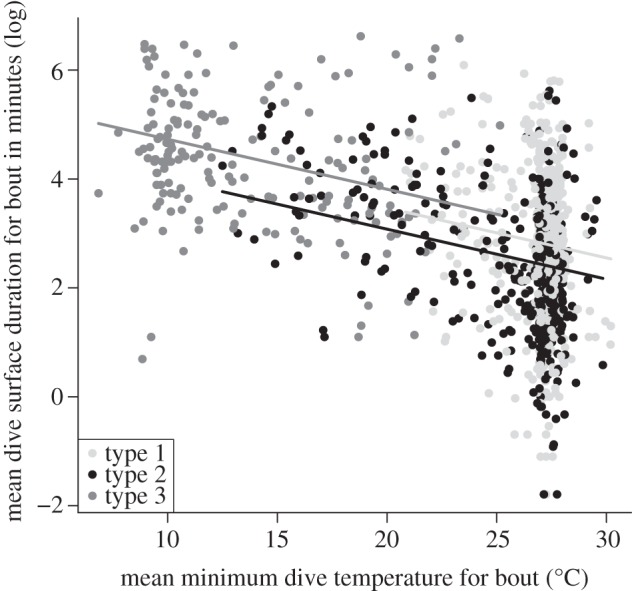
Relationship between the mean post-dive surface duration and mean minimum temperature. Shown are the fitted lines (of the top-ranked model) among sharks for each bout type.
4. Discussion
Our results are consistent with the thermoregulation hypothesis, which suggests that surface intervals are required to return the body temperature of ectothermic fishes to levels necessary to regulate physiological processes after time spent in cooler, deep waters [4,8]. Regular, deep descents by animals inhabiting the open ocean are thought to be driven by foraging [13,21–23]. Because of their filtering mode of feeding (which involves the use of gills to filter small plankton and fishes from the water column), it might be expected that despite their size, whale sharks should cool relatively swiftly when feeding in deeper waters, at least in comparison with piscivorous species that must respire, but are not required to pass large volumes of water over a gill in order to obtain their food. This problem would be compounded for whale sharks that have a physiology adapted to the warm (generally > 20°C) surface waters where they are usually found.
The relationship we found between the mean minimum temperature of the bout and the mean surface duration broke down when sharks were diving in minimum temperatures above 25°C. In these warm waters, more than one factor may be responsible for extended surface durations such as satiation, predator avoidance or rest. Alternatively, or in addition, in warm surface waters, the need for behavioural thermoregulation could be reversed, so that descents to cooler deep waters are necessary to dissipate excess heat gained from swimming at the surface [24]. Perhaps in this case, a strict thermoregulatory explanation might not be applicable, but rather behavioural thermoregulation is used to reduce metabolic losses and increase foraging efficiency [25].
Available technology did not permit measurement of body temperatures using internal sensors, because this would have required restraint of the subject animals, which was not possible owing to ethical and logistic constraints. However, the assumption that the external temperatures measured by the sensors provided a measure of body temperature of the sharks is reasonable, given that these animals are ectotherms.
Type 3 bouts largely consisted of one long, deep dive and were associated with the longest surface durations. Three of the sharks exhibited the deep type 3 bouts, but only one of these had large quantities (the Christmas Island shark). This was likely due to the record being much longer for this shark (88 days). Unlike the other individuals, during most of the record, the shark travelled over open oceans beyond continental shelves, where it was not bathymetrically constrained in vertical movements by shallow water.
Despite the increasing sophistication of archival and satellite tags, particularly in terms of the variety of physiological and environmental variables that can now be recorded [26], time–depth records are probably the most common form of data retrieved from tag deployments on fishes inhabiting the open ocean; in historical terms, they certainly form some of the largest data archives from deployments of earlier model tags. Our study shows how these data records might be analysed and interpreted to provide insights into the physiological drivers of vertical movement in fishes.
References
- 1.Nathan R. 2008. An emerging movement ecology paradigm. Proc. Natl Acad. Sci. USA 105,19 050–19 051 10.1073/pnas.0808918105 (doi:10.1073/pnas.0808918105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sims DW. 2010. Tracking and analysis techniques for free-ranging shark movements and behavior. In Sharks and their relatives II (eds Carrier JC, Musick JA, Heithaus MR.), pp. 351–392 Boca Raton, FL: CRC Press [Google Scholar]
- 3.Schreer JF, Kovacs KM, Hines RJO. 2001. Comparative diving patterns of pinnipeds and seabirds. Ecol. Monogr. 71, 137–162 10.1890/0012-9615(2001)071[0137:CDPOPA]2.0.CO;2 (doi:10.1890/0012-9615(2001)071[0137:CDPOPA]2.0.CO;2) [DOI] [Google Scholar]
- 4.Carey FG, Scharold JV. 1990. Movements of blue sharks (Prionace glauca) in depth and course. Mar. Biol. 106, 329–342 10.1007/BF01344309 (doi:10.1007/BF01344309) [DOI] [Google Scholar]
- 5.Graham RT, Roberts CM, Smart JCR. 2006. Diving behaviour of whale sharks in relation to a predictable food pulse. J. R. Soc. Interface 3, 109–116 10.1098/rsif.2005.0082 (doi:10.1098/rsif.2005.0082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heithaus MR, Dill LM, Marshall GJ, Buhleier B. 2002. Habitat use and foraging behaviour of tiger sharks (Galeocerdo cuvier) in a seagrass ecosystem. Mar. Biol. 140, 237–248 10.1007/s00227-001-0711-7 (doi:10.1007/s00227-001-0711-7) [DOI] [Google Scholar]
- 7.Klimley AP, Beavers SC, Curtis TH, Jorgensen SJ. 2002. Movements and swimming behaviour of three species of sharks in La Jolla Canyon, California. Environ. Biol. Fishes 63, 117–135 10.1023/A:1014200301213 (doi:10.1023/A:1014200301213) [DOI] [Google Scholar]
- 8.Holland KN, Brill RW, Chang RKC, Sibert JR, Fournier DA. 1992. Physiological and behavioural thermoregulation in bigeye tuna (Thunnus obesus). Nature 358, 410–412 10.1038/358410a0 (doi:10.1038/358410a0) [DOI] [PubMed] [Google Scholar]
- 9.Sims DW. 2003. Tractable models for testing theories about natural strategies: foraging behaviour and habitat selection of free-ranging sharks. J. Fish Biol. 62(Suppl. A), 53–73 10.1111/j.1095-8649.2003.00207.x (doi:10.1111/j.1095-8649.2003.00207.x) [DOI] [Google Scholar]
- 10.Gleiss AC, Norman B, Wilson RP. 2011. Moved by that sinking feeling: variable diving geometry underlies movement strategies in whale sharks. Funct. Ecol. 25, 595–607 10.1111/j.1365-2435.2010.01801.x (doi:10.1111/j.1365-2435.2010.01801.x) [DOI] [Google Scholar]
- 11.Weihs D. 1973. Mechanically efficient swimming techniques for fish with negative buoyancy. J. Mar. Res. 31, 194–209 [Google Scholar]
- 12.Wilson SG, Polovina JJ, Stewart BS, Meekan MG. 2006. Movements of whale sharks (Rhincodon typus) tagged at Ningaloo Reef, Western Australia. Mar. Biol. 148, 1157–1166 10.1007/s00227-005-0153-8 (doi:10.1007/s00227-005-0153-8) [DOI] [Google Scholar]
- 13.Brunnschweiller JW, Baensch H, Pierce SJ, Sims DW. 2009. Deep-diving behaviour of a whale shark (Rhincodon typus) during long-distance movement in the western Indian Ocean. J. Fish Biol. 74, 706–714 10.1111/j.1095-8649.2008.02155.x (doi:10.1111/j.1095-8649.2008.02155.x) [DOI] [PubMed] [Google Scholar]
- 14.Eckert SA, Stuart BS. 2001. Telemetry and satellite tracking of whale sharks, Rhincodon typus, in the Sea of Cortez, Mexico, and the north Pacific Ocean. Environ. Biol. Fishes 60, 299–308 10.1023/A:1007674716437 (doi:10.1023/A:1007674716437) [DOI] [Google Scholar]
- 15.R Development Core Team 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 16.Luque SP. 2007. Diving behaviour analysis in R. R News 7, 8–14 [Google Scholar]
- 17.Luque SP, Arnould JPY, Guinet C. 2008. Temporal structure of diving behaviour in sympatric Antarctic and subantarctic fur seals. Mar. Ecol. Prog. Ser. 372, 277–287 10.3354/meps07689 (doi:10.3354/meps07689) [DOI] [Google Scholar]
- 18.Luque SP, Guinet C. 2007. A maximum likelihood approach for identifying dive bouts improves accuracy, precision and objectivity. Behaviour 144, 1315–1332 10.1163/156853907782418213 (doi:10.1163/156853907782418213) [DOI] [Google Scholar]
- 19.US Department of Commerce NOAA 2006. National Geophysical Data Center. 2-minute gridded global relief data (ETOPO2v2). See http://www.ngdc.noaa.gov/mgg/fliers/06mgg01.html.
- 20.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York, NY: Springer [Google Scholar]
- 21.Gore MA, Rowat D, Hall J, Gell FR, Ormond RF. 2008. Transatlantic migration and deep mid-ocean diving by basking shark. Biol. Lett. 4, 395–398 10.1098/rsbl.2008.0147 (doi:10.1098/rsbl.2008.0147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura I, Watanabe YY, Papastamatiou YP, Sato K, Meyer CG. 2011. Yo-yo vertical movments suggest a foraging strategy for tiger sharks Galeocerdo cuvier. Mar. Ecol. Prog. Ser. 424, 237–246 10.3354/meps08980 (doi:10.3354/meps08980) [DOI] [Google Scholar]
- 23.Sims DW, et al. 2008. Scaling laws of marine predator search behaviour. Nature 451, 1098–1102 10.1038/nature06518 (doi:10.1038/nature06518) [DOI] [PubMed] [Google Scholar]
- 24.Block BA, et al. 2001. Migratory movements, depth preferences, and thermal biology of Atlantic bluefin tuna. Science 293, 1310–1334 10.1126/science.1061197 (doi:10.1126/science.1061197) [DOI] [PubMed] [Google Scholar]
- 25.Campana SE, Dorey A, Fowler M, Joyce W, Wang Z, Wright D, Yashayaev I. 2011. Migration pathways, behavioural thermoregulation and overwintering grounds of blue sharks in the northwest Atlantic. PLoS ONE 6, e16854. 10.1371/journal.pone.0016854 (doi:10.1371/journal.pone.0016854) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ropert-Coudert Y, Wilson RP. 2005. Trends and perspectives in animal-attached remote sensing. Front. Ecol. Environ. 3, 437–444 10.1890/1540-9295(2005)003[0437:TAPIAR]2.0.CO;2 (doi:10.1890/1540-9295(2005)003[0437:TAPIAR]2.0.CO;2) [DOI] [Google Scholar]