Abstract
Adaptive reflective surfaces have been a challenge for both electronic paper (e-paper) and biological organisms. Multiple colours, contrast, polarization, reflectance, diffusivity and texture must all be controlled simultaneously without optical losses in order to fully replicate the appearance of natural surfaces and vividly communicate information. This review merges the frontiers of knowledge for both biological adaptive coloration, with a focus on cephalopods, and synthetic reflective e-paper within a consistent framework of scientific metrics. Currently, the highest performance approach for both nature and technology uses colourant transposition. Three outcomes are envisioned from this review: reflective display engineers may gain new insights from millions of years of natural selection and evolution; biologists will benefit from understanding the types of mechanisms, characterization and metrics used in synthetic reflective e-paper; all scientists will gain a clearer picture of the long-term prospects for capabilities such as adaptive concealment and signalling.
Keywords: adaptive coloration, reflective coloration, cephalopod, reflective displays
1. Introduction
In the scientific record, some of the earliest writings regarding adaptive coloration were by Aristotle [1], who wrote extensively about octopus tuneable coloration. Throughout our recorded history, there have always been diverse examples of tuneable coloration in nature for the purposes of both adaptive concealment and information communication [2,3]. Only in the past decade has humanity begun to master adaptive coloration for its own purposes, primarily in the form of reflective electronic paper (e-paper) devices such as the Amazon Kindle. This initial launch of e-paper products has stimulated investment in research and development, and a torrent of technological progress in more than a dozen disparate technologies vying for applications ranging from e-readers, to signage, to tuneable colour mobile phone casings [4,5]. However, e-paper still lags behind biological systems in optical performance, especially in colour generation, which is not surprising because biology has had more than a 100 million year head start. As a result, some e-paper technologies are now attempting to emulate optical effects already perfected in nature. Therefore, e-paper engineers should be examining equivalent biological systems in greater detail. On the other hand, intense e-paper research and development have now given us a mature understanding of the optics of adaptive coloration with synthetic materials, and the advanced ‘measurement standards’ required for scientific involvement. Although this framework now exists, it is far underused for analysis of biological adaptive coloration. It seems that now is an appropriate time for biologists and engineers to better inform each other, and therefore advance the state of the art across the wide spectrum of disciplines and applications.
This review aims to merge the frontiers of knowledge for both biological adaptive coloration and synthetic reflective e-paper within a consistent framework of scientific metrics. Merging these disparate fields is challenging, and our chosen approach for this review aims to develop common ground between biology and materials science. First, we review in a generic framework the optics of adaptive coloration. Next, because, out of the whole of the animal kingdom, the molluscan class Cephalopoda (squid, cuttlefish and octopus) is the most renowned for rapid adaptive coloration used for a variety of communication and camouflage tasks, we review the mechanisms of their adaptive coloration. This review includes detailed subsections on the chromatophore organs, iridophores and leucophore cells within the skin. Each of these structures will then be compared with synthetic technologies that provide similar functionality. Biological systems are too complex to compare directly with a single synthetic technology; for example, comparing a squid and an Amazon Kindle e-reader is not highly meaningful. However, important comparisons can be made between the function and performance of biological pigments and reflectors, and similarly functioning synthetic pixels and components.
The best approaches to adaptive colour generation in nature and artifice share two common aspects: adaptive colour is changed by compacting or spreading pigment (i.e. colourant transposition), and efficient reflection is achieved by optical interference/diffraction. The major outcome from this review is the foundation of a consistent set of scientific parameters that allows for a better understanding of how display engineers and biologists can efficiently coordinate a bio-inspired approach to advanced materials and devices.
2. A hierarchical view of adaptive coloration
A common, or at least interchangeable, set of terminology is needed for this review. There are three hierarchical levels that will be used in our analysis (figure 1). At the apex, there is the ‘organism’ or ‘system’ level that contains everything needed for self-sufficient operation. Even though an e-reader (figure 1c) is not truly autonomous, it is grouped with autonomous organisms (figure 1a) because it has all the features necessary to respond to stimuli and achieve an appropriate adaptation in colour or information communicated. One level deeper is the ‘organ’ or ‘device’ level. For organisms, the adaptive coloration ‘organ’ is often the skin, including the vascular and nervous system features within it. In electronic systems, there is no organ but instead the analogous ‘device’ that actually modulates the reflected colour. For example, an E ink ‘device’ has microcapsules of electronically switchable ink, and an array of electrodes for switching the ink capsules. Lastly, there is the ‘cellular’ or ‘pixel’ level, both of which also inherently require consideration of the materials used. The cellular level is where the basic biology and optical physics that enable adaptive coloration can be discussed in their greatest physical detail. The comparisons in this review will focus on this cellular/pixel level.
Figure 1.
Hierarchical levels for biological and synthetic adaptive coloration: organism/system (a) octopus rapidly transitioning out of concealment, (b) Kent Displays' multilayer cholesteric display, (c) Amazon Kindle e-reader using E ink film; organ/device (d) cephalopod skin, (e) E ink film; cellular/pixel, (f) chromatophore structure [6] and (g) E ink pixels.
3. The optics of adaptive coloration
Before we focus on the optics specific to the organisms/technologies of this review, we will introduce the reader to adaptive reflective coloration in its broadest context. The optics for reflective adaptive colour are unlike transmissive or emissive display approaches. In emissive or transmissive colour generation, there is an internal source to generate light, and optical inefficiency can be overcome by simply increasing the electrical power to the internal light generation. In the reflective mode, the only way to achieve proper coloration is through high optical efficiency for all layers and materials. Consider, for example, a conventional liquid-crystal display (LCD) in a laptop computer. The panel is designed for optimum efficiency; however, it emits a very small percentage of the light incident on the display (low optical efficiency). This is why the panel appears black if the backlight is turned off. Considering the low optical efficiency in LCDs (despite being designed for optimum performance), it is all the more amazing that biological organisms are able to achieve bright adaptive coloration solely in the reflective mode. Simply stated, animal pigments and structurally coloured reflectors are very efficient at using available light. There are well-established visual standards for reflective efficiency (brightness) and colour [4].
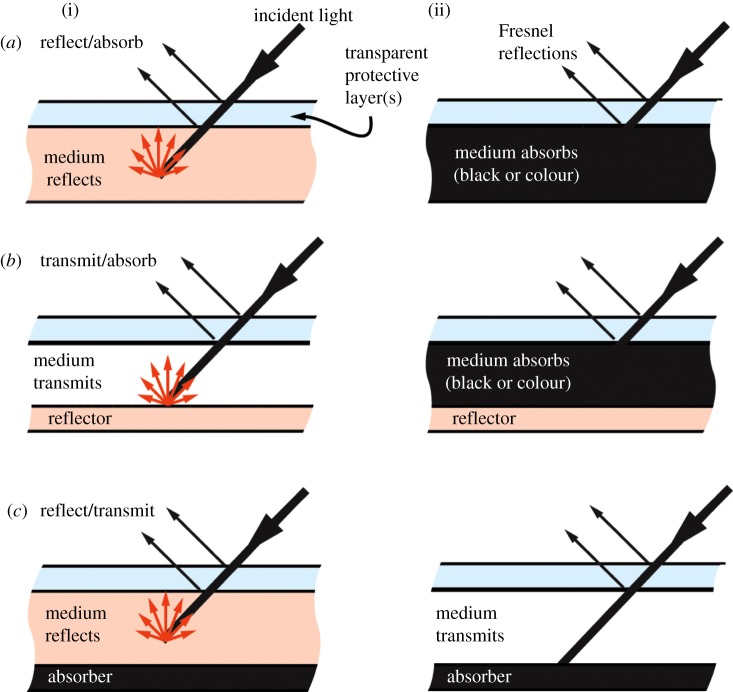
Whether biological or electronic, the optical efficiency of adaptive coloration involves several chronological steps that are common regardless of which approach is used (figure 2). (i) Light must be effectively coupled into the device or organ. Generally, this means that various layers of the organ or device should have low optical absorption and as small a refractive index mismatch as possible. Layers with different refractive indices can cause Fresnel reflection [7] which reflects the light before it can couple (transmit) into the device or organ. (ii) Light must be efficiently reflected inside the pixel. A mechanism for efficient reflection is needed, and generally the reflection must be at least semi-diffuse (semi-Lambertian) to appear like natural surfaces. (iii) The diffusely reflected light must be outcoupled. Once the incident light is diffused, it must also be outcoupled (i.e. escape the device or organ). Some light does not escape due to total internal reflection [7] and must be diffusely reflected again, introducing further optical loss. For example, an organ or device that is internally 80 per cent reflective and has a refractive index of n ∼ 1.5 can lose more than 20 per cent of the light owing to total internal reflection [7].
Figure 2.
Fundamental approaches for reflective adaptive coloration. (a) Medium can switch from a reflective mode (i) to an absorbing mode (ii); (b) medium can switch from a transmitting mode with a reflector behind it (i) to an absorbing mode (ii); (c) medium can switch from a reflective mode with an absorber behind it (i) to a transmitting mode where the absorber prevents light from reflecting out of the device (ii).
Three important optical parameters for comparison include contrast ratio, colour gamut and the number of grey-scale levels. Contrast ratio is the optical ratio between colour state and black (or non-colour colour state). Colour gamut is defined as the subset of complete colours that a surface can achieve. Reflective colour is typically defined by the L*a*b* (laboratory colour space) coordinates that can be derived from the wavelength spectrum of the reflected light, when compared with a perfectly diffuse white reflector [4]. L* is an intensity measurement that closely matches human perception of lightness. This is distinct from a reflectivity measurement in that reflectivity measures the amount of photons reflected from a surface that is not linear with the human visual system. a* and b* are the colour-opponent dimensions. Greyscale relates to the number or shades of a colour.
While one can use optical parameters for comparative discussion, importantly, a human-made system (e-paper) is optimized for a fundamentally different purpose than the evolved, biological adaptive colour systems, i.e. it is optimized specifically for the human visual system. The primate (e.g. human) visual system is distinct from many other organisms. Humans possess three cone visual pigments for conveying colour information that is said to allow humans to be able to detect approximately 10 million unique colours [8,9] but only distinguish about 30 shades of grey [10]. With regard to spectral sensitivity, there are enormous variations in visual abilities across the animal kingdom. Some simpler animal eyes have only one visual pigment; others, such as mantis shrimp, have as many as 12, covering the human visible spectrum as well as UV and IR [11–13]. While there are several methods available to analyse how colours are perceived from the perspective of the human visual system (e.g. CIE 1931 XYZ colour space), there have to date been very few attempts to extend these methods to include animal visual systems.
Nevertheless, various modelling methods have been developed [14–17] that allow us to assess how particular colours are perceived by a given animal's visual system. This is particularly important because the (L*a*b) method is devised for human vision and is not applicable to other visual systems. These modelling methods [14–17] take into consideration how many photons (i.e. light reflected from a surface) are absorbed by a given set of photoreceptors in the retina, and how these photon catches are represented in a specific colour space. However, precise knowledge of the ratio of different photoreceptor types present in the retina of di-, tri- or even tetra-chromats are required for these models to give an accurate estimation of what an animal sees. While such models are helpful when assessing species for which these data exist, for many others, these modelling techniques remain speculation.
4. The cephalopod system for adaptive coloration
Of all of the organisms in the animal kingdom capable of colour modulation, cephalopods (squid, cuttlefish and octopus) are able to produce the widest range of colours and patterns to help them adapt to their visually diverse marine environments as well as signal and communicate with their own species and others. There are more than 700 species of cephalopods, and many of the organisms within this class are able to adapt their coloration to their environment to various degrees (figure 3). Cephalopods are the focus in this review, although other organisms (such as chameleons) [18–20] have similar mechanisms for adaptive colour and will be briefly discussed in a later section. The cephalopod is best presented in terms of the organism/organ/cellular hierarchy (figure 1). Organism—for the cephalopod organism to modulate skin colour, it first needs to sense its surroundings to determine what colours and patterns it needs to create. This sensing is done by the visual system of the cephalopod [21]. The visual data are processed by the brain, which then sends control signals to the skin, which is the Organ that enables adaptive coloration. The skin contains three distinct structures—chromatophores, iridophores and leucophores—that contribute to the colour and pattern adaptation. The skin is also capable of flattening or wrinkling on demand, providing surface texturing (see octopus in figure 1a). As shown in figure 3, the epidermis and dermis are transparent, allowing light to pass through to impact all three skin structures before light is modified through interaction at a Cellular level. At the cellular level, the chromatophore pigments impart variable optical transmission, and the iridophores and leucophores serve as a rear reflector (similar to the basic approach of figure 2b). The chromatophore has a pigment-filled sac that has dozens of radial muscle fibres attached around its periphery. As shown in the photographs of figure 3, these structures work in combination to produce stark changes in the organisms' coloration and patterning (via colour transposition).
Figure 3.
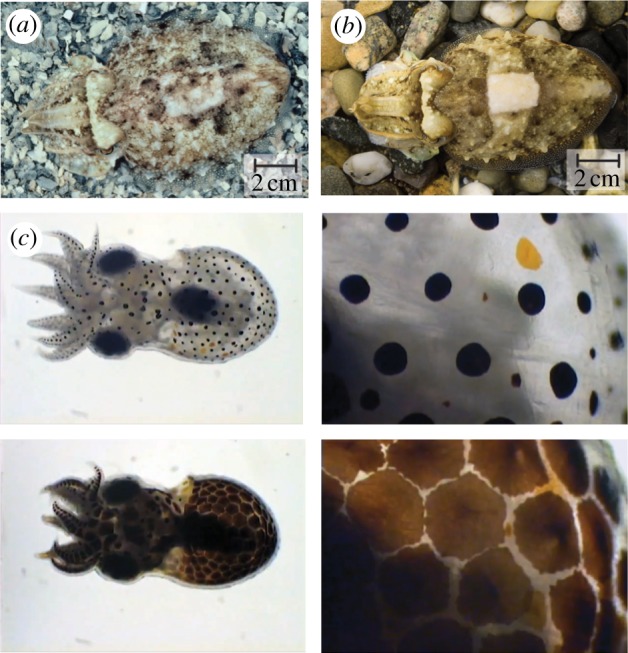
Cuttlefish, Sepia officinalis, showing (a) mottle and (b) disruptive camouflage. (c) Low magnification and close-up photographs of a hatchling blue-ringed octopus (Hapalochlaena lunulata), credit: Roy Caldwell.
The cumulative mechanism behind adaptive coloration for cephalopods is more sophisticated than anything synthetic (figure 4). There are separate mechanisms for the physics of how the cephalopod's colour modulation works. The pigmented chromatophores are punctate when retracted (i.e. barely visible) and are expanded into a thin disc of colour by the radial muscle fibres. This allows the pigments to go from almost invisible to full coverage on the organism. The function of chromatophores is to reflect, block and transmit light, as well as act as a spectral filter. A red-pigmented chromatophore, for example, absorbs all other wavelengths except red. The iridophores function differently: they produce structural colour based on constructive interference (i.e. there is no absorption of light). There is a difference in refractive index between the reflectin protein in each iridophore platelet and the interplatelet spaces within the entire iridophore cell [22–24]. Moreover, the platelet thicknesses and adjacent spaces have precise and periodic arrangement that influence which wavelengths are reflected (a dielectric mirror) [25]. The plate spacing for some iridophores can also be controlled by the organism, thereby varying the peak reflected wavelength [26]. Recently, it has been discovered that not only the iridophore plate spacing can be controlled but also the arrangement of the reflectin proteins through a reversible phosphorylation process [23]. The rearrangement of these proteins also results in changes to the peak reflected wavelength. Cuttlefish leucophores (leuco means ‘white’) also reflect light, but from wavelengths of 300 to 900 nm giving them a diffuse white appearance (like paper). In some skin areas, reflectance is as high as 70 per cent, and the intensity of the reflected white light is the same, regardless of the viewing and incident light angles (similar to a Lambertian surface). Cuttlefish leucophores are composed of spherical protein assemblages of varying diameters (200–2000 nm) that scatter light whether the surrounding medium is water or air. Moreover, these leucophores are very flexible: they do not lose their optical properties when mechanically deformed. They are physiologically passive: with no associated musculature or innervation, their energy requirement is nil [27].
Figure 4.
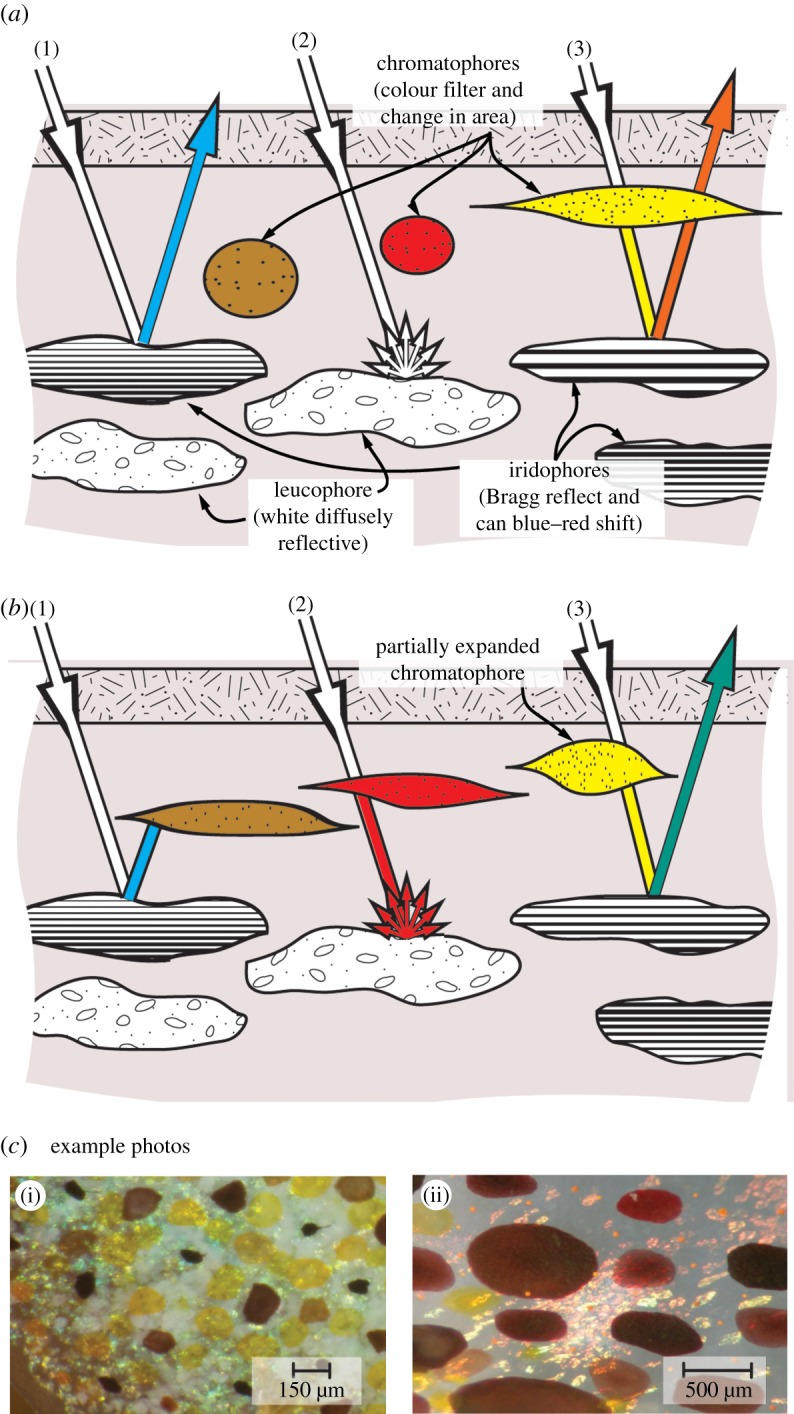
Diagram of cephalopod skin detailing the three main skin structures (chromatophores, iridophores and leucophores), two example states (a,b) and three distinct ray traces (1, 2, 3) show the sophisticated means by which cephalopods can change reflective colour. (c) (i) Cuttlefish skin, showing leucophores (white), chromatophores (yellow, red dark brown) and iridophores (green); (ii) squid skin, showing chromatophores and iridophores.
The sophistication of biological adaptive coloration can be fully appreciated by a detailed examination of the ray traces (1, 2, 3) in figure 4a,b. Ray trace (1a) shows a ray of white light incident on an iridophore cell. Owing to constructive interference, the reflected light is blue (shorter wavelength), given by the periodic spacing for the iridophore. Ray trace (1b) shows the expanded brown chromatophore absorbing most of the reflected blue light. Ray trace (2a) shows diffusely reflected white light from a leucophore, which in (2b) is coloured red by the expanded red chromatophore. Ray trace (3a) depicts incident light filtered by a yellow chromatophore, and then reflected from an iridophore with a plate spacing shifted closer to the red spectrum such that the reflected light is orange. Ray trace (3b) shows the same yellow-filtered light appearing greenish as the iridophore plate spacing decreased (wavelength shift towards cyan/blue). These are just several examples and the coordination of all these cell types gives rise to an enormous range of available colours. It should be noted that the ray traces in figure 4 are meant to convey the level of sophistication and interplay for light interactions in the skin, and do not represent the dominant ray paths/colour nor partial transparency of the skin (figure 3c).
A quantitative optical model for the biological system has not been developed. CIE colour coordinates have been theoretically mapped in detail by Sutherland et al. [28]. The contrast ratio has not been directly measured. For example, a measure of contrast ratio would be the colour state from the combined chromatophore and iridophore divided by ‘off-state’ retracted chromatophore states. An adaptive colour biological system has evolved to optimize colour contrast ratio relative to their environment. As such, a comparison between the biological optical parameters and environment is equally important. For example, for concealment, biological systems should generally resemble the contrast ratio of environment or a dark shadow, and the colour coordinates for the majority of environment colours. For signalling, the contrast ratio is usually high so that the signal is highly conspicuous, but of course colour plays a role in some species by targeting certain wavelengths in the receiver's visual system [29].
5. Cephalopod adaptive coloration for signalling
The most well-known application of adaptive coloration in cephalopods is predator avoidance through camouflage. However, cephalopods also use the same skin elements used in camouflage to create visual patterns of high contrast for vivid unambiguous signalling and communication [30,31]. Biological signalling is an important topic, because it parallels the foremost human use of adaptive coloration, which is for visual communication of information. The leucophores can play a particularly important role here because their highly reflective whiteness provides the strongest contrast to the overlying dark brown/black pigmented chromatophores (figure 4) [32,33]. For example, the Zebra display, shown by cuttlefish males during reproductive behaviour, is created by maximally expanding the black chromatophores to achieve a dark striped pattern, while simultaneously retracting the chromatophores in-between and allowing the bright white scattering from leucophores to produce maximum contrast with the dark stripes. In addition, the cuttlefish amplifies the signal with arm postures that maximize visibility of the pattern. Other more subtle signals, including the use of polarized reflections, may use the iridophores to achieve displays of varying conspicuousness [30,31,33]. It is worth noting that synthetic technologies also have the ability to reflect polarized light but owing to limitations of the human visual system, it is of little use to humanity, unless technological aid is provided.
6. Synthetic adaptive colour technology
There has been a plethora of ‘bio-inspired’ technology development, most of which has only a weak connection to the actual optics of cephalopod skin [34,35]. Therefore, we have judiciously selected the very few technologies that are more closely biomimetic to the cephalopod skin. Next, detailed subsections on the chromatophore, iridophore and leucophore structures within the skin will be compared with synthetic technologies that provide similar functionality. The skin in cephalopods is more complex and sophisticated than any synthetic technology. Therefore, meaningful comparisons must be made at the cellular level (biological) to the pixel level (synthetic).
7. Chromatophores versus synthetic technology
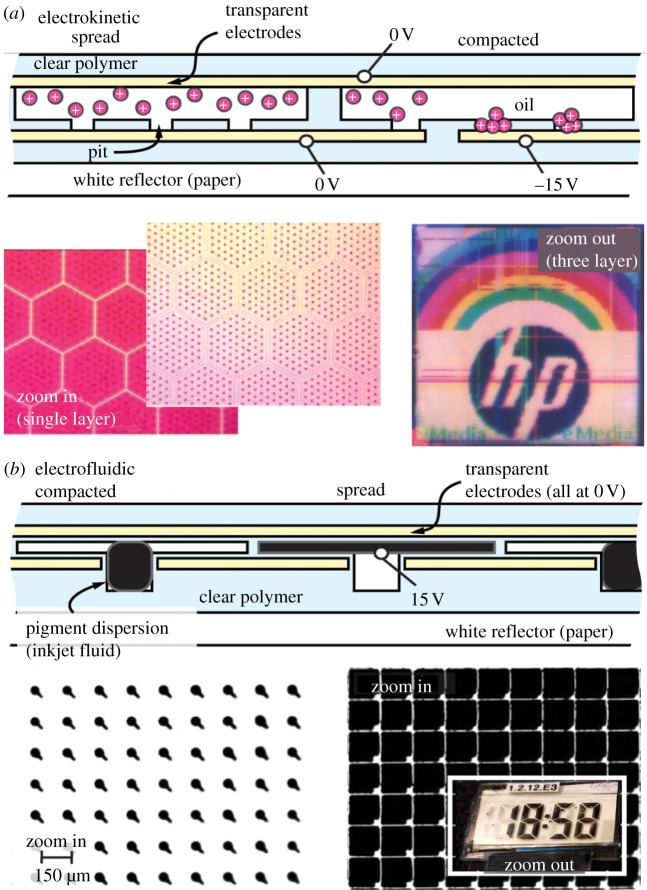
Cephalopod chromatophores all function similarly, compacting from as small as a tenth of a millimetre to 2 mm in diameter (20 : 1 expansion factor) [30]. The time that it takes to go from fully retracted to fully expanded varies and is based on the organism, but recent work has reported it to be typically around 300 ms [36]. The colour of the pigments inside the sac can be grouped into two or three colour classes, depending on species—red, yellow/orange and brown/black [31,37]. When selecting the most closely related synthetic technologies, the limiting criteria are: (i) use of pigment; (ii) transposing pigment between a spread and compacted state; and (iii) in the zero energy stage, pigment is compacted. There are two technologies in development for e-paper [4] which closely match these functions: electrokinetic [38], which provides criteria (i,ii), and electrofluidic [39], which matches all three criteria (figure 5).
Figure 5.
Synthetic technologies for adaptive reflective coloration. (a) Electrokinetic approach; (b) electrofluidic approach.
Like a chromatophore, the electrokinetic technology is able to spread or compact pigment, but using an electric field instead of muscle fibres. The pigment particles have an electrical charge, and if, for example, the pigment particles have positive charge, then they will be attracted to electrodes at which negative voltage is applied. As shown in figure 5a, voltage can be used to compact the pigment particles in tiny pits, with a speed of 100s of milliseconds, allowing transparency and therefore 60 per cent white reflectance from a layer of paper beneath the pixel. In the absence of voltage, the pigment particles will spread and colour the surface. Expansion factors of 10 : 1–20 : 1 have been achieved. Also shown in figure 5a, cyan, magenta and yellow electrokinetic films can be layered and create full colour images by subtractive colour [40]. The colour performance is currently close to that of colour newsprint, which is the highest performance shown for any synthetic adaptive colour technology.
Even closer to a chromatophore's function are the electrofluidic pixels. Here a droplet of pigmented ink is bound not by biological membranes, but by surface tension. With no applied voltage, surface tension compacts the ink in a semi-spherical shape (similar to the shape a droplet of water forms), allowing approximately 60–70% reflection from paper behind the pixel. When voltage is applied to the droplet, the electric field spreads it into a thin channel, where it becomes highly visible. Switching between these two states is fast (approx. 10s of ms). Currently, only monochrome versions of this technology have been demonstrated, but subtractive colour stacking is possible similar to that shown for electrokinetic technologies (figure 5a).
7.1. Summary
Optically, synthetic electrokinetic and electrofluidic technologies compare well with chromatophores. It is interesting to note that compacting and spreading a pigment is one of the highest performance techniques developed by both nature and humans [4] for creating adaptive colour. Only recently have humans been able to create the function and performance of reflective surfaces similar to what nature has refined over hundreds of millions of years. There are several additional factors of interest, but they will be presented in §11.
8. Iridophores versus synthetic technology
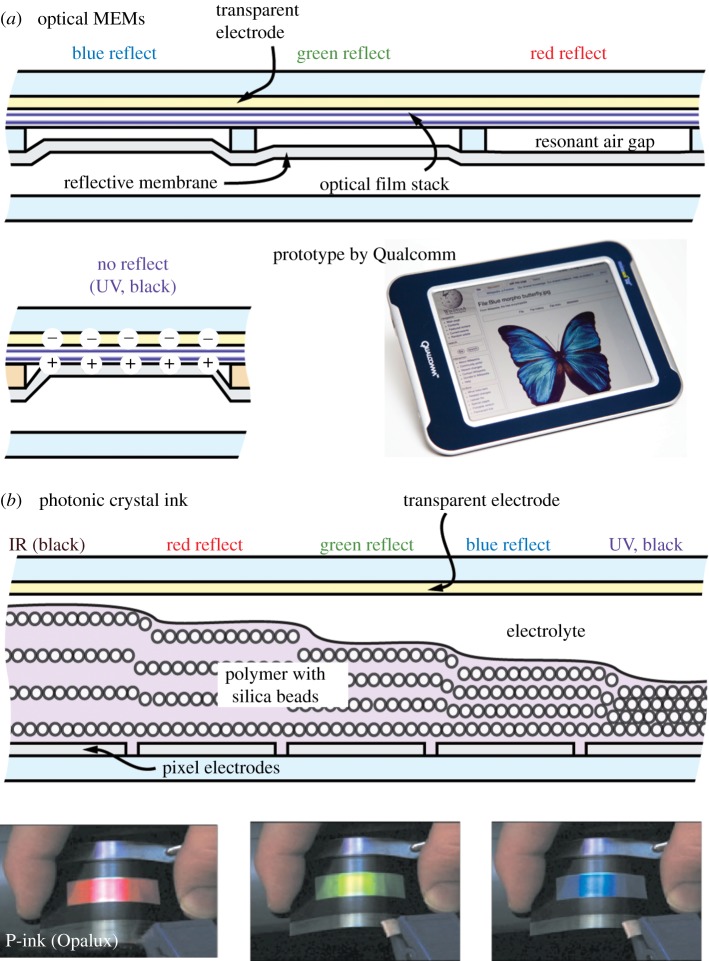
Iridophores are typically less than 1 mm wide and are composed of several periodic layers of cytoplasm (n ∼ 1.36) and reflectin protein (n ∼ 1.59) or guanine crystals (n ∼ 1.86) [41,42]. The thickness of each reflective layer and the spacing in-between is typically a fraction of the reflected wavelength (e.g. in an ‘ideal’ quarter-wave stack the reflective layers and spaces have an optical thickness of a quarter of the wavelength the stack reflects). The thicker the reflective layers and the wider the spaces, the longer the wavelength of reflected light. This multilayer reflector system is similar to a dielectric mirror [25], and is highly efficient because, unlike metal mirrors, the materials used have far less optical absorption. In cephalopods, iridophores are even more powerful than a conventional dielectric mirror because with acetylcholine stimulation, the reflectin protein can change in refractive index and plate spacing, typically producing a shift in 60–80% peak reflection over roughly 100 nm, with subsets of iridophores specialized for bands ranging from the UV through infrared [32]. Recently, it has been reported that this shift may actually well exceed 100 nm for certain organisms [43]. This process is much slower than the muscle-controlled chromatophores and can take anywhere from seconds to minutes to complete. When selecting the most closely related synthetic technologies, the limiting criteria are: (i) constructive interference from periodical modulations in refractive index and (ii) a shift in spectrum. There are two technologies in development for e-paper [4] that closely match these functions: optical microelectromechanical systems (MEMs) [44] and photonic crystal ink [45] (figure 6).
Figure 6.
Synthetic technologies for adaptive iridescence. (a) Optical microelectromechanical systems; (b) photonic crystal ink.
Like an iridophore, optical MEMs [4,44] use constructive interference; however, even though MEMs is a multilayer technology, the resonant-reflected wavelength is set by a single cavity. As shown in figure 6a, the shorter the cavity height, the shorter the reflected wavelength, with a collapsed cavity reflecting only UV light (which appears black to the human eye). The membrane actuates with electrical field at microseconds speeds between the open and collapsed states. Because intermediate positions are not possible, one pixel can only display a single colour or black. Additional colours are created by side-by-side mixing, but as a result, pure colours can only be created at 33 per cent of the area. The peak reflectance is very high at approximately 50 per cent, and like iridophores is highly dependent on the angles of illumination and viewing.
The technology most similar to an iridophore is photonic crystal ink [4,46], which can electrochemically tune its reflected wavelength over an amazing range, spanning the UV through the infrared. The ink contains an ordered array of approximately 200 nm silica microspheres surrounded by a ferrocene-based metallopolymer that swells or shrinks with a highly reversible redox process [47]. Stripping electrons from the iron atoms renders them positively charged, and causes negative counter-anions from the surrounding electrolyte to enter and swell the film. Wider bead spacing causes reflections at longer wavelengths (figure 6b). Currently, peak performance is 60–70% reflectance with approximately 100 ms switching speed. There are additional technologies that leverage the use of structural coloration that include altering the localized concentration of colloidal particles in a fluid medium [48,49] and self-assembling block copolymers [35]. However, these technologies are still early in their development.
The colours and intensity produced by constructive interference techniques using regularly spaced arrays (as discussed in this section) are angularly dependent (will appear different as viewing angle changes). However, not all forms of constructive interference produce angularly dependent colours and intensity. Amorphous or quasi-ordered nanostructures (as can be found in bird feathers) [50] produce a non-iridescent structural coloration. While the iridescent form of structural coloration is desirable for applications like banknotes and identification documents, it imposes an extra challenge for displays where appearance should not vary with viewing angle [4].
8.1. Summary
Like chromatophores, optically synthetic technology appears to outperform iridophores. However, all synthetic technologies exhibit numerous limitations that will be highlighted in §11. Moreover, the synthetic technologies mentioned in this section have the ability to reflect UV and/or IR wavelengths. Many organisms (including cephalopods) use these wavelengths for a variety of signalling activities. Thus, technology can actually replicate the appearance of some biological organisms.
9. Leucophores versus synthetic technology
Leucophores also use protein to reflect light. However, in leucophores, the reflection is not from layered sheets but from spherical granules with varying diameters (200–2000 nm) inside the cell. This reflection is similar in appearance and function to diffuse white paint (which typically contains approx. 100 nm-sized granules of TiO2). This reflection involves significant scattering, and is far less efficient than reflection due to multilayer interference. Therefore, thicker sheets of cells (approx. 100–250 µm) are needed to increase the total reflectance to approximately 70 per cent (as in the Sepia cuttlefish fin spots). The function of the leucophore is assumed to be in aiding both wavelength and intensity matching at a localized level within the skin [27,51].
There are numerous synthetic examples of highly efficient white reflectors, including the scattering properties of bleached wood fibre in paper, microvoided polymer films and polymer : TiO2 composite films. For example, 3M has developed less than 60 µm thick films of multilayer bi-refringent polyethylene terephthalate or polyethylene naphthalate that reflect the entire visible spectrum (white light) with more than 99 per cent efficiency over nearly all angles and polarizations [52].
9.1. Summary
In terms of diffuse reflection, humanity clearly outperforms nature. However, again, nature has several advantages that will be highlighted in §11.
10. Other biological colour-changing organisms
Cephalopods are not the only organisms capable of colour modulation. In fact, chameleons have historically been the most well-known organism capable of adaptive coloration. The mechanisms of colour change in the chameleon differ greatly from the cephalopod. In cephalopods, colour change occurs under direct neural control and is therefore fast (milliseconds to seconds). In lizards, such as the chameleon, colour change is regulated by a neuro-hormonal mechanism, which generally takes longer (seconds to minutes, and in some species hours or days) [18]. The structures responsible for colour change also differ between the two animal groups. Lizards also have structures called chromatophores, but they are distinct from those of cephalopods. Lizard chromatophores are cells, not organs. Three types of lizard chromatophores are known: melanophores, guanophores and xanthophores. Melanophores are large cells deep in the dermis. They have numerous long dendritic processes (like small channels) that run towards the skin surface. Melanin granules travel along these processes to darken the skin when they are at the skin surface, or lighten it, when they are concentrated at the centre of the cell. Guanophores are above the melanophores and contain guanine crystals that reflect light by constructive interference and are responsible for blue coloration. Xanthophores are the most superficial cells and lack dendritic processes. They contain red and yellow pigments. Similar to cephalopods, the final skin coloration is the end result of the interactions among all skin structures [18–20]. It is worth noting that the function of the melanophores is similar to vertical electrophoretic displays [4,5] used in the Amazon Kindle: coloured particles move either closer to, or farther away from, the viewer to elicit a change in reflection.
Chameleons change colour for a variety of reasons, such as thermoregulation, camouflage and communication. In the dwarf chameleon, it has been shown that the camouflage body patterns are even tailored to the visual system of the predator they are aiming to avoid [53,54]. However, adaptable colour change of cephalopods is much more diverse in patterning, speed and optical effects [21,33].
Other biological organisms, such as birds [55,56] and insects, [55,57] commonly produce coloration through the use of submicron structures and pigmentation. These coloration strategies can also be described as coherent (i.e. structural interference/diffraction) and incoherent (i.e. scattering) [58,59]. Which means are used is dependent on the specific species of interest. Although not tuneable like the cephalopod system, the structural means of coloration used by these organisms are equally sophisticated and can achieve a similarly wide gamut of colours.
11. Discussion: who can inform whom?
Synthetic technology was shown to equal or outperform biological systems (cephalopods) when making direct comparison with chromatophores, iridophores and leucophores.
This is only a very recent achievement for humanity (past several years) [4]. The current applications of synthetic technology more closely match the signalling/communication functionality of the cephalopod system than camouflage. However, the cephalopod skin systems are far more efficient at handling ambient light, whereas many synthetic technologies have thus far relied on large amounts of electrical power to create emissive light and there has been much less focus on looking for reflective materials that may produce comparable results.
Regarding signalling, the requirements for electronic signage and e-readers are still very different from cephalopod signalling and communication. The primary difference is in the desired colour gamut and level of information content. Synthetic technology often displays information-rich and dense groupings of high-resolution symbols and characters, while cephalopods and other animals achieve signalling using simple vivid static or flashing patterns.
Regarding camouflage, few synthetic technologies aim to replicate the mechanisms and functions found in biological systems. However, robust adaptive coloration is the basic enabler of high performance in all applications (camouflage, signalling and communication). For example, having good dark states, bright maximum light states and ability to adapt quickly benefits nearly all uses for both technology and cephalopods.
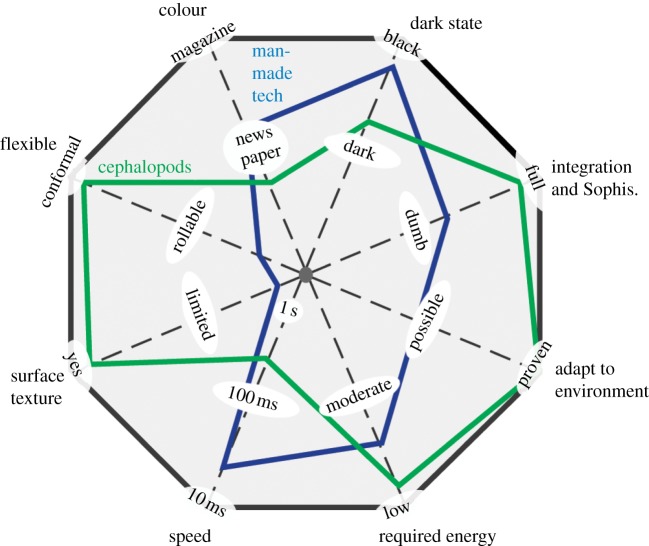
In terms of overall performance, we will now revisit the organisms/system performance (figure 1a–c) and plot performance in the spider chart of figure 7. For biology, it is cephalopods such as squid, cuttlefish and octopus, for synthetic technology the focus will be the technologies of figure 5 (electrokinetic/electrofluidic), because they have been shown to produce some of the brightest/widest ranges of colours and patterns in a single device.
Figure 7.
Spider chart comparing the important metrics to both cephalopod (green) and synthetic (blue) adaptive coloration. The newspaper/magazine metrics in the colour category refer to ‘SNAP and specifications for web offset publications (SWOP)’ standards, respectively [4]. Regarding the dark state metric, cephalopods are capable of changing their colour to dark brown, but not to black.
Colour. Although not commercially available, Hewlett-Packard's electrokinetic displays (figure 5a) now provide colour quality comparable to that found in printed newspapers (specifications for newsprint advertising production (SNAP) printing standard) [4]. Cephalopods might exceed SNAP standards for a few colours, but do not provide all the colours and greyscale found in newspaper quality colour. This comparison may not be fair, however, because cephalopods have adapted over 200 million years to a more limited colour set relevant to their underwater habitat. Had their habitat been as diverse and colourful as magazine print, cephalopods may have evolved to exceed current synthetic technology in terms of colour.
Dark state. Here, technology exceeds, providing dark state reflection values of less than 5–10%. However, like colour, this comparison may be unfair as most cephalopods do not require adaptation to a pitch black background.
Integration and sophistication. Cephalopods far outperform technology in this category, not only in terms of intelligence (compared with a computer that lacks intelligence), but also in elegant integration of numerous cell types in a single organ. Humanity has never developed anything as complex nor sophisticated as the biology and physics of cephalopod skin. For optical integration, biological systems have one major advantage, in that tissue layers can be extremely clear and have a low and homogeneous refractive index such that light is very efficiently coupled into and out of the tissue.
Adapt to environment. Although in theory, technology could use sensors and computing to adapt in coloration to its local environment, this requires numerous additional electronic components and integration of all such components. Compared with cephalopods, colour adaptation to the environment with technology is unproven.
Required energy. Both synthetic and biological systems exhibit very low power consumptions; however, biological systems are superior in how they are self-reliant for gathering energy compared with synthetic systems, which typically require batteries. However, a few displays have had solar-cells integrated into them to harvest energy [60,61].
Speed. Ideally, speed of adaptation should be close to or exceed the response time of biological vision systems (typically 10 s of ms). Here, technology is far superior to cephalopods.
Surface texture. Displaying texture on an e-reader or laptop screen is most always noticeably fake. Texture provides additional light scattering and shadowing that is difficult to dynamically display with technology. However, cephalopods have developed the ability to selectively adapt or ‘crinkle’ their skin to match a variety of textures.
Flexible. Technology companies have prototypes that are not mass-produced and sold, including flexible electrophoretic displays [62–64], and electrokinetic displays [38] to name a few. These flexible display technologies currently conform in one-dimension to adapt to the printed-paper technologies, which is far from the flexible capability that cephalopods can achieve. Several groups have demonstrated electronics that conform (stretch) in two-dimensions, which has allowed synthetic electronics to be integrated on human skin [65]. By contrast, biological organisms have adapted to a highly conformal environment that requires a system that can stretch many times the original size.
Scalable. For an adaptive reflective surface to be scalable, it must be able to be increased in size while keeping an acceptably low number of defects. In this respect, cephalopods also outperform technology. While humans have been able to scale up rigid panels (100″ rigid panels are being manufactured) flexible scalability has been far less (less than 10″). Cephalopods have been successful at scaling up their fully conformal method of adaptive coloration over a wide range of sizes of organisms.
It is noteworthy that although not included in figure 7, the ratio of the colour state to dark state (contrast ratio) is very important. A good dark state will make a colour state look brighter, and vice versa. There are other additional metrics, like polarization of light, resolution (points per inch) and ability to self-generate light, to name a few. These metrics are beyond the scope of this review but may be worthy of consideration based on application.
So, how can nature better inform the development of improved synthetic technology for adaptive coloration? For some applications, nature has already influenced synthetic technology. For example, biological coloration strategies used by cephalopods have inspired engineering approaches to create devices that exhibit tuneable optical properties. One example is that the use of layered materials has resulted in the fabrication of Bragg mirrors that have a rapid reversible optical reflectance [66]. This is analogous to the colour-changing mechanism used by cephalopods, using the change in thickness of iridophore platelets. Another synthetic device inspired by cephalopods is the electrochemically tuneable block copolymer full colour pixels demonstrated by Thomas and co-workers [35]. Again, this is analogous to the iridophore platelets in that changing the distance in the optical elements (platelets in the cephalopod) results in tuneable optical properties. The iridophores are not the only component of the cephalopod that humans have taken inspiration from. Recently, a group at the University of Bristol used inspiration from the underlying mechanisms of chromatophore actuation to create artificial elastomeric chromatophores that undergo optical modulation in response to electrical stimuli [67]. In addition, a simple soft machine constructed from polymers was equipped with microfluidics to change the color and pattern of the small robot; this work was inspired by cephalopods and other animals with adaptive coloration [68]. Inspiration has also been derived from organisms other than cephalopods. For example, the structural coloration created by butterfly wings has been used by L'Oreal for cosmetics [69].
For the existing applications of e-paper, such as e-readers, humanity has explored most of the physics relevant to reflective coloration. Furthermore, the performance of emerging e-paper technologies is now superior in optical performance to biological adaptive colour. However, there are emergent designs for applications where humanity is lacking technology that nature has mastered. For example, consider reconfigurable keyboards used in touch-based smart phones and tablets. It would be highly desirable to provide both visual display of the keyboard and texture/tactile feedback. The ability of cephalopods to modulate the texture of their skin could, in theory, provide a form of tactile feedback. Furthermore, there is the desire to have adaptive colour, and reconfigurable input capability, on compound curves of electronics. Although the development of rollable displays is well underway [70], conformal technology is much farther behind [65]. In addition, humanity can learn from the many other performance deficiencies described in figure 7. We note that our speculations certainly do not represent a complete set of opportunities.
So, how can technology better inform our understanding of adaptive coloration in nature? Firstly, it is difficult to isolate and analyse cellular components of adaptive coloration. Work has been done in direct acetylcholine stimulation of iridophores [71] and sophisticated spectroscopic models of chromatophore/iridophore layers have been developed [72]. However, synthetic devices can be easily controlled electronically and cycled millions if not billions of times, and, in some cases, might provide suitable biomimetic measurements. Secondly, there is a clear lack of measurement standards, metrics and advanced measurement techniques in analysis of natural adaptive colour. Synthetic e-paper is part of a much larger display industry that has developed sunlight, diffuse light and other illumination standards. In addition, measurement techniques are now quite advanced for diffuse versus specular reflection, and optical models for light-outcoupling are now available [73]. Furthermore, biologists could go beyond simple plots of ‘reflected intensity versus angle’ to cephalopod equivalents of L* and a*,b* colour space [4] (caveat, these are for human perception of colour; suitable colour-space models for other organisms need further development [74]). It seems that the value that technology can provide to nature is in optical characterization of natural adaptive coloration.
12. Conclusions
Adaptive reflective surfaces have been developed through natural selection by biological organisms for hundreds of millions of years for the purposes of adaptive coloration and communication. Only recently have synthetic technologies (e-paper) attempted to achieve similar adaptive reflective properties. In order to achieve the most robust adaptive reflective platforms, both biological organisms and synthetic technologies have to control patterns, textures, colours, contrast, diffuseness, reflectance and polarization all while minimizing optical losses. Because biology has a significant head start on humanity in terms of adaptive reflectance, it is imperative to study biology closely to help direct the development of technology.
Owing to the complexity of biological systems, it is not easy to make a direct comparison between an organism and a synthetic technology. By breaking down both the organism and technology into a functional hierarchy, much more can be learned. Cephalopods are useful candidates for study, as they achieve the widest range of adaptive coloration. From studying cephalopods, two main outcomes about adaptive reflectance can be derived. The first is that the best method for changing coloration is by compacting and spreading out pigments. The second is that efficient reflection and wavelength modulation can be achieved by thin-film interference. Most importantly, animal systems such as cephalopods that have the most diverse and changeable skin patterning always use a combination of pigments and reflectors in various combinations of layers, and studying these systems will certainly yield new ideas about how to engineer synthetic systems.
Technologies exist that begin to imitate the individual colour adapting structures of cephalopods and outperform them in terms of speed (microseconds to milliseconds), coloration (SNAP achieved) and dark states (<5% reflective in dark state). However, cephalopods have major advantages in terms of flexibility (fully conformal), texturing (technology has yet to commercially prove this capability), adapting to environments, integration and scalability. As a result, humanity can learn much from biology in terms of how to increase the sophistication of their technology, but implemented in a simple and self-reliant system. On the other hand, science as a whole can learn from the various standards and methods for optical characterization that have been developed for measuring the performance of synthetic technology. By using these methods and metrics, a more complete database of information regarding adaptive coloration in organisms can be created.
Acknowledgements
E.K., J.H. and E.F. created content on technological (synthetic) aspects of adaptive coloration while L.M.M., R.T.H., P.B.D. and R.R.N. focused on the biological content. E.K. organized and compiled the sections into the main paper, and worked with J.H. on the comparisons section at the end. All authors discussed the information presented in the paper at all stages, with the exception of E.F. who made significant contributions at later stages. The University of Cincinnati authors gratefully acknowledge partial support from AFRL (contract no. 5408-25-SC-0003) NSF Career award (no. 0640964; University of Cincinnati), NSF IHCS award (no. 1001141) and ARL (grant no. W9111NF-09-2-0034). MBL authors acknowledge support from AFOSR grant no. FA9550-09-0346, ARL grant no. W911NF-09-2-0043, DARPA (DSO) grant no. W911NF-10-1-0113 and ONR grant no. N00014-10-1-0989. R.R.N. acknowledges support from AFOSR.
References
- 1.Aristotle. 1883. Aristotle's history of animals. In ten books. London, UK: George Bell [Google Scholar]
- 2.Cott H. B. 1940. Adaptive coloration in animals. London, UK: Methuen & Co. Ltd. [Google Scholar]
- 3.Thayer G. H. 1896. The law which underlies protective coloration. Auk 13, 124–129 [Google Scholar]
- 4.Heikenfeld J., Drzaic P., Yeo J. S., Koch T. 2011. Review paper: a critical review of the present and future prospects for electronic paper. J. SID 19, 129–156 10.1889/JSID19.2.129 (doi:10.1889/JSID19.2.129) [DOI] [Google Scholar]
- 5.Comiskey B., Albert J. D., Yoshizawa H., Jacobson J. 1998. An electrophoretic ink for all-printed reflective electronic displays. Nature 394, 253–255 10.1038/28349 (doi:10.1038/28349) [DOI] [Google Scholar]
- 6.Cloney R. A., Florey E. 1968. Ultrastructure of cephalopod chromatophore organs. Zeitschrift fur Zellforschung 89, 250–280 10.1007/BF00347297 (doi:10.1007/BF00347297) [DOI] [PubMed] [Google Scholar]
- 7.Yang S., Hagedon M., Heikenfeld J. 2011. Light out-coupling for reflective displays: simple geometrical model, MATLAB simulation, and experimental validation. J. Display Technol. 7, 473–477 10.1109/JDT.2011.2130510 (doi:10.1109/JDT.2011.2130510) [DOI] [Google Scholar]
- 8.Judd D. B., Wyszecki G. 1975. Color in business, science and industry, 3rd edn. New York, NY: Wiley Interscience [Google Scholar]
- 9.Sharma G., Trussell H. J. 1997. Digital color imaging. IEEE Trans. Image Process 6, 901–932 10.1109/83.597268 (doi:10.1109/83.597268) [DOI] [PubMed] [Google Scholar]
- 10.Glover D. M., Jenkins W. J., Doney S. C. 2011. Scientific visualization. In Modeling methods for marine science, p. 398 New York, NY: Cambridge University Press [Google Scholar]
- 11.Kelber A., Vorobyev M., Osorio D. 2003. Colour vision in animals: behavioural tests and physiological concepts. Biol. Rev. 78, 81–118 10.1017/S1464793102005985 (doi:10.1017/S1464793102005985) [DOI] [PubMed] [Google Scholar]
- 12.Lythgoe J. N., Partridge J. C. 1989. Visual pigments and the acquisition of visual information. J. Exp. Biol. 146, 1–20 [DOI] [PubMed] [Google Scholar]
- 13.Land M. F., Nilsson D. E. 2012. Animal eyes, 2nd edn. Oxford, UK: Oxford University Press [Google Scholar]
- 14.Vorobyev M., Brandt R., Peitsch D., Laughlin S. B., Menzel R. 2001. Colour tresholds and receptor noise: behaviour and physiology compared. Vision Res. 41, 639–653 10.1016/S0042-6989(00)00288-1 (doi:10.1016/S0042-6989(00)00288-1) [DOI] [PubMed] [Google Scholar]
- 15.Vorobyev M., Marshall J., Osorio D., de Ibarra N. H., Menzel R. 2001. Colourful objects through animal eyes. Color Res. Appl. 26, S214–S217 (doi:10.1002/1520-6378(2001)26:1+<::AID-COL45>3.0.CO;2-A) [DOI] [Google Scholar]
- 16.Vorobyev M., Osorio D. 1998. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. Lond. B 265, 351–358 10.1098/rspb.1998.0302 (doi:10.1098/rspb.1998.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoddard M. C., Prum R. O. 2008. Evolution of avian plumage color in a tetrahedral color space: a phylogenetic analysis of New World buntings. Am. Nat. 171, 755–776 [DOI] [PubMed] [Google Scholar]
- 18.Bagnara J. T., Hadley M. E. 1973. Chromatophores and color change: the comparative physiology of animal pigmentation. Englewood Cliffs, NJ: Prentice-Hall [Google Scholar]
- 19.Fox D. L. 1976. Animal biochromes and structural colours. Berkeley, CA: University of California Press [Google Scholar]
- 20.Nečas P. 2001. Chameleons: nature's hidden jewels. Malabar, FL: Krieger Publishing [Google Scholar]
- 21.Hanlon R. T. 2007. Cephalopod dynamic camouflage. Curr. Biol. 17, R400–R404 10.1016/j.cub.2007.03.034 (doi:10.1016/j.cub.2007.03.034) [DOI] [PubMed] [Google Scholar]
- 22.Crookes W. J., Ding L., Huang Q. L., Kimbell J. R., Horwitz J., Mcfall-Ngai M. J. 2004. Reflectins: the usual proteins of squid reflective tissues. Science 303, 235–238 10.1126/science.1091288 (doi:10.1126/science.1091288) [DOI] [PubMed] [Google Scholar]
- 23.Izumi M., et al. 2009. Changes in reflectin protein phosphorylation are associated with dynamic iridescence in squid. J. R. Soc. Interface 7, 549–560 10.1098/rsif.2009.0299 (doi:10.1098/rsif.2009.0299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kramer R. M., Crookes-Goodson W. J., Naik R. R. 2007. The self-organizing properties of squid reflectin protein. Nat. Mater. 6, 533–538 10.1038/nmat1930 (doi:10.1038/nmat1930) [DOI] [PubMed] [Google Scholar]
- 25.Hecht E. 2002. Optics, 4th edn. Reading, MA: Addison-Wesley [Google Scholar]
- 26.Cooper K. M., Hanlon R. T., Budelmann B. U. 1990. Physiological color change in squid iridophores. II. Ultrastructural mechanisms in Lolliguncula brevis. Cell Tissue Res. 259, 15–24 10.1007/BF00571425 (doi:10.1007/BF00571425) [DOI] [PubMed] [Google Scholar]
- 27.Mäthger L. M., et al. In preparation Bright white passive diffusion from sorft organic spheres in color changing cuttlefish. [Google Scholar]
- 28.Sutherland R. L., Mäthger L. M., Hanlon R. T., Urbas A. M., Stone M. O. 2008. Cephalopod coloration model. I. Squid chromatophores and iridophores. J. Opt. Soc. Am. A 25, 588–599 10.1364/JOSAA.25.000588 (doi:10.1364/JOSAA.25.000588) [DOI] [PubMed] [Google Scholar]
- 29.Endler J. A., Westcott D. A., Madden J. R., Robson T. 2005. Animal visual systems and the evolution of color patterns: sensory processing illuminates signal evolution. Evolution 59, 1795–1818 [DOI] [PubMed] [Google Scholar]
- 30.Hanlon R. T. 1982. The functional organization of chromatophores and iridescent cells in the body patterning of Loligo plei (Cephalopoda: Myopsida). Malacologia 23, 89–119 [Google Scholar]
- 31.Hanlon R. T., Messenger J. B. 1996. Cephalopod behaviour. Cambridge, UK: Cambridge University Press [Google Scholar]
- 32.Mäthger L. M., Hanlon R. T. 2007. Malleable skin coloration in cephalopods: selective reflectance, transmission and absorbance of light by chromatophores and iridophores. Cell Tissue Res. 329, 179–186 10.1007/s00441-007-0384-8 (doi:10.1007/s00441-007-0384-8) [DOI] [PubMed] [Google Scholar]
- 33.Mäthger L. M., Denton E. J., Marshall N. J., Hanlon R. T. 2009. Mechanisms and behavioral functions of structural colouration in cephalopods. J. R. Soc. Interface 6, S149–S163 10.1098/rsif.2008.0311 (doi:10.1098/rsif.2008.0311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manakasettharn S., Taylor J. A., Krupenkin T. N. 2011. Bio-inspired artificial iridophores based on capillary origami: fabrication and device characterization. Appl. Phys. Lett. 99, 144102. 10.1063/1.3646394 (doi:10.1063/1.3646394) [DOI] [Google Scholar]
- 35.Walish J. J., Kang Y., Mickiewicz R. A., Thomas E. L. 2009. Bioinspired electrochemically tunable block copolymer full color pixels. Adv. Mater. 21, 3078–3081 10.1002/adma.200900067 (doi:10.1002/adma.200900067) [DOI] [Google Scholar]
- 36.Mäthger L. M., Bell G., Kuzirian A. M., Allen J. J., Hanlon R. T. In press How does the blue-ringed octopus (Hapalochlaena lunulata) flash its blue rings? J. Exp. Biol. [DOI] [PubMed] [Google Scholar]
- 37.Messenger J. B. 2001. Cephalopod chromatophores: neurobiology and natural history. Biol. Rev. 76, 473–528 10.1017/S1464793101005772 (doi:10.1017/S1464793101005772) [DOI] [PubMed] [Google Scholar]
- 38.Yeo J. S., et al. 2010. Novel flexible reflective color media integrated with transparent oxide TFT backplane. SID Symp. Digest 41, 1041. 10.1889/1.3499827 (doi:10.1889/1.3499827) [DOI] [Google Scholar]
- 39.Heikenfeld J., Zhou K., Kreit E., Raj B., Yang S., Sun B., Milarcik A., Clapp L., Schwartz R. 2009. Electrofluidic displays using Young–Laplace transposition of brilliant pigment dispersions. Nat. Photonics 3, 292–296 10.1038/nphoton.2009.68 (doi:10.1038/nphoton.2009.68) [DOI] [Google Scholar]
- 40.Koch T. R., Liu Q., Benson B., Mabeck J., Hoffman R., Mourey D., Combs G., Zhou Z.-L., Henze D. Reflective full color electrokinetic displays. In Proc. of the 18th Int. Display Workshops (IDW ‘11). 7–9 December 2011, Nagoya, Japan.
- 41.Romney A. K. 2008. Relating reflectance spectra space to Munsell color appearance space. J. Opt. Soc. Am. A 25, 658–666 10.1364/JOSAA.25.000658 (doi:10.1364/JOSAA.25.000658) [DOI] [PubMed] [Google Scholar]
- 42.Levy-Lior A., Shimoni E., Schwartz O., Gavish-Regev E., Oron D., Oxford G., Weiner S., Addadi L. 2010. Guanine-based biogenic photonic-crystal arrays in fish and spiders. Adv. Funct. Mater. 20, 320–329 10.1002/adfm.200901437 (doi:10.1002/adfm.200901437) [DOI] [Google Scholar]
- 43.Tao A. R., DeMartini D. G., Izumi M., Sweeney A. M., Holt A. L., Morse D. E. 2010. The role of protein assembly in dynamically tunable bio-optical tissues. Biomaterials 31, 793–801 10.1016/j.biomaterials.2009.10.038 (doi:10.1016/j.biomaterials.2009.10.038) [DOI] [PubMed] [Google Scholar]
- 44.Miles M. W. 1997. A new reflective FPD technology using interferometric modulation. J. SID 5, 379–382 10.1889/1.1985183 (doi:10.1889/1.1985183) [DOI] [Google Scholar]
- 45.Arsenault A. C., Puzzo D. P., Manners I., Ozin G. A. 2007. Photonic-crystal full-colour displays. Nat. Photonics 1, 468–472 10.1038/nphoton.2007.140 (doi:10.1038/nphoton.2007.140) [DOI] [Google Scholar]
- 46.Wang H., Kerins F., Kamp U., Bonifacio L., Arsenault A. C., Ozin G. A. 2011. Photonic-crystal display materials. Inf. Display 7, 26–29 [Google Scholar]
- 47.Kulbaba K., et al. 2001. Polyferrocenylsilane and magnetic ceramic microspheres. Adv. Mater. 13, 732–736 (doi:10.1002/1521-4095(200105)13:10<732::AID-ADMA732>3.0.CO;2-2) [DOI] [Google Scholar]
- 48.Lee I., et al. 2010. Quasi-amorphous colloidal structures for electrically tunable full-color photonic pixels with angle-independency. Adv. Mater. 22, 4973–4977 10.1002/adma.201001954 (doi:10.1002/adma.201001954) [DOI] [PubMed] [Google Scholar]
- 49.Harun-Ur-Rashid M., Imran A. B., Seki T., Ishii M., Nakamura H., Takeoka Y. 2010. Angle-independent structural color in colloidal amorphous arrays. ChemPhysChem 11, 579–583 10.1002/cphc.200900869 (doi:10.1002/cphc.200900869) [DOI] [PubMed] [Google Scholar]
- 50.Saranathan V., Forster J. D., Noh H., Liew S. F., Mochrie S. G. J., Cao H., Dufresne E. R., Prum R. O. 2012. Structure and optical function of amorphous photonic nanostructures from avian feather barbs: a comparative small angle X-ray scattering (SAXS) analysis of 230 bird species. J. R. Soc. Interface 9, 2563–2580 10.1098/rsif.2012.0191 (doi:10.1098/rsif.2012.0191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Froesch D., Messenger J. B. 1978. On leucophores and chromatic unit of Octopus vulgaris. J. Zool. Lond. 186, 163–173 10.1111/j.1469-7998.1978.tb03363.x (doi:10.1111/j.1469-7998.1978.tb03363.x) [DOI] [Google Scholar]
- 52.Weber M. F., Stover C. A., Gilbert L. R., Nevitt T. J., Ouderkirk A. J. 2000. Giant birefringent optics in multilayer polymer mirrors. Science 287, 2451–2456 10.1126/science.287.5462.2451 (doi:10.1126/science.287.5462.2451) [DOI] [PubMed] [Google Scholar]
- 53.Stuart-Fox D., Moussalli A. 2009. Camouflage, communication and thermoregulation: lessons from colour changing organisms. Phil. Trans. R. Soc. B 364, 463–470 10.1098/rstb.2008.0254 (doi:10.1098/rstb.2008.0254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stuart-Fox D., Moussalli A., Whiting M. J. 2008. Predator-specific camouflage in chameleons. Biol. Lett. 4, 326–329 10.1098/rsbl.2008.0173 (doi:10.1098/rsbl.2008.0173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vukusic P., Sambles J. R. 2003. Photonic structures in biology. Nature 424, 852–855 10.1038/nature01941 (doi:10.1038/nature01941) [DOI] [PubMed] [Google Scholar]
- 56.Prum R. O., Dufresne E. R., Quinn T., Waters K. 2009. Development of colour-producing beta-keratin nanostructures in avian feather barbs. J. R. Soc. Interface 6(Suppl. 2), S253–S265 10.1098/rsif.2008.0466.focus (doi:10.1098/rsif.2008.0466.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Srinivasarao M. 1999. Nano-optics in the biological world: beetles, butterflies, birds, and moths. Chem. Rev. 99, 1935–1962 10.1021/cr970080y (doi:10.1021/cr970080y) [DOI] [PubMed] [Google Scholar]
- 58.Shawkey M. D., Morehouse N. I., Vukusic P. 2009. A protean palette: colour materials and mixing in birds and butterflies. J. R. Soc. Interface 6, S221–S231 10.1098/rsif.2008.0459.focus (doi:10.1098/rsif.2008.0459.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vukusic P., Sambles J. R., Lawrence C. R., Wootton R. J. 1999. Quantified interference and diffraction in single morpho butterfly scales. Proc. R. Soc. Lond. B. 266, 1403–1411 10.1098/rspb.1999.0794 (doi:10.1098/rspb.1999.0794) [DOI] [Google Scholar]
- 60.Nozawa T. 2011. TechOn. See http://techon.nikkeibp.co.jp/english/NEWS_EN/20111027/199890/
- 61.Heikenfeld J. 2010. IEEE spectrum. See http://spectrum.ieee.org/computing/hardware/the-electronic-display-of-the-future/0.
- 62.Gelinck G. H., et al. 2006. A rollable, organic electrophoretic QVGA display with field-shielded pixel architecture. J. SID 14, 113–118 10.1889/1.2176112 (doi:10.1889/1.2176112) [DOI] [Google Scholar]
- 63.Johnson M. T., Zhou G., Zehner R., Amundson K., Henzen A., Kamer J. v. d. 2006. High-quality images on electrophoretic displays. J. Soc. Info. Display 14, 175–180 10.1889/1.2176120 (doi:10.1889/1.2176120) [DOI] [Google Scholar]
- 64.O'Rourke S. M., et al. 2008. Direct fabrication of a-Si:H thin film transistor arrays on flexible substrates: critical challenges and enabling solutions. ECS Transactions 16, 49–54 [Google Scholar]
- 65.Kim D. H., et al. 2011. Epidermal electronics. Science 333, 838–843 10.1126/science.1206157 (doi:10.1126/science.1206157) [DOI] [PubMed] [Google Scholar]
- 66.Karaman M., Kooi S. E., Gleason K. K. 2008. Vapor deposition of hybrid organic-inorganic dielectric Bragg mirrors having rapid and reversibly tunable optical reflectance. Chem. Mater. 20, 2262–2267 10.1021/cm703107d (doi:10.1021/cm703107d) [DOI] [Google Scholar]
- 67.Rossiter J., Yap B., Conn A. 2012. Biomimetic chromatophores for camouflage and soft active surfaces. Bioinspiration Biomimetics 7, 036009. 10.1088/1748-3182/7/3/036009 (doi:10.1088/1748-3182/7/3/036009) [DOI] [PubMed] [Google Scholar]
- 68.Morin S. A., Shepherd R. F., Kwok S. W., Stokes A. A., Nemiroski A., Whitesides G. M. 2012. Camouflage and display for soft machines. Science 337, 828–832 10.1126/science.1222149 (doi:10.1126/science.1222149) [DOI] [PubMed] [Google Scholar]
- 69.Vukusic P. 2010. Contact lens spectrum. See http://www.clspectrum.com/printarticle.aspx?articleID=104164 (accessed 19 July 2012).
- 70.Huitema E., van Veenendaal E., van Aerle N., Touwslager F., Hamers J., van Lieshout P. 2008. Rollable displays: a technology development enabling breakthrough mobile devices. SID Symp. Digest 39, 927. 10.1889/1.3069827 (doi:10.1889/1.3069827) [DOI] [Google Scholar]
- 71.Mäthger L. M., Collins T. F. T., Lima P. A. 2004. The role of muscarinic receptors and intercellular Ca2+ in the spectral reflectivity changes of squid iridophores. J. Exp. Biol. 207, 1759–1769 10.1242/jeb.00955 (doi:10.1242/jeb.00955) [DOI] [PubMed] [Google Scholar]
- 72.Sutherland R. L., Mäthger L. M., Hanlon R. T., Urbas A. M., Stone M. O. 2008. Cephalopod coloration model. II. Multiple layer skin effects. J. Opt. Soc. Am. A 25, 2044–2054 10.1364/JOSAA.25.002044 (doi:10.1364/JOSAA.25.002044) [DOI] [PubMed] [Google Scholar]
- 73.Yang S., et al. 2011. Electrofluidic displays: fundamental platforms and unique performance attributes. J. SID 19, 608–613 10.1889/JSID19.9.608 (doi:10.1889/JSID19.9.608) [DOI] [Google Scholar]
- 74.Chiao C. C., Wickiser J. K., Allen J. J., Genter B., Hanlon R. T. 2011. Hyperspectral imaging of cuttlefish camouflage indicates good color match in the eyes of fish predators. Proc. Natl Acad. Sci. USA 108, 9148–9153 10.1073/pnas.1019090108 (doi:10.1073/pnas.1019090108) [DOI] [PMC free article] [PubMed] [Google Scholar]