Abstract
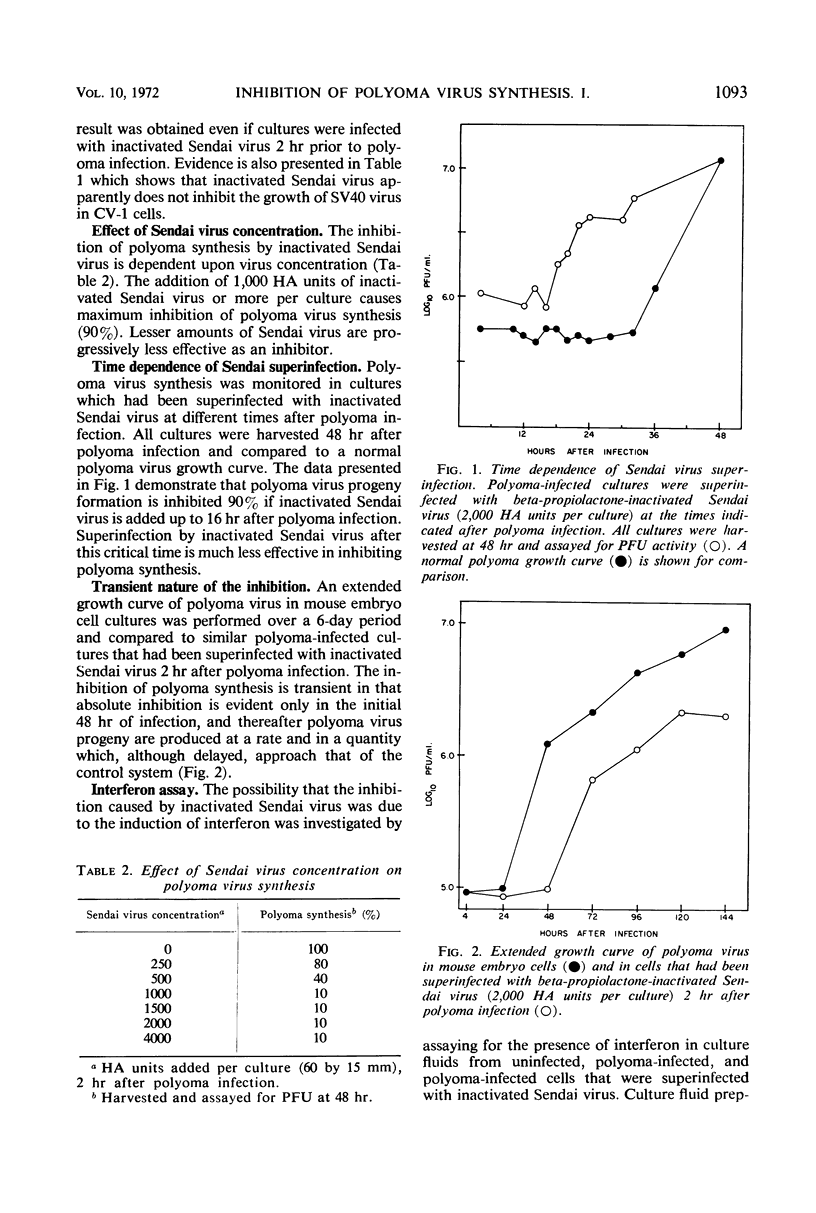
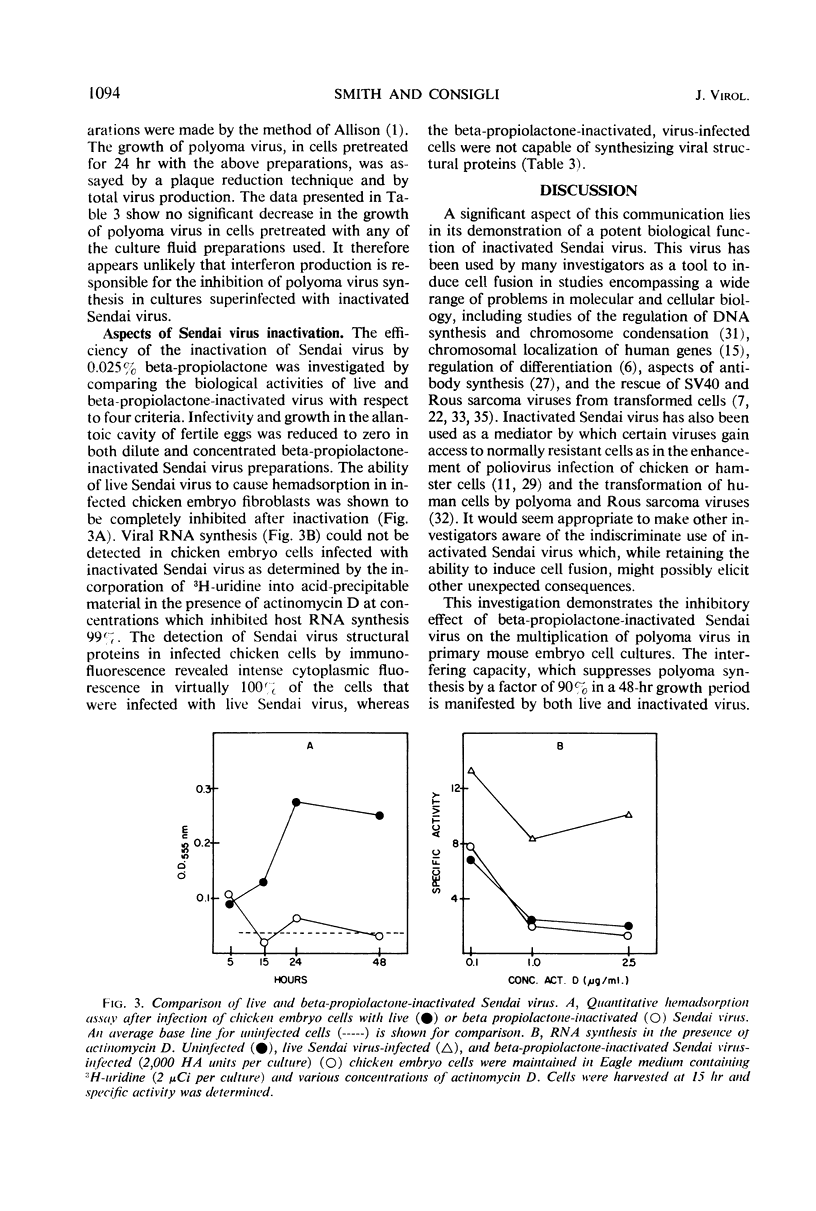
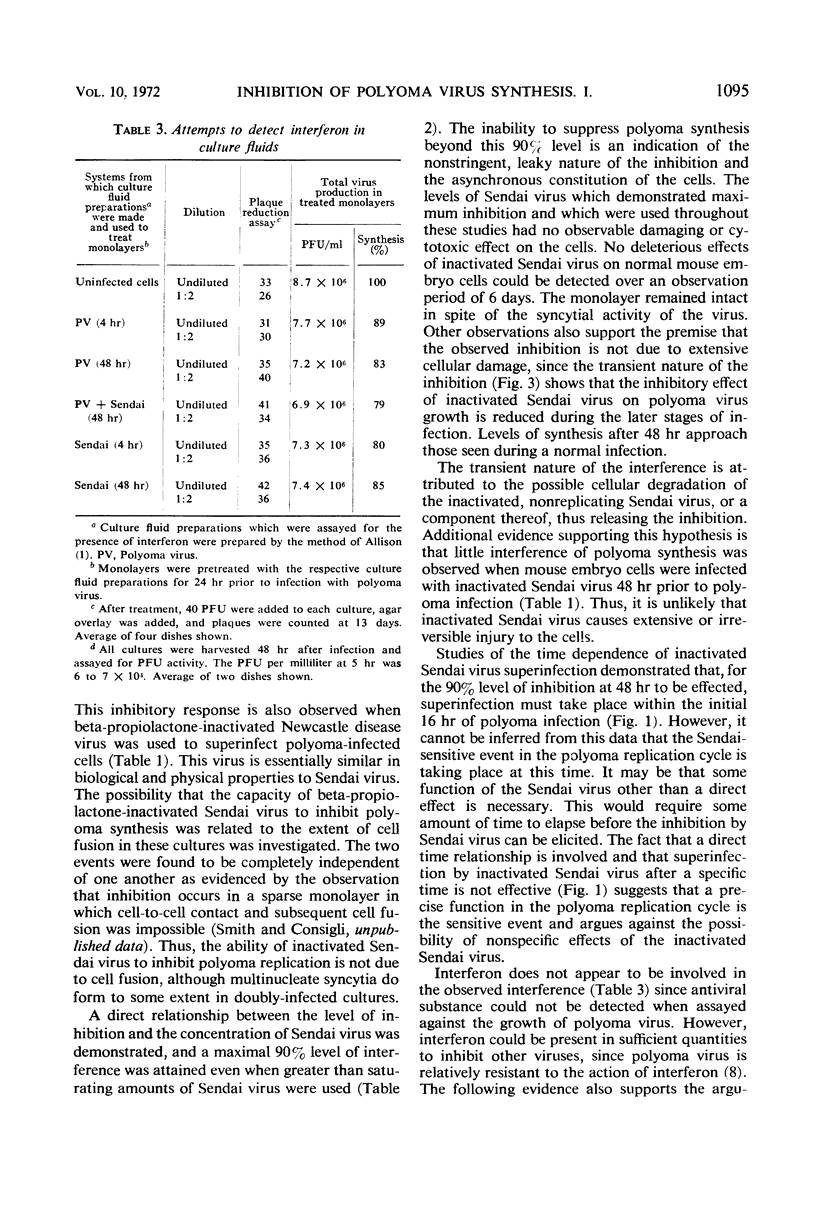
Superinfection of polyoma virus-infected mouse embryo cells by beta-propiolactone-inactivated Sendai virus resulted in a 90% inhibition of the synthesis of infectious polyoma progeny. The interference is dependent upon the time of superinfection and the concentration of the inactivated virus. The inhibition of polyoma virus synthesis is transient in nature since normal synthesis of polyoma progeny virus is seen upon prolonged incubation. Interferon does not appear to be implicated in the interference. Various aspects of the biological and synthetic capabilities of beta-propiolactone-inactivated Sendai virus are also described.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C. Interference with, and interferon production by, polyoma virus. Virology. 1961 Sep;15:47–51. doi: 10.1016/0042-6822(61)90075-7. [DOI] [PubMed] [Google Scholar]
- Aubertin A. M., Guir J., Kirn A. The inhibition of vaccinia virus DNA synthesis in KB cells infected with frog virus 3. J Gen Virol. 1970 Aug;8(2):105–111. doi: 10.1099/0022-1317-8-2-105. [DOI] [PubMed] [Google Scholar]
- Basilico C., Wang R. Susceptibility to superinfection of hybrids between polyoma "transformed" BHK and "normal" 3T3 cells. Nat New Biol. 1971 Mar 24;230(12):105–107. doi: 10.1038/newbio230105a0. [DOI] [PubMed] [Google Scholar]
- Consigli R. A., Minocha H. C., Abo-Ahmed H. Multiplication of polyoma virus. II. Source of constituents for viral deoxyribonucleic acid and protein synthesis. J Bacteriol. 1966 Sep;92(3):789–791. doi: 10.1128/jb.92.3.789-791.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubbs D. R., Kit S. Isolation of defective lysogens from Simian virus 40-transformed mouse kidney cultures. J Virol. 1968 Nov;2(11):1272–1282. doi: 10.1128/jvi.2.11.1272-1282.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R., Johnson T. Interferon-sensitivity of the enhanced incorporation of thymidine into cellular DNA induced by polyoma virus. Virology. 1970 Oct;42(2):368–374. doi: 10.1016/0042-6822(70)90280-1. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Nutrition needs of mammalian cells in tissue culture. Science. 1955 Sep 16;122(3168):501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- Enders J. F., Holloway A., Grogan E. A. Replication of poliovirus I in chick embryo and hamster cells exposed to sendai virus. Proc Natl Acad Sci U S A. 1967 Mar;57(3):637–644. doi: 10.1073/pnas.57.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINTER N. B. QUANTITATIVE HAEMADSORPTION, A NEW ASSAY TECHNIQUE. I. ASSAY OF INTERFERON. Virology. 1964 Dec;24:589–597. doi: 10.1016/0042-6822(64)90212-0. [DOI] [PubMed] [Google Scholar]
- Fuchs P., Kohn A. Nature of transient inhibition of deoxyribonucleic acid synthesis in HeLa cells by parainfluenza virus 1 (Sendai). J Virol. 1971 Nov;8(5):695–700. doi: 10.1128/jvi.8.5.695-700.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber P. Studies on the transfer of subviral infectivity from SV40-induced hamster tumor cells to indicator cells. Virology. 1966 Apr;28(4):501–509. doi: 10.1016/0042-6822(66)90234-0. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J. INHIBITION OF HOST CELL MACROMOLECULAR SYNTHESIS BY HIGH MULTIPLICITIES OF POLIOVIRUS UNDER CONDITIONS PREVENTING VIRUS SYNTHESIS. J Mol Biol. 1964 Apr;8:574–581. doi: 10.1016/s0022-2836(64)80012-7. [DOI] [PubMed] [Google Scholar]
- Hatano M., Yoshie C. Interfering action of myxovirus on polyoma virus in primary mouse embryo cell cultures. Arch Gesamte Virusforsch. 1967;20(3):314–326. doi: 10.1007/BF01241951. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Inhibition of cellular RNA synthesis by nonreplicating vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1579–1584. doi: 10.1073/pnas.54.6.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare G. P., Consigli R. A. Cytologic studies in polyoma virus-infected mouse embryo cells. Am J Vet Res. 1967 Sep;28(126):1527–1536. [PubMed] [Google Scholar]
- Khoobyarian N., Fischinger P. J. Role of heated adenovirus 2 in viral interference. Proc Soc Exp Biol Med. 1965 Nov;120(2):533–538. doi: 10.3181/00379727-120-30582. [DOI] [PubMed] [Google Scholar]
- Knowles B. B., Jensen F. C., Steplewski Z., Koprowski H. Rescue of infectious SV40 after fusion between dfferent SV 40-transformed cells. Proc Natl Acad Sci U S A. 1968 Sep;61(1):42–45. doi: 10.1073/pnas.61.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H., Jensen F. C., Steplewski Z. Activation of production of infectious tumor virus SV40 in heterokaryon cultures. Proc Natl Acad Sci U S A. 1967 Jul;58(1):127–133. doi: 10.1073/pnas.58.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levine A. J., Ginsberg H. S. Mechanism by which fiber antigen inhibits multiplication of type 5 adenovirus. J Virol. 1967 Aug;1(4):747–757. doi: 10.1128/jvi.1.4.747-757.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuslan B. R., Smith W. R. Deoxyribonucleic acid synthesis in FV-3-infected mammalian cells. J Virol. 1968 Oct;2(10):1006–1015. doi: 10.1128/jvi.2.10.1006-1015.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekler L. B., Shlyankevich M. A., Shevliaghyn V. J. Sendai virus RNA as messenger RNA determining the synthesis of early virus specific proteins. Arch Gesamte Virusforsch. 1970;30(4):309–315. doi: 10.1007/BF01258361. [DOI] [PubMed] [Google Scholar]
- Mohit B., Fan K. Hybrid cell line from a cloned immunoglobulin-producing mouse myeloma and a nonproducing mouse lymphoma. Science. 1971 Jan 8;171(3966):75–77. doi: 10.1126/science.171.3966.75. [DOI] [PubMed] [Google Scholar]
- Moss B. Inhibition of HeLa cell protein synthesis by the vaccinia virion. J Virol. 1968 Oct;2(10):1028–1037. doi: 10.1128/jvi.2.10.1028-1037.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff J. M., Enders J. F. Poliovirus replication and cytopathogenicity in monolayer hamster cell cultures fused with beta propiolactone-inactivated Sendai virus. Proc Soc Exp Biol Med. 1968 Jan;127(1):260–267. doi: 10.3181/00379727-127-32668. [DOI] [PubMed] [Google Scholar]
- POHJANPELTO P., COOPER P. D. INTERFERENCE BETWEEN POLIOVIRUSES INDUCED BY STRAINS THAT CANNOT MULTIPLY. Virology. 1965 Mar;25:350–357. doi: 10.1016/0042-6822(65)90054-1. [DOI] [PubMed] [Google Scholar]
- Rao P. N., Johnson R. T. Mammalian cell fusion: studies on the regulation of DNA synthesis and mitosis. Nature. 1970 Jan 10;225(5228):159–164. doi: 10.1038/225159a0. [DOI] [PubMed] [Google Scholar]
- Shevliaghyn V. J., Karazas N. V. Transformation of human cells by polyoma and Rous sarcoma viruses mediated by inactivated Sendai virus. Int J Cancer. 1970 Sep 15;6(2):234–244. doi: 10.1002/ijc.2910060210. [DOI] [PubMed] [Google Scholar]
- Svoboda J., Dourmashkin R. Rescue of Rous sarcoma virus from virogenic mammalian cells associated with chicken cells and treated with Sendai virus. J Gen Virol. 1969 Jun;4(4):523–529. doi: 10.1099/0022-1317-4-4-523. [DOI] [PubMed] [Google Scholar]
- Vilaginès R., McAuslan B. R. Interference with viral messenger RNA and DNA synthesis by superinfection with a heterologous deoxyvirus. Virology. 1970 Dec;42(4):1043–1053. doi: 10.1016/0042-6822(70)90352-1. [DOI] [PubMed] [Google Scholar]
- Watkins J. F., Dulbecco R. Production of SV40 virus in heterokaryons of transformed and susceptible cells. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1396–1403. doi: 10.1073/pnas.58.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W., Youngner J. S. Inhibition of protein synthesis in L cells infected with vesicular stomatitis virus. J Virol. 1972 Jan;9(1):85–89. doi: 10.1128/jvi.9.1.85-89.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaoi Y., Amano M. Inhibitory effect of ultraviolet-inactivated vesicular stomatitis virus on initiation o DNA synthesis in cultured chick embryo cells. J Gen Virol. 1970 Oct;9(1):69–75. doi: 10.1099/0022-1317-9-1-69. [DOI] [PubMed] [Google Scholar]


