Abstract
Glycine transporter 1 (GlyT1) is a potential pharmacological target to ameliorate memory deficits attributable to N-methyl-d-aspartate receptor (NMDAR) hypofunction. Disruption of glycine-reuptake near excitatory synapses is expected to enhance NMDAR function by increasing glycine-B site occupancy. Genetic models with conditional GlyT1 deletion restricted to forebrain neurons have yielded several promising promnesic effects, yet its impact on working memory function remains essentially unanswered because a previous attempt had yielded un-interpretable outcomes. The present study clarified this important outstanding lacuna using a within-subject multi-paradigm approach. Here, a consistent lack of effects was convincingly demonstrated across three working memory test paradigms – the radial arm maze, the cheeseboard maze, and the water maze. These null outcomes contrasted with the phenotype of enhanced working memory performance seen in mutant mice with GlyT1 deletion extended to cortical/hippocampal glial cells. It follows that glial-based GlyT1 might be more closely linked to the modulation of working memory function, and raises the possibility that neuronal and glial GlyT1 may regulate cognitive functions via dissociable mechanisms.
Keywords: cheeseboard maze, GlyT1, learning, NMDA receptor, radial arm maze, water maze
1. INTRODUCTION
N-methyl-d-aspartate receptors (NMDARs) are critical for long-term synaptic modifications believed to underlie some forms of learning and memory [1, 2]. Hence, augmentation of NMDAR activity may boost memory formation [3]. However, direct NMDAR stimulation can induce neurotoxicity and seizures, but the modulatory sites on NMDAR may offer more viable alternative targets. One potential site receiving increasing attention is the glycine-B site. Binding to the glycine-B site is mandatory for NMDAR activation by glutamate. Extracellular glycine levels near glycine-B sites are governed by glycine transporter 1 (GlyT1), which co-localizes with NMDAR in neurons and adjacent glial cells. GlyT1 mediated re-uptake of glycine from the synaptic clefts thus maintains glycine at sub-saturated levels [4, 5, 6, 7]. Due to the ensuing elevation in extracellular glycine, GlyT1 disruption is expected to facilitate NMDAR activation and memory function. Systemic pharmacological blockade [8, 9, 10] or genetic deletion [11, 12, 13] of the GlyT1 gene have yielded some support for this hypothesis. GlyT1 disruption yielded pro-cognitive effects in wild type animals and reversed behavioural deficits produced by NMDAR antagonists.
The most promising mouse model thus far is based on the conditional deletion of GlyT1 from forebrain neurons generated using the CamKIIαCre loxP system [13, 14]. This gene deletion enhances Pavlovian conditioning, learned inattention, object recognition and reversal learning [13, 14, 15]. However, our previous attempt to evaluate working memory in this mouse line using the water maze paradigm had failed to yield a clearly interpretable outcome [14]. In this test, the hidden escape platform assumes a new position in the water maze on each day, and indexation of working memory relies on within-day performance improvement seen from trial 1 to trial 2. However, mutants already outperformed controls in trial 1 while not differing from them in trial 2. This divergence in baseline performance (i.e., in trial 1 when the platform location was essentially unknown to the animals) has precluded a simple interpretation in terms of enhancement or impairment.
The present study was designed to clarify this outstanding issue. Here, a within-subject multi-paradigm approach was adopted. The animals were first evaluated in the radial arm maze and cheeseboard paradigms of working memory, before being subjected to a replication of the water maze working memory test based on Singer et al. [14]. Here, this multi-paradigms within-subjects approach convincingly shows that disruption of forebrain neuronal GlyT1 did not substantially alter the cognitive processes underlying effective working memory performance. The contrast of these null results with the promnesic effects on working memory function previously demonstrated in mutant mice with GlyT1 deletion extended to cortical glial cells [16] highlight the possibility that cell-specificity and/or the magnitude of GlyT1 deletion may critically determine whether working memory would be enhanced or not.
2. METHODS
2.1. Subjects
A full description of the generation of the CamKIIαCre; GlyT1tm1.2fl/fl mouse line has been provided previously (see [10]). The experimental subjects were generated by crossing CamKIIαCre:Glyt1tm1.2fl/fl mice with Glyt1tm1.2fl/fl mice (both maintained on a pure C57BL/6J background) to yield a 1:1 mixture of offspring comprising the CamKIIαCre:Glyt1tm1.2fl/fl (“mutant”) and Glyt1tm1.2fl/fl (“control”) genotype. This breeding strategy maintained the CamKIIαCre allele in a heterozygous state for the mutant, whereas all mice were homozygous for the Glyt1tm1.2fl/fl allele. Breeding was performed in a specific-pathogen free breeding facility (Laboratory of Behavioural Neurobiology, Swiss Federal Institute of Technology Zurich, Schwerzenbach, Switzerland). Genotype was determined by standard polymerase chain reaction on postnatal Days 21–30 as described by Yee et al. [10]. Litters were weaned at postnatal day 21, and a mixture of mutant and control littermates of the same sex were housed in groups of 4–6 in Macrolon Type-III cages (Techniplast, Milan, Italy). At the age of 12 weeks, the subjects were transferred to a separate mouse vivarium (21 ± 1 C°; relative humidity, 55 ± 5%) and single caged in Macrolon Type-II cages (Techniplast, Milan, Italy) for the duration of behavioural testing. Mice had ad libitum access to water and food (Kliba 3430, Klibamuhlen, Kaiseraugst, Switzerland), unless otherwise specified, and kept under a reversed light–dark cycle with lights off from 0700 to 1900 hrs. All behavioural experiments were conducted in the dark phase.
A cohort of male mice consisting of 10 mutant and 10 littermate controls was used in the present study. The animals were approximately 24 weeks old at the beginning of behavioural testing, and were subjected to working memory assessment in the radial arm maze, cheeseboard maze and water maze.
All experimental procedures described had been previously approved by the Zürich Veterinary Office. All manipulations conformed to the ethical standards stipulated by the Swiss Act and Ordinance on Animal Protection, and were in accordance with the European Council Directive 86/609/EEC as well as the National Institutes of Health publication no. 86-23 (revised 1985) on animal experimentation.
2.2. Apparatus & Procedures
2.2.1. Experiment 1: Radial Arm Maze
The apparatus consisted of an automated maze made of grey acrylic (Imetronic, Pessac, France) position in the middle of a well-lit room enriched with extra-maze cues. The maze comprised eight identical arms radiating from a common central platform. The maze was elevated 73 cm above floor level. Each arm measured 55.5 cm long and 12 cm wide, and each was guided by transparent Plexiglas walls (16 cm long and 11 cm high) mounted on either side to prevent mice from jumping across adjacent arms. The entrance to each arm from the central platform was guided by a movable transparent Plexiglas door (12cm × 16cm), which could be retracted into the floor of the maze to allow access to specific arms. A food pellet dispenser was located at the far end of each arm delivering 20 mg Noyes pellets (Noyes Precision Pellets PJPPP-0020, Research Diets Inc., New Brunswick, NJ, USA). The entrance doors and pellet dispensers were individually controlled by a PC that was connected to a digital camera mounted directly above the maze for continuously tracking the animal’s position. The test procedures consisted of four consecutive phases as specified below.
Habituation: The animals were gradually introduced to a restricted food diet, reducing access to food in the home cage from 12 h per day (day 1) to 2 h per day (from day 5 onwards). Body weights were monitored daily and prevented from falling below 86% of their ad lib weight with additional feeding whenever necessary. In the same period, habituation to the maze was conducted over six consecutive days. On the first two days, mice were placed in the maze in pairs and allowed to explore freely the entire maze surface for 5 min where food pellets were scattered along the eight arms. On the next two days, mice were placed individually on the maze for 5 min with food pellets scattered only towards the end of each arm. On the last two days, access was restricted to 4 arms forming a “+” configuration to be used in the next stage. The two possible configurations were balanced between mutant and control animals. They were allowed to collect one food pellet at the end of each arm within 5 min. Two mutant mice (out of the initial ten) failed to explore the maze or did not consume any reward, and were dropped from further testing.
Working memory test (4-arm): Working memory performance was first assessed in a 4-arm design with the arms spaced 90° apart. A trial began by confining the mouse in the centre of the maze with all doors closed. Ten seconds later, the doors to the four designated arms were open and the animals were allowed to enter in any one arm. A choice was deemed to be made when the body of the mouse reached 4 cm into the chosen arm. All doors were then closed and they remained so except the door to the chosen arm was re-opened as the mouse reached the food dispenser. Only the first visit to a given arm was rewarded by the delivery of one reward pellet. The mouse returned to the central platform and, again, was confined there for 10 s before the next choice. The mice were allowed a maximum of 10 min to collect all four rewards, and all mice succeeded in doing so across the 8 days of test.
Working memory test (8-arm): The procedures were identical to the previous phase except that all eight arms were employed and the maximum session time was extended to 30 min. The animals were now required to collect a total of 8 rewards, one from the end of each arm. Re-entries like before were never rewarded. This phase include 5 consecutive daily sessions.
Working memory test (8-arms) with an intra-session time-out: Memory load was increased from the 4-arm to the 8-arm procedures. Here, the retention load was taxed by introducing a time-out following the collection of the fourth reward in a session. When the mouse returned to the central platform after consuming the fourth reward, it was removed from the maze and placed inside an opaque box in the experimental room for the time-out period, which lasted 2 min in the first two sessions, and 15 min in the next two sessions. At the end of the time out period, the mouse was returned to the central platform and allowed to collect the remaining four pellets from the unvisited arms.
Working memory performance was indexed by scoring the number of working memory errors defined as re-entries to any arms. The session time was also recorded.
2.2.2. Experiment 2: Cheeseboard Maze
The apparatus has been fully described previously [17]. It was made from a 3-cm thick circular wooden table measuring 1.1 m in diameter. The maze was elevated 123cm above the floor and positioned in the same testing room used for the radial arm experiments without any alteration to the extra maze cues. One side of the board was smooth and was used in the habituation phase. The other was prepared with 32 food wells, arranged in a radial pattern resembling the arrangement of arms in the radial arm maze (see [17]). There were thus eight rows of food wells radiating from the board centre, each comprising four food wells spaced equally apart, spanning 20 to 45cm from the board centre. Each food well measured 3.1cm in diameter and 1.3cm deep, into which a removable plastic food cup could be fitted. A reward of 75μl condensed milk solution (25% dilution of the commercial condensed milk manufactured by Alicommerce SAS, Liebefeld-Bern, Switzerland) could be placed in the centre of the removable food cup. A digital camera mounted directly above the maze was connected to a PC running the Ethovision tracking system (Noldus Information Technology, Wageningen, The Netherlands) to record distance travelled at a sampling rate of 5Hz.
Habituation: The animals were already familiar with the testing room, and they were therefore only habituated to the cheeseboard maze using the smooth side of the cheeseboard. To begin, the mice were individually placed in the centre confined under a semitransparent plastic beaker (diameter: 15cm, height: 25cm) for 5 s before being released and allowed two minutes to explore the maze surface.
Working Memory test: The test procedures resembled that of the water maze working memory protocol and consisted of discrete visits (or trials) to the maze within a day. A different food well was baited every day, but the same food well was repeatedly rewarded across visits within a day. The baited well positions were counterbalanced across days, delays and animals but only the wells located at 37.5 cm from the centre were selected. Each visit began with the mouse placed in the board centre and confined under a beaker for 5 s. If an animal failed to collect the reward within 120s, it was guided to it by the experimenter. After locating the reward, the animals were allowed 20s to consume the reward. Over the first four days of test, animals were given four trials/visits per day with an inter-trial interval (ITI) of 2 min. The ITI delay was extended to 15 min over the next four days and the number of trials reduced to two. The animals were kept in an opaque box during the ITIs.
Working memory performance was indexed by the improvement in efficiency of collecting the food reward from trial 1 to trial 2 on each day. Further improvement from trials 3 onwards (in the first 4 days) might incorporate elements of reference memory and was not included in the analysis, but they were effective in instilling the matching or ‘win-stay’ rule implicit in this working memory procedure, which in contrast to the ‘win-shift’ strategy intrinsic to the radial arm maze procedure.
2.2.3. Experiment 3: Water Maze
The water maze apparatus, procedures and testing room were essentially similar to those employed by Singer et al. [14, 16] to facilitate comparison with the previous studies. Briefly, the maze was a white circular fibreglass tank, 102 cm in diameter and 36 cm deep. It was filled to a depth of 19 cm with tap water which temperature was strictly maintained at 24±1°C. A transparent Plexiglas cylinder (diameter 7 cm, height 18.5 cm) served as the escape platform, with its surface submerged 0.5 cm below the water surface and therefore invisible to the animals. A local cue (a black wooden arrow measuring 10 × 2 × 2 cm) could be mounted 7 cm directly above the platform to allow visual detection of its location during the cued test. A digital camera was installed above the water maze and was connected to a PC running the Ethovision (version 3.1, Noldus Information Technology, Wageningen, The Netherlands), which tracked the animal’s position at a sampling rate of 5 Hz and calculated the escape latency, distance moved and average swim speed on each trial. Four points, spaced equally along the circumference of the WM, served as the cardinal points: N, E, S and W, dividing the maze surface into four quadrants (NE, SE, NW and SW) of equal size.
Cued task: After the completion of the cheeseboard experiment, the mice were returned to an ad libitum diet and left undisturbed for one day before commencement of the water maze experiment. On the first day, the mice were evaluated on a ‘cued task’ in which a local cue indicated the platform’s location. This served to familiarize the animals with the escape response. The platform was positioned in the maze centre and each animal underwent two consecutive trials, with the starting positions randomly selected from four release points (N, E, S, and W). In the first trial, the mouse was released from the starting point with its head facing the platform location. In the second trial, it was released from the starting point facing the wall of the maze. The two trials were separated by 15s. When an animal failed to locate the platform within the 60s limit, an escape latency of 60s was assigned and the animal was guided to the platform by the experimenter and allowed to stay on it 15 s.
Working memory test: The platform now always remained hidden underneath the water surface, and assumed a new position on each test day but remained in the same position across trials within the same day. The animals were first trained on a 4-trial-per-day procedure to facilitate acquisition of the matching rule over the first four days with an ITI of 2 min, which matched the design adopted in Experiment 2 with the cheeseboard maze. Over the next days, the ITI was extended to 15 min, and returned to a 2-trial-per-day procedure. The mice were kept in a waiting cage during the ITI located in the same experimental room. Working memory was indexed by the reduction in time spent and path length in trial 2 in comparison with trial 1 when the platform location was unknown. As in the cheeseboard experiment, the third and fourth trials included in the first four days of training were not included in the analysis. Twelve platform positions were defined: six located at a distance of 35cm (in the N, S, NE, SE, SW, and NW directions), and six more at a distance of 15cm from the centre (in the N, E, S, W, NW and SE directions). These locations were counterbalanced across days and genotype factor. The start position differed between consecutive trials, and was randomly selected from eight possible release points (N, E, S, W, NE, NW, SE and SW) for each mouse.
2.2.4. Statistical analysis
All data were analysed by parametric analysis of variance (ANOVA) with the between-subject factor Genotype (mutant vs. littermate control) and any within-subject factors deemed appropriate for the specific experiment. All statistical analyses were carried out using SPSS for Windows (version 18, SPSS Inc. Chicago IL, USA) implemented on a PC running the Windows 7 operating system.
3. RESULTS
3.1. Experiment 1: Working memory in the Radial arm maze
The experiment comprised four separate stages spanning across 17 days. These were separately analyzed. Performance was indexed by working memory errors throughout as defined by any re-entry.
Four-arm protocol: Learning was reflected by the decrease of working memory errors progressively across days, which was similarly observed in both mutant and control mice (Figure 1A). A 2 × 8 (Genotype × Days) ANOVA of total errors only yielded a significant effect of days [F(7,112)=2.59; p<0.05]. Neither the effect of genotype nor its interaction was close to statistical significance [F’s < 1].
Eight-arm protocol: As shown in Figure 1B, working memory errors increased when the test was extended to the 8-arm protocol. This was followed by the gradual reduction of working memory across the five test days. Performance was again largely comparable between mutants and controls. A 2 × 5 (Genotype × Days) ANOVA of working memory errors yielded a significant main effect of days [F(4,64)=10.24; p<0.01] without any statistical evidence for a genotype effect [F(1,16)=1.76; p=0.20] or its interaction [F(4,64)=1.32; p=0.27].
Eight-arm protocol with 2 min intra-session timeout: Consistent with the high level of performance already established by the end of the previous stage, the animals committed very few working memory error in collecting the first four rewards in the first half of the test session. Next, there was as expected an increase in errors when the animals were returned to the maze following the 2-min timeout (Figure 1C). This pattern of outcome was comparably observed in mutant and control mice. A 2 × 2 × 2 (Genotype × Half-session × Days) ANOVA of working memory errors only yielded a significant main effect of Half-session [F(1,16)=34.42; p<0.001].
Eight-arm protocol with 15 min intra-session timeout: A similar pattern of results was obtained with the intra-session timeout extended to 15 min (Figure 1D), again without any evidence for any genotypic difference. A 2 × 2 × 2 (Genotype × Half-session × Days) ANOVA of working memory errors again only revealed a significant effect of half-session [F(1,16)=14.78; p<0.01] without any statistical significance for any genotype effect [all F’s <1].
Figure 1.
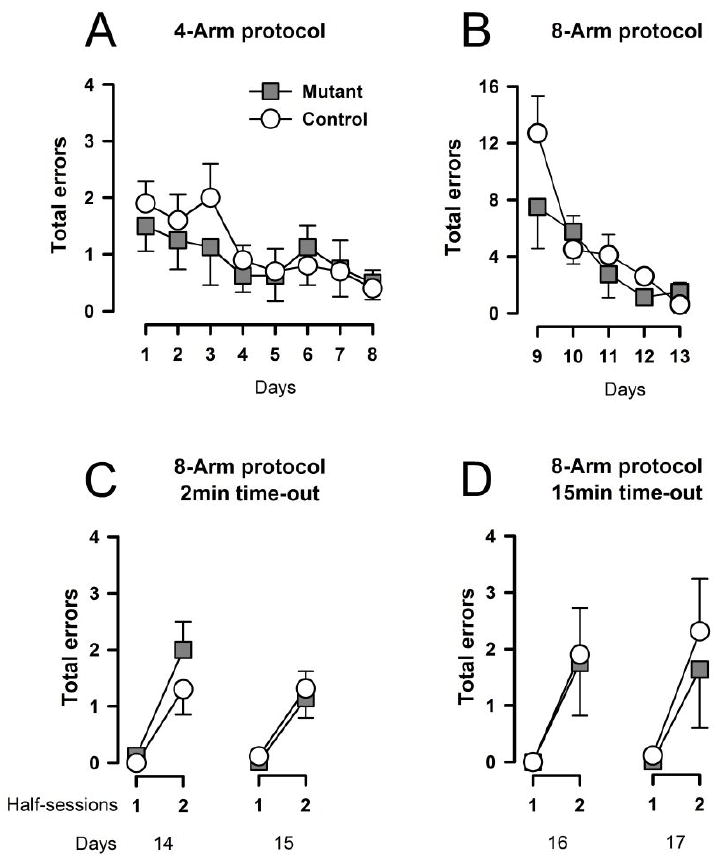
Working memory performance on the Radial Arm Maze. The total number of errors (re-entry into a previously visited arm) is shown across days for both groups in the four arms and the eight arms configuration (A&B). There is a reduction of the total number of errors across days for both genotypes reflecting learning of the tasks and improvement of working memory performance. In (C&D), working memory performance on the eight arms baited configuration when a time-out is imposed after four collected rewards. For each delay is depicted the total number of errors per half-sessions on each day. In all the tasks (A, B, C & D) the mutation did not affect the performance. Error bars refer to ±SEM, mutant (n=8), control (n=10).
3.2. Experiment 2: Working memory (delayed matching-to-place) in the Cheeseboard maze
In this experiment, working memory performance was indexed by the reduction of latency or distance travelled to reach the food reward in trial 2 relative to trial 1 when the location of the food reward was essential not known to the animals.
2-min ITI: Averaged across the four test days, both mutant and control mice were more efficient in reaching the food reward in trial 2 compared to trial 1 (right panel of Figure 2A). Separate 2 × 2 × 4 (Genotype × Trials × Days) ANOVAs of the latency and distance measures yielded a clear trial effect [latency: F(1,16)=15.61, p<0.01, distance: F(1,16)=15.78; p<0.01].
15-min ITI: As expected, extending the ITI to 15 min imposed additional retention demand and had affected performance accordingly, while the overall improvement from trial 1 to 2 appeared weaker than before (Figure 2B). Separate 2 × 2 × 4 (Genotype × Trials × Days) ANOVAs distance and time to reach the reward location only yielded near-significant effects of trials [latency: F(1,16)=4.20; p=0.06, distance: F(1,16)=4.45; p=0.05]. The analysis yielded no support for any genotype effect or its interaction [All F’s<1].
Figure 2.
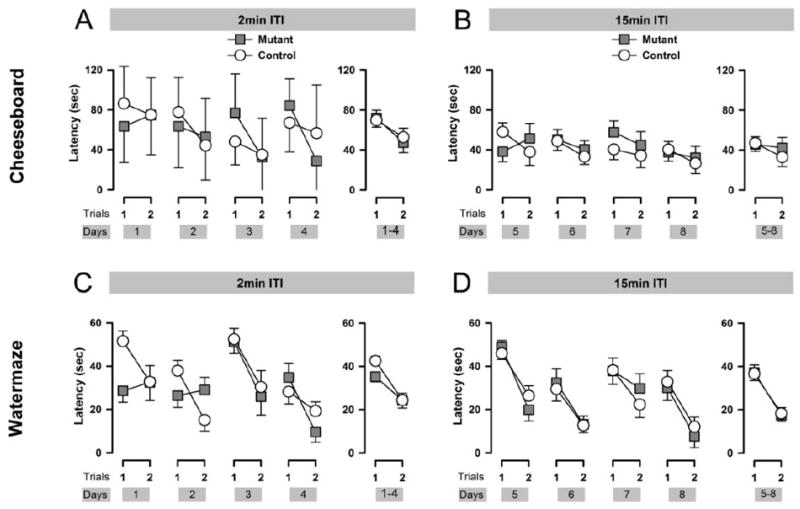
Working memory performance was assessed in the cheeseboard and the watermaze. Performance in the working memory task in the cheeseboard maze indexed by latency to reach the reward location, across days at each trial is depicted in A when the ITI was 2min and B when the ITI was 15 min. Latency averaged across days as a function of trials are depicted at the right of each graphs A and B. The latency to reach the reward was significantly reduced from Trial 1 to Trial 2 when the delay was 2min, but failed to achieve significance when the delay was 15min. The performance never statistically differed between genotypes (A and B). Working memory performance in the water maze indexed by latency to reach the platform location, across days, for each trial is depicted in A when the ITI was 2min and B when the ITI was 15 min. The latency averaged across days as a function of trials is depicted for each ITI in the right of the graphs (C and D). In both delays, mice escaping performance statistically progressed from Trial 1 to Trial 2 without any differences between genotypes. Error bars refer to ±SEM.
The consistency of the latency and distance measures across the two ITI conditions was in agreement with the lack of any genotypic effect on the separate analysis of speed of movement (data not shown).
3.3. Experiment 3: Working memory in the Water maze
Cued task: This was uneventful and all mice acquiring the escape response (data not shown) without any suggestion of any genotypic difference, which is consistent with the previous study by Singer et al. (2009b). The outcomes of the subsequent working memory tests approximated closely to those obtained using the cheeseboard maze (Experiment 2) described above.
2-min ITI: Over the first four test days when the ITI was limited to 2 min, both mutant and control mice showed a similar magnitude of overall reduction in escape latency and associated path length from trial 1 to 2 (right panel of Figure 2C). Separate 2 × 2 × 4 (Genotype × Trials × Days) ANOVAs of both performance measures yielded a significant effect of trials [escape latency: F(1,16)=45.49; p<0.01; path length: F(1,16)=41.10; p<0.01].
15-min ITI: When the ITI was extended to 15 min over the next four days, performance had been stabilized in both groups. A consistent trial-dependent reduction in escape latency and path length was detected across all days (Figure 2D), yielding a highly significant trials effect in both performance measures [latency: F(1,16)=77.51; p<0.0001, path length: F(1,16)=26.73; p<0.0001]. There was no longer any indication for a genotype effect or its interactions [All F’s<1].
Similar to the cheeseboard experiment, there was no evidence for any significant group difference in speed of movement (swim speed) across all stages of the watermaze experiment (data not shown).
4. DISCUSSION
4.1 Working memory performance is insensitive to forebrain neuronal GlyT1 disruption
One impetus of the present study was to clarify the impact of forebrain neuronal GlyT1 deletion on working memory, which has remained ill-defined until now. In the previous report [14], the interpretation of the data was problematic because mutants already outperformed controls in trial 1 while not differing from them in trial 2. This divergence in baseline performance (when the platform location was essentially unknown to the animals) has precluded a simple interpretation in terms of enhancement or impairment. Singer et al. [14] speculated that this divergence in baseline performance might have been promoted by a non-mnemonic search strategy used by the mutants. As a remedy for this shortcoming, they [14] proposed that increasing the number of trials within a day (while still keeping platform location constant only within a day) should encourage the mutants to adopt a working memory strategy operating according to a “win-stay” rule.
Here, the extension to a 4-trial-per-day protocol had been effective in instilling an adherence to the matching rule underlying the water maze working memory paradigm as evidenced by the elimination of the “trial-1 effect” reported by Singer et al. [14]. However, it can be noted that the pattern of outcome reported by Singer et al. [14] could also be seen here on the first two test days (Figure 2C) even though the critical 3-way interaction term marginally failed to attain outright statistical significance [p=0.06]. Transient as it might be, its re-appearance argued against the possibility that Singer et al.’s [14] original outcome merely reflect a sampling error.
We included in addition the spatial working memory test using the cheeseboard maze (Experiment 2) and obtained the same outcomes. Thus, the null effect in working memory is demonstrably extendable to the non-aversive alternative test that relies on positive (as opposed to negative) reinforcement but carried essentially the same spatial and cognitive demand – viz., both the cheeseboard maze and water maze paradigms followed a matching-to-place (or “win-stay”) rule within a circular search area of similar sizes. When the Olton’s [18] radial arm maze test (Experiment 1) was introduced and again yielded the same outcomes, we can further conclude that the null effect in working memory function is generalizable to a task that relies on a non-matching-to-place rule that emphasizes a “shift” instead of “stay” strategy.
The radial arm maze test here not only has provided us a working memory paradigm based on a win-shift rule, its application in the solution of the task further enabled us to examine the capacity of the working memory buffer. In the water maze and cheeseboard experiments, the task simply required the animals to return to one specific location made known to them a short while ago (hence, ‘win-stay’). In the radial arm maze task, however, efficient performance depends on the recollection of multiple locations - i.e., the ability to judge the familiarity of many locations at the same time; the animals must effectively distinguish novel arms from familiar (visited) arms so as to avoid the latter. A reduction in the capacity of the working memory buffer and/or persistence of the memory trace of the visited arms may impair performance. The ability to avoid entering an arm that the animal has just visited would be less demanding than avoiding an arm visit earlier, and therefore fewer errors of the former kind should be made. This relative advantage could give rise to a form of “recency effect” in memory recall [19], because more recent experiences have less time for decay (forgetting) and suffers less from retroactive interference (viz., any intervening arm-visits). We attempted to examine this by classifying working memory errors (i.e., re-entries into already visited arms) by the number of intervening choices preceding each error in question, i.e., the degree of disparity between two visits into the same arm. The frequency of errors is expected to increase with increasing disparity. This was equally observed in mutant and controls mice (Figure 3) based on the data taken from the 8-arm protocol (i.e., those illustrated in Figure 1B). Taken together, the radial arm maze convincingly showed that forebrain neuronal GlyT1 disruption exerted no significant impact on the operation of working memory in this test. Given that this was the first test examined in this cohort, one can also be confident that this conclusion is free from any possible confounds due to transfer effects between tests.
Figure 3.
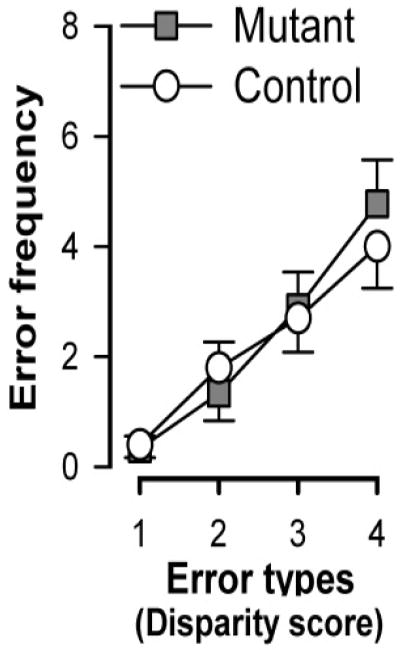
Frequency of errors classified as a function of the degree of disparity between two visits in the same arm for control and mutant mice based on the data obtained across the five days of test with the 8-arm protocol (Fig. 1B). The working memory errors were classified depending on the number of arms visited (disparity) between two visits into the same arm. For example, in the sequence of arm entries: ➀→➄→➂→➁→➁→➃→➈→➆→➃→➇ (where re-entry or working memory errors are underlined), the re-entry into arm ➁ is an error with disparity = 0 whereas the re-entry into arm ➃ is an error with disparity = 2. With increasing disparity, the frequency of error rises. This monotonic increase of error frequency as a function of the disparity did not differ between genotype. Error bars refer to ±SEM.
4.2 A new synthesis based on neuron-glial distinction
The resounding null effect here in mice lacking specifically GlyT1 in forebrain neurons might seem intriguing. First, the CamKIIα/GlyT1 KO mouse line has yielded the most promising promnesic effects in comparisons to other GlyT1 models [20]. The demonstration that these mice are not generally better learners suggest that the promnesic effects previously identified in these mutant mice are confined to specific psychological domains. Second, hippocampal NMDAR-mediated current is demonstrably enhanced in CamKIIα/GlyT1 KO mice [13] but not the EMX/GyT1-KO mice [16]. Yet, working memory performance in the water maze was enhanced in the latter but not the former mutant line. Thus, augmenting hippocampal NMDAR-dependent synaptic potentiation need not always lead to working memory enhancement [c.f., 21].
Interestingly, comparison between these two specific conditional GlyT1 knock out models may also be instructive in delineating the sufficient condition whereby GlyT1 blockade would improve working memory function. Given that Cre recombination is restricted to forebrain neurons in CamKIIα/GlyT1 KO mice (lacking working memory phenotype) but extended to both neurons and glial cells of the telencephalon in the EMX /GyT1-KO mice (showing working memory enhancement), we may speculate that the critical determinant is the disruption of telencephalonic glial GlyT1.
The emphasis on this specific subpopulation of GlyT1 may first imply the importance of achieving a sufficiently strong disruption of GlyT1 in the telencephalon. Because GlyT1 is mainly expressed in glial cells [19] their inclusion would produce a stronger disruption of glycine uptake. Extending the deletion to glial cells (as in the EMX /GlyT1-KO model) doubled the reduction of GlyT1-mediated glycine uptake in the brain in comparison with the current CamKIIα/GlyT1 KO model in which the deletion was confined to the forebrain neuronal component (see [20]). At glutamatergic synapses where neuronal GlyT1’s and NMDARs are co-localized, neighbouring glial-GlyT1s provide additional clearance of glycine from the synaptic cleft. Hence, the additional loss of glial-GlyT1 would be expected to further increase glycine-B site occupancy and consequently NMDAR-dependent neuronal activity than that achieved in CamKIIα/GlyT1 mice. However, glial-GlyT1 also assumes a major role in the uptake of glycine in forebrain glycinergic synapses [7, 22, 23]. One may therefore expect that deletion of brain glial-GlyT1 might additionally potentiate inhibitory glycinergic transmission that depends on the binding of glycine to strychnine-sensitive glycine-A site located on glycinergic receptors (GlyRs). This consideration thus raises the interesting possibility that enhanced glycinergic inhibitory activity might be partly responsible for working memory enhancement reported in EMX /GlyT1-KO mice [16] and perhaps also following acute systemic GlyT1 blockade by SSR504734 [10].
Disrupting glial-GlyT1 may therefore lead to complementary changes on neuronal excitation specifically mediated by NMDARs and network excitability via glycinergic inhibition [24]. The importance of inhibitory regulation in the maintenance of network stability is well recognized (e.g. [25]). For instance, the relevance of GABAergic inhibition to learning and memory has been most extensively examined (e.g. [26, 27]). Glycinergic inhibition may assume a similar regulatory role [24, 28], and this certainly warrants direct investigation. The two mechanisms (NMDAR-based and GlyR-based) are therefore not mutually exclusive. It is possible that both contribute to the observed effects on working memory reported by Singer et al. [10, 16]. This hypothesis can be readily tested in mouse models with selective disruption of brain glial-GlyT1 (c.f., [29]). Although glial-GlyT1 function is essential for early life survival, its deletion in adult mice is not associated with any gross abnormality [29] and would be congenial for cognitive assays. It would be even more instructive to target separately glial-GlyT1 associated with NMDAR versus those near glycinergic synapses when the relevant molecular technique becomes available. This possibility certainly deserves consideration even though glycine re-uptake at glycinergic synapses is also governed by GlyT2 in addition to glial-GlyT1 [7, 22].
Lastly, developmental compensation needs to be taken into consideration in any transgenic mouse model. The two mutant lines also differed in the onset time of GlyT1 deletion: around gestational day 10 [30] and near postnatal day 21 [31], in EMX /GlyT1-KO mice and CamKIIα/GlyT1 KO mice, respectively. EMX /GlyT1-KO mice might therefore be more prone to developmental compensation. However, we can safely conclude that such compensatory changes were unlikely by themselves be responsible for the working memory phenotype, because acute systemic GlyT1 inhibitor, SSR504734, was able to facilitate working memory in adult wild type mice [10].
5. Conclusion
The comparative exposé above incorporates a long line of investigations with multiple models. This is instrumental in illustrating that multiple molecular and pharmacological models are necessary for us to fully appreciate the potential and limitations of a possible therapeutic target. This approach enables the full and effective exploit of any potential drug target. Here, we show that divergences as much as convergences are instructive towards the building of a more complete mechanistic picture, and the synthesis of further hypotheses taking into account relevant cell biology, anatomy and physiology.
RESEARCH HIGHLIGTS.
Working memory is improved by disruption of neuronal and glial glycine transporter 1 → Is loss of neuronal GlyT1 that co-localized with NMDA receptors critically involved? → But, working memory is not affected by forebrain neuronal GlyT1 loss alone → Striatal GlyT1 and/or cortical glial GlyT1 may assume a more important role → Targeting them instead of neuronal GlyT1 may maximally facilitate working memory
Acknowledgments
The authors thank Peter Schmid for electronic and software maintenance of the behavioural equipments, the animal husbandry staffs for their excellent services. The present study was supported by the Swiss Federal Institute of Technology Zurich. BKY and DB had received additional support from the National Institutes of Health (MH083973). Sylvain Dubroqua was partly supported by a studentship awarded by the Neural Plasticity & Repair –National Centre for Competence in Research (NCCR) funded by the Swiss National Science Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319(6056):774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 2.Morris RG. Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J Neurosci. 1989;9(9):3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 4.Wood PL. The co-agonist concept: is the NMDA-associated glycine receptor saturated in vivo? Life Sci. 1995;57(4):301–310. doi: 10.1016/0024-3205(95)00288-h. [DOI] [PubMed] [Google Scholar]
- 5.Danysz W, Parsons CG. Glycine and N-methyl-D-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol Rev. 1998;50(4):597–664. [PubMed] [Google Scholar]
- 6.Aragon C, Lopez-Corcuera B. Glycine transporters: crucial roles of pharmacological interest revealed by gene deletion. Trends Pharmacol Sci. 2005;26(6):283–286. doi: 10.1016/j.tips.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Eulenburg V, Armsen W, Betz H, Gomeza J. Glycine transporters: essential regulators of neurotransmission. Trends Biochem Sci. 2005;30(6):325–333. doi: 10.1016/j.tibs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Bergeron R, Meyer TM, Coyle JT, Greene RW. Modulation of N-methyl-D-aspartate receptor function by glycine transport. Proc Natl Acad Sci U S A. 1998;95(26):15730–15734. doi: 10.1073/pnas.95.26.15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Depoortère R, Dargazanli G, Estenne-Bouhtou G, Coste A, Lanneau C, Desvignes C, et al. Neurochemical, electrophysiological and pharmacological profiles of the selective inhibitor of the glycine transporter-1 SSR504734, a potential new type of antipsychotic. Neuropsychopharmacology. 2005;30(11):1963–1985. doi: 10.1038/sj.npp.1300772. [DOI] [PubMed] [Google Scholar]
- 10.Singer P, Feldon J, Yee BK. The glycine transporter 1 inhibitor SSR504734 enhances working memory performance in a continuous delayed alternation task in C57BL/6 mice. Psychopharmacology (Berl) 2009;202(1-3):371–384. doi: 10.1007/s00213-008-1286-5. [DOI] [PubMed] [Google Scholar]
- 11.Tsai G, Ralph-Williams RJ, Martina M, Bergeron R, Berger-Sweeney J, Dunham KS, et al. Gene knockout of glycine transporter 1: characterization of the behavioral phenotype. Proc Natl Acad Sci U S A. 2004;101(22):8485–8490. doi: 10.1073/pnas.0402662101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabernet L, Pauly-Evers M, Schwerdel C, Lentz M, Bluethmann H, Vogt K, et al. Enhancement of the NMDA receptor function by reduction of glycine transporter-1 expression. Neurosci Lett. 2005;373(1):79–84. doi: 10.1016/j.neulet.2004.09.064. [DOI] [PubMed] [Google Scholar]
- 13.Yee BK, Balic E, Singer P, Schwerdel C, Grampp T, Gabernet L, et al. Disruption of glycine transporter 1 restricted to forebrain neurons is associated with a procognitive and antipsychotic phenotypic profile. J Neurosci. 2006;26(12):3169–3181. doi: 10.1523/JNEUROSCI.5120-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singer P, Boison D, Möhler H, Feldon J, Yee BK. Deletion of glycine transporter 1 (GlyT1) in forebrain neurons facilitates reversal learning: enhanced cognitive adaptability? Behav Neurosci. 2009a;123(5):1012–1027. doi: 10.1037/a0016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singer P, Boison D, Möhler H, Feldon J, Yee BK. Enhanced recognition memory following glycine transporter 1 deletion in forebrain neurons. Behav Neurosci. 2007;121:815–825. doi: 10.1037/0735-7044.121.5.815. [DOI] [PubMed] [Google Scholar]
- 16.Singer P, Yee BK, Feldon J, Iwasato T, Itohara S, Grampp T, et al. Altered mnemonic functions and resistance to N-METHYL-d-Aspartate receptor antagonism by forebrain conditional knockout of glycine transporter 1. Neuroscience. 2009c;161(2):635–654. doi: 10.1016/j.neuroscience.2009.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llano Lopez L, Hauser J, Feldon J, Gargiulo PA, Yee BK. Evaluating spatial memory function in mice: a within-subjects comparison between the water maze test and its adaptation to dry land. Behav Brain Res. 2010;209(1):85–92. doi: 10.1016/j.bbr.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Olton DS, Samuelson RJ. Remembrance of Places Passed - Spatial Memory in Rats. Journal of Experimental Psychology-Animal Behavior Processes. 1976;2(2):97–116. [Google Scholar]
- 19.Lund FH. The Psychology of Belief IV: The Law of Primacy in Persuasion. Journal of Abnormal Psychology and Social Psychology. 1925;20(2):183–191. [Google Scholar]
- 20.Möhler H, Boison D, Singer P, Feldon J, Pauly-Evers M, Yee BK. Glycine transporter 1 as a potential therapeutic target for schizophrenia-related symptoms: Evidence from genetically modified mouse models and pharmacological inhibition. Biochem Pharmacol. 2011;81(9):1065–1077. doi: 10.1016/j.bcp.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Bannerman DM, Good MA, Butcher SP, Ramsay M, Morris RG. Distinct components of spatial learning revealed by prior training and NMDA receptor blockade. Nature. 1995;378(6553):182–186. doi: 10.1038/378182a0. [DOI] [PubMed] [Google Scholar]
- 22.Gomeza J, Ohno K, Betz H. Glycine transporter isoforms in the mammalian central nervous system: structures, functions and therapeutic promises. Curr Opin Drug Discov Devel. 2003;6(5):675–682. [PubMed] [Google Scholar]
- 23.Gomeza J, Armsen W, Betz H, Eulenburg V. Lessons from the knocked-out glycine transporters. Handb Exp Pharmacol. 2006;(175):457–483. doi: 10.1007/3-540-29784-7_19. [DOI] [PubMed] [Google Scholar]
- 24.Zhang LH, Gong N, Fei D, Xu L, Xu TL. Glycine uptake regulates hippocampal network activity via glycine receptor-mediated tonic inhibition. Neuropsychopharmacology. 2008;33(3):701–711. doi: 10.1038/sj.npp.1301449. [DOI] [PubMed] [Google Scholar]
- 25.Möhler H, Rudolph U, Boison D, Singer P, Feldon J, Yee BK. Regulation of cognition and symptoms of psychosis: focus on GABA(A) receptors and glycine transporter 1. Pharmacol Biochem Behav. 2008;90(1):58–64. doi: 10.1016/j.pbb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, et al. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J Neurosci. 2002;22(13):5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yee BK, Hauser J, Dolgov VV, Keist R, Möhler H, Rudolph U, et al. GABA receptors containing the alpha5 subunit mediate the trace effect in aversive and appetitive conditioning and extinction of conditioned fear. Eur J Neurosci. 2004;20(7):1928–1936. doi: 10.1111/j.1460-9568.2004.03642.x. [DOI] [PubMed] [Google Scholar]
- 28.Xu TL, Gong N. Glycine and glycine receptor signaling in hippocampal neurons: diversity, function and regulation. Prog Neurobiol. 2010;91(4):349–361. doi: 10.1016/j.pneurobio.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Eulenburg V, Retiounskaia M, Papadopoulos T, Gomeza J, Betz H. Glial glycine transporter 1 function is essential for early postnatal survival but dispensable in adult mice. Glia. 2010;58(9):1066–1073. doi: 10.1002/glia.20987. [DOI] [PubMed] [Google Scholar]
- 30.Iwasato T, Nomura R, Ando R, Ikeda T, Tanaka M, Itohara S. Dorsal telencephalon-specific expression of Cre recombinase in PAC transgenic mice. Genesis. 2004;38(3):130–138. doi: 10.1002/gene.20009. [DOI] [PubMed] [Google Scholar]
- 31.Dragatsis I, Zeitlin S. CaMKIIalpha-Cre transgene expression and recombination patterns in the mouse brain. Genesis. 2000;26(2):133–135. doi: 10.1002/(sici)1526-968x(200002)26:2<133::aid-gene10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]


