Abstract
The objective of this study was to evaluate the potential benefit of utilizing a pharmacogenomic testing report to guide the selection and dosing of psychotropic medications in an outpatient psychiatric practice. The non-randomized, open label, prospective cohort study was conducted from September 2009 to July 2010. In the first cohort, depressed patients were treated without the benefit of pharmacogenomic testing (the unguided group). A DNA sample was obtained from patients in the unguided group, but the results were not shared with either the physicians or patients until the end of the 8-week study period. In the second cohort (the guided group), testing results were provided at the beginning of the 8-week treatment period. Depression ratings were collected at baseline and after 2 weeks, 4 weeks and 8 weeks of treatment using the Quick Inventory of Depressive Symptomatology, Clinician Rated (QIDS-C16) and the 17-item Hamilton Rating Scale for Depression (HAM-D17). Clinician and patient satisfaction was also assessed. The reduction in depressive symptoms achieved within the guided treatment group was greater than the reduction of depressive symptoms in the unguided treatment group using either the QIDS-C16 (P=0.002) or HAM-D17 (P=0.04). We concluded that a rapidly available pharmacogenomic interpretive report provided clinical guidance that was associated with improved clinical outcomes for depressed patients treated in an outpatient psychiatric clinic setting.
Keywords: depression, genomics, personalized medicine, pharmacogenomics, psychiatric treatment
Introduction
There is considerable inter-individual variability in therapeutic drug response, required dosage and adverse effects in antidepressant treatment. Only 35–45% of depressed patients experience a complete remission of their illness when initially treated with antidepressant medications.1, 2 In those who remain depressed, less than half will experience a significant improvement with a change in antidepressant monotherapy or with the addition of an alternative antidepressant.2 Variation in drug response is complex and is dependent on a number of factors, including diagnostic accuracy, the potential for drug–drug interactions, age, gender, renal and hepatic functioning, medical and psychiatric comorbidity, nutritional status, coincident substance use, genomic and related downstream translational factors and patient compliance.
In recent studies examining the use of antidepressants, antipsychotics and mood stabilizers, substantial proportions of study patients discontinue treatment as a consequence of side effects or symptom relapse.3, 4, 5 Similarly in community practice settings, nearly half of the patients make no follow-up visits, and only a fourth return to pursue regular primary treatment.6, 7
Prolonged times to remission or response whether caused by side-effects or by other factors are associated with substantial increased risk for morbidity or mortality. Pharmacogenomic testing is expected to improve time to response and remission, as well as minimize the likelihood of side-effects associated with patient nonadherence and extended morbidity.8, 9, 10
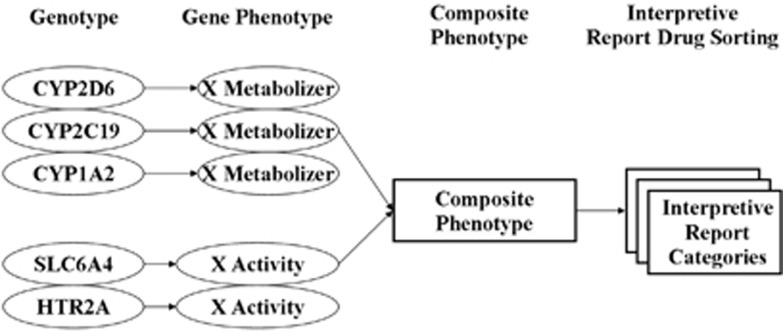
While the scientific understanding of pharmacogenomics is quickly accelerating, its translation to clinical decision-making in practice has progressed more slowly.11, 12 In an effort to begin to bridge this translational gap, a psychopharmacogenomic algorithm has been developed. This algorithm has been primarily designed to improve the safety of prescribing antidepressant and antipsychotic medications. This pharmacogenomic-based interpretive report is based on genotyping both copies of five informative genes (Figures 1 and 2). These five genes are (1) the cytochrome P450 2D6 gene (CYP2D6); (2) the cytochrome P450 2C19 gene (CYP2C19); (3) the cytochrome P450 1A2 gene (CYP1A2); (4) the serotonin transporter gene (SLC6A4) and (5) the serotonin 2A receptor gene (HTR2A). The three cytochrome P450 genes code for the enzymes that are responsible for metabolism of most antidepressant medications. The SLC6A4 and the serotonin 2A receptor have been associated with differential treatment response to specific antidepressants. To increase the clarity of the implications of the genotyping results for both clinicians and patients, a user-friendly software-based product was used to report the genotyping results.13
Figure 1.
Composite phenotyping.
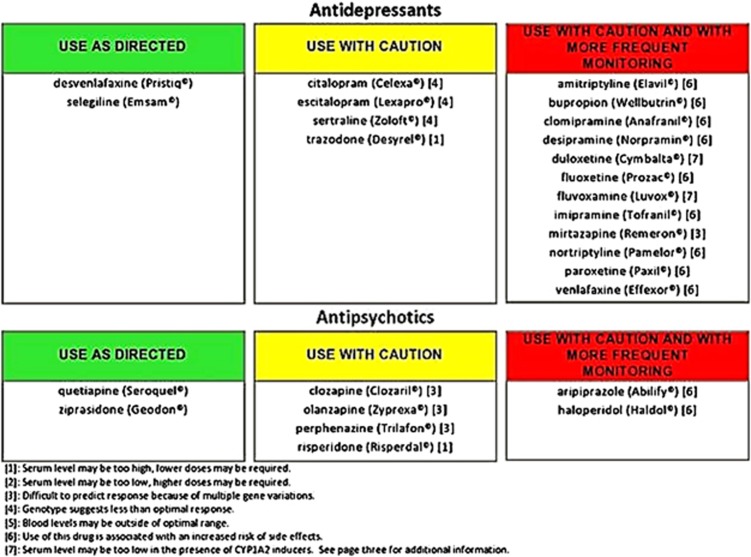
Figure 2.
An example of a pharmacogenomic-based interpretive report.
This prospective pilot study was designed to evaluate the clinical impact of the pharmacogenomic-based interpretive report in an outpatient behavioral health clinic (Figure 2). This clinic provides multidisciplinary integrated services that are delivered by mental health professionals including psychiatrists, psychologists and masters level therapists. In this setting, both pharmacological and non-pharmacological treatment options are offered to patients based on individual needs as determined by initial and ongoing treatment planning with active patient participation in the process. The clinic serves individuals from diverse socio-economic backgrounds. Prior to the initiation of the trial, study psychiatrists had little exposure to clinical pharmacogenomics, which allowed for the opportunity to study the translation and adoption of such technology in a novel setting.
Materials and methods
This non-randomized, open label, prospective cohort study took place at a non-profit outpatient behavioral health clinic in St Paul, MN, from September 2009 until July 2010. Genotyping was performed at no cost to patients. The trial was approved by the Mayo Clinic Institutional Review Board.
Interpretive report
This investigation describes the implementation of a pharmacogenomic algorithm designed to improve the safety and efficacy of prescribing antidepressant and antipsychotic medication in an outpatient psychiatric clinic. This algorithm is based on the genotyping of both copies of five genes selected either for their pharmacokinetic prominence in the metabolism of most antidepressants and antipsychotics or for reports of differential treatment response based on pharmacodynamic considerations. These five genes include (1) the CYP2D6; (2) the CYP2C19; (3) the CYP1A2; (4) the SLC6A4 and (5) the HTR2A T102C single nucleotide polymorphism (SNP). To improve the clinical relevance of the genotyping results for clinicians, genotype results were applied using a proprietary interpretive report, in which 26 psychiatric medications were placed in the advisory categories of ‘use as directed' (green bin), ‘use with caution' (yellow bin) and ‘use with caution or more frequent monitoring' (red bin). The report incorporates the genetic information with the known pharmacological profile for each of the medications in the panel (Figure 2).
Genotyping procedure
CYP2D6, CYP2C19 and CYP1A2 were genotyped using the Luminex xTAG system. Relevant regions were amplified using PCR (polymerase chain reaction) and clarified using Exonuclease I and Shrimp Alkaline Phosphotase. Individual mutations were identified using allele-specific primer extension primers tagged for hybridization to Luminex xTAG beads.
The following CYP2D6 alleles were identified: *1,*2, *2A, *3, *4, *5, *6, *7, *8, *9, *10, *11, *12, *14, *15, *17, *41 and duplications. The following CYP2C19 alleles were identified: *1, *2, *3, *4, *5, *6, *7, *8. *17 was not identified. The following CYP1A2 SNPs were identified: −3860G>A, −2467delT, −739T>G, −729C>T, −163C>A, 125C>G, 558C>A, 2116G>A, 2473G>A, 2499A>T, 3497G>A, 3533G>A, 5090C>T, 5166G>A, 5347C>T. The CYP1A2 SNPs were converted into star nomenclature as defined by the Karolinska Institute, which included the *1B,*1F, *1L and *1N alleles.14
Relevant regions of SLC6A4 and HTR2A (T102C) were amplified using PCR. HTR2A was then digested with the restriction enzyme MSPI. The PCR product and digested HTR2A (T102C) PCR product were then run on a 2% gel to determine genotype. The short and long forms of the SLC6A4 gene were identified and the HTR2A (T102C) SNP was identified.
Study criteria and description
The study sample for this pilot study consisted of 51 adult subjects between the ages of 25 and 75, with a primary diagnosis of a major depressive disorder as determined by board certified psychiatrists utilizing DSM-IV criteria. Study subjects were consecutively selected by their treating physicians based on meeting study criteria and completion of informed consent. A minimum score of 14 was required on the 17-item Hamilton Rating Scale for Depression (HAM-D17) for study enrollment.15, 16 The trial excluded subjects with a diagnosis of bipolar disorder type I, schizophrenia and schizoaffective disorder.
The trial was designed to compare two treatment conditions. In the unguided treatment group, 26 subjects were enrolled and treatment was provided without pharmacogenomic testing. While DNA was collected for these subjects using buccal swabs at the onset of treatment, the pharmacogenomic-based interpretive report was not provided to the clinician until the completion of 8 weeks of treatment. In the guided treatment group, 25 new subjects were enrolled. DNA was collected before the onset of treatment, and the pharmacogenomic-based interpretive report was provided to the treating physicians. Patients in the guided group were informed that their medication selection was being guided by DNA testing. Consequently, the development of the treatment plan for each subject could be individualized on the basis of the report.
Data collection
Data collection was performed at baseline and at 2 weeks, 4 weeks and 8 weeks. Selection of measurements was guided by the design of the STAR*D trial.17 At baseline, subjects were evaluated using the Quick Inventory of Depressive Symptomatology, Clinician Rated (QIDS-C16), the HAM-D17, the Patient Rated Inventory of Side Effects, and the Frequency, Intensity, Burden Side Effect Rating.18, 19, 20, 21, 22, 23 Demographic information, a psychotropic medication history and DNA samples were also collected at the baseline visit. Subsequent study visits were conducted via telephone to reduce participatory burden. Medication history was updated at each study visit. Compliance confirmation was not included. At the 8-week visit, a patient satisfaction survey was administered. The three psychiatrists who provided the treatment also received a satisfaction survey that evaluated their assessment of the usefulness of the pharmacogenomic-based interpretive report.
Statistical analysis
Categorical data (for example, genotype frequencies) are presented as n (%). Distributions of categorical variables were compared between the unguided and guided treatment groups using Fisher's exact test. Distributions of continuous variables (for example, depression rating scores) are summarized as mean±s.d. and were compared between groups using Wilcoxon's rank sum test.
The primary analysis assessed the change in the depression rating score (measuring the raw change in number, using QIDS-C16 and the HAM-D17) over the course of the study (weeks 0, 2, 4 and 8). Due to the longitudinal nature of the data, and in order to control for differences between subjects, repeated measures analysis using a mixed model approach was performed on the data using the subject as a random effect, and with group (guided versus unguided), time (number of weeks) and time2 as fixed effects. The intent of the study was to identify the effect of group, the duration of treatment (weeks 0, 2, 4 and 8) and the interaction between group and time.
Secondary analyses assessed the difference in overall percent reduction in depression scores from baseline to 8 weeks between the unguided group and the guided groups. The reductions in scores at 2 and 4 weeks were also investigated. For these analyses, assuming on average an overall 20% reduction in symptom scores over 8 weeks, a sample size of 22 subjects per group will provide 66% power to detect a difference of 20 and 84% power to detect a difference of 25% in the overall percent reduction of depression scores with an α level of 0.05.
We also compared the proportion of subjects taking medications in the three advisory categories at 8 weeks in the unguided and the guided treatment groups using a Fisher's exact test. All tests were two-sided, and effects were declared significant if P⩽0.05. Analyses were performed using the JMP 9.0 (SAS Institute Inc., Cary, NC, USA) and R software packages (R 2.15.1, The R Foundation for Statistical Computing c/o Institute for Statistical and Mathematics, Wirtschaftsuniversität Wien, Vienna, Austria).
Results
Description of sample
Of the 51 original subjects, seven were excluded from the analysis due to missing data (four in the unguided treatment group and three in the guided treatment group). The subjects almost exclusively identified themselves to be of European ancestry. In both the unguided and guided groups, there were 12 women and 10 men. The mean age for subjects in the unguided treatment group was 42.1, and the mean age for subjects in the guided treatment group was 42.6. The mean QIDS-C16 score at baseline was 15.4 for the unguided treatment group and 16.0 for the guided treatment group. The two groups did not differ significantly in terms of age or baseline QIDS-C16 or HAM-D17 scores. Previous and current medication treatment of the unguided and guided treatment groups are summarized in Table 1.
Table 1. Demographic characteristics of study populationa.
| Unguided (n=22) | Guided (n=22) | P-valueb | |
|---|---|---|---|
| Age (years) | 42.6 (13.1) | 42.1 (13.6) | NS |
| QIDS-C16 score at baseline | 15.4 (4.46) | 16.0 (3.56) | NS |
| Previous psychiatric medication trials | 6.7 (6.73) | 6.0 (5.95) | NS |
| Previous antidepressant trials | 4.4 (3.13) | 4.4 (3.4) | NS |
| Current psychiatric medications | 2.2 (1.4) | 1.7 (0.84) | NS |
Abbreviations: QIDS-C16, Quick Inventory of Depressive Symptomatology, Clinician Rated.
Values are expressed as mean±s.d. There were no statistically significant differences for any of these variables between the two groups.
P-values were calculated using the Wilcoxon test.
The drug metabolizing enzyme genotypes
The genotypes for CYP2D6, CYP2C19 and CYP1A2 were obtained. The distribution of the corresponding phenotypes was typical of a population sample of European ancestry and identified subjects with poor, intermediate, extensive and ultrarapid metabolic capacity.
The subjects in the study's unguided group received their genotypes at the end of the 8-week study. Their 2D6 metabolic capacity phenotypes were 18.2% poor metabolizers, 31.8% intermediate metabolizers, 45.5% extensive metabolizers and 4.5% ultrarapid metabolizers. Their 2C19 metabolic capacity phenotypes were 4.5% poor metabolizers, 40.9% intermediate metabolizers and 54.6% extensive metabolizers. Their 1A2 metabolic capacity phenotypes were 54.5% extensive metabolizers and 45.5% ultrarapid metabolizers. Table 2 summarizes the metabolic capacities of the unguided and guided treatment groups.
Table 2. Metabolic capacity phenotype frequency by treatment group.
| Gene | Group | Poor | Intermediate | Extensive | Ultrarapid |
|---|---|---|---|---|---|
| CYP2D6 | Unguided | 4 | 7 | 10 | 1 |
| Guided | 1 | 6 | 12 | 3 | |
| CYP2C19 | Unguided | 1 | 9 | 12 | N/A |
| Guided | 0 | 4 | 18 | N/A | |
| CYP1A2 | Unguided | 0 | 0 | 12 | 10 |
| Guided | 0 | 0 | 13 | 9 |
Abbreviations: CYP1A2, cytochrome P450 1A2 gene; CYP2C19, cytochrome P450 2C19 gene; CYP2D6, cytochrome P450 2D6 gene.
There were no statistically significant differences for any of these variables between the two groups.
The subjects in the study's guided group received their genotypes prior to the onset of treatment. Their 2D6 metabolic capacity phenotypes were 4.5% poor metabolizers, 27.3% intermediate metabolizers and 68.2% extensive metabolizers. Their 2C19 metabolic capacity phenotypes were 18.2% intermediate metabolizers and 81.8% extensive metabolizers. Their 1A2 metabolic capacity phenotypes were 59.1% extensive metabolizers and 40.9% ultrarapid metabolizers. There were no significant differences in CYP2D6, CYP2C19 and CYP1A2 metabolic capacity phenotype distributions between the two study groups.
Target genotypes
The genotypes for SLC6A4 and HTR2A (T102C SNP) were obtained. The distribution of genotypic results was typical of a population of European ancestry and identified subjects who may be less likely to respond to treatment with some selective serotonin reuptake inhibitors.
The unguided treatment patients were genotyped but did not receive their results until after the 8-week study. Their SLC6A4 genotypes were 31.8% L/L, 63.6% L/S and 4.5% S/S. Their HTR2A T102C genotypes were 22.7% T/T, 40.9% T/C and 36.4% C/C.
The guided treatment patients were genotyped for SLC6A4 and HTR2A genes prior to the onset of treatment. Their SLC6A4 genotypes were 50% L/L, 45.5% L/S and 4.5% S/S. Their HTR2A T102C genotypes were 4.5% T/T, 72.7% T/C and 22.7% C/C. There were no statistically significant differences in genotype distributions for the SLC6A4 and HTR2A genotypes between the two study groups.
Testing accessibility and implementation
This pilot study was designed to evaluate the potential benefit of pharmacogenomic testing in an outpatient psychiatric clinic. The pharmacogenomic-based interpretive report was available in just over 25 h from the receipt of the DNA sample at the genotyping laboratory. Results were provided electronically to the study physician. Physicians reported that they were consistently satisfied with the availability of the interpretive report. Motivation to participate in the study on the part of subjects was high. Only one patient who was screened as being appropriate to participate in the study declined to participate.
For each subject, the pharmacogenomics report assigned psychotropic medications to one of the three categories. These categories were (1) ‘use as directed,' (2) ‘use with caution' and (3) ‘use with caution and more frequent monitoring.' Utilization of the pharmacogenomic-based interpretive report to guide the selection of medications was assessed by measuring the percentage of subjects taking medications within each advisory category that was being prescribed to each subject at the 8-week visit.
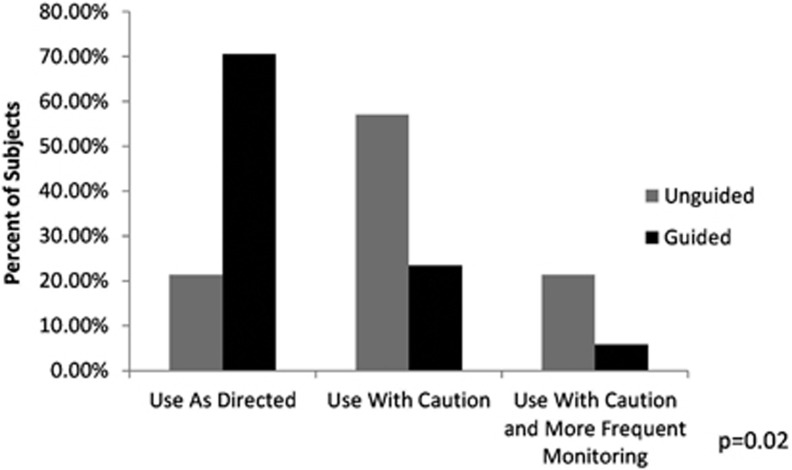
In order to account for subjects on more than one of the 26 medications in the interpretive report, subjects were categorized based on the most severe advisory category of their medication regimen. All subjects with at least one medication in the ‘Use With Caution or More Frequent Monitoring' advisory category were designated as a consequence of taking this medication. Subjects who were not taking a medication in the ‘Use With Caution or More Frequent Monitoring' category but were taking at least one medication in the ‘Use With Caution' advisory category were designated as a consequence of taking this medication. Subjects taking only medications in the ‘Use As Directed' category were designated as a consequence of taking this medication. At the 8-week visit, there were more subjects categorized in the ‘use as directed' category in the guided treatment group than in the unguided group. Furthermore, fewer subjects were prescribed medications in the ‘use with caution' classification in the guided treatment group when compared with the unguided group. While only 5.9% of the subjects in the guided treatment group were prescribed a medication in the ‘use with caution and more frequent monitoring' category, 21.4% of the subjects in the unguided group were in this category (Figure 3). These differences in prescribing patterns at 8 weeks between the guided and unguided treatment groups were significant at P=0.02.
Figure 3.
Subjects by advisory category at 8 weeks in the unguided treatment group and the guided treatment group.
Medications listed on the interpretive report that were used to categorize subjects included 18 antidepressants and 8 antipsychotics. Psychotropic medications that were not listed on the panel and were not used to categorize subjects included anti-anxiety medications and mood stabilizers. Subjects in the study had been treated with an average of 5.1 of the 26 possible psychotropic medications over the course of their illnesses.
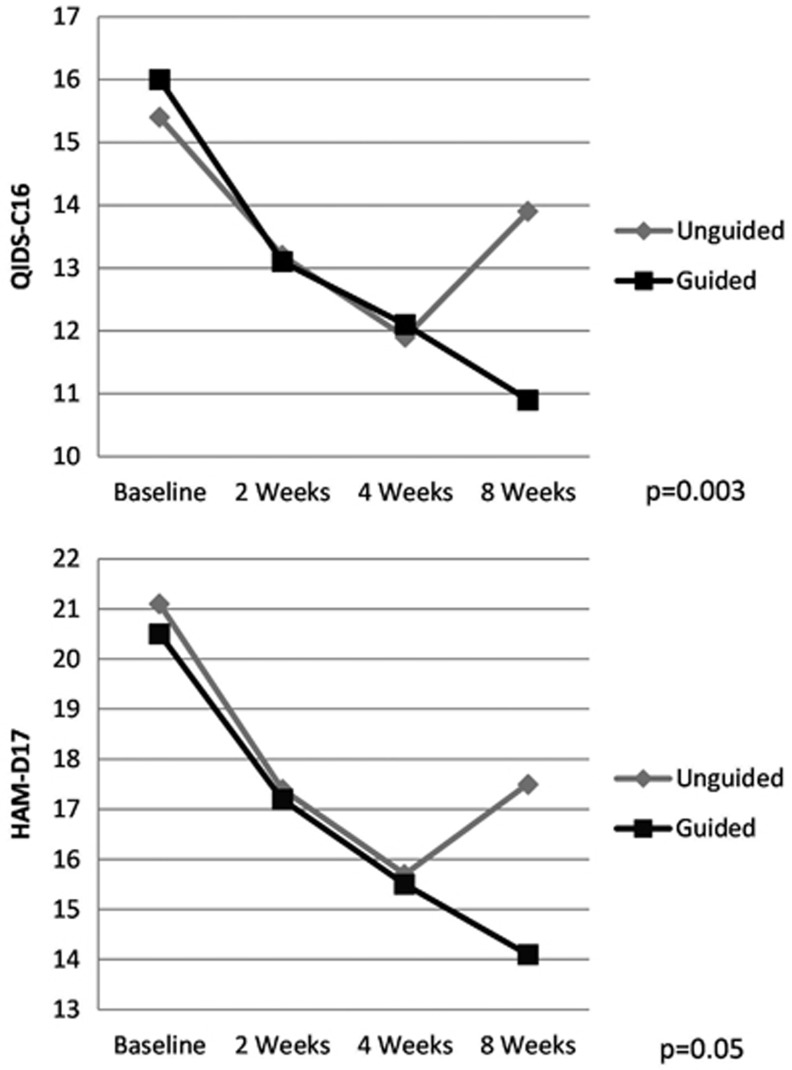
In the repeated measures analysis, we examined the effect of time and group (guided versus unguided treatment) on the reduction of depression rating score. Using the QIDS-C16 tool, a greater reduction of symptoms was observed across the duration of the study for the guided group subjects compared with the unguided group subjects (P=0.003). Similarly, using the HAM-D17 tool, a greater reduction of symptoms was observed for the guided group subjects compared with the unguided group subjects across the duration of the study (P=0.05). These results are illustrated in Figure 4.
Figure 4.
Depression scores using the Quick Inventory of Depressive Symptomatology, Clinician Rated (QIDS-C16) and the 17-item Hamilton Rating Scale for Depression (HAM-D17) over the duration of the 8-week trial.
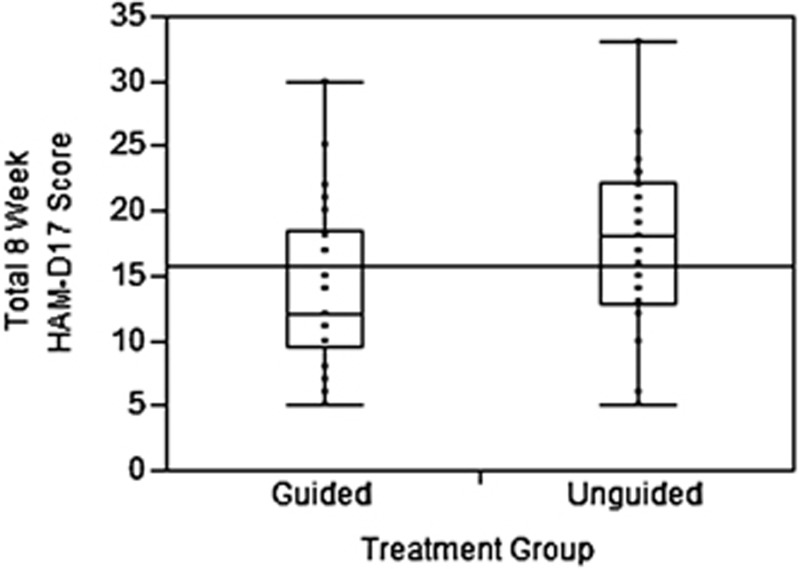
There was no statistically significant difference in the reduction in QIDS-C17 or HAM-D17 scores between the unguided and guided treatment groups at week 2 and week 4. Using both instruments, the unguided group had an increase in depressive scores after week 4, whereas in the guided group, there was continued improvement in depression scores at week 8. The reduction in depression scores from the baseline to the 8-week visit was greater in the guided group than in the unguided group. On average, there was a 7.2% reduction in the QIDS-C16 score for study subjects in the unguided treatment group compared with a 31.2% reduction in overall score for subjects in the guided group (P=0.002). Similarly, there was an 18.2% reduction in HAM-D17 ratings for study subjects in the unguided group compared with a 30.8% reduction for subjects in the guided group (P=0.04). Figure 4 illustrates the mean depression measurement scores in each group at each study visit for the QIDS-C16 and HAM-D17. Figure 5 illustrates the 8-week HAM-D scores for each subject in the unguided and guided group. The continued response in the guided group is consistent with known pharmacologic response times.
Figure 5.
Depression scores using the 17-item Hamilton Rating Scale for Depression (HAM-D17) at the 8-week time point (box plot indicates minimum, maximum and median scores).
Discussion
The goal of this prospective pilot study was to evaluate the potential benefit of introducing a pharmacogenomic algorithm to practitioners who were unfamiliar with the use of such technology. As an exploratory, ‘real-world' study designed to evaluate translational issues as well as the clinical impact of a multigenetic panel, we saw value in observing a clinical practice as it evolves from treatment as usual (that is, complete lack of clinical pharmacogenomic knowledge) to pharmacogenomic-guided treatment. The cohort, non-blinded design allowed us to observe this evolution. We believed it was important to observe how pharmacogenomic-naive physicians would utilize this new tool with their patients.
A greater reduction in overall QIDS-C16 and HAM-D17 scores were achieved with pharmacogenomically guided treatment. Decreasing the time-to-response is an important treatment goal. However, it is a challenge to demonstrate a differential outcome in patients suffering from complex chronic psychiatric illnesses. The potential benefits of testing must be considered within the context of the costs of testing.
There are a number of limitations of the study. First, it is a small pilot study largely restricted to subjects of European ancestry with relatively heterogeneous patients. Second, although all patients were enrolled in psychotherapy, there was no control for the modality of therapy involved. Third, these subjects had a long treatment history. Over the course of their treatment, they had received more than five medications on average that were categorized by the pharmacogenomic-based interpretive report. Given the chronicity of the illnesses of these patients, it is not surprising that unguided treatment would result in relatively modest improvement. Indeed, this chronicity may explain the unguided group's apparent worsening of symptoms (Figure 5), as this is a common feature of chronic depression.24 The documentation of a greater improvement in the patients with guided treatment is encouraging, but further research is necessary to evaluate whether pharmacogenomic testing can protect against worsening depression following improvement of symptoms in a chronic population. Finally, because of the non-blinded pilot study design, placebo effect may have influenced these findings, as the guided group patients' knowledge of their DNA testing results could have contributed to the greater treatment response observed in this group. However, the observation that both groups had similar depression ratings through week 4 suggests that this is less likely.
A critical factor in the introduction of any new technology into clinical practice is the effect that this may have on the physician–patient relationship. The goal of pharmacogenomic testing is to synthesize and tailor genomic information to provide patient-specific guidance, and feedback from physicians utilizing the pharmacogenomics testing report was uniformly positive.
Replication in a larger sample would increase the confidence in the validity of these findings. Additionally, it will be important to study patients from other ancestral origins. However, these results provide a preliminary suggestion that there may be benefit in implementing pharmacogenomic testing in clinical outpatient population settings that treat patients with complex and severe mood disorders.
Conclusions
Pharmacogenomic-based interpretive reports are currently being used in clinical practice. The introduction of these newly available reports represents an advance for translational medicine and provides an example of evidence-based individualized molecular medicine. However, more research is needed to fully demonstrate the benefit of these newly developed strategies to guide treatment. While a number of barriers to the introduction of new technologies into routine clinical practice exist, this pilot study illustrates that studies in a ‘real-world' setting can contribute to our understanding of the effectiveness of treatment. Future studies are needed to better understand the interaction of the multiple factors that may be associated with the better outcomes that may be achieved using pharmacogenomic testing.
Acknowledgments
We thank Barbara A Hall, BSW, for graphics preparation and Lori l Solmonson and Jessica Sagen for manuscript preparation. AssureRx Health provided in-kind services consisting of genotyping, the GeneSightRx® report and shipping of buccal samples. Research was funded by a Mayo Clinic Discovery Translation Grant and the Samuel C Johnson Genomics of Addiction Program.
Dr Winner and Mr Allen were employed by the Mayo Clinic during the design phase of the study and Mr Allen performed the data collection. Both are now employed by AssureRx Health. Dr Mrazek has developed intellectual property that has been licensed by AssureRX Health, and subsequently incorporated into physician decision support software. He has also received research funding from AssureRX Health to create and maintain a bibliographic system designed to regularly monitor the scientific literature.
References
- Kemp AH, Gordon E, Rush AJ, Williams LM.Improving the prediction of treatment response in depression: integration of clinical, cognitive, psychophysiological, neuroimaging, and genetic measures CNS Spectr 2008131066–1086.quiz 87–88. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Stewart JW, Nierenberg AA, Thase ME, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354:1231–1242. doi: 10.1056/NEJMoa052963. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Thase ME. STEP-BD and bipolar depression: what have we learned. Curr Psychiatry Rep. 2007;9:497–503. doi: 10.1007/s11920-007-0068-9. [DOI] [PubMed] [Google Scholar]
- Simon GE, Perlis RH. Personalized medicine for depression: can we match patients with treatments. Am J Psychiatry. 2010;167:1445–1455. doi: 10.1176/appi.ajp.2010.09111680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GE, Von Korff M, Rutter CM, Peterson DA. Treatment process and outcomes for managed care patients receiving new antidepressant prescriptions from psychiatrists and primary care physicians. Arch Gen Psychiatry. 2001;58:395–401. doi: 10.1001/archpsyc.58.4.395. [DOI] [PubMed] [Google Scholar]
- Flockhart DA, Skaar T, Berlin DS, Klein TE, Nguyen AT. Clinically available pharmacogenomics tests. Clin Pharmacol Ther. 2009;86:109–113. doi: 10.1038/clpt.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrazek DA. Psychiatric Pharmacogenomics. New York, NY: Oxford University Press; 2010. [Google Scholar]
- Zandi PP, Judy JT. The promise and reality of pharmacogenetics in psychiatry. Psychiatr Clin North Am. 2010;33:181–224. doi: 10.1016/j.psc.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Flavin DK, Schneekloth TD, Allen JD. Translational psychiatry: bringing pharmacogenomic testing into clinical practice. Prim Psychiatry. 2010;17:39–44. [Google Scholar]
- Mrazek DA, Lerman C. Facilitating clinical implementation of pharmacogenomics. JAMA. 2011;306:304–305. doi: 10.1001/jama.2011.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AssureRx Health, Mason, OH; Available fromwww.assurerxhealth.com .
- Sim SC.Home Page of the Human Cytochrome P450 (CYP) Allele Nomenclature Committee; Solna, Sweden: Karolinska Institutet; 2011 [updated Last updated 9 September 2008; 15 December 2011] Available fromhttp://www.cypalleles.ki.se/Access date December 152011 [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Rush AJ.Sequenced treatment alternatives to relieve depression (STAR*D) protocol. NIMH Contract GMO-010111 2001. Available from http://www.edc.gsph.pitt.edu/stard/ .
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon AC, Olfson M, Portera L, Farber L, Sheehan DV. Assessing psychiatric impairment in primary care with the Sheehan Disability Scale. Int J Psychiatry Med. 1997;27:93–105. doi: 10.2190/T8EM-C8YH-373N-1UWD. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Bernstein IH, Trivedi MH, Carmody TJ, Wisniewski S, Mundt JC, et al. An evaluation of the quick inventory of depressive symptomatology and the Hamilton rating scale for depression: a sequenced treatment alternatives to relieve depression trial report. Biol Psychiatry. 2006;59:493–501. doi: 10.1016/j.biopsych.2005.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34:73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- Wisniewski SR, Rush AJ, Balasubramani GK, Trivedi MH, Nierenberg AA. Self-rated global measure of the frequency, intensity, and burden of side effects. J Psychiatr Pract. 2006;12:71–79. doi: 10.1097/00131746-200603000-00002. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Husain MM, Trivedi MH, Fava M, Warden D, Wisniewski SR, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. 2010;40:41–50. doi: 10.1017/S0033291709006011. [DOI] [PMC free article] [PubMed] [Google Scholar]