Abstract
In this paper, we study the response of zebrafish to a robotic-fish whose morphology and colour pattern are inspired by zebrafish. Experiments are conducted in a three-chambered instrumented water tank where a robotic-fish is juxtaposed with an empty compartment, and the preference of live subjects is scored as the mean time spent in the vicinity of the tank's two lateral sides. The tail-beating of the robotic-fish is controlled in real-time based on feedback from fish motion to explore a spectrum of closed-loop systems, including proportional and integral controllers. Closed-loop control systems are complemented by open-loop strategies, wherein the tail-beat of the robotic-fish is independent of the fish motion. The preference space and the locomotory patterns of fish for each experimental condition are analysed and compared to understand the influence of real-time closed-loop control on zebrafish response. The results of this study show that zebrafish respond differently to the pattern of tail-beating motion executed by the robotic-fish. Specifically, the preference and behaviour of zebrafish depend on whether the robotic-fish tail-beating frequency is controlled as a function of fish motion and how such closed-loop control is implemented.
Keywords: animal behaviour, bioinspiration, closed-loop control, ethorobotics, zebrafish
1. Introduction
Nature is frequently being used to draw inspiration for new design concepts [1]. Borrowing ideas from nature allows for the realization of better performing mechanical systems for human-centred applications [2]. Nevertheless, seldom has the feasibility of integrating such systems within their source of inspiration been investigated. In this context, the integration of bioinspired robots with their animal counterparts may allow a better understanding of animal behaviour [3] and may find application in agriculture [4], alien and pest species control [5], and animal bypass systems [6].
This interdisciplinary research field is generally referred to as ‘ethorobotics’ and is currently receiving more and more attention by both the biology and the robotics communities. Specifically, the interaction of robotic platforms, with various degrees of biomimicry, has been explored across a wide spectrum of animal taxa. Studies can be generally grouped in two classes depending on whether the robotic platform operates irrespective of the animal with which it is interacting or whether it is controlled based on feedback from animal response. We refer to the former class as ‘open-loop’ and the latter as ‘closed-loop’ ethorobotics. Open-loop control strategies have been implemented for crustaceans [7], honeybees [8], fish [9–14], quails [15], brush-turkeys [16], songbirds [17,18] and squirrels [19]. Closed-loop control has instead been implemented on cockroaches [20], fish [21], chickens [22], ducks [23], bowerbirds [24], dogs [25] and rats [26]. More specifically, ground-wheeled vehicles have been used to engage animals in earlier studies [20,22,23,26]; the interaction between a commercially available quadrupedal robot with dogs has been studied in Kubinyi et al. [25]; the response of shoals of golden shiners to a replica rigidly translating in a water tank has been investigated in Swain et al. [21]; and the posture and movement of a robotic female satin bowerbird remotely controlled based on courtship behaviours of males has been explored in Patricelli et al. [24].
In this study, the response of zebrafish to a robotic-fish controlled based on feedback from the animals' motion is studied. We consider a canonical preference test where zebrafish are confronted with competing stimuli in a three-chambered instrumented tank [9,13]. Specifically, the experimental conditions in this work comprise an empty compartment juxtaposed with a bioinspired robotic-fish exhibiting various tail-beating motions. The target species used in this experiment is the ‘wild-type’ phenotypical variety of zebrafish (Danio rerio), a fresh water fish species commonly used as an animal model in genetic and neurobiological laboratory studies [27,28]. Zebrafish have a high reproduction rate and short intergenerational time, as well a natural propensity to form social groups [29,30]. To influence zebrafish behaviour, the design of the robotic-fish incorporates salient determinants of attraction based on morphological similarities [31–33]. Specifically, the aspect ratio of the robotic-fish is similar to that of a zebrafish with an enlarged abdomen that simulates a fertile female, a feature that is shown to produce a high attraction in both sexes [33]. The colour pattern of the robotic-fish resembles the stripes and yellow pigmentation on live subjects, features that have been shown to be determinants of attraction in zebrafish through computer-animated images [31,32] and experiments on different phenotypes [33]. In addition, the robot's motility is selected to replicate typical locomotory patterns of carangiform/subcarangiform swimmers to which zebrafish are typically assimilated [34].
Differently from earlier studies [9,13], where the behaviour of zebrafish in response to a predetermined (open-loop) stimulus has been analysed, in this work, fish motion is acquired through an image-based tracking software to drive the tail-beating frequency of the robotic-fish in real-time (closed-loop). The tail section is composed of a compliant passive caudal fin and a rigid part actuated by a servomotor to undulate at a desired amplitude and angular speed. Drawing inspiration from the work of Kohler [35] on the interaction between conditioned and naive fish schools, we control the angular speed of the servomotor to vary the tail-beating frequencies as a function of the fish distance from the robot's compartment. In Kohler [35], it has indeed been demonstrated that trained juvenile carp can influence the behaviour of untrained individuals in response to a hidden food resource through the exhibition of a series of specific behavioural patterns involving changes in speed and direction of swimming. Here, we keep the amplitude of the servomotor oscillation fixed and we consider an array of strategies to control in real-time the tail-beating frequency of the robotic-fish. We focus on proportional and integral closed-loop control systems, where the tail-beating frequency of the robotic-fish depends on either the distance of the fish from it or the time spent by the fish in its vicinity. For each control system, we study positive and negative gains, that is, we consider both positive and negative correlations between the tail-beating frequency of the robotic-fish and fish distance or residence time. In addition to these four closed-loop control strategies, we present results for two additional conditions in which the servomotor's angular speed is held constant or varies in time independently of the fish motion. The hypothesis that zebrafish respond differently to the pattern of tail-beating motion executed by the robotic-fish is investigated in this study. By comparing fish response across conditions, we also expect to dissect a set of determinants of zebrafish attraction towards the robotic-fish. Results are analysed in terms of both fish preference and locomotory patterns as they differ from the reference condition, where both stimulus compartments are empty.
2. Material and methods
The experiment described in this work was approved by Polytechnic Institute of New York University (NYU-Poly) Animal Welfare Oversight Committee AWOC-2011-101 and AWOC-2012-102.
2.1. Animals and housing
Twenty zebrafish (Danio rerio) procured from a local aquarium store (Petland Discounts, Brooklyn, NY) and an online aquaria source (www.LiveAquaria.com, Rhinelander, WI, USA) were used for this study, which was performed between September and December 2011. Zebrafish involved in this study were approximately six- to eight-months old with a mean body length of ca. 3 cm. Individuals of this age have been shown to display prominent shoaling tendencies [36]. Fish were acclimated for a minimum of 12 days in the facility vivarium housed in the Department of Mechanical and Aerospace Engineering at NYU-Poly prior to the experimental campaign. Owing to their identical shoaling preference, both male and female wild-type zebrafish were selected in this study for almost identical shoaling preference of male and female subjects [33]. Fish were housed in groups of 10 in separate holding tanks each 50 cm long, 25 cm wide and 30 cm high, with a capacity of 36 l, during both the acclimatization and the experimental phases. Water temperature was maintained at 26 ± 1°C, and the illumination was provided by fluorescent lights for 10 h each day in accordance with the circadian rhythm of zebrafish [29]. Fish were fed with commercial flake food (Hagen Corp., Nutrafin max, USA) once a day, after the conclusion of the daily experimental session.
2.2. Apparatus
The instrumented test-tank included a 65 l glass aquarium situated in a larger Acrylic tank supported by an aluminium frame structure. The dimensions of the glass aquarium were 74 × 30 × 30 cm in length, height and width, respectively, whereas the Acrylic tank's dimensions were 120 × 20 × 120 cm. The aluminium frame structure (135 × 180 × 120 cm in length, height and width, respectively) was modular, which allowed for simple instrument upgrades and provided self-contained lighting and video-capture features.
The glass aquarium consisted of three compartments: a large focal compartment and two smaller stimulus compartments. The focal compartment was 54 cm long and centred in the middle of the aquarium. The remaining space on the sides of the aquarium was partitioned, using 0.5 cm thick transparent Acrylic panels. In other words, each of the two stimulus regions was 10 cm long and was alternatively used to house the robot stimulus, if present. The fish were free to explore the entire focal compartment, but the Acrylic panels restricted them from entering the stimulus areas, with the twofold intent of dissecting visual stimulation from other cues and facilitating fish real-time tracking. Technical details on the role of the panels on fish visual perception are presented in the electronic supplementary material.
The water condition in the housing and experimental tanks was regulated with external overflow filters (Aqueon, QuietFlow 10–100 GPH) to maintain water quality and a heater (Elite, A750) for temperature control. The heater and filter were removed from the experimental tank during the experimental periods to facilitate identification of fish.
A webcam, interfacing with a computer via a universal serial bus (USB), was implemented as the overhead camera to provide a bird's eye view of the experimental tank. The camera was positioned 100 cm above the water's free surface to decrease the effects of barrel distortion owing to the curvature of the lens, while still being close enough to provide ample resolution for fine position tracking.
Two 50 W fluorescent lights illuminated the test tank from the direction of the longitudinal walls of the glass aquarium at a distance of 50 cm from the walls and were approximately levelled with the top edge of the tank. Dark fabric curtains were suspended from the top of the aluminium frame structure and covered the perimeter of the tank. The curtains isolated the experimental set-up from external visual disturbances and allowed the precise control of stimuli introduced during the experiment.
2.3. Robotic-fish
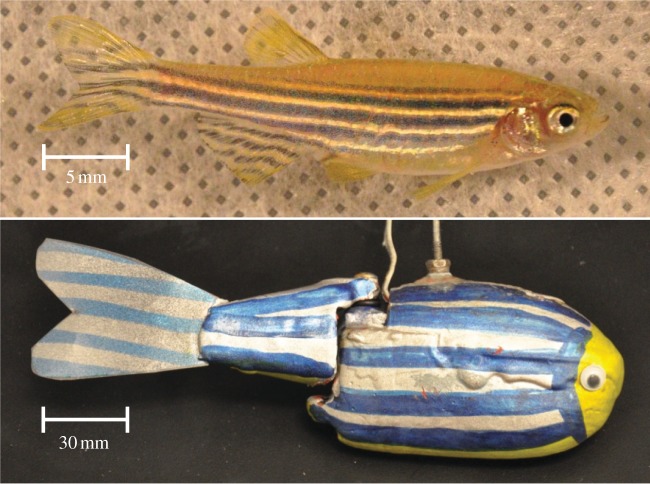
The robotic-fish used in this study was adapted from a miniature, free-swimming and remotely controlled bioinspired robot designed for ethorobotics [37] and for K-12 education and outreach [38]. The robot's tail, including a flexible caudal fin, was controlled by an Arduino microcontroller to obtain a bending of the flexible fin inspired by carangiform/subcarangiform swimming typical of zebrafish [34]. The robot was 15 cm long, 4.8 cm high and 2.6 cm wide, which was approximately five times larger than the live subjects to house the electronics needed for autonomous operation if it were left untethered (figure 1).
Figure 1.
Comparison of the robotic-fish to a zebrafish individual. (Online version in colour.)
Following earlier studies [9,13], the robot was rubberized and painted to resemble the colour and stripe pattern of zebrafish. Further details on the chromatic contrast of the robotic fish when compared with live subjects are presented in the electronic supplementary material. However, the robot considered in this study is not recognized as a conspecific by zebrafish; indeed, live subjects, when confronted with the robotic-fish and a conspecific, preferred to spend time in the vicinity of a conspecific [13].
The robot was anchored to a thin stainless steel rod in one of the stimulus compartments. For the purpose of uninterrupted operation owing to battery depletion, power was provided to the servomotor through a wire extension running along the stainless steel rod. To ensure a homogeneous background between the two stimulus areas, an identical rod was inserted in the empty compartment. The electronics received power from a computer USB port, which also allowed serial communication with the host computer for control of the tail-beating frequency f along with the amplitude B and mean value α of the servomotor oscillation with respect to the neutral axis.
2.4. Visual tracking
Real-time acquired data were collected through a vision system comprising a computer (Dell, Vostro 220 s, 3 GB of memory, 2.5 GHz Pentium dual core e5200 processor, Ubuntu 11.04 32-bit) and the webcam (Webcam Pro 9000, Logitech) mounted on the experimental apparatus. A tracking program, developed in OpenCV 2.3.1 (opencv.willowgarage.com), was used to automatically mark the in-plane position of the fish in the experimental tank.
The two-dimensional position (x,y) of the fish was measured relative to the origin o of the xy-coordinate system located at the centre of the experimental tank (figure 2). Figure 2 shows a snapshot from a sample experimental trial, as seen from the webcam, with a red point marking the online-tracked position of the fish. Experimental conditions that did not require real-time tracking were recorded with the webcam, using the manufacturer's supplied software (Logitech, QuickCam Pro 9000) through a secondary computer (Hewlett Packard Compaq, 8100 Elite Small Form Factor). These videos were analysed offline, using a similar tracking algorithm to obtain the fish position-data.
Figure 2.
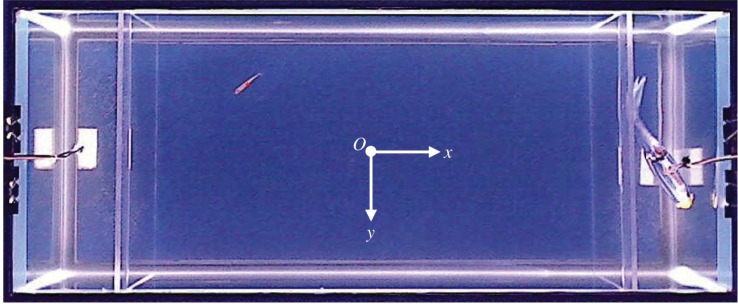
Snapshot from a sample experimental trial showing online tracking of a fish, marked with a red point, along with an overlayed coordinate system. (Online version in colour.)
The tracking algorithm detected the location of the fish in the test tank by using a combination of colour- and movement-based tracking. A similar method was used in Balch et al. [39] to track the in-plane positions of large groups of live insects using an overhead camera. A static background image of the experimental set-up was created prior to the start of a trial. Variations between experimental conditions, such as lighting, position of the tank with respect to the camera, were accounted for by updating the background image before each trial. The location of the fish for each captured frame was determined by comparing each frame with the static background image. More specifically, the static background image was subtracted from each frame and the resulting image was converted to greyscale and then to binary using a threshold value, tuned by the user. The centroid of the largest blob present in the image was marked as the position of the fish. To attenuate noise, a Gaussian filter was sometimes applied to the greyscale image with a resolution of 5 × 5 pixels2 to smoothen noise and improve tracking speed. We comment that, due to the fact that fish deform their shape and their trajectories cannot generally be embedded in planes parallel to the xy-plane, the method cannot be adapted to retrieve the position of the fish in the water column. More sophisticated methods have been presented in Butail & Paley [40], where three-dimensional positions and bending motions were tracked using a dual-camera set-up, yet their real-time implementation is limited by computational costs.
The computational load for fish localization during frames was reduced by using the previously known position of the fish to create a 128 × 128 pixels2 search window, centred about the previous location, to look for the fish's position. If the fish position was not known, the entire 1280 × 720 pixels2 region was scanned until the fish was found. Typical time between fish localizations was 0.14 s, yielding an average frame-rate of seven frames per second. These values normally fluctuated owing to variation in the time needed by the program to find the fish, yet these variations were small.
The tracked position of the fish was used to modulate the tail-beating frequency f of the robotic-fish. This modulation differed for the several control-based strategies implemented in this study, referred to as experimental conditions and discussed in what follows. Prior to commanding the robotic-fish to alter its tail-beating via a USB connection, five previous positions of the fish were averaged to yield the averaged distance from the robot compartment.
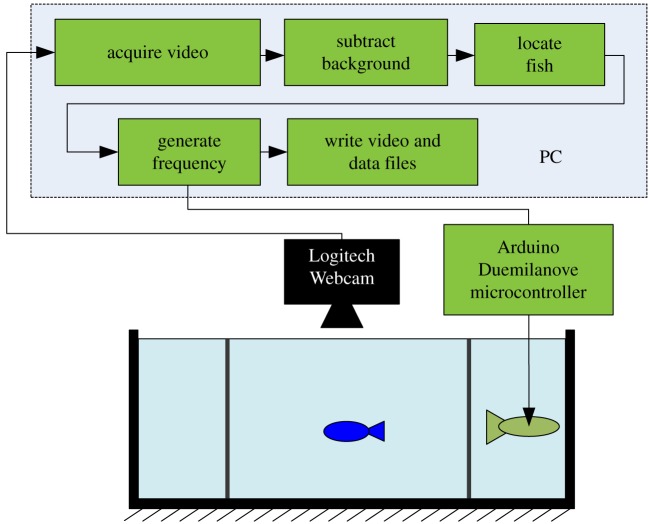
The x and y positions of the fish for the tracked ith frame were saved in a data file along with other information such as the start time t0 and current time ti of the trial, frame number, number of frames for which the fish position could not be determined and the location of the robotic-fish (left or right compartment) and its tail-beating parameters. The overall process is further illustrated with a schematic in figure 3.
Figure 3.
Schematic of the experimental set-up showing representations of a fish in the focal compartment being tracked with a webcam and the robotic-fish in one of the stimulus compartments receiving commands from a computer via a microcontroller. (Online version in colour.)
2.5. Experimental conditions
Six experimental conditions for the modulation of the tail-beating frequency f of the robotic-fish were studied. Four conditions used closed-loop control to regulate f as a function of the fish response, whereas two conditions did not consider fish motion to control f. In all these conditions, the robot was juxtaposed with the empty compartment.
The closed-loop conditions applied classical proportional and integral controllers using the distance of the fish from the wall of the stimulus compartment containing the robot, along the x-axis, as the control input [41]. More specifically, the closed-loop conditions P– and P+ proportionally modulated f in the range fmin = 1 to fmax = 3.6 Hz based on the distance of the fish d from the robot compartment using a positive and a negative gain, respectively. This frequency range was selected to provide a visibly different tail-motion as the fish progresses through the experimental tank, keeping a frequency of fn = 2.3 Hz when the fish was in the centre of the tank. The frequency fn would maximize the swimming speed if the robot were left untethered [37] and was used in Polverino et al. [13], where open-loop response of zebrafish was first characterized. The direction of frequency modulation was alternated between the two conditions. In particular, when the fish was immediately next to the robot compartment (d = 0), fP– = fmin and fP+ = fmax, where, here and henceforth, we use superscripts to identify conditions. The robot's tail-beating frequency for the two conditions was
| 2.1 |
and
| 2.2 |
where L = 54 cm was the length of the focal compartment, and
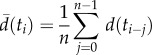 |
2.3 |
was the average distance from five previous frames (n = 5).
Conditions I+ and I– implemented integral controllers using the fish time spent in the further or closer half of the experimental tank's focal compartment. Depending on the condition, spending time on the side of tank close to the robot or far from it would either increase or decrease f. Specifically, the tail-beating frequencies for I– were
with
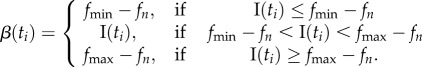 |
2.4 |
Here,
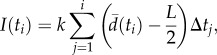 |
2.5 |
where Δtj = tj−tj−1 is the time difference between data samples, and k = 0.08 cm−1 s−2 is a control gain. Condition I+ was obtained by setting k = −0.08 cm−1 s−2 in (2.5). An experiment for each of these conditions is reported in the electronic supplementary material, videos S1–S4.
The open-loop experimental conditions C and U did not consider the fish position for varying the robot's tail-beating frequency. In particular, C, also executed in earlier studies [9,13], prescribed a constant tail-beating frequency of 2.3 Hz irrespective of the fish position in the tank, that is,
| 2.6 |
while U executed a tail-beating response to a ‘pre-recorded’ video from a trial of P+, for all trials in this condition. That particular trial was selected owing to its considerable variation of the tail-beating frequency.
In summary, in P+, the robotic-fish beats its tail faster if the fish is closer and slower if it is further; in P–, the robotic-fish beats its tail faster if the fish is further and slower if it is closer; in I+, the robotic-fish beats its tail faster if the fish spends more time in its vicinity and slower if it resides more away; in I–, the robotic-fish beats its tail faster if the fish spends more time away from it and slower if it resides more in its proximity; in C, the robotic-fish beats its tail at a constant frequency; and in U, the robotic-fish varies its tail-beating frequency irrespective of fish preference.
A supplementary control condition in which the fish was confronted with two empty compartments was also executed. This reference condition, referred to as O, is aimed at assessing bias in the experimental set-up and defining a baseline for fish behaviour.
2.6. Experimental procedure
Experiments were performed in an isolated facility at the Department of Mechanical and Aerospace Engineering at NYU-Poly under controlled conditions.
The robotic-fish was fixed in one of the stimulus compartments and oriented at approximately 45° with respect to the longitudinal wall of the glass aquarium. This configuration allowed a clear view of the robot's beating tail to the fish in the focal compartment. The tail-beating frequency was controlled by the host computer to which the robot was connected during the experiment. The robotic-fish was systematically alternated between the two stimulus compartments during each experimental condition in order to reduce the risk of bias in the data due by a persistent preference of the zebrafish for a side of the test tank.
For each experimental condition, fish were selected at random from the same holding tank, manually captured by a net, and placed into the focal compartment of the experimental set-up. Each fish was allowed to habituate for 10 min prior to data acquisition, which consisted of a 5 min experimental period. The initial 10 min allowed the fish to acclimate to the new environment and recuperate after being transferred from its holding tank, and its duration exceeded the amount of time typically considered sufficient for excluding novelty effects [42].
For the closed-loop experimental conditions P–, P+, I+ and I–, fish position was tracked in real-time and the relative position of the fish with respect to the robot controlled its tail-beating frequency during the entire 15 min trial. Fish positions were stored for the whole 15 min, yet only the last 5 min were used for analysis. Each condition was tested in four repetitions of 10 trials each, so that a fish was tested four times per condition. To assure that in each repetition fish were not tested multiple times, they were isolated from their holding tank after being tested. Each fish was tested no more than two times per day to minimize stress.
Conditions P+, P–, C, I– and I+ were executed (in this temporal order) on fish from one holding tank, while fish from the other tank were used to perform O and U (in this temporal order).
2.7. Data processing and behavioural classification
MathWorks Matlab (www.mathworks.com/products/matlab/index.html) was used to analyse preference and behaviour of the fish.
Fish preference was scored in terms of their positions in the focal compartment. For the analysis, data on two-dimensional positions of fish during the experimental trial were converted into one-dimensional distances along the tank's longitudinal axis. Behavioural analysis was instead based on two-dimensional positions.
A script for extraction of fish behaviour was created adapting the ethograms described by recent studies [32,43] to include the following behaviours: ‘freezing’ (a lack of mobility), ‘thrashing’ (rapid changing of swimming direction next to a wall or while in contact with the wall) and ‘swimming’ (locomotion in any direction). This script was devised to automatically classify fish behaviour, which was normally analysed using commercially available software such as Observer v. 2.0 (www.noldus.com/human-behaviour-research/products/theobserver-xt). Details on the implementation of the script are reported in the electronic supplementary material.
For each trial, both the fish position and the behavioural patterns exhibited were used to ascertain fish preference within the 5 min experimental session. The three partitions of the focal compartment included two near-stimulus areas, each within four fish body-lengths from the stimulus compartment wall and a central region, comprising the remaining space of the focal compartment.
2.8. Statistical analysis
As mentioned earlier, 40 trials were performed for each experimental condition and analysed to compute the time spent by the fish exhibiting each of the three behavioural patterns in the three focal compartments. In other words, each 300 s trial was partitioned into nine intervals that represent the time spent exhibiting each behaviour in each focal compartment. These nine numbers were resolved into three by first considering the total time spent in each focal compartment, and then by considering the total time spent by fish exhibiting each behaviour. Finally, we considered the time spent exhibiting each behaviour in both of the stimulus compartments, that is, near the robot and near the empty stimulus. Fish preference for a given condition was taken as proportional to the time spent near the robot in any of the three behaviours.
Data analysis was carried out using Statview v. 5.0. A one-way analysis of variance (ANOVA) was used for assessing variations in the time spent in each focal compartment or exhibiting each behaviour. Specifically, the time spent in each focal compartment (combining all three behaviours) or behaviour (combining all three focal compartments) from each of the 40 trials was the dependent variable and the condition was the independent variable. Furthermore, to study the repetition-effect on the time spent near the robot in a given condition, a one-way ANOVA was used with the repetition taken as the independent variable. Finally, a one-way ANOVA was used to assess the effect of the condition on the time spent in each stimulus compartment and behaviour simultaneously, with condition as the independent variable and compartment and behaviour as the dependent variables. Data between repetitions were unmatched as the order of testing of fish was not retained. The significance level was set at p ≤ 0.05. Fisher's protected least significant difference (PLSD) post hoc tests were used where a significant main effect of the condition variable was observed. Condition O was included in the swimming analysis as a baseline to ascertain differences in fish behaviour caused by the robot's presence and tail-beating.
3. Results
3.1. Zebrafish preference
Across all the experiments, fish were never consistently found away from the robot, that is, they always spent a portion of their time in the proximity of the robot. The mean amount of time that the fish spent in each of the three areas of the focal compartment was generally different between the experimental conditions (figure 4).
Figure 4.
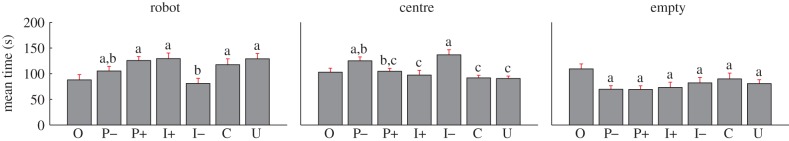
Histograms of the mean time spent by the fish in each of three areas in the focal compartment of the experimental tank for each experimental condition. Error bars refer to the s.e. Means not sharing a common superscript are significantly different (Fisher's PLSD, p < 0.05). We note that for O, the ‘robot’ region refers to the left side of the tank and that such condition is not part of the statistics due to the arbitrariness in the selection of the juxtaposed stimuli. See the end of §2.5 for a description of experimental conditions. (Online version in colour.)
The time spent near the robot was found to significantly vary between conditions (F5,216 = 3.50, p ≤ 0.01). Specifically, condition I+ showed the highest mean time spent in the vicinity of the robot (129.3 s). Post hoc comparisons revealed a statistical difference between condition I– and conditions P+, I+, C and U, which showed an increase in the mean time spent in the vicinity of the robot of 44.8, 48.4, 36.6 and 48.1 s, respectively.
For the time spent by fish in the central region, an effect of the condition was also observed (F5,216 = 6.39, p ≤ 0.01). In contrast to the analysis of the time spent in the vicinity of the robot, condition I– showed the highest mean time spent in the central area (136.6 s), which was found to be statistically different from P+, I+, C and U by post hoc comparisons. Specifically, the decrease in the time spent in the central region was found to be 32.0, 39.5, 45.0 and 46.1 s, respectively. Post hoc comparisons also revealed a significant decrease in the time spent in the central region in condition P– than in condition I+, C and U, which showed a decrease in mean time spent in this region of 27.8, 33.3 and 34.4 s, respectively.
The time spent in the empty region was found instead to not significantly differ between conditions. However, the highest amount of time spent in the empty compartment was observed in condition C.
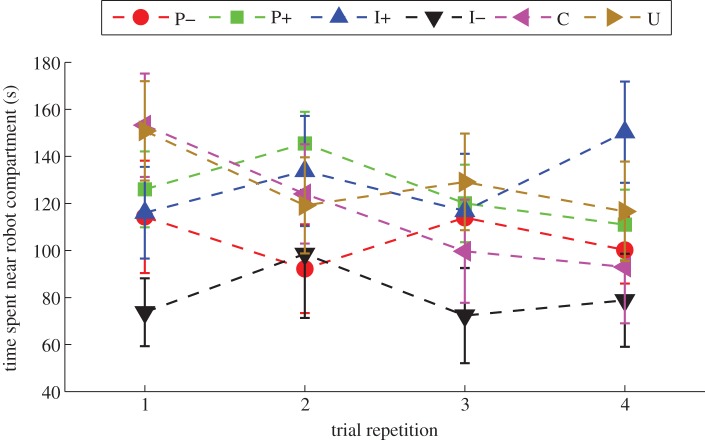
In figure 5, the mean time spent near the robot compartment along with the standard error mean for each of the six experimental conditions as a function of the trial repetition is reported. The attraction for the robot was the strongest in C in the first trial repetition, with a mean time spent near the robot compartment of 153.3 s. Attraction for the robot became weakest in C in the last trial repetition, with a mean time spent near the robot compartment of 93.0 s. Yet, the repetition-effect in C was not found to be significant (F3,36 = 3.20, p = 0.08).
Figure 5.
Mean time spent near robot compartment split into four 10-trial repetitions, here 10 distinct fish appear in each repetition exactly once. Error bars refer to the s.e. See the end of §2.5 for a description of experimental conditions. (Online version in colour.)
3.2. Zebrafish swimming
The mean amount of time the fish spent swimming varied significantly between the experimental conditions (F6,252 = 9.55, p ≤ 0.01; figure 6). Specifically, fish minimized their mean time spent swimming in I+ and I– (237.9 and 242.0 s, respectively). The time spent not swimming mirrors the time spent swimming, which implies, for example, that I+ and I– displayed the largest mean time spent non-swimming. Post hoc comparisons showed a significant increase in the time spent swimming when comparing I+ and I– with P+ (32.4, and 28.3 s, respectively), P– (50.1 and 46.0 s, respectively), C (38.2 and 34.1 s, respectively), U (54.7 and 50.6 s, respectively) and O (51.2 and 47.1 s, respectively). Furthermore, the mean swimming time observed in U was found to be significantly higher compared with P+, where the time was reduced by 22.3 s.
Figure 6.
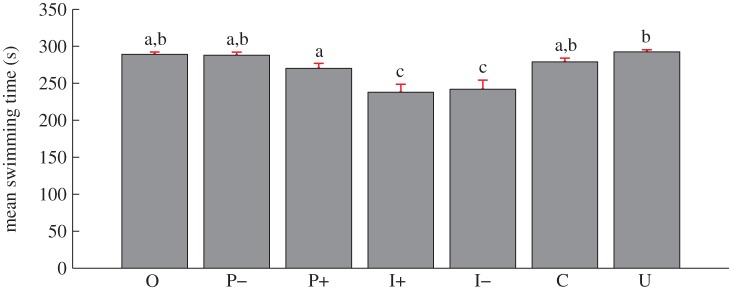
Histograms of the mean time spent by the fish exhibiting swimming behaviour for each experimental condition. Error bars refer to the s.e. Means not sharing a common superscript are significantly different (Fisher's PLSD, p < 0.05). See the end of §2.5 for a description of experimental conditions. (Online version in colour.)
3.3. Zebrafish behavioural response in the near-stimulus regions
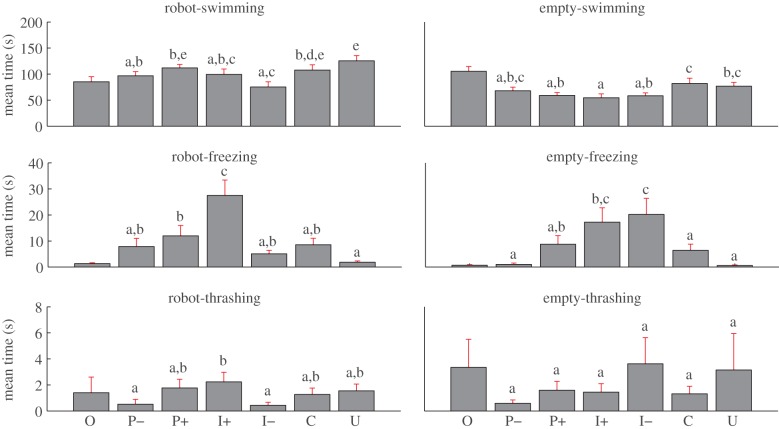
The time spent by fish exhibiting swimming, freezing and thrashing behaviours near the two stimulus compartments was found to be generally different between experimental conditions (figure 7).
Figure 7.
Histograms of the mean time spent by the fish exhibiting each of the three behaviours near each of the two stimulus compartments of the experimental tank. Error bars refer to the s.e. Means not sharing a common superscript are significantly different (Fisher's PLSD, p < 0.05). We note that for O, the ‘robot’ region refers to the left side of the tank and that such condition is not part of the statistics owing to the arbitrariness in the selection of the juxtaposed stimuli. See the end of §2.5 for a description of experimental conditions. (Online version in colour.)
In relation to the robot stimulus region, a significant condition-dependent effect was observed for the swimming behaviour (F5,234 = 3.37; p ≤ 0.01). In other words, the mean time spent by fish swimming in the vicinity of the robot was influenced by the experimental condition, with the highest swimming level observed in U (125.5 s). Post hoc comparison revealed a decrease in the swimming time in the vicinity of the robot between U and P–, I+ and I– by 28.8, 26.0, and 50.2 s, respectively. A significant increase was conversely found between I– and both P+ and C by 36.5 and 32.3 s, respectively. For the case of freezing, a significant condition-dependent effect was also found in the area adjacent to the robot stimulus (F5,234 = 7.04; p ≤ 0.01). Furthermore, the highest time spent freezing was found in I+ (27.5 s) and post hoc comparisons revealed a significant decrease in this time than in P+, P–, I–, C and U by 15.5, 19.6, 22.4, 18.9 and 25.6 s, respectively. Post hoc comparison also showed that the time spent freezing in P+ was significantly higher than in U by 10.1 s. Differently, for the thrashing behaviour near the robot a condition-effect was not found. In other words, the time spent by fish thrashing in the robot region was not significantly different among experimental conditions. However, post hoc comparisons showed that the time spent thrashing in I+ was significantly higher than in P– and I–, by 1.7 and 1.8 s, respectively.
For the empty stimulus region, the time spent swimming was also found to be condition-dependent (F5,234 = 2.31; p ≤ 0.05). Post hoc comparisons revealed significant differences between the time spent swimming among the different experimental conditions, with C that showed the highest time spent swimming in this stimulus region (82.0 s). Such time was found to be significantly higher than in P+, I+ and I– by 23.1, 27.5 and 23.8 s, respectively, as well as for U that compared with I+ showed a mean time swimming in the empty region 22.2 s higher. For the case of freezing, a significant condition-dependent effect was also found in the area adjacent to the empty stimulus (F5,234 = 4.67; p ≤ 0.01). In particular, I– showed the highest freezing time (20.2 s) that post hoc comparison revealed significantly different than in P+, P–, C and U, by 11.5, 19.2, 13.8 and 19.6 s, respectively. In addition, the time spent freezing in I+ was also found to be significantly higher than in P–, C and U, by 16.2, 10.8 and 16.6 s, respectively. As for the robot stimulus region, the thrashing behaviour near the empty stimulus was not condition-dependent, that is, the time spent by fish thrashing in the empty region was not significantly different among experimental conditions.
4. Discussion
The results of this study confirm that a robotic-fish whose morphology and colour pattern are designed by drawing inspiration from zebrafish social behaviour is able to differently attract live subjects depending on its pattern of tail-beating motion. Specifically, the degree of attraction of zebrafish for the robot depends on whether its tail-beating frequency is controlled as a function of fish response and how such closed-loop control is implemented.
The robotic-fish used in this study is considerably larger than live subjects (approx. five times) to accommodate for the requisite electronics for remotely controlled untethered operations and maintain the aspect ratio of a fertile female [33]. Yet, zebrafish attraction for the robotic-fish is probably not explained as an instance of ‘predator inspection’ to gain information about a putative predator [44]. Indeed, this explanation would not be compatible with the selected experimental protocol that featured a 10 min habituation to the stimuli [42]. Another explanation of zebrafish preference for the robotic-fish may hinge on a novelty effect; yet, this hypothesis would also conflict with the extended habituation period used in this study. The potential view of the larger robotic-fish as a shelter for zebrafish is also unlikely to be feasible given the presence of solid panels that do not allow the live subjects to find shelter behind the robot [45]. Thus, the preference of zebrafish for the robotic-fish is likely to be based on the gregarious nature of this species and on salient features purposefully displayed by the robot, that is, a bright yellow pigment, comparable stripe pattern, curbed shape and carangiform/subcarangiform undulations whose influence on zebrafish response has been dissected in earlier studies [9,13]. Reducing the size of the robotic-fish is likely to enhance zebrafish attraction in light of the fact that zebrafish prefer a conspecific to the robotic-fish beating its tail at a prescribed frequency [13]. Nevertheless, the latter evidence may also be explained by considering that, in open-loop conditions, the robotic-fish was not able to balance the visual feedback offered by the conspecific.
The visual features incorporated in the design of the robotic-fish have been largely based on biological studies on zebrafish interaction with computer-animated stimuli and heterospecifics [31–33]. Differently from computer-animated stimuli, the robotic-fish offers a wide spectrum of sensory cues to zebrafish; thus, the observed preference may a priori be attributed to the complex interplay between such cues. Nevertheless, the presence of solid Acrylic panels minimizes the effect of flow-based sensory feedback, which could result in hydrodynamic advantages [12], along with chemical or electrical cues. The presence of a servomotor within the robotic-fish produces a high-frequency noise associated with mechanical friction between moving parts, measured to be on the order of 2–5 kHz [13], and thus perceived by zebrafish [46]. Yet, such high-frequency noise is largely independent of the low-frequency actuation and is thus expected to be consistent across the conditions studied in this work. Therefore, the evidence that conditions are generally different and, in particular, that condition U (in which the tail-beating frequency of the robotic-fish is uncorrelated to fish response) is different from other conditions seem to hamper a possible explanation of zebrafish attraction based on the auditory cue. In agreement with previous findings supporting the dominance of visual cues in zebrafish response [31–33], we favour an explanation of the attraction of live subjects towards the robotic-fish based on visual perception.
The attraction of zebrafish towards the robotic-fish depends on how the robot modulates its tail-beating frequency. Such modulation is performed by following closed- and open-loop schemes; namely correlating tail motion in real-time to fish behaviour or independently modulating it, respectively. Among the closed-loop approaches, experimental conditions in which the feedback gain is positive, that is, the tail-beating frequency of the robot increases as either fish approach, condition P+, or spend more time close to the robot, I+, are generally preferred. Preference towards a robotic-fish that beats its tail faster as live subjects are closer is in accordance with observations on attractive strategies used by trained fish to influence naive conspecifics [35,47,48]. More specifically, three types of behaviour have been documented in juvenile carps trying to influence a shoal of naive conspecifics [35,48] and similar evidence has been found in golden shiners [47]. From Kohler [35], such behaviours include: (i) increase in tail-beating frequency connected with an increase of swimming speed; (ii) swimming in the direction of the desired location back to the shoal repetitively; and (iii) repeated movements in front of the shoal. Conditions P+ and I+ share both similarities with such behavioural patterns as they both feature an increase in tail-beating frequency of the robot in front of the fish as they become closer. If the robotic-fish were left untethered, such increase in the frequency would result in increased swimming speeds. While both conditions P+ and I+ display a strong preference of zebrafish for the robotic-fish, they may differ in terms of the locomotory patterns they induce on the live subjects. For example, high values of preference for the robotic-fish in condition I+ are accompanied by significant portions of time freezing, which are not observed in condition P+. Such behaviour is generally related to anxiety and fear [32], suggesting that condition P+ should be preferred for its ability to enhance fish preference while minimizing anxiety and fear in experimental conditions.
Open-loop conditions, where either the robot beats its tail at a constant frequency, condition C, or varies the frequency following an a priori defined time history, condition U, display the levels of attractions comparable to condition P+. Yet, a progressive loss of fish preference for the robotic-fish is observed as more trials are executed. This may suggest that repeated exposure to the robot, under open-loop control, yields a gradual loss of preference, which may be attributed to long-term habituation or other memory effects [27,49]. Indeed, while condition C is initially superior to all closed-loop conditions, it is consistently outperformed by them as the number of trial repetitions increase; nevertheless, a repetition-effect was not found to be statistical significant.
Nature is a growing source of inspiration for engineers. This study has demonstrated that real-time visual feedback from the robotic-fish has a significant role in determining the feasibility of attracting live zebrafish in preference tests and influencing their behaviour. Introducing robots in the laboratory may aid addressing fundamental questions in animal behaviour, pertaining to perception, fear, memory and anxiety in functional and dysfunctional scenarios for its multisensory feedback coupled to its closed-loop control. Introducing robots in the wild may open new horizons for conservation studies, wherein closed-loop control can be used to modulate the response of live subjects for alien and pest species control as well as animal bypass systems.
Acknowledgements
The authors gratefully acknowledge Drs F. Chiarotti and N. Abaid for valuable help on the statistical analysis, S. Macrì for a useful discussion and for reviewing the manuscript, T. Y. Tsang for his assistance in performing reflectance measurements at the Brookhaven National Laboratory and D. M. Parichy for providing reflectance data on zebrafish. This research was supported by the National Science Foundation (under grant no. CMMI-0745753), GK-12 Fellows (grant no. DGE-0741714) and through a Graduate Research Fellowship to Vladislav Kopman (under grant no. DGE-1104522). This research has also been supported in part by the Honors Center of Italian Universities (H2CU) through a scholarship to Giovanni Polverino. The authors would also like to thank the anonymous reviewers for their careful reading of the manuscript and for giving useful suggestions that have helped improve the work and its presentation.
References
- 1.Fujita M. 2001. AIBO: toward the era of digital creatures. Int. J. Robot. Res. 20, 781–794 10.1177/02783640122068092 (doi:10.1177/02783640122068092) [DOI] [Google Scholar]
- 2.Bar-Cohen Y. 2005. Biomimetics: biologically inspired technologies. Boca Raton, FL: CEC [Google Scholar]
- 3.Krause J, Winfield AFT, Deneubourg J-L. 2011. Interactive robots in experimental biology. Trends Ecol. Evol. 26, 369–375 10.1016/j.tree.2011.03.015 (doi:10.1016/j.tree.2011.03.015) [DOI] [PubMed] [Google Scholar]
- 4.Goldburg R, Naylor R. 2005. Future seascapes, fishing, and fish farming. Front. Ecol. Environ. 3, 21–28 10.1890/1540-9295(2005)003[0021:FSFAFF]2.0.CO;2 (doi:10.1890/1540-9295(2005)003[0021:FSFAFF]2.0.CO;2) [DOI] [Google Scholar]
- 5.Pyke GH. 2008. Plague minnow or mosquito fish? A review of the biology and impacts of introduced Gambusia species. Annu. Rev. Ecol. Evol. Syst. 39, 171–191 10.1146/annurev.ecolsys.39.110707.173451 (doi:10.1146/annurev.ecolsys.39.110707.173451) [DOI] [Google Scholar]
- 6.Schilt CR. 2007. Developing fish passage and protection at hydropower dams. Appl. Anim. Behav. Sci. 104, 295–325 10.1016/j.applanim.2006.09.004 (doi:10.1016/j.applanim.2006.09.004) [DOI] [Google Scholar]
- 7.Rashid MT, Frasca M, Ali AA, Ali RS, Fortuna L, Xibilia MG. 2012. Artemia swarm dynamics and path tracking. Nonlinear Dyn. 68, 555–563 10.1007/s11071-011-0237-6 (doi:10.1007/s11071-011-0237-6) [DOI] [Google Scholar]
- 8.Michelsen A, Andersen BB, Storm J, Kirchner WH, Lindauer M. 1992. How honeybees perceive communication dances, studied by means of a mechanical model. Behav. Ecol. Sociobiol. 30, 143–150 10.1007/BF00166696 (doi:10.1007/BF00166696) [DOI] [Google Scholar]
- 9.Abaid N, Bartolini T, Macrì S, Porfiri M. 2012. What zebrafish want: aspect ratio, motility, and color modulate robot-fish interactions. Behav. Brain Res. 233, 545–553 10.1016/j.bbr.2012.05.047 (doi:10.1016/j.bbr.2012.05.047) [DOI] [PubMed] [Google Scholar]
- 10.Aureli M, Fiorilli F, Porfiri M. 2012. Portraits of self-organization in fish schools interacting with robots. Physica D: Nonlinear Phenom. 241, 908–920 10.1016/j.physd.2012.02.005 (doi:10.1016/j.physd.2012.02.005) [DOI] [Google Scholar]
- 11.Faria JJ, Dyer J, Clément R, Couzin I, Holt N, Ward A, Waters D, Krause J. 2010. A novel method for investigating the collective behaviour of fish: introducing ‘Robofish’. Behav. Ecol. Sociobiol. 64, 1211–1218 10.1007/s00265-010-0988-y (doi:10.1007/s00265-010-0988-y) [DOI] [Google Scholar]
- 12.Marras S, Porfiri M. 2012. Fish and robots swimming together: attraction towards the robot demands biomimetic locomotion. J. R. Soc. Interface 9, 1856–1868 10.1098/rsif.2012.0084 (doi:10.1098/rsif.2012.0084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polverino G, Abaid N, Kopman V, Macrì S, Porfiri M. 2012. Zebrafish response to robotic fish: preference experiments on isolated individuals and small shoals. Bioinspiration Biomimetics 7, 036019 10.1088/1748-3182/7/3/036019 (doi:10.1088/1748-3182/7/3/036019) [DOI] [PubMed] [Google Scholar]
- 14.Rossi C, Coral W, Barrientos A. 2012. Swimming physiology of fish: towards using exercise for farming a fit fish in sustainable aquaculture, chapter Robotic fish to lead the school. Berlin, Germany: Springer [Google Scholar]
- 15.de Margerie E, Lumineau S, Houdelier C, Richard Yris M-A. 2011. Influence of a mobile robot on the spatial behaviour of quail chicks. Bioinspiration Biomimetics 6, 034001 10.1088/1748-3182/6/3/034001 (doi:10.1088/1748-3182/6/3/034001) [DOI] [PubMed] [Google Scholar]
- 16.Göth A, Evans CS. 2004. Social responses without early experience: Australian brush-turkey chicks use. J. Exp. Biol. 207, 2199–2208 10.1242/jeb.01008 (doi:10.1242/jeb.01008) [DOI] [PubMed] [Google Scholar]
- 17.Fernández-Juricic E, Gilak N, McDonald JC, Pithia P, Valcarcel A. 2006. A dynamic method to study the transmission of social foraging information in flocks using robots. Anim. Behav. 71, 901–911 10.1016/j.anbehav.2005.09.008 (doi:10.1016/j.anbehav.2005.09.008) [DOI] [Google Scholar]
- 18.Fernández-Juricic E, Kowalski V. 2011. Where does a flock end from an information perspective? A comparative experiment with live and robotic birds. Behav. Ecol. 22, 1304–1311 10.1093/beheco/arr132 (doi:10.1093/beheco/arr132) [DOI] [Google Scholar]
- 19.Partan SR, Larco CP, Owens MJ. 2009. Wild tree squirrels respond with multisensory enhancement to conspecific robot alarm behaviour. Anim. Behav. 77, 1127–1135 10.1016/j.anbehav.2008.12.029 (doi:10.1016/j.anbehav.2008.12.029) [DOI] [Google Scholar]
- 20.Halloy J, et al. 2007. Social integration of robots into groups of cockroaches to control self-organized choices. Science 318, 1155–1158 10.1126/science.1144259 (doi:10.1126/science.1144259) [DOI] [PubMed] [Google Scholar]
- 21.Swain DT, Couzin ID, Leonard NE. 2012. Real-time feedback-controlled robotic fish for behavioral experiments with fish schools. Proc. IEEE 100, 150–163 10.1109/JPROC.2011.2165449 (doi:10.1109/JPROC.2011.2165449) [DOI] [Google Scholar]
- 22.Bohlen M. 1999. A robot in a cage-exploring interactions between animals and robots. In Proc. IEEE Int. Symp. on Computational Intelligence in Robotics and Automation, Monterey, CA, November 1999, pp. 214–219 Piscataway, NJ: IEEE [Google Scholar]
- 23.Vaughan R, Sumpter N, Henderson J, Frost A, Cameron S. 2000. Experiments in automatic flock control. Robot. Auton. Syst. 31, 109–117 10.1016/S0921-8890(99)00084-6 (doi:10.1016/S0921-8890(99)00084-6) [DOI] [Google Scholar]
- 24.Patricelli GL, Uy AC, Walsh G, Borgia G. 2002. Sexual selection: male displays adjusted to female's response. Nature 415, 279–280 10.1038/415279a (doi:10.1038/415279a) [DOI] [PubMed] [Google Scholar]
- 25.Kubinyi E, Miklosi A, Kaplan F, Gacsi M, Topal J, Csanyi V. 2004. Social behaviour of dogs encountering AIBO, an animal-like robot in a neutral and in a feeding situation. Behav. Proc. 65, 231–239 10.1016/j.beproc.2003.10.003 (doi:10.1016/j.beproc.2003.10.003) [DOI] [PubMed] [Google Scholar]
- 26.Takanishi A, Aoki T, Ito M, Ohkawa Y, Yamaguchi J. 1998. Interaction between creature and robot: development of an experiment system for rat and rat robot interaction. In Proc. IEEE/RSJ Int. Conf. on Intelligent Robots and Systems, Victoria, BC, October 1998, vol. 3, pp 1975–1980 Piscataway, NJ: IEEE [Google Scholar]
- 27.Gerlai R. 2010. High-throughput behavioral screens: the first step towards finding genes involved in vertebrate brain function using zebrafish. Molecules 15, 2609–2622 10.3390/molecules15042609 (doi:10.3390/molecules15042609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miklósi A, Andrew R. 2006. The zebrafish as a model for behavioral studies. Zebrafish 3, 227–234 10.1089/zeb.2006.3.227 (doi:10.1089/zeb.2006.3.227) [DOI] [PubMed] [Google Scholar]
- 29.Cahill G. 2002. Clock mechanisms in zebrafish. Cell Tissue Res. 309, 27–34 10.1007/s00441-002-0570-7 (doi:10.1007/s00441-002-0570-7) [DOI] [PubMed] [Google Scholar]
- 30.Quera V, Beltran FS, Dolado R. 2011. Determining shoal membership: a comparison between momentary and trajectory-based methods. Behav. Brain Res. 225 363–366 10.1016/j.bbr.2011.07.017 (doi:10.1016/j.bbr.2011.07.017) [DOI] [PubMed] [Google Scholar]
- 31.Rosenthal GG, Ryan MJ. 2005. Assortative preferences for stripes in danios. Anim. Behav. 70, 1063–1066 10.1016/j.anbehav.2005.02.005 (doi:10.1016/j.anbehav.2005.02.005) [DOI] [Google Scholar]
- 32.Saverino C, Gerlai R. 2008. The social zebrafish: behavioral responses to conspecific, heterospecific, and computer animated fish. Behav. Brain Res. 191, 77–87 10.1016/j.bbr.2008.03.013 (doi:10.1016/j.bbr.2008.03.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snekser JL, Ruhl N, Bauer K, McRobert SP. 2010. The influence of sex and phenotype on shoaling decisions in zebrafish. Int. J. Comp. Psychol. 23, 70–81 [Google Scholar]
- 34.Plaut I. 2000. Effects of fin size on swimming performance, swimming behaviour and routine activity of zebrafish Danio rerio. J. Exp. Biol. 203, 813–820 [DOI] [PubMed] [Google Scholar]
- 35.Kohler D. 1976. The interaction between conditioned fish and naive schools of juvenile carp (Cyprinus carpio, pisces). Behav. Processes 1, 267–275 10.1016/0376-6357(76)90027-9 (doi:10.1016/0376-6357(76)90027-9) [DOI] [PubMed] [Google Scholar]
- 36.Buske C, Gerlai R. 2011. Shoaling develops with age in Zebrafish (Danio rerio). Prog. Neuro Psychopharmacol. Biol. Psychiatry 35, 1409–1415 10.1016/j.pnpbp.2010.09.003 (doi:10.1016/j.pnpbp.2010.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kopman V, Porfiri M. In press. Design, modeling, and characterization of a miniature robotic-fish for research and education in biomimetics and bioinspiration. IEEE/ASME Trans. Mechatronics. 10.1109/TMECH.2012.2222431 (doi:10.1109/TMECH.2012.2222431) [DOI] [Google Scholar]
- 38.Abaid N, Kopman V, Porfiri M. 2012. The story of a Brooklyn outreach program on biomimetics, underwater robotics, and marine science for K-12 students. IEEE Robot. Autom. Mag. 10.1109/MRA.2012.2184672 (doi:10.1109/MRA.2012.2184672) [DOI] [Google Scholar]
- 39.Balch T, Khan Z, Veloso M. 2001. Automatically tracking and analyzing the behavior of live insect colonies. In Proc. 5th Int. Conf. on Autonomous Agents, pp. 521–528, Montreal, Canada [Google Scholar]
- 40.Butail S, Paley DA. 2012. Three-dimensional reconstruction of the fast-start swimming kinematics of densely schooling fish. J. R. Soc. Interface 9, 77–88 10.1098/rsif.2011.0113 (doi:10.1098/rsif.2011.0113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogata K. 2010. Modern control engineering, 5th edn Upper Saddle River, NJ: Prentice Hall [Google Scholar]
- 42.Wong K, et al. 2010. Analyzing habituation responses to novelty in zebrafish (Danio rerio). Behav. Brain Res. 208, 450–457 10.1016/j.bbr.2009.12.023 (doi:10.1016/j.bbr.2009.12.023) [DOI] [PubMed] [Google Scholar]
- 43.Gerlai R, Fernandes Y, Pereira T. 2009. Zebrafish (Danio rerio) responds to the animated image of a predator: towards the development of an automated aversive task. Behav. Brain Res. 201, 318–324 10.1016/j.bbr.2009.03.003 (doi:10.1016/j.bbr.2009.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maximino C, de Brito T, da Silva Batista A, Herculano A, Morato S, Gouveia A., Jr 2010. Measuring anxiety in zebrafish: a critical review. Behav. Brain Res. 214, 157–171 10.1016/j.bbr.2010.05.031 (doi:10.1016/j.bbr.2010.05.031) [DOI] [PubMed] [Google Scholar]
- 45.Dempster T, Taquet M. 2004. Fish aggregation device (FAD) research: gaps in current knowledge and future directions for ecological studies. Rev. Fish Biol. Fisheries 14, 21–42 10.1007/s11160-004-3151-x (doi:10.1007/s11160-004-3151-x) [DOI] [Google Scholar]
- 46.Higgs DM, Rollo AK, Souza MJ, Popper AN. 2003. Development of form and function in peripheral auditory structures of the zebrafish (Danio rerio). J. Acoust. Soc. Am. 113, 1145–1154 10.1121/1.1536185 (doi:10.1121/1.1536185) [DOI] [PubMed] [Google Scholar]
- 47.Reebs SG. 2000. Can a minority of informed leaders determine the foraging movements of a fish shoal? Anim. Behav. 59, 403–409 10.1006/anbe.1999.1314 (doi:10.1006/anbe.1999.1314) [DOI] [PubMed] [Google Scholar]
- 48.Zion B, Barki A, Grinshpon J, Rosenfeld L, Karplus I. 2007. Social facilitation of acoustic training in the common carp Cyprinus carpio (L.). Behaviour 144, 611–630 10.1163/156853907781347781 (doi:10.1163/156853907781347781) [DOI] [Google Scholar]
- 49.Pather S, Gerlai R. 2009. Shuttle box learning in zebrafish. Behav. Brain Res. 196, 323–327 10.1016/j.bbr.2008.09.013 (doi:10.1016/j.bbr.2008.09.013) [DOI] [PMC free article] [PubMed] [Google Scholar]