Abstract
Strontium-releasing injectable bone cements may have the potential to prevent implant-related infections through the bactericidal action of strontium, while enhancing bone formation in patients suffering from osteoporosis. A melt-derived bioactive glass (BG) series (SiO2–CaO–CaF2–MgO) with 0–50% of calcium substituted with strontium on a molar base were produced. By mixing glass powder, poly(acrylic acid) and water, cements were obtained which can be delivered by injection and set in situ, giving compressive strength of up to 35 MPa. Strontium release was dependent on BG composition with increasing strontium substitution resulting in higher concentrations in the medium. Bactericidal effects were tested on Staphylococcus aureus and Streptococcus faecalis; cell counts were reduced by up to three orders of magnitude over 6 days. Results show that bactericidal action can be increased through BG strontium substitution, allowing for the design of novel antimicrobial and bone enhancing cements for use in vertebroplasty or kyphoplasty for treating osteoporosis-related vertebral compression fractures.
Keywords: antibacterial, antimicrobial, bioactive glass, bone cement, strontium
1. Introduction
In osteoporotic bone osteoclasts resorb too much bone, while osteoblasts do not form enough bone to counterbalance this, resulting in reduced bone mineral density and an increased risk of fractures. About 700 000 vertebral compression fractures occur each year in the United States, and about a third of these fractures are persistently painful after non-operative care [1]. Vertebroplasty and kyphoplasty are common procedures which restore fractured vertebrae by injecting a bone cement such as poly(methyl methacrylate) (PMMA), and the aim is to relieve the pain while restoring dimensions and strength of the vertebrae. Glass ionomer cements (GICs) have the potential to overcome some of the limitations of acrylic cements, such as shrinkage during polymerization and a highly exothermic setting reaction, as they set by an acid–base reaction between a polymeric acid (such as poly(acrylic acid), PAA) and a degradable glass [2]. Furthermore, GICs form a chemical bond to bone [3], which is in contrast to acrylic cements, where mechanical stability is achieved by mechanical interlocking only.
Bioactive glasses (BGs) are recognized as biomaterials for hard tissue repair, and have been in clinical use since 1985 [4], e.g. under the trade name of NovaBone for orthopaedic or PerioGlas for periodontal applications. Strontium-substituted BGs have recently been shown to combine the known bone regenerative properties of BG with the anabolic and anticatabolic effects of Sr2+ cations in vitro, making them of interest for bone regeneration in patients suffering from osteoporosis [5]. An ideal bone cement for treatment of vertebral compression fractures would combine the benefits of strontium-substituted BGs with handling properties allowing for minimally invasive delivery, and GICs based on strontium-containing BGs are of great interest for such applications.
Antimicrobial materials are attractive in a wide variety of medical applications. The use of invasive devices increases the risk of infection as they bypass normal host defences and allow micro-organisms to access normally sterile environments [6,7], while the surfaces of the implants act as substrata that allow micro-organisms to adhere and form biofilms. Gram-positive bacteria such as Streptococcus spp., Staphylococcus spp. and Bacillus spp. play an important role in implant-related infections [8]. In recent years, strontium has been incorporated into dental and orthopaedic biomaterials to reduce this microbial contamination [9,10], and the antimicrobial activity of Sr2+ ions can be used to enhance the use of medical devices by inhibiting bacterial growth and reproduction and impeding permeability of cytoplasmic membrane, cell wall synthesis, replication of bacterial chromosomes and cell metabolism. Although the importance of strontium and its biological role in the treatment of osteoporosis has been recognized in recent years [11,12], its mechanism of antimicrobial action still remains unclear, but a possible synergistic antimicrobial effect of fluoride and Sr2+ ions has been suggested [13].
In this study, BG (SiO2–CaO–CaF2–MgO) with 0, 2.5, 10 or 50 per cent of calcium replaced by strontium on a molar base were produced, and novel injectable materials were formed by mixing with PAA and deionized water (dH2O). The aim was to study the influence of strontium on the cement properties and antimicrobial activity in vitro.
2. Material and methods
2.1. Glass synthesis
BGs (SiO2–CaO–CaF2–MgO) where either no calcium (Sr0) or 2.5, 10 or 50 per cent of calcium (Sr2.5, Sr10, Sr50) was replaced by strontium on a molar base (table 1) were produced using a standard melt-quench route. Mixtures of analytical grade SiO2 (Prince Minerals Ltd, Stoke-on-Trent, UK), CaCO3, SrCO3, CaF2, SrF2 and MgO light (all Sigma-Aldrich, Gillingham, UK) were melted in a platinum/rhodium crucible for 1 h at 1430°C in an electric furnace (EHF 17/3, Lenton, Hope Valley, UK); a batch size of 100 g was used. After melting, the BG was rapidly quenched into water to prevent crystallization. After drying at 80°C, the BG frit was ground using a vibratory mill (Gy-Ro mill, Glen Creston, London, UK) for 7 min and sieved using a 38 µm mesh analytical sieve (Endecotts Ltd, London, UK).
Table 1.
Glass composition (mol%) and strontium for calcium substitution (%).
| glass | SiO2 | CaO | CaF2 | SrO | SrF2 | MgO | Sr substitution |
|---|---|---|---|---|---|---|---|
| Sr0 | 47.32 | 10.41 | 11.04 | — | — | 31.23 | 0 |
| Sr2.5 | 47.32 | 10.15 | 10.76 | 0.26 | 0.28 | 31.23 | 2.5 |
| Sr10 | 47.32 | 9.37 | 9.93 | 1.04 | 1.10 | 31.23 | 10 |
| Sr50 | 47.32 | 5.21 | 5.52 | 5.21 | 5.52 | 31.23 | 50 |
2.2. Cement formation
Cement formulations are given in table 2. The amount of BG+PAA was 0.6 g, and the dH2O : PAA ratio was 1.5 : 1. The weight of glass powder was changed with composition to account for differences in atomic weights of calcium and strontium. Cements were prepared by weighing and mixing BG and PAA powder (Advanced Healthcare Ltd, Tonbridge, UK), then weighing dH2O and hand-mixing on a Perspex sheet with a stainless steel spatula. After mixing, plastic moulds of 7.6 mm inner diameter and 0.7 mm height were filled with cement, covered with acetate sheets, clamped between stainless steel plates and kept at 37°C for 1 h; afterwards each specimen was stored for 60 min in 1000 µl dH2O at room temperature under UV light for disinfection. Working and setting times of the cements were determined using an oscillating rheometer at 37°C. Cement capsules (Advanced Healthcare Ltd) were prepared using 0.650 g glass–PAA mixture (ratio 10 : 3) and 0.200 g H2O in a liquid chamber. Mixing time was 10 s using a rotating mixer (ESPE RotoMix).
Table 2.
Cement composition accounting for differences in atomic weights of calcium and strontium.
| weight (g) |
|||
|---|---|---|---|
| sample | glass | PAA | H2O |
| Sr0 | 0.5 | 0.1 | 0.15 |
| Sr2.5 | 0.5 | 0.1 | 0.149 |
| Sr10 | 0.502 | 0.098 | 0.148 |
| Sr50 | 0.507 | 0.093 | 0.139 |
2.3. Solid-state magic angle spinning nuclear magnetic resonance
Glass structure was analysed using 29Si magic angle spinning nuclear magnetic resonance (MAS NMR) spectroscopy; experiments were performed using a Bruker 200 MHz (4.7 T) spectrometer at a Larmor frequency of 39.7 MHz and spinning speed of 4.5 kHz in a 4 mm rotor using a Hahn-echo pulse sequence with a π/2 pulse of 5.25 and 76.5 µs echo delay. The −1.5 ppm peak of tetrakis(trimethylsilyl)methane was used for reference. Data were processed with 200 Hz line broadening.
For MAS NMR characterization of structural changes during setting, cement samples (10 mm inner diameter and 1 mm height) were allowed to set for 1 h, 1 day, one week and one month, respectively. Afterwards, specimens were quenched using liquid nitrogen, dehydrated in ethanol and allowed to dry. Cement structure at each time point was analysed using 29Si MAS NMR as described above.
2.4. Mechanical testing
For compressive strength testing, cements were mixed as described above; stainless steel moulds with an inner diameter of 4 and 6 mm in height were used. Per composition, eight samples were prepared and tested (n = 8). Samples were stored in 10 ml of deionized water at 37°C for four weeks, then compressive strength was tested using a hydraulic testing machine (Instron 5567) with a 1 kN load cell. Results were analysed using analysis of variance (ANOVA; Spss Statistics v. 17.0, SPSS Inc., Chicago, IL, USA) followed by Bonferroni post hoc test; p < 0.05 was considered significant. Results are presented as mean ± confidence interval (CI).
2.5. Bactericidal studies
Two Gram-positive micro-organisms Staphylococcus aureus (NCIMB6571) and Streptococcus faecalis (NCIMB775) were obtained from the University of Wolverhampton culture collection. The stock cultures were freeze-dried and kept at −20°C. Before use, bacteria were cultivated on a plate overnight.
Tryptone soya agar (TSA) and tryptone soya broth (TSB) (both Lab M Ltd, Bury, UK) were prepared following the manufacturer's protocol. One-quarter strength Ringer solution was prepared by dissolving one tablet in 500 ml of dH2O. All media were sterilized by autoclaving at 121°C for 15 min.
A single bacterial colony for each micro-organism was used to inoculate a 100 ml starter culture, which was grown overnight at 37°C in a rotary shaker (150 r.p.m.), then diluted to obtain a concentration of 1 × 106 cells ml−1. One microlitre of culture was transferred to a test tube and a sterile cement pellet was added. Tubes containing S. aureus or S. faecalis only in TSB were used as negative controls; tubes containing a strontium-free cement pellet (Sr0) with S. aureus or S. faecalis in TSB were used as positive controls. Samples were incubated at 37°C and 150 r.p.m. Experiments were performed in triplicate.
The viable count was monitored by withdrawing 0.5 ml of each culture at 0, 48 and 144 h. Each sample was serially diluted in 10-fold steps from 10−1 to 10−8 by dispensing 0.5 ml of each sample in 4.5 ml of sterile one-quarter strength Ringer solution; then 0.5 ml of this solution was further diluted in 4.5 ml. The procedure was repeated until a dilution factor (DF) of 10−8 was reached. The Miles and Misra technique was used to calculate the viable cell count in colony forming units per millilitre (CFU ml−1). Twenty microlitres of each dilution were dispensed onto TSA plates under aseptic conditions. This was done in triplicate. The plates were then incubated at 37°C for 24 h after which plates with less than 20 colonies were selected and used for viable count determination. CFU ml−1 was calculated according to equation (2.1):
| 2.1 |
where n is the number of colonies and DF is a dilution factor. Results are presented as mean±standard deviation (s.d.).
The remaining 0.5 ml TSB in each tube was diluted by a factor 1 : 10 for elemental analysis by inductively coupled plasma atomic emission spectroscopy (ICP-AES; Spectro Ciros, Spectro GmbH, Kleve, Germany). Results are presented as mean±s.d.
3. Results
3.1. Bioactive glass formation
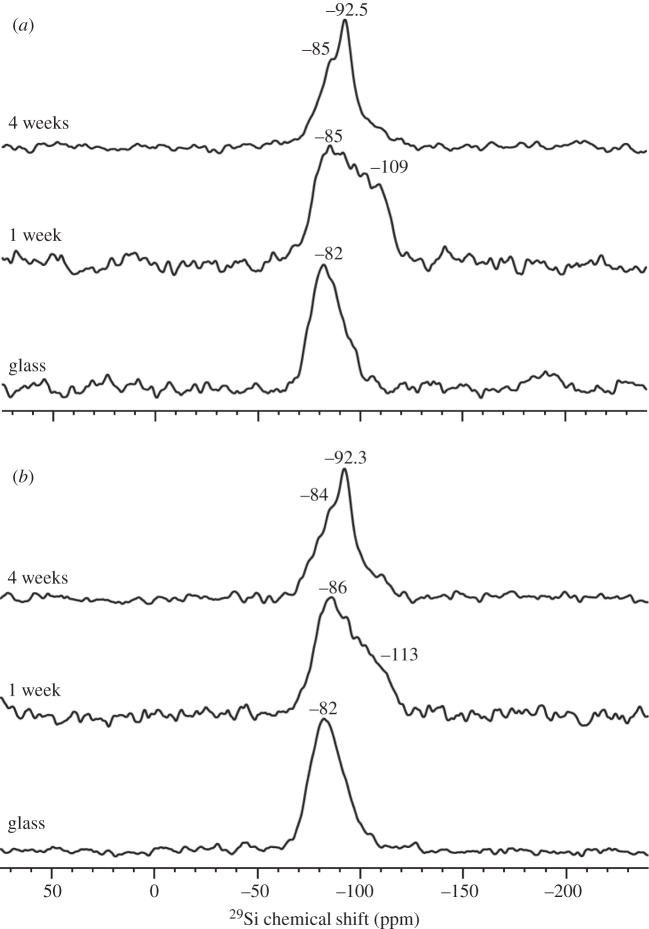
The BG frits were optically clear and were shown to be amorphous by powder X-ray powder diffraction (Phillips PW1700; results not shown). 29Si solid-state MAS NMR spectra of glasses (shown in figure 1 for glasses Sr0 and Sr50 only) showed a single peak at a chemical shift of −82 ppm with a shoulder between −90 and −100 ppm, indicating a predominantly Q2 silicate structure (i.e. two bridging oxygen atoms, BO, per silicon atom, corresponding to a silicate chain structure) with smaller amounts of Q3 silicate units (i.e. three BO per silicon, corresponding to branching silicate units), which is typical for BG [14–17]. Strontium for calcium substitution did not significantly alter the structure of the silicate network.
Figure 1.
29Si MAS NMR spectra of glass and cements (at 1 and 4 weeks) for compositions (a) Sr0 and (b) Sr50.
3.2. Cement formation and properties
Cements mixed well, showing working times between 90 s and 2 min when hand-mixed. Only glass Sr50 showed shorter mixing and working times (<1 min). These mixing properties allowed for preparation of cements in capsules using a commercial rotary mixer and extrusion of cements through the 2 mm diameter nozzle of the capsules. All cements set hard within 60 min. Although they turned from brittle to slightly rubbery in dH2O, they remained stable and hardened further over time.
At one week of setting of the cements, 29Si solid-state MAS NMR spectra showed a broad shoulder appearing at about −113 ppm, corresponding to the presence of both Q3 and Q4 units (figure 1). At four weeks the shoulder had disappeared again, suggesting the absence of Q4 units, but a peak at −92 ppm suggested that Q3 units were still present in significant concentrations.
Compressive strength (figure 2) of cements after setting in deionized water at 37°C for four weeks did not show a direct relationship with strontium substitution in the glass. Compositions Sr0 and Sr10 gave average compressive strengths of approximately 29 MPa, which were significantly higher than those of compositions Sr2.5 (20 MPa) and Sr50 (19 MPa) (ANOVA, Bonferroni, p < 0.05; figure 2).
Figure 2.
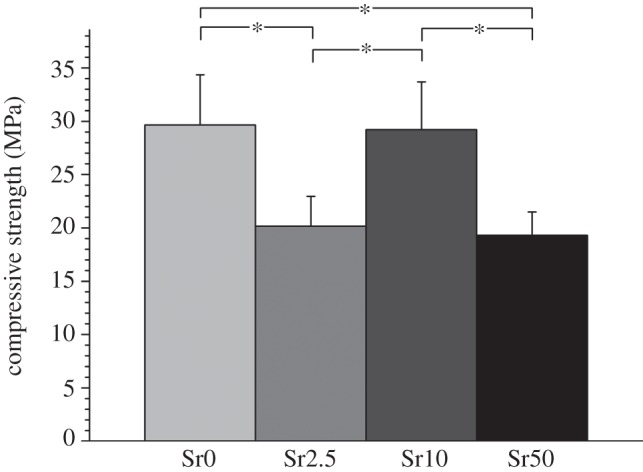
Mean compressive strength±95% CI at 4 weeks; *significantly different (ANOVA, Bonferroni, p < 0.05).
3.3. Bactericidal studies
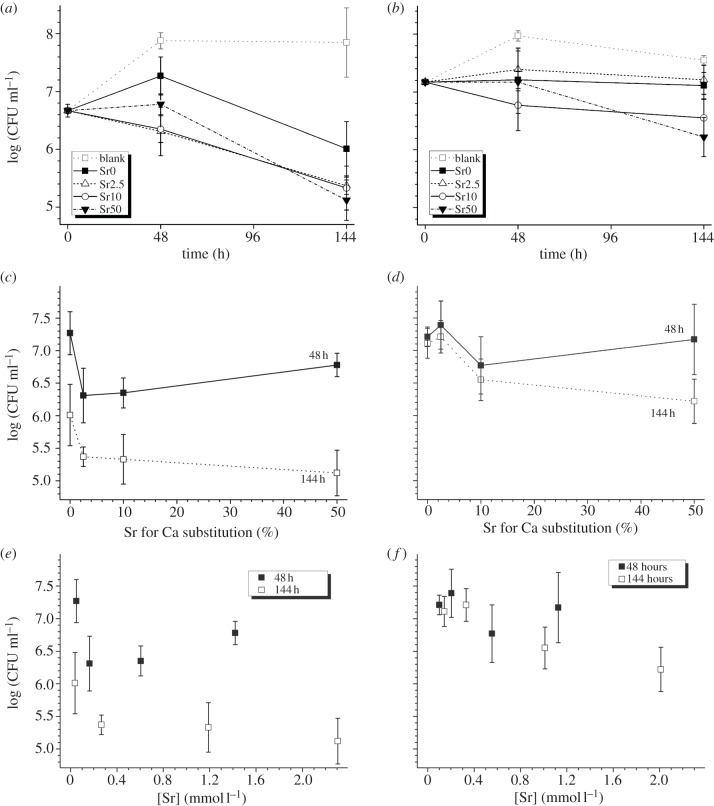
The negative controls (blank, figure 3a,b) showed progressive growth of both organisms over the first 48 h (2 days); afterwards cell counts remained constant (S. faecalis) or decreased slightly (S. aureus) until 144 h (6 days). The presence of strontium cements (Sr2.5, Sr10 and Sr50) resulted in inhibition of both micro-organisms within 48 h (figure 3a,b), with cell counts for S. faecalis and S. aureus reduced between 0.5 and 1.5 log(CFU ml−1) compared with blank. At 144 h, the action of the strontium cements was more pronounced with S. faecalis (bactericidal counts reduced up to 2.7 log(CFU ml−1) compared with the blank) than in S. aureus (bactericidal cell counts reduced up to 1.3 log(CFU ml−1) compared with the blank). The strontium-free positive control (Sr0) also reduced cell counts compared with the blank with 1.84 log(CFU ml−1) reduction for S. faecalis and 0.44 log(CFU ml−1) for S. aureus at 144 h.
Figure 3.
Antimicrobial activity (± s.d.) of (a) S. faecalis and (b) S. aureus over time, (c) S. faecalis and (d) S. aureus versus Sr for Ca substitution in the glass and (e) S. faecalis and (f) S. aureus versus Sr2+ concentration in the culture medium.
Cell counts of S. faecalis (figure 3c) reduced when substituting 2.5 per cent strontium for calcium; however, cell counts did not decrease further with increasing strontium substitution. For S. aureus (figure 3d), this trend was less pronounced owing to larger variation of the cell numbers. Figure 3e,f shows a reduction in cell counts with increased strontium concentration in solution; however, owing to cell count variability the correlation was not significant.
3.4. Ion release
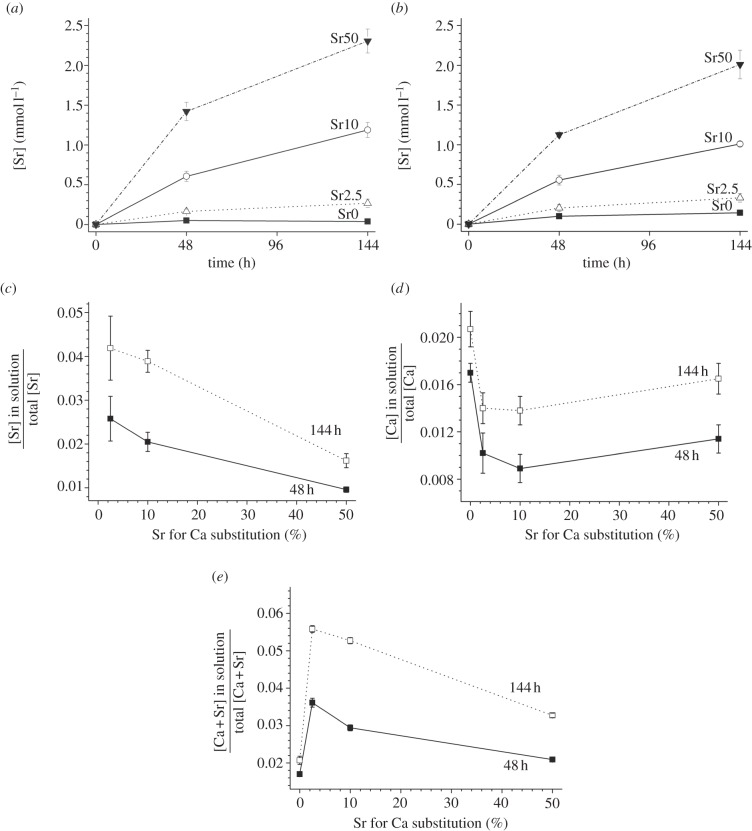
Strontium release into the medium increased over time (figure 4a,b) but was also dependent on BG composition, with increasing strontium in solution for increasing strontium for calcium substitution (from Sr0 to Sr50; figure 4a,b). Figure 4c–e shows dissolved ions (Ca, Sr and their sum) relative to their total amount (i.e. relative to the total amount per cement disc before immersion in culture medium) as a function of strontium for calcium substitution. The amount of strontium in solution relative to the total strontium concentration decreased for compositions Sr2.5 to Sr50; calcium showed an initial decrease upon first strontium incorporation in the BG (from composition Sr0 to Sr2.5), but for the other compositions (from Sr2.5 to Sr50) error bars overlapped, so concentration differences were not significant. The amount of Ca+Sr in solution relative to their total amount increased for 2.5 per cent strontium substitution, but decreased with further strontium for calcium substitution.
Figure 4.
Strontium concentrations (±s.d.) after immersion of cements in TSB with (a) S. faecalis and (b) S. aureus versus time. (c) Strontium, (d) calcium and (e) combined strontium+calcium concentration versus Sr for Ca substitution in the glass.
4. Discussion
Calcium and strontium are typical network modifiers, and owing to similarities in their ionic radius they can be substituted for each other without significantly altering the silicate network [18]. The lower charge-to-size ratio of Sr2+ (owing to a slightly larger ionic radius), however, was shown to cause an expansion of the silicate network, making it less strongly ionically cross-linked [18], which improves glass dissolution [19] and would be expected to facilitate cement formation.
Magnesium-containing BGs degrade when mixed with PAA and dH2O, allowing for making novel injectable bone cements which can be delivered via minimally invasive methods. The setting mechanism of these materials is similar to that of dental glass polyalkenoate (ionomer) cements (GPC) which occur by the PAA chains being cross-linked by cations released from the glass upon degradation [2]. Dental GPCs contain aluminium, which plays an important role in both glass degradation (hydrolysis of Si–O–Al bonds) and in stability of the cements (cross-linking of PAA chains by Al3+); however, as aluminium is a known neurotoxin [20] and also negatively affects bone mineralization [21–23], dental GPCs are not ideal for orthopaedic applications. Efforts have been made to replace aluminium with iron [24] or zinc [25]; however, these also have drawbacks and particularly too high a zinc release has been shown to result in cytotoxic reactions [26]. Materials based on magnesium-containing BG can therefore provide a promising alternative.
The BG used in this study contained more than 30 mol% MgO, an intermediate oxide [27] that can either act as a network modifier (such as Ca and Sr) in the glass (creating non-bridging oxygens) or enter the silicate network, forming Si–O–Mg bonds [28]. These Si–O–Mg bonds can be hydrolysed under acidic conditions (e.g. when mixing glass powder, PAA and dH2O), resulting in degradation of the glass matrix and the release of cations such as Ca2+, Mg2+ and Sr2+ from the glass. These ions subsequently form ionic cross-links with the carboxylate groups of the PAA chains, causing the cement to set. Our results show that it is possible to produce cements based on bioactive, i.e. aluminium-free, glasses.
The BGs studied have a silicate chain structure consisting of mostly Q2 units, as shown by 29Si MAS NMR, allowing for the glasses to degrade by exchanging cations for protons from the surrounding solutions and resulting in the transformation of Si–O− (non-bridging oxygen) to Si–OH groups [29]. 29Si MAS NMR measurements on the cements show that subsequently these Si–OH groups undergo condensation polymerizsation, resulting in increasing numbers of Q3 and Q4 groups (in addition to Q2) at one week. At about four weeks, 29Si MAS NMR shows presence of Q3 and Q2 units mostly, with only small amounts of Q4, suggesting further structural rearrangement. 29Si MAS NMR allows us to follow the glass degradation during the setting of cement. Cement properties, on the other hand, will depend on ionic cross-links between PAA chains and therefore on the amounts of different ions in the cement matrix. Further studies focussing on 13C MAS NMR will hopefully help us to elucidate changes in cross-linking over time.
When substituting the larger Sr2+ ions (ionic radius 1.12 Å) for the smaller Ca2+ (0.99 Å), the silicate network, although not changing its Q speciation, expands [18], facilitating glass degradation and the release of ions [19]. This allows for faster and more efficient cross-linking of the PAA chains owing to larger amounts of cations being available, and it explains the short mixing and working times observed for composition Sr50.
Although BG becomes more degradable with strontium substitution, the cements in this study were more hydrolytically stable with increasing strontium substitution, shown by decreasing ion release (relative to their total amount). This is somewhat surprising, as one might expect the ionic cross-linking of the PAA chains to also become less effective with decreasing charge-to-size ratio of the cations (i.e. when substituting strontium for Ca) in analogy to observations for Sr-BG. However, the observed increased cement stability with Sr substitution can be explained by the possibly larger concentrations of cations being available for cross-linking. As strontium-substituted glasses are more degradable than the strontium-free glass [19], more ions are available for cross-linking of the PAA chains, and subsequently the strontium cements are more stable.
The shorter working times with strontium substitution, on the one hand, and the increased cement stability with strontium substitution, on the other, may be the explanation for the nonlinear (but statistically significant) variation in cement compressive strength, suggesting that the relationship between cement performance and strontium content is a complicated one and needs some more detailed investigation.
The cements in this study reduced bacterial cell counts by several orders of magnitude. The bactericidal action of strontium-substituted compositions can be explained by Sr2+ release and subsequent presence of Sr2+ cations in the culture medium, as shown previously for calcium phosphate bone cements [9] and dental ionomer cements [10]. However, even the Sr-free composition (Sr0) reduced bacterial cell counts by up to two orders of magnitude compared with the blank. This bactericidal effect of Sr0 can be explained by the combined effect of PAA and fluoride. The BG contain 11.04 mol% of calcium fluoride or strontium fluoride. Fluoride is known to have bactericidal effects [30], particularly under acidic conditions [31], and as PAA reduces the pH of the surrounding medium compared to the blank it may be this combined bactericidal action of fluoride and acidic pH which works for all materials in this study, including the strontium-free positive control, thereby affecting cells and reducing cell numbers.
Although the relative Sr2+ release (compared with its total amount) decreased with strontium substitution, the absolute strontium concentrations in solution increased with increasing strontium substitution. These differences in strontium concentration in the culture medium, however, did not seem to affect bacterial cell counts, and there was no correlation of either strontium substitution or strontium concentration in solution with bacterial cell counts. Small strontium substitutions (Sr2.5) resulted in cell counts of S. faecalis being reduced by between 0.5 and 1 order of magnitude compared with the strontium-free composition, but cell counts did not reduce further with increasing strontium substitution. This suggests that strontium concentrations above 0.16 mmol l−1 (14 ppm) do not further improve bactericidal action.
While increasing strontium concentrations in the culture medium did not result in further improved bactericidal effects, varying the strontium release is of interest for other reasons. Cements in this study gave strontium concentrations between 0.16 mmol l−1 (14 ppm) and 2.5 mmol l−1 (219 ppm) in the culture medium. Concentrations in a similar range were recently shown to stimulate osteoblasts (25 ppm) and inhibit osteoclasts (80 ppm) [5], suggesting that the incorporation of strontium into these materials may allow for a dual action of strontium: inhibition of bacteria and enhancement of bone formation.
5. Conclusions
Novel injectable strontium-releasing bone cements based on BGs and PAA were shown to be bactericidal, reducing cell counts of S. faecalis and S. aureus by up to three orders of magnitude over 6 days. Strontium for calcium substitution in the glass was shown to be an efficient way to adjust the bactericidal properties of the bone cements, making strontium a promising bactericidal component of BGs and related biomaterials. As strontium-substituted BGs are also known to stimulate osteoblasts and inhibit osteoclasts, these bone cements may have the potential to be used for treating osteoporosis-related vertebral compression fractures, enhancing bone formation and preventing implant-related infections.
Acknowledgements
Financial support by the Department of Trade and Industry (now the Technology Strategy Board, grant HO669), UK, is gratefully acknowledged.
References
- 1.Department of Research & Scientific Affairs, American Academy of Orthopaedic Surgeons. 2010. Rosemont, IL, USA.
- 2.Wilson AD. 1996. A hard decade's work: steps in the invention of the glass-ionomer cement. J. Dental Res. 75, 1723–1727 10.1177/00220345960750100301 (doi:10.1177/00220345960750100301) [DOI] [PubMed] [Google Scholar]
- 3.Wilson AD, Prosser HJ, Powis DM. 1983. Mechanism of adhesion of poly-electrolyte cements to hydroxyapatite. J. Dental Res. 62, 590–592 10.1177/00220345830620051801 (doi:10.1177/00220345830620051801) [DOI] [PubMed] [Google Scholar]
- 4.Hench LL. 2006. The story of Bioglass. J. Mater. Sci. Mater. Med. 17, 967–978 10.1007/s10856-006-0432-z (doi:10.1007/s10856-006-0432-z) [DOI] [PubMed] [Google Scholar]
- 5.Gentleman E, Fredholm YC, Jell G, Lotfibakhshaiesh N, O'Donnell MD, Hill RG, Stevens MM. 2010. The effects of strontium-substituted bioactive glasses on osteoblasts and osteoclasts in vitro. Biomaterials 31, 3244–3252 10.1016/j.biomaterials.2010.01.121 (doi:10.1016/j.biomaterials.2010.01.121) [DOI] [PubMed] [Google Scholar]
- 6.Kwakye-Awuah B, Williams C, Kenward MA, Radecka I. 2008. Antimicrobial action and efficiency of silver-loaded zeolite X. J. Appl. Microbiol. 104, 1516–1524 10.1111/j.1365-2672.2007.03673.x (doi:10.1111/j.1365-2672.2007.03673.x) [DOI] [PubMed] [Google Scholar]
- 7.Schierholz JM, Fleck C, Beuth J, Pulverer G. 2000. The antimicrobial efficacy of a new central venous catheter with long-term broad-spectrum activity. J. Antimicrob. Chemotherapy 46, 45–50 10.1093/jac/46.1.45 (doi:10.1093/jac/46.1.45) [DOI] [PubMed] [Google Scholar]
- 8.Farrar D, Benson R, Milner R. 2010. Antibiotic-loaded bone cements. In Drug-device combination products: delivery technologies and applications. (ed. Lewis A.). Cambridge, UK: Woodhead Publishing [Google Scholar]
- 9.Alkhraisat MH, Rueda C, Cabrejos-Azama J, Lucas-Aparicio J, Mariño FT, Torres Garcia-Denche J, Jerez LB, Gbureck U, Cabarcos EL. 2010. Loading and release of doxycycline hyclate from strontium-substituted calcium phosphate cement. Acta Biomater. 6, 1522–1528 10.1016/j.actbio.2009.10.043 (doi:10.1016/j.actbio.2009.10.043) [DOI] [PubMed] [Google Scholar]
- 10.Dabsie F, Gregoire G, Sixou M, Sharrock P. 2009. Does strontium play a role in the cariostatic activity of glass ionomer? Strontium diffusion and antibacterial activity. J. Dentistry 37, 554–559 10.1016/j.jdent.2009.03.013 (doi:10.1016/j.jdent.2009.03.013) [DOI] [PubMed] [Google Scholar]
- 11.Marie PJ, Ammann P, Boivin G, Rey C. 2001. Mechanisms of action and therapeutic potential of strontium in bone. Calcified Tissue Int. 69, 121–129 10.1007/s002230010055 (doi:10.1007/s002230010055) [DOI] [PubMed] [Google Scholar]
- 12.Marie PJ. 2005. Strontium as therapy for osteoporosis. Curr. Opin. Pharmacol. 5, 633–636 10.1016/j.coph.2005.05.005 (doi:10.1016/j.coph.2005.05.005) [DOI] [PubMed] [Google Scholar]
- 13.Guida A, Towler MR, Wall JG, Hill RG, Eramo S. 2003. Preliminary work on the antibacterial effect of strontium in glass ionomer cements. J. Mater. Sci. Lett. 22, 1401–1403 10.1023/A:1025794927195 (doi:10.1023/A:1025794927195) [DOI] [Google Scholar]
- 14.Elgayar I, Aliev AE, Boccaccini AR, Hill RG. 2005. Structural analysis of bioactive glasses. J. Non-Cryst. Solids 351, 173–183 10.1016/j.jnoncrysol.2004.07.067 (doi:10.1016/j.jnoncrysol.2004.07.067) [DOI] [Google Scholar]
- 15.Lockyer MWG, Holland D, Dupree R. 1995. NMR investigation of the structure of some bioactive and related glasses. J. Non-Cryst. Solids 188, 207–219 10.1016/0022-3093(95)00188-3 (doi:10.1016/0022-3093(95)00188-3) [DOI] [Google Scholar]
- 16.O'Donnell MD, Watts SJ, Law RV, Hill RG. 2008. Effect of P2O5 content in two series of soda lime phosphosilicate glasses on structure and properties. Part I. NMR. J. Non-Cryst. Solids 354, 3554–3560 10.1016/j.jnoncrysol.2008.03.034 (doi:10.1016/j.jnoncrysol.2008.03.034) [DOI] [Google Scholar]
- 17.Brauer DS, Karpukhina N, Law RV, Hill RG. 2009. Structure of fluoride-containing bioactive glasses. J. Mater. Chem. 19, 5629–5636 10.1039/b900956f (doi:10.1039/b900956f) [DOI] [Google Scholar]
- 18.Fredholm YC, Karpukhina N, Law RV, Hill RG. 2010. Strontium containing bioactive glasses: glass structure and physical properties. J. Non-Cryst. Solids 356, 2546–2551 10.1016/j.jnoncrysol.2010.06.078 (doi:10.1016/j.jnoncrysol.2010.06.078) [DOI] [Google Scholar]
- 19.Fredholm YC, Karpukhina N, Brauer DS, Jones JR, Law RV, Hill RG. 2012. Influence of strontium for calcium substitution in bioactive glasses on degradation, ion release and apatite formation. J. R. Soc. Interface 9, 880–889 10.1098/rsif.2011.0387 (doi:10.1098/rsif.2011.0387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshi JG. 1990. Aluminum a neurotoxin which affects diverse metabolic reactions. Biofactors 2, 163–170 [PubMed] [Google Scholar]
- 21.Blades MC, Moore DP, Revell PA, Hill R. 1998. In vivo skeletal response and biomechanical assessment of two novel polyalkenoate cements following femoral implantation in the female New Zealand White rabbit. J. Mater. Sci. Mater. Med. 9, 701–706 10.1023/A:1008990516159 (doi:10.1023/A:1008990516159) [DOI] [PubMed] [Google Scholar]
- 22.Boyce BF, Elder HY, Elliot HL, Fogelman I, Fell GS, Junor BJ, Beastall G, Boyle IT. 1982. Hypercalcemic osteomalacia due to aluminum toxicity. Lancet 2, 1009–1013 10.1016/S0140-6736(82)90049-6 (doi:10.1016/S0140-6736(82)90049-6) [DOI] [PubMed] [Google Scholar]
- 23.Cournot-Witmer G, et al. 1981. Aluminum localization in bone from hemodialyzed patients: relationship to matrix mineralization. Kidney Int. 20, 375–385 10.1038/ki.1981.149 (doi:10.1038/ki.1981.149) [DOI] [PubMed] [Google Scholar]
- 24.Hurrell-Gillingham K, Reaney IM, Brook I, Hatton PV. 2006. In vitro biocompatibility of a novel Fe2O3 based glass ionomer cement. J. Dentistry 34, 533–538 10.1016/j.jdent.2005.07.011 (doi:10.1016/j.jdent.2005.07.011) [DOI] [PubMed] [Google Scholar]
- 25.Boyd D, Towler MR, Law RV, Hill RG. 2006. An investigation into the structure and reactivity of calcium–zinc–silicate ionomer glasses using MAS-NMR spectroscopy. J. Mater. Sci. Mater. Med. 17, 397–402 10.1007/s10856-006-8465-x (doi:10.1007/s10856-006-8465-x) [DOI] [PubMed] [Google Scholar]
- 26.Brauer DS, Gentleman E, Stevens MM, Hill RG. 2011. Benefits and drawbacks of zinc in glass ionomer bone cements. Biomed. Mater. 6, 045007. (doi:10.1088/1748–6041/6/4/045007) [DOI] [PubMed] [Google Scholar]
- 27.Dietzel A. 1942. The cation field strengths and their relation to devitrifying processes, to compound formation and to the melting points of silicates. Z. Elektrochem. Angew. Phys. Chemie 48, 9–23 [Google Scholar]
- 28.Watts SJ, O'Donnell MD, Law RV, Hill RG. 2010. Influence of magnesia on the structure and properties of bioactive glasses. J. Non-Cryst. Solids 356, 517–524 10.1016/j.jnoncrysol.2009.04.074 (doi:10.1016/j.jnoncrysol.2009.04.074) [DOI] [Google Scholar]
- 29.Hill RG, Brauer DS. 2011. Predicting the bioactivity of glasses using the network connectivity or split network models. J. Non-Cryst. Solids 357, 3884–3887 10.1016/j.jnoncrysol.2011.07.025 (doi:10.1016/j.jnoncrysol.2011.07.025) [DOI] [Google Scholar]
- 30.Featherstone JDB. 1999. Prevention and reversal of dental caries: role of low level fluoride. Commun. Dentistry Oral Epidemiol. 27, 31–40 10.1111/j.1600-0528.1999.tb01989.x (doi:10.1111/j.1600-0528.1999.tb01989.x) [DOI] [PubMed] [Google Scholar]
- 31.Caufield PW, Wannemuehler Y. 1984. pH-Dependent bactericidal effects of acidulated fluoride gels on preformed plaque aggregates of Streptococcus mutans 6715. Antimicrob. Agents Chemotherapy 26, 807–810 10.1128/AAC.26.6.807 (doi:10.1128/AAC.26.6.807) [DOI] [PMC free article] [PubMed] [Google Scholar]