Abstract
Kidney transplant and liver transplant are the treatments of choice for patients with end-stage renal disease and end-stage liver disease, respectively. Pancreas transplant is most commonly performed along with kidney transplant in diabetic end-stage renal disease patients. Despite a steady increase in the numbers of kidney and liver transplants performed each year in the United States, a significant shortage of kidneys and livers available for transplant remains. Organ allocation is the process the Organ Procurement and Transplantation Network (OPTN) uses to determine which candidates are offered which deceased donor organs. OPTN is charged with ensuring the effectiveness, efficiency and equity of organ sharing in the national system of organ allocation. The policy has changed incrementally over time in efforts to optimize allocation to meet these often competing goals. This review describes the history, current status and future direction of policies regarding the allocation of abdominal organs for transplant, namely the kidney, liver and pancreas, in the United States.
Keywords: Kidney allograft, liver allograft, organ allocation, pancreas allograft, transplant waiting list, transplantation
Introduction
Kidney transplant and liver transplant are the treatments of choice for patients with end-stage renal disease and end-stage liver disease, respectively, because transplant improves quality of life and survival. Pancreas transplants are commonly performed as simultaneous kidney–pancreas transplants for diabetic patients with end-stage renal disease. The numbers of kidney and liver transplants have increased over the years, but this increase has not kept pace with the growing number of patients who need these transplants. As of June 15, 2012, more than 99 000 people were waiting for a deceased donor kidney and more than 16 773 were waiting for a deceased donor liver (1). The shortage of kidneys and livers for transplant has made allocation of deceased donor organs an important subject of debate and controversy.
To address the nation’s critical organ donation shortage and improve the organ matching and placement process, the US Congress passed the National Organ Transplant Act (NOTA) in 1984. The act established the Organ Procurement and Transplantation Network (OPTN) to maintain a national registry for organ matching, and called for the network to be operated by a private, nonprofit organization under federal contract. The United Network for Organ Sharing (UNOS) is the OPTN contractor.
Organ allocation is the process OPTN uses to determine which candidates are offered which deceased donor organs. The goal of deceased donor organ allocation policy in the US has been to balance utility and equity in the distribution of deceased donor organs. The policy has changed incrementally over time in efforts to optimize allocation to meet these often competing goals. We describe kidney, pancreas and liver allocation policy historically and currently, and discuss potential future policy changes. We do not address all local variances that may be in place.
Kidney Allocation and Distribution
History of kidney allocation policy
Initially, allocation of kidneys was heavily dictated by how closely donor and recipient HLA subtypes were matched. Over time, advances in immunosuppression and the associated decrease in acute rejection rates permitted a shift in allocation priority away from HLA matching and toward waiting time (first come, first served). In 1995, for example, the allocation points awarded based on HLA-A matching were eliminated (Table 1).
Table 1.
Summary of major changes to kidney allocation policy
| Date | Policy Change |
|---|---|
| February 28, 1989 | Four points for candidates with PRA = 80%. |
| August 1, 1995 | Point system modified to assign 1 point for longest waiting time with fractions for shorter times, plus 1 point for each year of waiting beyond the first year. |
| September 2, 1996 | Extra points assigned for living organ donors who subsequently develop end-stage renal disease. |
| January 19, 1998 | Established defined medical criteria for initiating waiting time for adult kidney and kidney/pancreas candidates. |
| November 23, 1998 | Extra points for pediatric candidates modified to assign additional priority for children who did not undergo transplant within time goals. |
| October 30, 2002 | Defined expanded criteria donors. |
| May 7, 2003 | Revised 0MM from blood type O donors to give priority first to blood type O candidates, then to B candidates, then to A or AB candidates. |
| May 7, 2003 | Eliminated priority for HLA-B matching points. |
| May 7, 2003 | Additional priority assigned at the local level of distribution for prior living organ donors who subsequently develop end-stage renal disease. |
| November 5, 2003 | Candidates accrue waiting time even in inactive status from this point forward. |
| November 18–19, 2004 | Time goals eliminated for pediatric candidates, new policy prioritized donors aged < 35 years to pediatric candidates after 0MM, highest scoring high CPRA, and prior living organ donors but before paybacks. |
| January 21, 2009 | Elimination of 0MM sharing for nonsensitized adult candidates. |
| October 1, 2009 | Change from PRA to CPRA. |
0MM = zero antigen mismatch; CPRA = calculated panel-reactive antibody; PRA = panel-reactive antibody.
Some changes in allocation policy have been made specifically to improve equitable access to kidney transplants. Numerous studies have documented disparities in access to deceased donor kidney transplants between African American and non-Hispanic white patients. Due to racial differences in the frequency of alleles at each locus, allocation policy that heavily weighted HLA subtype matching was shown to be disadvantageous to minorities and to limit their access to deceased donor transplants (2). On May 7, 2003, kidney allocation policy was changed to eliminate the allocation points assigned for HLA-B similarity. Studies have shown that this policy has been effective in reducing, but not eliminating, racial disparities in rates of deceased donor kidney transplant with no adverse effect on graft survival (3,4).
Other changes in allocation policy have been made to address the shortage of kidneys. One strategy was to expand the deceased donor kidney pool to include kidneys previously deemed unsuitable (5). In 2002, the concept of expanded criteria donors (ECD) was introduced. ECD kidneys are defined as kidneys from any donor aged 60 years or older or aged 50 to 59 years with at least two of the following conditions: hypertension history, serum creatinine >1.5 mg/dL, or cause of death from cerebrovascular accident (OPTN Policy 3.5.1). These criteria define a donor population with an estimated risk of graft failure 70% higher than the risk associated with donors aged 10–39 years who were not hypertensive, did not die of cerebrovascular accident, and had terminal creatinine <1.5 mg/dL. Despite this, multiple studies have shown that kidney transplant using ECD kidneys is associated with lower morbidity and improved life expectancy compared with dialysis (6,7). Policy dictates that ECD kidneys must be offered first to candidates who have agreed to receive them (OPTN Policy 3.5.8). The purpose of this policy is to avoid delays in placement of ECD kidneys that result from multiple refusals, under the theory that if ECD kidneys were offered to candidates known to be willing to accept them, cold ischemia times could be reduced. However, some transplant centers exercise their option to be informed of all ECD kidneys available for their candidates, even if the center rarely accepts ECD kidneys (8).
In addition to expanded use of ECD kidneys, use of kidneys from donors after circulatory death (DCD) has increased; these donors now represent 12.9% of all deceased donors. Kidneys from DCD donors are associated with 1-year graft and patient survival rates equivalent to kidneys from brain-dead donors (9–11).
Current kidney allocation and distribution policy
The current kidney allocation system categorizes donors into four mutually exclusive groups: standard criteria donors (SCD) aged < 35 years, SCD aged ≥ 35 years, DCD and ECD (Figure 1). Within these categories, several principles dictate current allocation priority. First, allocation policy gives priority to candidates listed for simultaneous kidney and nonkidney organ transplants, including kidney–pancreas, kidney–liver and kidney–heart. Kidney–pancreas allocation policy is described in detail later. Priority for allocation of kidneys to kidney–heart and kidney–liver candidates follows the allocation priority for the nonrenal organ (12).
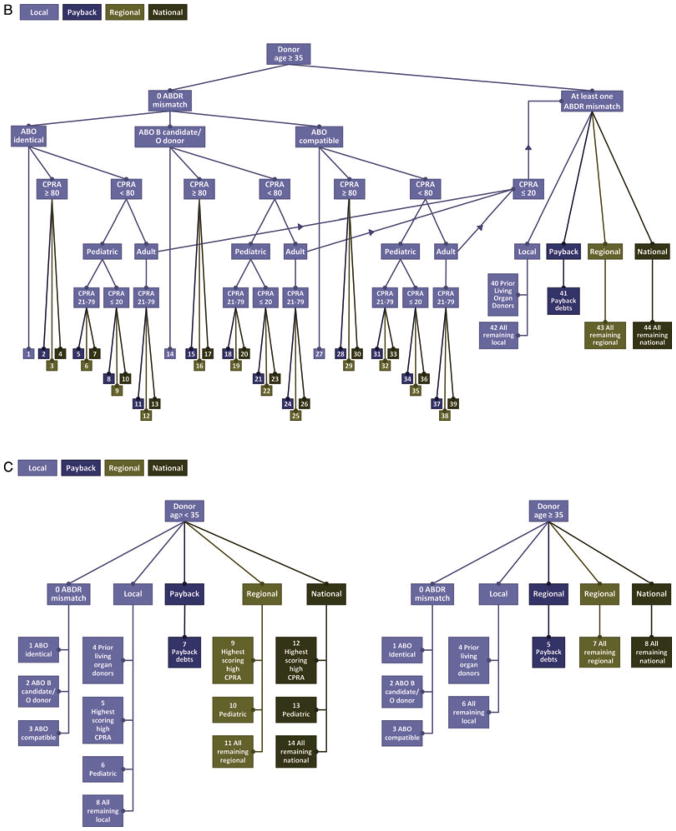
Figure 1. Organ Procurement and Transplantation Network’s allocation algorithm for kidney transplants for (A) standard criteria donors aged < 35 years; (B) standard criteria donors aged ≥ 35 years; (C) donation after cardiac death; (D) expanded criteria donors.
CPRA = calculated panel-reactive antibodies; OPO = organ procurement organization; UNOS = United Network for Organ Sharing. This figure can be downloaded in color from www.srtr.org.
Second, allocation policy for deceased donor kidneys gives priority to candidates with zero antigen mismatch with the donor kidney (mandatory sharing of zero antigen mismatched kidneys, OPTN Policy 3.5.3). A zero antigen mismatch is defined as ABO blood type being compatible between a candidate and a donor, and the absence of any donor antigens (among the six HLA A, B and DR antigens) not native to the candidate (OPTN Policy 3.5.3.1).
Finally, allocation policy adheres to a geographic sequence (OPTN Policy 3.5.6). Kidneys are distributed initially to local candidates, defined as candidates listed at transplant centers within the donation service area (DSA) of the organ procurement organization (OPO) that procured the deceased donor organ (OPTN Policy 3.5.6.1). Regional distribution follows local distribution and occurs if a kidney is not accepted by any local transplant center for a local candidate (OPTN Policy 3.5.6.2). The US is divided into 11 regions (Figure 2). When a kidney is distributed regionally, it is offered for specific candidates in the region. National distribution occurs if a kidney is not accepted by any transplant center in the region that procured the kidney (OPTN Policy 3.5.6.3). At each geographic level of distribution, candidates are rank-ordered according to the point system described later.
Figure 2.
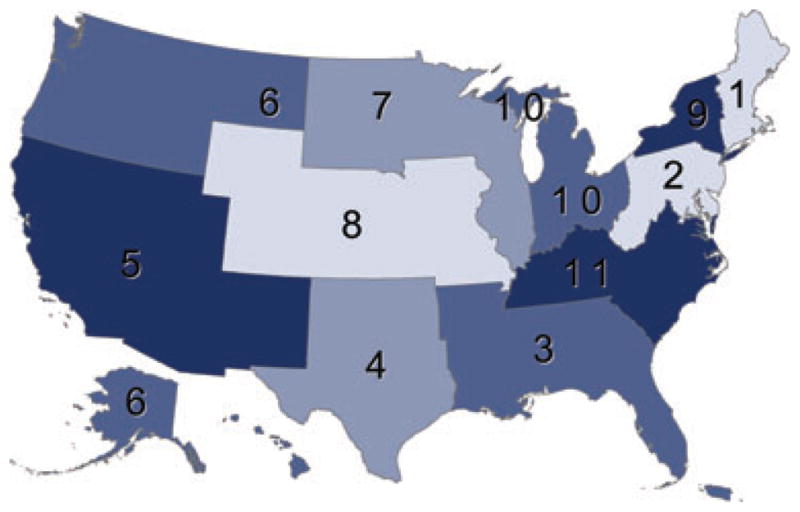
Regions of the Organ Procurement and Transplantation Network.
Distribution of standard criteria donor kidneys
SCD kidney allocation policy is categorized by donor age < 35 years or ≥ 35 years (Figures 1A and B). After allocation of deceased donor kidneys for simultaneous kidney and nonkidney organ transplant, zero antigen mismatched SCD kidneys (from donors aged < 35 years and ≥ 35 years) are distributed first to candidates with ABO blood group identical to the donors in the following sequence (OPTN Policy 3.5.3.3.1):
Local candidates (bin 1).
-
Candidates with ≥80% calculated panel-reactive antibody (CPRA) on the list of OPOs that are owed a payback kidney (bin 2).
CPRA expresses the likelihood (% chance) that a donor selected randomly from the population of donors is incompatible with this particular candidate, based on the unacceptable HLA antigens to which the candidate has been sensitized. If present in the donor, these antigens would represent an unacceptable risk. CPRA is determined using an established algorithm (13,14) and HLA frequencies derived from the HLA phenotypes of more than 12 000 donors entered into the OPTN/UNOS registry.
A payback debt is generated in the setting of (1) a zero antigen mismatch kidney allocated to a nonlocal OPO, (2) a voluntary arrangement for sharing the kidney and an organ other than a kidney from the same donor for transplant into the same recipient or (3) a voluntary arrangement for sharing the kidney for a candidate with panel-reactive antibody (PRA) ≥80% and a negative preliminary crossmatch with the donor (Policy 3.5.5).
Candidates with ≥80% CPRA on the regional (bin 3), then national (bin 4) waiting lists.
Pediatric (age < 18 years at the time of distribution) candidates with CPRA 21–79% who are on the list of OPOs owed a payback kidney (bin 5).
Pediatric (age < 18 years at the time of distribution) candidates with CPRA 21–79% who are on the regional (bin 6), then national (bin 7) waiting lists.
Pediatric (age < 18 years at the time of distribution) candidates with CPRA ≤20% who are on the list of OPOs owed a payback kidney (bin 8).
Pediatric (age < 18 years at the time of distribution) candidates with CPRA ≤ 20% who are on the regional (bin 9), then national (bin 10) waiting lists.
Adult candidates with CPRA 21–79% who are on the list of OPOs owed a payback kidney (bin 11).
Adult candidates with CPRA 21–79% who are on the regional (bin 12), then national (bin 13) waiting lists.
Next, zero antigen-mismatched kidneys are allocated to ABO compatible candidates following the same distribution sequence as above (OPTN Policy 3.5.3.3.2). This includes blood type O donor kidneys distributed to blood type B zero antigen mismatched candidates, then to blood type A and AB zero antigen mismatched candidates (bins 14–26). Blood type A, B and AB donor kidneys are distributed in the same sequence described above to candidates who are blood type compatible (bin 27–39).
If no candidates among the zero antigen mismatch categories accept the kidney, it is distributed to candidates with 1 or more HLA mismatches in the following sequence for donors aged < 35 years (Figure 1A).
Local candidates who are prior living donors (bin 40).
Local candidates in the highest scoring, high CPRA category (bin 41). (Candidates are determined to be in this category by the following logic: Candidates are sorted according to total score from highest to lowest. All candidates with CPRA ≥ 80% and total score greater than the total score of the first candidate with CPRA <80% are in the highest scoring, high CPRA category.)
Local pediatric candidates (age < 18 years at time of listing) (bin 42).
Candidates in OPOs with payback credits (bin 43).
All remaining local candidates (bin 44).
Regional candidates in the highest scoring, high CPRA category (bin 45).
Regional pediatric (age < 18 years at time of listing) candidates (bin 46).
All remaining regional candidates (bin 47).
National candidates in the highest scoring, high CPRA category (bin 48).
National pediatric (age < 18 years at time of listing) candidates (bin 49).
All remaining national candidates (bin 50).
For SCDs aged ≥ 35 years (Figure 1B), kidneys with at least one ABDR mismatch are distributed in order to prior living donors (bin 40), OPOs owed a payback kidney (bin 41) and all remaining local, regional and national candidates (bins 42–44).
Distribution of kidneys recovered by donation after circulatory death
DCD kidney allocation is categorized by donor age < 35 years and ≥ 35 years (Figure 1C). The distribution sequence for kidneys from DCD donors aged < 35 years is as follows
Zero antigen mismatch candidates who are ABO identical (bin 1), blood type B candidate/O donor (bin 2) and ABO compatible (bin 3).
Prior living donor candidates (bin 4).
Local candidates in the highest scoring, high CPRA category (bin 5).
Local pediatric (age < 18 years at time of listing) candidates (bin 6).
Payback debt and credits (bin 7).
All remaining local candidates (bin 8).
Regional candidates in the highest scoring, high CPRA category (bin 9).
Regional pediatric (age < 18 years at time of listing) candidates (bin 10).
All remaining regional candidates (bin 11).
National candidates in the highest scoring, high CPRA category (bin 12).
National pediatric (age < 18 years at time of listing) candidates (bin 13).
All remaining national candidates (bin 14).
Distribution of DCD kidneys from donors aged ≥ 35 years follows a similar sequence but without pediatric and highest scoring, high CPRA priority (Figure 1C).
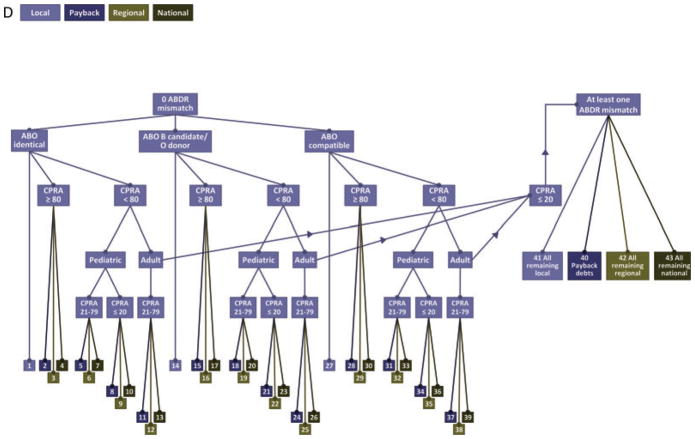
Distribution of expanded criteria donor kidneys
Zero antigen mismatched ECD kidneys are distributed first to ABO identical recipients in the following sequence (Figure 1D)
Local candidates (bin 1).
Candidates with CPRA ≥80% who are on the list of OPOs owed a payback kidney (bin 2).
Candidates with CPRA ≥80% who are on the regional (bin 3), then national (bin 4) waiting lists.
Pediatric (age < 18 years at the time of allocation) candidates with CPRA 21–79% who are on the list of OPOs owed a payback kidney (bin 5).
Pediatric (age < 18 years at the time of allocation) candidates with CPRA 21–79% who are on the regional (bin 6), then national (bin 7) waiting lists.
Pediatric (age < 18 years at the time of allocation) candidates with CPRA ≤ 20% who are on the list of OPOs owed a payback kidney (bin 8).
Pediatric (age < 18 years at the time of allocation) candidates with CPRA ≤ 20% who are on the regional (bin 9), then national (bin 10) waiting lists.
Adult candidates with CPRA 21–79% who are on the list of OPOs owed a payback kidney (bin 11).
Adult candidates with CPRA 21–79% who are on the regional (bin 12), then national (bin 13) waiting lists.
Next, zero antigen mismatched ECD kidneys are distributed to ABO compatible candidates following the same sequence. This includes blood type O donor kidneys distributed to blood type B zero antigen mismatched candidates and then to blood type A and AB zero antigen mismatched candidates (bins 14–26). Blood type A, B and AB donor kidneys are distributed in the same sequence described above to blood type compatible candidates (bins 27–39). If no candidates with zero antigen mismatch accept the ECD kidney, it is distributed to candidates with one or more HLA mismatches in the following sequence:
OPOs with payback credits (bin 40).
All remaining local, regional and national candidates (bins 41–44).
For kidneys that meet both DCD and ECD criteria, distribution follows the DCD sequence. Within the ECD/DCD sequence, the points used to sort candidates are based solely on waiting time, as dictated by ECD allocation policy. If any candidate on the waiting list has a zero antigen mismatch with the donor, the kidney from that donor is offered to the candidate listed locally with the zero antigen mismatch, first to blood group identical candidates, then to B candidates/O donors, and then to blood group compatible candidates. Payback obligations come next, followed by all other (non zero-mismatch) local candidates in point sequence according to the ECD policy (OPTN Policy 3.5.11), then all regional, then all national candidates in point sequence for ECD allocation (OPTN Policy 3.5.12).
The point system for distribution of kidney allografts
Points are assigned to wait-list candidates based on several factors, enabling priority ranking of candidates within each bin in Figure 1 (OPTN Policy 3.5.11). The same point assignments apply to ECD and SCD allocation, irrespective of donor age. The first factor is time on the waiting list (OPTN Policy 3.5.11.1). For candidates aged ≥ 18 years, waiting time begins when an active candidate listed for an isolated kidney or combined kidney/pancreas transplant meets the minimum criteria of (1) measured (urinary collection) or calculated creatinine clearance or glomerular filtration rate (Cockcroft-Gault or other reliable formula) ≤ 20 mL/min; or (2) initiation of maintenance dialysis. For candidates aged < 18 years, waiting time begins when the candidate is registered on the waiting list; there are no minimum listing criteria for pediatric candidates. Regardless of age, since November 2003, all candidates continue to accrue waiting time when they are inactive on the waiting list. Once waiting time begins to accrue, one point is assigned to the candidate who has waited the longest in each of these four high-level categories: zero antigen mismatches, local, regional and national. Fractions of a point are assigned proportionately to all other candidates in the high-level category according to their relative ranking regarding waiting time. An additional point is assigned for each full year of waiting time accrued. Additional points are assigned based on number of antigen mismatches between candidate and donor at the DR locus: two points if there are no DR mismatches and one point if there is one DR mismatch (OPTN Policy 3.5.11.2). Sensitized wait-list candidates with defined unacceptable HLA antigens that yield a CPRA ≥ 80% are assigned four points (OPTN Policy 3.5.11.3).
In contrast to point systems for other organs, the kidney point system assigns no points based on medical urgency for regional or national allocation of kidneys. At the local level, the candidate’s physician has the authority to use medical judgment to assign medical urgency points (OPTN Policy 3.5.11.4).
Pediatric candidates aged <11 years are assigned four additional points for distribution of zero antigen mismatch kidneys (OPTN Policy 3.5.11.5). Candidates aged ≥11 but < 18 years are assigned three additional points for distribution of zero antigen mismatch kidneys. These points are assigned when the candidate is registered on the waiting list and retained until the candidate reaches age 18 years. Prior living donors are assigned four points.
Payback requirements
To prevent net loss or gain of kidneys by any OPO due to sharing, the OPO that accepts a regionally or nationally shared zero antigen mismatched kidney must pay back a kidney to a common national pool (OPTN Policy 3.5.5). The OPO that receives the kidney must then offer a kidney from the next suitable standard donor who does not meet criteria for DCD, is aged 6–59 years, and has the same ABO blood type as the donor from whom the shared kidney was procured. In an exception to the payback requirement, kidneys procured from SCDs are allocated locally first to prior living organ donors before other obligations. To maintain system balance, if an OPO has accumulated six or more payback obligations, it is no longer permitted to retain a kidney for a local kidney/nonrenal organ transplant. Finally, policy dictates that an OPO accumulate no more than nine kidney payback debts (all blood groups combined) at any point in time.
Blood type
Organs are distributed first to ABO identical candidates, then to ABO compatible candidates. This includes blood type O donor kidneys distributed to blood type B zero antigen mismatched candidates and then to blood type A and AB candidates. Kidneys from a blood type O donor are distributed only to blood type O candidates and kidneys from a blood type B donor are allocated only to blood type B candidates, with the exception for zero antigen mismatched candidates (OPTN Policy 3.5.3.1). This policy does not nullify the physician’s responsibility to use appropriate medical judgment in an extreme circumstance.
Pediatric kidney transplant organ allocation
Children have long been recognized as deserving priority in kidney allocation. Candidates listed before their 18th birthday are considered to be pediatric until they undergo transplant or are otherwise removed from the waiting list (OPTN Policy 3.5.11.1). In 1993, OPTN/UNOS formed an Ad Hoc Pediatric Advisory Committee. The committee prepared a white paper giving evidence of the detrimental effects of end-stage renal disease and dialysis on growth and development, and describing technical problems with dialysis in pediatric patients. This led to policy changes that awarded additional points to pediatric candidates in an effort to allow them to undergo transplant sooner.
In 1998, the OPTN/UNOS Pediatric Committee reviewed the effect of the additional points on pediatric transplant rates and found the rates to be unacceptably low. Therefore, the committee determined that time-to-transplant goals be put in place for pediatric candidates. The goals stipulated that candidates aged ≤5 years undergo transplant within 6 months of listing, candidates aged 6–11 years undergo transplant within 12 months, and candidates aged >11 years undergo transplant within 18 months. Unfortunately, the time goal policy did not improve pediatric transplant rates. For pediatric candidates, expediency in offers had to be balanced with donor quality. Under the time goal policy, pediatric candidates received offers but often did not undergo transplant due to concerns about the potential longevity of the offered kidney.
Congress passed the Children’s Health Act of 2000, which was incorporated as an amendment to NOTA. This Act specifically stated that organ allocation policy is to recognize the differences in health and organ transplant issues between children and adults throughout the system, and adopt criteria, policies, and procedures that address the unique health care needs of children.
On September 28, 2005, the kidney allocation system was modified to give priority to pediatric candidates ahead of adult candidates within each distribution category locally, regionally and nationally for nonzero mismatch kidney offers from donors aged < 35 years (OPTN Policy 3.5.11.5.1). This priority is illustrated by bins 42, 46 and 49 in Figure 1. The intent of this modification, referred to as “Share 35”, was to prioritize allocation of younger donor kidneys, which are better suited to children, to address established goals of rapidly providing transplants to pediatric candidates with minimal impact on adult transplant rates. Pediatric candidates aged < 18 years at the time of organ allocation also receive priority over adults for zero-antigen mismatch offers (OPTN Policy 3.5.3.3.1), as well as pediatric points for zero-antigen mismatch kidney offers. Younger candidates (aged 0–10 years) receive four points for zero-antigen mismatch kidney offers, and adolescent candidates (aged 11–17 years) receive three points (OPTN Policy 3.5.11.5). In cases of nonzero antigen mismatch offers, pediatric candidates aged <10 years at the time of organ distribution receive one additional point for kidneys from donors aged < 35 years (OPTN Policy 3.5.11.5.1).
Future direction of kidney allocation policy
Current allocation policy is limited in several ways. Prioritizing candidate waiting time does not necessarily allow for the sickest patients to undergo transplant sooner. Current policy is not designed to differentiate a candidate’s ability to survive on the waiting list, and therefore does not minimize wait-list mortality. In addition, current allocation policy does not attempt to match donor kidney and candidate characteristics to optimize survival posttransplant and minimize unrealized graft years and unnecessarily high retransplant rates.
To address these limitations, the OPTN/UNOS Kidney Transplantation Committee drafted a concept document in 2010 that proposed use of age matching, survival matching and a kidney donor profile index (KDPI) for kidney allocation. This document was released for public comment in February 2011. The objectives of the revised kidney allocation system include: (1) to improve graft and recipient longevity; (2) to improve offer system efficiency and organ utilization through the introduction of a new scale for kidney quality, the KDPI; (3) to improve availability of comprehensive data for patients and transplant programs to guide renal replacement therapy choices and (4) to reduce differences in transplant access for populations described in NOTA (e.g. candidates from racial/ethnic minority groups, pediatric candidates and sensitized candidates).
The KPDI is a continuous scale for measuring the association of deceased donor kidney quality with expected allograft failure rates in a typical recipient. The KDPI summarizes the risk of graft failure after kidney transplant by combining several donor factors into a single number. The KDPI is based on the following donor characteristics: age, race/ethnicity, hypertension status, diabetes status, serum creatinine level, cause of death (cerebrovascular, cardiac, etc.), height, weight, DCD and hepatitis C status. Use of the KDPI is proposed to optimize allocation of the highest quality kidneys to the candidates with the highest estimated posttransplant survival. The calculation for estimating posttransplant survival is based on four factors: candidate age, dialysis duration, any prior organ transplant and diabetes status. The shift from waiting time to dialysis time may help address disparities in transplant access.
Pancreas Allocation and Distribution
History of pancreas allocation policy
Historically, there was no uniform national allocation policy for pancreas transplants. Pancreas-alone allografts were allocated in the same way as kidney-pancreas allografts until 1995, when islet candidates were added to the pancreas allocation policy. Similar to kidney allocation policy, OPTN policy for deceased donor pancreas allocation has given first preference to zero HLA-mismatch candidates for simultaneous kidney–pancreas and pancreas-alone transplants locally, then regionally, then nationally since 2000. Subsequent policy limited first preference to zero HLA-mismatch candidates with PRA ≥ 80% for simultaneous kidney–pancreas transplants in 2002 and for pancreas-alone transplants in 2005.
In 2007, Stegall et al. (15) evaluated the rationale for changing pancreas allocation policy, studying the disposition and outcome of deceased donor pancreata from January 1, 2000, to December 31, 2003. Organs for 90% of whole-organ pancreas transplants were from donors aged ≤50 years with body mass index (BMI) ≤30 kg/m2; organs from older, more obese donors were more often recovered for islet transplant or research. Starting in 2007, OPTN adopted the current allocation algorithm, in which pancreas allografts from donors aged >50 years or with BMI > 30 kg/m2 are preferentially allocated for islet transplant, but only if the pancreas has not been allocated to a zero HLA-mismatch recipient with PRA ≥ 80% or to a local recipient.
Current pancreas allocation and distribution policy
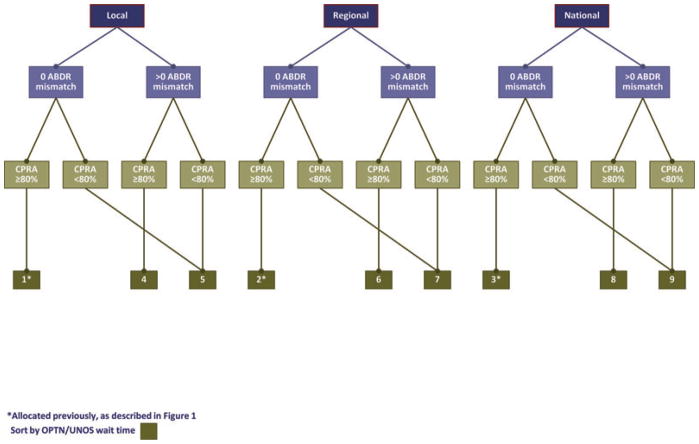
Currently, allocation of a deceased donor pancreas is determined by OPTN Policy 3.8, Pancreas Allocation (16). However, it is also dependent on Policy 3.5, Allocation of Deceased Donor Kidneys. Figure 3 illustrates this point; the allocation sequence that an OPO must follow depends first on how many kidneys are available at the time of allocation. If two kidneys and a pancreas are available, one kidney will be allocated to kidney-alone candidates per Policy 3.5 (5). The remaining kidney–pancreas must then be offered to zero HLA-mismatch kidney–pancreas candidates who are highly sensitized (Figure 3). After these zero-mismatch offers, the kidney–pancreas can be allocated to kidney–pancreas or pancreas candidates per policy and the OPO’s discretion. However, if only one kidney is available, it must first be offered in tandem with the pancreas to zero HLA-mismatch kidney–pancreas candidates who are highly sensitized with CPRA ≥ 80%. If no offers are accepted, the kidney must be distributed to kidney-alone candidates through kidney allocation policy (Policy 3.5) up to local payback debts (bin 43 of Figure 1A). If the kidney is not allocated through the payback system, the OPO can resume allocation of the kidney–pancreas per the pancreas allocation policy. While the kidney is being offered through the kidney-alone allocation policy, the OPO can proceed with allocating the pancreas to pancreas-alone candidates.
Figure 3. Pancreas allocation policy and the required sequence of kidney allocation.
This figure can be downloaded in color from www.srtr.org.
The OPO also has some degree of choice in whether to allocate the pancreas alone or in conjunction with the kidney. As shown in Figure 3, the OPO may choose to give kidney–pancreas candidates absolute priority (Figure 4), combine kidney–pancreas and pancreas-alone candidates (Figure 5), or give pancreas-alone candidates absolute priority and offer the remaining kidney to kidney-alone candidates (Figures 6A and B). The OPO can choose any of these three waiting lists and switch among them, but no candidate on the selected waiting list is skipped (Figure 3). Figures 4–6 show these options in detail; the figures are annotated to describe how organs are allocated within each bin (the end of each sequence), via allocation score, waiting time, or both.
Figure 4. Simultaneous kidney–pancreas allocation.
This figure can be downloaded in color from www.srtr.org.
Figure 5. Pancreas-alone allocation, stratified by donor age and body mass index.
This figure can be downloaded in color from www.srtr.org.
Figure 6. Combined kidney–pancreas/pancreas allocation for (A) donors aged ≤ 50 years with body mass index ≤ 30 kg/m2 and (B) combined kidney–pancreas/pancreas allocation for donors aged > 50 years or with body mass index > 30 kg/m2.
This figure can be downloaded in color from www.srtr.org.
Simultaneous kidney-pancreas waiting list
A simultaneous kidney–pancreas transplant is first offered locally to candidates with zero HLA mismatches and CPRA ≥ 80% (bin 1, Figure 4), then regionally (bin 2), then nationally (bin 3). These three bins have priority, as also shown in Figure 3. Next in the sequence are kidney–pancreas candidates with one or more HLA mismatches and CPRA ≥ 80% (bin 4), then candidates with CPRA < 80% with zero or more HLA mismatches, locally (bin 5), regionally (bins 6 and 7), then nationally (bins 8 and 9). Within each bin, candidates are prioritized by waiting time for simultaneous kidney–pancreas transplant. At any point, the OPO may choose to switch to the individual pancreas or kidney waiting list, as long as no candidate is skipped on a particular waiting list. For instance, many OPOs prefer to offer the kidney–pancreas to all local candidates, then switch to the pancreas waiting list to offer the pancreas alone to all local candidates. The OPO may continue to allocate to the pancreas waiting list or switch back to the kidney–pancreas waiting list.
Kidney-alone waiting list and pancreas-alone waiting list
An OPO can allocate to the kidney-alone and the pancreas-alone waiting lists. The current allocation sequence for pancreas-alone allografts considers donor age and BMI (Figure 5). The first match sequence is for donors aged ≤ 50 years with BMI ≤ 30 kg/m2; a separate match sequence is for donors aged > 50 years or BMI > 30 kg/m2. Candidates with zero HLA mismatches and CPRA ≥ 80% receive preference in both match sequences. Candidates are categorized into bins, then prioritized by OPTN/UNOS waiting time for pancreas alone. Islet candidates are prioritized by islet points, then by OPTN/UNOS waiting time. One point is assigned to the islet candidate who has waited the longest within a geographic division; fractions of points are assigned proportionally to other candidates in that same geographic division by waiting time relative to the longest waiting time. The geographic divisions for islets are local, regional and national. For example, if there are 25 islet candidates in a local geographic division, the candidate with the longest waiting time will be given one point. The candidate with the next-longest waiting time will be given a fraction of one point defined by the following equation: 24/25 × 1 = 0.98.
Donor age ≤ 50 years and BMI ≤ 30 kg/m2
First in the sequence for pancreas allografts from donors aged ≤50 years with BMI ≤ 30 kg/m2 are local pancreas-alone candidates with zero HLA mismatches and CPRA ≥ 80% (bin 1, left side of Figure 5). Next are local candidates with one or more HLA mismatches and CPRA ≥ 80% (bin 2), then regional candidates with zero HLA mismatches and CPRA ≥ 80% (bin 3), then national candidates with zero HLA mismatches and CPRA ≥ 80% (bin 4). Next in the sequence are local candidates with CPRA < 80% regardless of HLA mismatch (bin 5). If no suitable local candidates are identified, the pancreas allografts are allocated regionally, first to candidates with one or more HLA mismatches and CPRA ≥ 80% (bin 6), then to candidates with CPRA < 80% regardless of HLA mismatches (bin 7). If no suitable regional candidates are identified, the pancreas allografts are allocated nationally, first to candidates with one or more HLA mismatches and CPRA ≥ 80% (bin 8), then to candidates with CPRA < 80% regardless of HLA mismatch (bin 9). Finally, islets are allocated locally (bin 10), regionally (bin 11) and nationally (bin 12).
Donor age ≥ 50 years or BMI ≥ 30 kg/m2
Policy for these donors is similar to policy for donors aged ≤50 years and BMI ≤ 30 kg/m2, except for allocation to islets candidates earlier in the order, as follows: If no suitable candidate is identified when the pancreas alone is offered to local candidates with one or more HLA mismatches and CPRA ≥ 80% (bin 2 of Figure 5), then regional candidates with zero HLA mismatches and CPRA ≥ 80% (bin 3), then national candidates with zero HLA mismatches and CPRA ≥ 80% (bin 4), then local candidates with CPRA < 80% regardless of HLA mismatches (bin 5), the pancreas is allocated to islet candidates. The match run uses the islet waiting list, first for local (bin 6), then regional (bin 7), then national (bin 8) candidates. If no suitable islet candidates are identified, the match run returns to regional pancreas candidates.
Combined kidney-pancreas/pancreas allocation
The second option for an OPO is a combined kidney–pancreas/pancreas allocation strategy. Preference is always given to simultaneous kidney–pancreas candidates with zero HLA mismatches and CPRA ≥ 80% locally (bin 1, Figures 6A and B), then regionally (bin 2), then nationally (bin 3). Again, donor age ≤50 years and BMI ≤ 30 kg/m2 affect the allocation sequence. Candidates are categorized into bins and prioritized by OPTN/UNOS waiting time. Islets candidates are prioritized by islet points and OPTN/UNOS waiting time.
Donor age ≤50 years and BMI ≤ 30 kg/m2
Next in sequence are local pancreas candidates with zero HLA mismatches and CPRA ≥ 80% (bin 4, Figure 6A), then local pancreas or kidney–pancreas candidates with one or more HLA mismatches and CPRA ≥ 80% (bin 5). If no suitable candidate is identified, the pancreas alone is offered to regional (bin 6), then to national (bin 7) candidates with zero HLA mismatches and CPRA ≥ 80%. Next in sequence are local pancreas and kidney–pancreas candidates with CPRA < 80% regardless of HLA mismatches (bin 8), regional pancreas candidates with 1 or more HLA mismatches and CPRA ≥ 80% (bin 9) and regional pancreas candidates with CPRA < 80% regardless of HLA mismatches (bin 10).
Regional pancreas-alone candidates are followed in sequence by regional kidney–pancreas candidates, first those with one or more HLA mismatches and CPRA ≥ 80% (bin 11), then those with CPRA < 80% regardless of HLA mismatches (bin 12). If no suitable regional candidate is identified, national pancreas and kidney–pancreas candidates are next in sequence. Nationally, pancreas-alone candidates with one or more HLA mismatches and CPRA ≥ 80% (bin 13) are first, then those with CPRA < 80% regardless of HLA mismatches (bin 14). If no suitable pancreas-alone candidates are identified, the match run considers national kidney–pancreas candidates, first those with one or more HLA mismatches and CPRA ≥ 80% (bin 15), then those with CPRA < 80% regardless of HLA mismatches (bin 16). Finally, the pancreas is allocated to islet candidates locally (bin 17), regionally (bin 18), and nationally (bin 19).
Donor age ≥ 50 years and BMI ≥ 30 kg/m2
Policy for these donors is similar to policy for donors aged ≤50 years and BMI ≤ 30 kg/m2 except for allocation to islets candidates earlier in the order, as follow: If no suitable candidate is identified when the pancreas alone is offered to regional (bin 6 of Figure 6B) then national (bin 7) candidates with zero HLA mismatches and CPRA ≥ 80%, then to local pancreas and kidney–pancreas candidates with CPRA < 80% regardless of HLA mismatches (bin 8), the pancreas is allocated to islet candidates. The pancreas allograft is distributed to islet candidates locally (bin 9), regionally (bin 10) and nationally (bin 11). If an islet candidate is not found, the match run returns to pancreas and kidney–pancreas candidates.
Future pancreas allocation policy
A revision of OPTN Policy 3.8, Pancreas Allocation, was approved by the OPTN Board of Directors on November 9, 2010 (16). However, it has not yet been implemented.
Criteria for accruing wait-list time for simultaneous kidney–pancreas transplant
The revised pancreas allocation policy explicitly defines eligibility criteria for kidney–pancreas candidates in OPTN policy that will be implemented along with the revised kidney allocation policy that is currently being developed. To be eligible to accrue simultaneous kidney–pancreas waiting time, candidates must qualify for waiting time for a kidney transplant as is currently required. However, under future policy, candidates must also meet one of the following criteria: on insulin and C-peptide ≤ 2 ng/mL, or on insulin and C-peptide ≥ 2 ng/mL with BMI less than the maximum allowable BMI. Maximum allowable BMI will be 28 kg/m2, but can change based on BMI of active candidates on the kidney–pancreas waiting list. If BMI is 28 kg/m2 for more than 15% of the simultaneous kidney–pancreas wait-list candidates, the maximum allowable BMI will be reduced by 2 kg/m2. Similarly, if BMI is 28 kg/m2 for less than 10% of the wait-list candidates, the maximum allowable BMI will be increased to 30 kg/m2. Once a candidate becomes eligible to accrue waiting time, eligibility will remain in effect regardless of policy changes regarding maximum allowable BMI or changes to the candidate’s BMI. Exceptions to waiting time criteria will be automatically granted to all candidates listed for simultaneous kidney–pancreas transplant before their 18th birthday. Candidates accrue pancreas-alone waiting time beginning at the time they are added to the pancreas-alone waiting list.
Allocation sequence
Another major change to pancreas allocation policy is creation of a national system that is virtually independent of kidney allocation. OPOs will no longer be able to give preference to simultaneous kidney–pancreas candidates or pancreas-alone candidates; instead, the two types of candidates will be given equal priority within geographic region, HLA mismatch status and CPRA status. As is the case in current policy, organs from blood type-O donors can only be allocated to type-O simultaneous kidney–pancreas recipients; the same restriction does not apply to pancreas-alone candidates. An exception to this requirement occurs if the simultaneous kidney–pancreas candidate has zero HLA mismatches and CPRA ≥ 80%.
Donor age ≤ 50 years and BMI ≤ 30 kg/m2
The pancreas is first allocated to local pancreas and kidney–pancreas candidates with zero HLA mismatches and CPRA ≥ 80%, then to local pancreas and kidney–pancreas candidates with one or more HLA mismatches and CPRA ≥ 80%. If no suitable candidate is identified, regional, then national pancreas and kidney–pancreas candidates with zero HLA mismatches and CPRA ≥ 80% are next, followed by local pancreas and kidney–pancreas candidates with CPRA < 80% regardless of HLA mismatches. At this point, the OPO has a choice in how to continue to allocate the kidney and pancreas, if both organs remain. The OPO can either continue to use the kidney–pancreas/pancreas waiting list, or it can switch to the kidney-alone waiting list to allocate the kidney, and offer the remaining pancreas to pancreas-alone candidates on the kidney–pancreas/pancreas list. This is true regardless of the number of kidneys available. If the OPO continues to allocate from the kidney–pancreas/pancreas list, regional pancreas and kidney–pancreas candidates with one or more HLA mismatches and CPRA ≥ 80% will receive offers next, followed by regional pancreas and kidney–pancreas candidates with CPRA < 80% regardless of HLA mismatches. If no suitable regional candidate is identified, the match run uses the national waiting list, first seeking pancreas and kidney–pancreas candidates with one or more HLA mismatches and CPRA ≥ 80%, then pancreas and kidney–pancreas candidates with CPRA < 80% regardless of HLA mismatches. Finally, islets are allocated locally, regionally and nationally.
Donor age ≥ 50 years or BMI ≥ 30 kg/m2
The allocation sequence for donors who are aged >50 years or whose BMI is >30 kg/m2 is initially identical to the sequence for donors aged ≤50 years with BMI ≤ 30 kg/m2 through the local allocation bins, except for earlier allocation to islet candidates. If no suitable candidate is identified among regional, then national pancreas and kidney–pancreas candidates with zero HLA mismatches and CPRA ≥ 80%, then local pancreas and kidney–pancreas candidates with CPRA < 80% regardless of HLA mismatches, the pancreas is allocated to local, then regional, then national islet candidates. If the pancreas is not allocated to an islet candidate, next in sequence are regional pancreas and kidney-pancreas candidates with one or more HLA mismatches and CPRA ≥ 80%, followed by regional pancreas and kidney–pancreas candidates with CPRA < 80% regardless of HLA mismatches. Next are national pancreas and kidney–pancreas candidates with one or more HLA mismatches and CPRA ≥ 80%, and last in the allocation sequence are national pancreas and kidney–pancreas candidates with CPRA < 80% regardless of HLA mismatches.
Waiting time
Future allocation policy also clarifies waiting time in order to create a uniform national policy. As with current allocation policy, waiting time for pancreas and pancreas islet candidates starts on the date the candidate is listed for the organ. As with current allocation policy, waiting time for simultaneous kidney–pancreas candidates starts on the date the candidate becomes eligible to accrue kidney transplant waiting time. However, in the future this waiting time will start provided the candidate is eligible to accrue simultaneous kidney–pancreas waiting time as per the future allocation policy. As with current allocation policy, candidates continue to accrue waiting time when they are registered as inactive.
Waiting time adjustments
Future allocation policy also clarifies adjustments to waiting time in order to create a uniform national policy. As under current allocation policy, waiting time accrued by pancreas-alone candidates will not be assigned to the listing for simultaneous kidney–pancreas or kidney-alone transplants. For simultaneous kidney–pancreas candidates, waiting time accrued will also be assigned to the listing for kidney-alone and pancreas-alone transplants. Similar to the current allocation policy, in the future waiting time accrued by kidney-alone candidates will be assigned to the listing for pancreas-alone transplant and to the listing for simultaneous kidney–pancreas transplant. However, this waiting time adjustment will occur only if the candidate is eligible to accrue simultaneous kidney-pancreas waiting time under future allocation policy. As under current policy, simultaneous kidney–pancreas candidates who undergo kidney-alone transplants and are listed for a pancreas alone are assigned waiting time based on the earliest of the following dates: listing for pancreas-alone transplant, listing for simultaneous kidney–pancreas transplant or starting to accrue simultaneous kidney–pancreas transplant waiting time. Waiting time accumulated by islet cell candidates will not be assigned to the listing for simultaneous kidney–pancreas or isolated kidney transplant.
Liver Allocation and Distribution
History of liver allocation policy
Early allocation systems in the US relied on candidate location (outpatient setting, hospital ward or intensive care unit [ICU]) as a surrogate for urgency of need for liver transplant. Subjective decisions regarding candidate management played a large role in prioritization and left large groups of candidates (all candidates in ICUs, for example) poorly differentiated. Time on the liver transplant waiting list, considered for further prioritization, was ultimately shown to be a poor predictor of mortality (17).
The Final Rule, adopted by the US Congress in 1998 (effective March 2000) to address these concerns, now guides organ allocation and OPTN policies (18). The Final Rule called for improved equitability by (1) deemphasizing waiting time, (2) focusing on disease severity and mortality risk measured by a continuous and objective scoring system and (3) geographic sharing over as broad a region as feasible. Subsequent changes to the allocation and distribution systems were made to satisfy Final Rule mandates.
To create an objective scoring system, the transplant community first used the Child-Turcotte-Pugh (CTP) score (19). OPTN/UNOS adopted this score in 1998 because of its predictive ability regarding candidates with complications of portal hypertension. Unfortunately, the score lacked the ability to discriminate severity of illness, used subjective variables and was not validated in candidates on the liver transplant waiting list. Its appropriateness as the allocation tool specified by the Final Rule was thus diminished.
Deceased donor liver allocation in the US has been based on model for end-stage liver disease (MELD) and pediatric end-stage liver disease (PELD) scores since February 27, 2002. This urgency-based system prioritizes candidates by mortality risk while they await liver transplant, and is recognized as a major improvement over previous allocation policy.
The MELD score was originally developed as a prognostic tool in cirrhotic candidates undergoing transjugular intrahepatic portosystemic shunts (TIPS) (20,21). The MELD score was subsequently validated in candidates on the liver transplant waiting list, and it predicted 3-month wait-list mortality more accurately than the CTP score (22). Using objective and continuous measures of renal function (creatinine level), coagulopathy (international normalized ratio [INR]) and cholestasis (total bilirubin level), the MELD score achieved a degree of reliability and reproducibility not seen in previous allocation tools, while using widely accessible variables.
To avoid negative scores, each parameter has a lower bound of 1.0 and creatinine has an upper bound of 4.0. Candidates who underwent two or more dialysis treatments during the prior week or who receive continuous renal replacement therapy are assigned a creatinine level of 4.0. For the purpose of allocation, the MELD score ranges from 6 to 40 (22).
The PELD score was derived from a population of children (ages < 18 years) in the Studies of Pediatric Liver Transplantation (SPLIT) database; the score predicts death or ICU admission within 3 months of listing for liver transplant (23). Of 17 potential variables evaluated, the four selected for model inclusion were: INR, total bilirubin level, age < 1 year, and height <2 standard deviations from the mean for age.
Like MELD, each parameter has a lower bound of 1.0. Growth failure is calculated based on age and sex using the Centers for Disease Control and Prevention growth chart. Scores for candidates listed before their first birthday include values assigned for age (<1 year) until the age of 24 months. Unlike the MELD score, the PELD score has no lower or upper bound.
After OPTN adopted the MELD and PELD scores for use in liver transplant allocation policy, the change led to fewer listings of low-MELD candidates, more deceased donor liver transplants and fewer wait-list deaths. Candidates who underwent transplant were sicker with higher MELD scores, yet survival after transplant was not worse than during the period before MELD/PELD adoption (24,25). Compared with before MELD/PELD implementation, the percentage of children undergoing liver transplants increased and the percentage of children dying while on the waiting list decreased (26).
Current liver allocation and distribution in the US
The current system uses a local, regional, national algorithm (Figures 7–9). Highest priority is given to candidates with acute liver failure, designated Status 1A, then to candidates designated Status 1B. Then prioritization, or allocation, is by MELD score for adult candidates (aged ≥ 18 years) and pediatric candidates aged 12–18 years, and by PELD score for candidates aged < 12 years. With rare exception, the local distribution unit is defined as the DSA of an OPO. After being offered to the sickest candidates (Status 1A) locally and regionally (Figure 7, bin 1), then to Status 1B candidates locally and regionally (bin 2), the graft is next offered to candidates with MELD/PELD score ≥ 15 locally (bin 3) and regionally (bin 4). If the liver is not accepted, it is offered to local candidates with MELD/PELD score ≤ 15 (bin 5), regional candidates with MELD/PELD score ≤ 15 (bin 6) and national candidates in the order Status 1A (bin 7), Status 1B (bin 8), and all others prioritized by MELD/PELD score (bin 9). The complete policy (Policy 3.6, Organ Distribution: Allocation of Livers) appears on the OPTN website (27).
Figure 7. Allocation of livers from adult deceased donors.

M/P, model for end-stage liver disease (MELD)/pediatric end-stage liver disease (PELD) score. This figure can be downloaded in color from www.srtr.org.
Figure 9. Allocation of livers from deceased donors aged 0–10 years.

M/P, model for end-stage liver disease (MELD)/pediatric end-stage liver disease (PELD) score. This figure can be downloaded in color from www.srtr.org.
Adult allocation
Adult candidates (aged > 18 years) with acute liver failure and life expectancy of 7 days or less without liver transplant can be designated Status 1A, the highest priority. Acute liver failure is defined as onset of hepatic encephalopathy within 8 weeks of the first symptom of liver disease. Absence of preexisting liver disease is critical to the diagnosis. Adult candidates with acute liver failure must be in the ICU and meet at least one of the following three criteria to be listed: (1) ventilator dependence, (2) requiring dialysis or continuous venous–venous hemodialysis or (3) INR > 2.0. Additionally, three specific diagnosis-driven definitions apply: (1) primary nonfunction of a transplanted liver within 7 days of implantation, defined as either (a) aspartate aminotransferase (AST) ≥ 3000 and one or both of INR ≥ 2.5 and acidosis (arterial pH ≤ 7.30 or venous pH ≤ 7.25 and/or lactate ≥ 4 mMol/L), or (b) anhepatic candidate; (2) hepatic artery thrombosis in a transplanted liver within 7 days of implantation with evidence of severe liver injury defined as for primary nonfunction and (3) acute decompensated Wilson’s disease. For recipients of segmental liver grafts from deceased or living donors, there is no AST requirement. Candidates who do not undergo transplant or die within 7 days require recertification as Status 1A or revert to priority by MELD score.
Adult candidates who do not meet criteria for Status 1A are prioritized by MELD score with highest priority to the highest score. Recertification with updated laboratory tests is required every 7 days for candidates with MELD ≥ 25, monthly with MELD 19–24, every 3 months with MELD 11–18 and yearly with MELD ≤ 10. Select diagnoses receive assigned priority apart from the MELD score calculated from laboratory test results. Candidates diagnosed with hepatocellular carcinoma (HCC) have been assigned such exceptional MELD scores since implementation of the MELD allocation system, but the assigned scores were reduced in April 2003 and again in January 2005. The HCC priority was intended to balance relative risk of progression to a stage beyond that amenable to transplant with risk of wait-list mortality in candidates without HCC (28,29). A diagnosis of HCC can be made by contrast-enhanced cross-sectional imaging with computerized tomography or magnetic resonance imaging with a vascular blush corresponding in the area of suspicion, alpha-fetoprotein >200, arteriogram confirming a tumor, biopsy confirmation or treatment with chemoembolization, radiofrequency, cryo-or chemical ablation of the lesion. Further reduction in HCC assigned priority and revisions to the imaging criteria for HCC have been evaluated, but not yet ratified (28,29).
To obtain a MELD exception, resection must not be an option and the HCC candidate must lack evidence of macrovascular invasion and extrahepatic metastatic lesions. Currently, candidates with stage II HCC and within the Milan criteria (1 lesion 2–5 cm in maximum diameter, 2–3 lesions each < 3 cm) are assigned a MELD score equivalent to a 3-month mortality risk of 15% (MELD 22) and are eligible for an increase equivalent to 10% mortality risk every 3 months (MELD 25, 27, 29, 31, 33, 35, 37, 40) until they undergo liver transplant, their tumor burden progresses beyond the Milan criteria, or they die. HCC exceeding stage II may be eligible for exceptional MELD, but this requires approval by the regional review board (RRB) serving the region where the candidate is registered. Some OPTN/UNOS regions have standing policies allowing MELD exceptions for tumor burden beyond Milan criteria but within UCSF criteria [1 lesion 2–6.5 cm, 2–3 lesions each <4.5 cm and total diameter ≤8 cm (30)]. MELD exceptions for HCC continue to be examined and changes may occur in the future.
Six additional diagnoses are granted standard MELD exception: (1) hepatopulmonary syndrome, (2) portopulmonary syndrome, (3) familial amyloid polyneuropathy, (4) cystic fibrosis, (5) hilar cholangiocarcinoma and (6) primary hyperoxaluria. Candidates meeting specific criteria for the first five of these diagnoses are eligible for a MELD exception schedule similar to that for HCC (Table 2). For candidates with primary hyperoxaluria, the initial MELD exception score is 28 and it increases by the equivalent of 10% morality risk every 3 months (MELD 30, 32, 34, 36, 39, 40). Candidates who do not meet these standard MELD exception criteria or have other complications of liver disease that may warrant additional exceptional priority can have a petition submitted to the RRB on their behalf. Although guidelines were developed for review of these nonstandard MELD exception petitions, the final decision is determined by the RRB. Candidates granted exceptional MELD priority are given the highest of their laboratory-calculated or exceptional MELD score (31).
Table 2.
Recognized diagnosis and criteria for exceptional priority for liver transplant
| Diagnosis | Criteria |
|---|---|
| Acute liver failure |
|
| Hepatocellular carcinoma |
|
| Hepatoblastoma |
|
| Hepatopulmonary syndrome |
|
| Portopulmonary syndrome |
|
| Familial amyloid polyneuropathy |
|
| Cystic fibrosis |
|
| Cholangiocarcinoma |
|
| Primary hyperoxaluria |
|
AGT = alanine glyoxylate aminotransferase; AST = aspartate aminotransferase; FEV1 = forced expiratory volume in 1 s; GFR = glomerular filtration rate; INR = international normalized ratio; MPAP = mean pulmonary artery pressure; UNOS = United Network for Organ Sharing.
Pediatric allocation
Pediatric candidates (aged < 18 years) can be listed as Status 1A if they meet criteria outlined above for adults with acute hepatic failure, hepatic artery thrombosis or acute decompensated Wilson’s disease. One exception to Status 1A listing for pediatric candidates compared with adults is that, as of February 2012, pediatric candidates are not required to be in the ICU to be listed as Status 1A. Pediatric candidates who do not meet Status 1A criteria can be listed as Status 1B if they have a calculated PELD score > 25 (ages 0–11 years) or MELD score > 25 (ages 12–17 years) and one of the following: (1) mechanical ventilator required, (2) gastrointestinal bleeding requiring at least 30 cc/kg of red blood cell replacement within prior 24 h, (3) renal replacement therapy required or (4) Glasgow coma score < 10 within 48 h of listing. Pediatric candidates not meeting Status 1A or 1B criteria are prioritized by PELD score if aged 0–11 years and by MELD score if aged 12–17 years. Recertification with updated laboratory tests is required every 7 days for candidates with MELD/PELD scores ≥ 25, monthly with scores 19–24, every 3 months with scores 11–18 and yearly with scores ≤ 10.
Pediatric candidates with specific diagnoses are also eligible for exceptional PELD or MELD priority. Priority for HCC is identical to that for adults. Pediatric candidates with a urea cycle disorder or organic acidemia are eligible for PELD (age < 12 years) or MELD (age 12–17 years) score of 30; if they do not undergo transplant within 30 days of this exceptional score, they may be listed as Status 1B. Pediatric candidates with nonmetastatic hepatoblastoma can be granted MELD/PELD 30 and may be listed as Status 1B if they do not undergo transplant within 30 days. A change recently approved by the OPTN/UNOS Board of Directors and awaiting implementation allows nonmetastatic hepatoblastoma to be directly granted Status 1B without the 30-day waiting period. Similar to adults, pediatric candidates who do not meet the above criteria may have petitions for nonstandard exceptional MELD priority submitted on their behalf to their RRBs. Like adults, pediatric candidates are granted the highest of laboratory-calculated or RRB-approved exceptional MELD/PELD score.
Distribution
The US distribution system is based on 11 OPTN/UNOS regions. The current regional organization evolved from seven regions in the 1970s, which split as OPTN developed. The regions are subdivided into 59 DSAs. OPOs are nonprofit organizations that recover donor organs; one OPO generally serves each DSA and operates under specific rules. The OPO/DSA boundaries evolved in an arbitrary way following their origin, which was based on historic organ sharing relationships. However, the boundaries rely on state and county lines, and thus have never completely satisfied the Final Rule, which requires that organ distribution be independent of geographic location. When an OPO recovers a liver graft from an adult deceased donor, the liver is generally offered locally first (within the DSA that the OPO serves), then regionally, then nationally (Figure 7), with the exception of the most urgent candidates (Status 1A/B).
In contrast, when an OPO recovers a liver graft from an adolescent deceased donor (age 11–17 years), the liver is offered to local Status 1A pediatric candidates (Figure 8, bin 1), regional Status 1A pediatric candidates (bin 2), local Status 1A adult candidates (bin 3) and regional Status 1A adult candidates (bin 4). Next, the liver is offered to local Status 1B pediatric candidates (bin 5), regional Status 1B pediatric candidates (bin 6) and local and regional candidates aged 0–11 years prioritized by PELD score (bin 7). The liver is then offered to local candidates aged 12–17 years with MELD ≥ 15 (bin 8), local adult candidates with MELD ≥ 15 (bin 9), regional candidates aged 12–17 years with MELD ≥ 15 (bin 10) and regional adult candidates with MELD ≥ 15 (bin 11). It is then offered to local candidates with MELD/PELD <15, first those aged 12–17 years (bin 12), then adults (bin 13), then to regional candidates with MELD/PELD <15, first those aged 12–17 years (bin 14), then adults (bin 15). Finally, the liver is offered to national candidates: Status 1A pediatric candidates (bin 16), Status 1A adults (bin 17), Status 1B pediatric candidates (bin 18), all other candidates aged 0–11 years by PELD score (bin 19), all candidates aged 12–17 years by MELD score (bin 20) and all adults by MELD score (bin 21).
Figure 8. Allocation of livers from deceased donors aged 11–17 years.
M/P, model for end-stage liver disease (MELD)/pediatric end-stage liver disease (PELD) score. This figure can be downloaded in color from www.srtr.org.
Livers from donors aged 0–10 years are distributed more widely than adult and adolescent donor livers because of the difficulty finding size-appropriate grafts for small pediatric candidates. Regional sharing for Status 1A and 1B candidates has been approved by the OPTN/UNOS Board of Directors but is pending implementation. Figure 9 shows a distribution scheme similar to that for livers from adult and adolescent donors shown in Figures 1 and 2. Bin 1 is for local and regional Status 1A pediatric candidates. Livers are next offered to national Status 1A candidates aged 0–11 years (bin 2), local Status 1A adult candidates (bin 3), regional Status 1A adult candidates (bin 4) and local/regional Status 1B pediatric candidates (bin 5). Bin 6 is for all candidates aged 0–11 years prioritized by PELD score. Next, the liver is offered to local candidates aged 12–17 years with MELD ≥ 15 (bin 7), local adults with MELD ≥ 15 (bin 8), regional candidates aged 12–17 years with MELD ≥ 15 (bin 9), regional adults with MELD ≥ 15 (bin 10), local candidates aged 12–17 years with MELD < 15 (bin 11) and adults with MELD < 15 (bin 12). Bins 13 and 14 are for regional candidates with MELD < 15, ages 12–17 years, then adults. Finally, the liver is offered nationally: Status 1A candidates aged 12–17 years (bin 15), Status 1A adults (bin 16), all Status 1B pediatric candidates (bin 17) and candidates prioritized by MELD/PELD (ages 0–11 years, bin 18; ages 12–17 years, bin 19; adults, bin 20).
The Share 15 distribution policy implemented in 2005 was intended to reduce wait-list deaths by directing livers to candidates who would most benefit. Analysis of national data showed that most candidates with a MELD score below 15 did not benefit from liver transplant, and the survival advantage with transplant increases as the MELD score increases (32). This research was the basis for the Share 15 policy, which allocates livers first locally, then regionally, to candidates with MELD/PELD scores > 15, before local candidates with lower scores. The goal is to redirect deceased donor livers to sicker candidates and away from less sick candidates (MELD/PELD < 15), who in general will live longer without a transplant. The transplant community developed the Share 15 policy to prioritize candidates with MELD score ≥ 15. After local and regional Status 1A or 1B candidates, the liver is offered to local candidates with MELD ≥ 15, then to regional candidates with MELD ≥ 15. It is next offered to local candidates with MELD < 15, then regional candidates with MELD < 15, then all candidates nationally.
Future modifications to liver allocation and distribution
Significant attention has been given to evaluating and optimizing the liver allocation and distribution systems over the last 2 years, including a request-for-information document survey (December 2009), a public forum (April 2010), presentations at professional society meetings (2010–2011), updates to the OPTN/UNOS Board of Directors (2010–2011), a concept paper and survey distributed in December 2010, a review of survey results (March 2011), a review of Scientific Registry of Transplant Recipients (SRTR) modeling (July 2011) by the OPTN/UNOS Liver and Intestine Committee, and a public comment period (December 2011), which included a public webinar in October 2011. Through these mechanisms, several modifications to the current system were considered. Proposals that have not been pursued include distribution by concentric circles, distribution by circles defined by population density, distribution by super regions that include several regions and net transplant benefit allocation using urgency- and outcome-based predictive models. Public feedback indicated that MELD-based allocation works well, but small incremental and practical changes in distribution and perhaps allocation may improve the current system (33).
Expansion of the current Share 15 regional policy to a Share 15 National policy received substantial support. Donor livers would be offered to candidates in Status 1A/B or listed with MELD ≥ 15 nationally before being offered to any candidates with MELD < 15 (Table 3). Through reasoning similar to the reasoning behind the Share 15 regional policy, the Share 15 National policy is expected to direct livers toward the sickest candidates and away from candidates whose relative transplant mortality risk versus wait-list mortality risk favors deferring transplant. Liver simulated allocation modeling by SRTR shows that Share 15 National could reduce total deaths (wait-list and posttransplant) by 50 per year, 40 of which would be wait-list deaths. Critics of this potential policy change raised concerns that wait-list mortality for a small subset of candidates with MELD < 15 may not be adequately reflected in MELD scores. One cited subset includes candidates with hyponatremia and low MELD score, whose mortality may be better estimated by the MELDNa score (34).
Table 3.
Outline of share 15 national liver policy

|
Another distribution policy change under active consideration is regional sharing for critically ill candidates with chronic liver disease. Similar to the policy proposal to distribute regionally to Status 1A/1B candidates before any local or regional non-Status 1A/1B candidates, this policy would create regional sharing for candidates with MELD scores ≥ 35. OPTN plans a review of public comments regarding the Share 15 National and Share 35 regional polices in spring 2012, with possible Board consideration in November 2012. If approved by the Board, both changes could be implemented as one algorithm.
Although feedback indicated that MELD score was an excellent allocation tool, some modifications were suggested. The observation that low serum sodium, particularly in candidates with low MELD scores, is a strong predictor of mortality in cirrhotic candidates (35–38) inspired development and validation of the MELDNa score (34). This score incorporates serum sodium into the MELD score and, if implemented as a priority score for liver transplant, is estimated to reduce wait-list mortality by 7% (20). The MELDNa score can be calculated with serum sodium 125–140 mg/dL using the following formula
Criticisms of MELDNa as an allocation tool include concerns about variability of serum sodium over time and about increased risk of complications related to potential rapid correction of serum sodium during liver transplant.
Another potential modification to liver allocation is refitting the MELD coefficients by reconsidering the relative statistical weights and upper and lower bounds of individual MELD parameters. The MELD score and its current coefficients were developed to predict mortality in a cohort of candidates undergoing TIPS. Sharma et al. (39) and more recently Leise et al. (40) found modest improvement in the accuracy of MELD by refitting the model coefficients to a cohort of candidates on the OPTN/UNOS liver transplant waiting list. The latter analysis, using more than 14 000 liver transplant candidates in the development and in the validation data set, found that the coefficients for INR and creatinine in the current MELD were over-weighted. Furthermore, modifying the bounds of creatinine to 0.8–3.0 mg/dL and of INR to 1.0–3.0 improved the model fit (40). Including serum sodium in this Refit-MELD modestly improved model accuracy (40). The OPTN/UNOS Liver and Intestine Committee is currently working with SRTR to optimize the fit of a Refit-MELD. Any proposal for a change to the MELD score would be circulated for public comment no earlier than fall 2012.
For candidates listed for liver transplant, policies for allocating and distributing lifesaving deceased donor liver grafts are of paramount importance. Policy makers are obliged to relentlessly pursue ways to better serve candidates in need with the scarce resources available. The current MELD/PELD-based allocation and distribution system was a tremendous advancement over prior systems and provides the foundation for further evidence-based improvements.
Conclusion
This article describes the historical and current kidney, pancreas, and liver allograft allocation policies and discusses potential future policies. SRTR will conduct analyses for the OPTN/UNOS committees to determine the impact of future policies after these policies are implemented.
Acknowledgments
The authors thank Scientific Registry of Transplant Recipients colleague Nan Booth, MSW, MPH, ELS, for manuscript editing.
This work was conducted under the auspices of the Minneapolis Medical Research Foundation, contractor for the Scientific Registry of Transplant Recipients, as a deliverable under contract no. HHSH250201000018C (US Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation). As a US Government-sponsored work, there are no restrictions on its use. The views expressed herein are those of the authors and not necessarily those of the US Government.
Abbreviations
- AST
aspartate aminotransferase
- BMI
body mass index
- CTP
Child-Turcotte-Pugh
- CPRA
calculated panel-reactive antibody
- DCD
donation after circulatory death
- DSA
donation service area
- ECD
expanded criteria donor
- HCC
hepatocellular carcinoma
- ICU
intensive care unit
- INR
international normalized ratio
- KDPI
kidney donor profile index
- MELD
model for end-stage liver disease
- NOTA
National Organ Transplant Act
- OPO
organ procurement organization
- OPTN
Organ Procurement and Transplantation Network
- PELD
pediatric end-stage liver disease
- PRA
panel-reactive antibody
- RRB
regional review board
- SCD
standard criteria donor
- SPLIT
Studies of Pediatric Liver Transplantation
- TIPS
transjugular intrahepatic portosystemic shunts
- UNOS
United Network for Organ Sharing
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation: By virtue of employment at or affiliation with a transplant program or an organization with an interest in transplant program performance, any author of this manuscript could be perceived to have a conflict of interest. Beyond that, no author has any conflict of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Organ Procurement and Transplantation Network. [Accessed June 22, 2012];National waiting list data. Available at: http://optn.transplant.hrsa.gov/latestData/step2.asp.
- 2.Roberts JP, Wolfe RA, Bragg-Gresham JL, et al. Effect of changing the priority for HLA matching on the rates and outcomes of kidney transplantation in minority groups. N Engl J Med. 2004;350:545–551. doi: 10.1056/NEJMoa025056. [DOI] [PubMed] [Google Scholar]
- 3.Ashby VB, Port FK, Wolfe RA, et al. Transplanting kidneys without points for HLA-B matching: Consequences of the policy change. Am J Transplant. 2011;11:1712–1718. doi: 10.1111/j.1600-6143.2011.03606.x. [DOI] [PubMed] [Google Scholar]
- 4.Hall EC, Massie AB, James NT, et al. Effect of eliminating priority points for HLA-B matching on racial disparities in kidney transplant rates. Am J Kidney Dis. 2011;58:813–816. doi: 10.1053/j.ajkd.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 5.Organ Procurement and Transplantation Network. [Accessed February 23, 2012];Allocation of deceased kidneys. 2011 Available at: http://optn.transplant.hrsa.gov/PoliciesandBylaws2/policies/pdfs/policy_7.pdf.
- 6.Ojo AO, Hanson JA, Meier-Kriesche H, et al. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol. 2001;12:589–597. doi: 10.1681/ASN.V123589. [DOI] [PubMed] [Google Scholar]
- 7.Stratta RJ, Rohr MS, Sundberg AK, et al. Increased kidney transplantation utilizing expanded criteria deceased organ donors with results comparable to standard criteria donor transplant. Ann Surg. 2004;239:688–697. doi: 10.1097/01.sla.0000124296.46712.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grams ME, Womer KL, Ugarte RM, Desai NM, Montgomery RA, Segev DL. Listing for expanded criteria donor kidneys in older adults and those with predicted benefit. Am J Transplant. 2010;10:802–809. doi: 10.1111/j.1600-6143.2010.03020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman J, Bock A, Dussol B, et al. Follow-up after renal transplantation with organs from donors after cardiac death. Transpl Int. 2006;19:715–719. doi: 10.1111/j.1432-2277.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- 10.Whiting JF, Delmonico F, Morrissey P, et al. Clinical results of an organ procurement organization effort to increase utilization of donors after cardiac death. Transplantation. 2006;81:1368–1371. doi: 10.1097/01.tp.0000179641.82031.ea. [DOI] [PubMed] [Google Scholar]
- 11.Saidi RF, Elias N, Kawai T, et al. Outcome of kidney transplantation using expanded criteria donors and donation after cardiac death kidneys: Realities and costs. Am J Transplant. 2007;7:2769–2774. doi: 10.1111/j.1600-6143.2007.01993.x. [DOI] [PubMed] [Google Scholar]
- 12.Colvin-Adams M, Valapour M, Hertz M, et al. Lung and heart allocation in the United States. Am J Transplant. 2012;12:3213–3234. doi: 10.1111/j.1600-6143.2012.04258.x. [DOI] [PubMed] [Google Scholar]
- 13.Zachary AA, Braun WE. Calculation of a predictive value for transplantation. Transplantation. 1985;39:316–318. doi: 10.1097/00007890-198503000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Zachary AA, Steinberg AG. Statistical analysis and applications of HLA population data. In: Rose NR, de Marcario EC, Folds JD, Lane HC, Nakamura RM, editors. Manual of Clinical Laboratory Immunology. 5. Washington, DC: ASM Press; 1997. pp. 132–140. [Google Scholar]
- 15.Stegall MD, Dean PG, Sung R, et al. The rationale for the new deceased donor pancreas allocation schema. Transplantation. 2007;83:1156–1161. doi: 10.1097/01.tp.0000261104.27113.05. [DOI] [PubMed] [Google Scholar]
- 16.Organ Procurement and Transplantation Network. [Accessed December 9, 2011];3.8 Pancreas Allocation. 2010 Available at: http://optn.transplant.hrsa.gov/policiesAndBylaws/policies.asp.
- 17.Freeman RB, Jr, Edwards EB. Liver transplant waiting time does not correlate with waiting list mortality: implications for liver allocation policy. Liver Transpl. 2000;6:543–552. doi: 10.1053/jlts.2000.9744. [DOI] [PubMed] [Google Scholar]
- 18.Organ Procurement and Transplantation Network–HRSA. Final rule with comment period. Fed Regist. 1998;63:16296–16338. [PubMed] [Google Scholar]
- 19.Christensen E, Schlichting P, Fauerholdt L, et al. Prognostic value of Child-Turcotte criteria in medically treated cirrhosis. Hepatology. 1984;4:430–435. doi: 10.1002/hep.1840040313. [DOI] [PubMed] [Google Scholar]
- 20.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 21.Freeman RB, Jr, Wiesner RH, Harper A, et al. The new liver allocation system: Moving toward evidence-based transplantation policy. Liver Transpl. 2002;8:851–858. doi: 10.1053/jlts.2002.35927. [DOI] [PubMed] [Google Scholar]
- 22.Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 23.McDiarmid SV, Anand R, Lindblad AS. Development of a pediatric end-stage liver disease score to predict poor outcome in children awaiting liver transplantation. Transplantation. 2002;74:173–181. doi: 10.1097/00007890-200207270-00006. [DOI] [PubMed] [Google Scholar]
- 24.Freeman RB., Jr MELD/PELD: One year later. Transplant Proc. 2003;35:2425–2427. doi: 10.1016/j.transproceed.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Freeman RB., Jr Model for end-stage liver disease (MELD) for liver allocation: a 5-year score card. Hepatology. 2008;47:1052–1057. doi: 10.1002/hep.22135. [DOI] [PubMed] [Google Scholar]
- 26.McDiarmid SV, Merion RM, Dykstra DM, Harper AM. Selection of pediatric candidates under the PELD system. Liver Transpl. 2004;10(Suppl 2):S23–S30. doi: 10.1002/lt.20272. [DOI] [PubMed] [Google Scholar]
- 27.HRSA/OPTN. [Accessed February 22, 2012];Policy management, policies. Available at: http://optn.transplant.hrsa.gov/policiesAndBylaws/policies.asp.
- 28.Pomfret EA, Washburn K, Wald C, et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transpl. 2010;16:262–278. doi: 10.1002/lt.21999. [DOI] [PubMed] [Google Scholar]
- 29.Washburn K, Edwards E, Harper A, Freeman R. Hepatocellular carcinoma patients are advantaged in the current liver transplant allocation system. Am J Transplant. 2010;10:1643–1648. doi: 10.1111/j.1600-6143.2010.03127.x. [DOI] [PubMed] [Google Scholar]
- 30.Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 31.Wiesner R, Lake JR, Freeman RB, Gish RG. Model for end-stage liver disease (MELD) exception guidelines. Liver Transpl. 2006;12(Suppl 3):S85–S87. doi: 10.1002/lt.20961. [DOI] [PubMed] [Google Scholar]
- 32.Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant. 2005;5:307–313. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 33.Washburn K, Pomfret E, Roberts J. Liver allocation and distribution: Possible next steps. Liver Transpl. 2011;17:1005–1012. doi: 10.1002/lt.22349. [DOI] [PubMed] [Google Scholar]
- 34.Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biggins SW, Kim WR, Terrault NA, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130:1652–1660. doi: 10.1053/j.gastro.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 36.Biggins SW, Rodriguez HJ, Bacchetti P, Bass NM, Roberts JP, Terrault NA. Serum sodium predicts mortality in patients listed for liver transplantation. Hepatology. 2005;41:32–39. doi: 10.1002/hep.20517. [DOI] [PubMed] [Google Scholar]
- 37.Heuman DM, Abou-Assi SG, Habib A, et al. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology. 2004;40:802–810. doi: 10.1002/hep.20405. [DOI] [PubMed] [Google Scholar]
- 38.Ruf AE, Kremers WK, Chavez LL, Descalzi VI, Podesta LG, Villamil FG. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl. 2005;11:336–343. doi: 10.1002/lt.20329. [DOI] [PubMed] [Google Scholar]
- 39.Sharma P, Schaubel DE, Sima CS, Merion RM, Lok AS. Re-weighting the model for end-stage liver disease score components. Gastroenterology. 2008;135:1575–1581. doi: 10.1053/j.gastro.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Leise MD, Kim WR, Kremers WK, Larson JJ, Benson JT, Therneau TM. A revised model for end-stage liver disease optimizes prediction of mortality among patients awaiting liver transplantation. Gastroenterology. 2011;140:1952–1960. doi: 10.1053/j.gastro.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]