Abstract
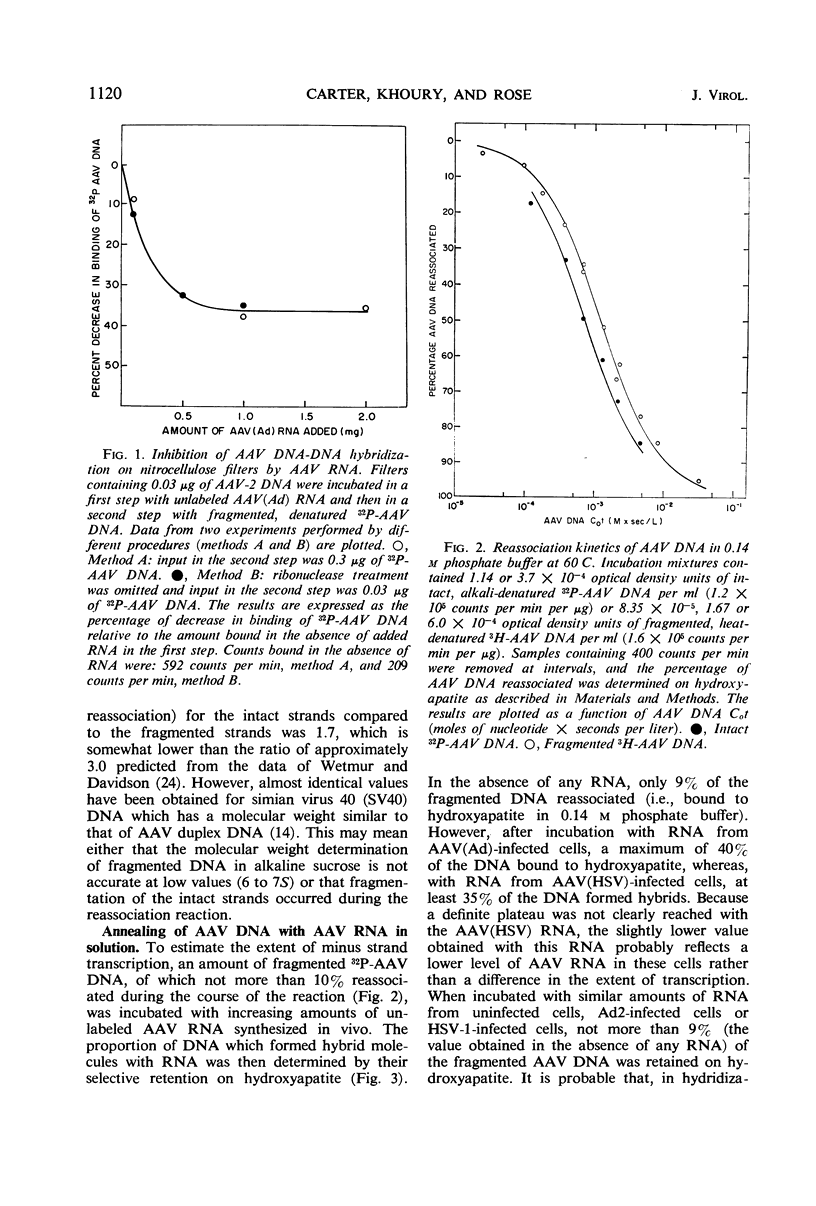
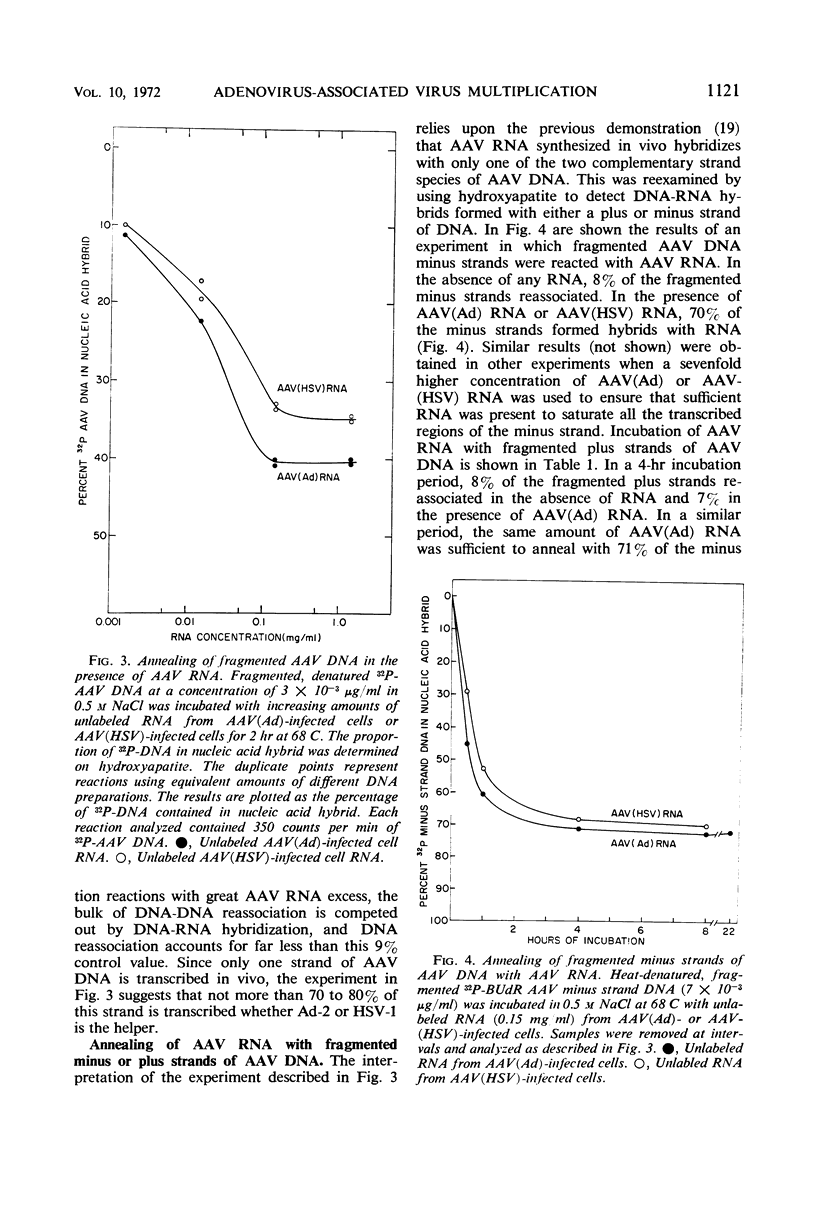
Nucleic acid hybridization procedures were used to measure the extent of transcription of adenovirus-associated virus (AAV) deoxyribonucleic acid (DNA) in KB cells in the presence of either adenovirus or herpes simplex virus as the helper. Annealing of AAV ribonucleic acid to AAV DNA was monitored by a hybridization inhibition assay on nitrocellulose filters or by hydroxyapatite chromatography. These experiments confirmed the previous observation that, in the presence of either type of helper virus, only one strand of AAV DNA (the thymidine-rich or “minus” strand) is transcribed in vivo. However, it was found that only 70 to 80% of this strand appears to be transcribed in vivo. Furthermore, studies with minus strands employing hydroxyapatite chromatography and nuclease S1, which specifically degrades single-stranded DNA, indicated that up to 20% of the minus strand is self-complementary. It seems likely that these self-complementary sequences account for the bulk of that portion of the minus strand (20 to 30%) which is not transcribed in vivo.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATCHISON R. W., CASTO B. C., HAMMON W. M. ADENOVIRUS-ASSOCIATED DEFECTIVE VIRUS PARTICLES. Science. 1965 Aug 13;149(3685):754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- Ando T. A nuclease specific for heat-denatured DNA in isolated from a product of Aspergillus oryzae. Biochim Biophys Acta. 1966 Jan 18;114(1):158–168. doi: 10.1016/0005-2787(66)90263-2. [DOI] [PubMed] [Google Scholar]
- Atchison R. W. The role of herpesviruses in adenovirus-associated virus replication in vitro. Virology. 1970 Sep;42(1):155–162. doi: 10.1016/0042-6822(70)90248-5. [DOI] [PubMed] [Google Scholar]
- BAUTZ E. K., HALL B. D. The isolation of T4-specific RNA on a DNA-cellulose column. Proc Natl Acad Sci U S A. 1962 Mar 15;48:400–408. doi: 10.1073/pnas.48.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns K. I., Rose J. A. Evidence for a single-stranded adenovirus-associated virus genome: isolation and separation of complementary single strands. J Virol. 1970 Jun;5(6):693–699. doi: 10.1128/jvi.5.6.693-699.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacklow N. R., Hoggan M. D., McClanahan M. S. Adenovirus-associated viruses: enhancement by human herpesviruses. Proc Soc Exp Biol Med. 1970 Sep;134(4):952–954. doi: 10.3181/00379727-134-34919. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Carter B. J., Rose J. A. Adenovirus-associated virus multiplication. 8. Analysis of in vivo transcription induced by complete or partial helper viruses. J Virol. 1972 Jul;10(1):9–16. doi: 10.1128/jvi.10.1.9-16.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon C. F., Berry K. W., Rose J. A. A unique form of terminal redundancy in adenovirus DNA molecules. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2391–2395. doi: 10.1073/pnas.69.9.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- Hoggan M. D., Blacklow N. R., Rowe W. P. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1467–1474. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob E., Hofschneider P. H. Replication of the single-stranded DNA bacteriophage M13: messenger RNA synthesis directed by M13 replicative form DNA. J Mol Biol. 1969 Dec 14;46(2):359–363. doi: 10.1016/0022-2836(69)90429-x. [DOI] [PubMed] [Google Scholar]
- Martin M. A., Axelrod D. Polyoma virus gene activity during lytic infection and in transformed animal cells. Science. 1969 Apr 4;164(3875):68–70. doi: 10.1126/science.164.3875.68. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Green M., Piña M., Melnick J. L. Physicochemical characterization of adeno-associated satellite virus type 4 and its nucleic acid. J Virol. 1967 Oct;1(5):980–987. doi: 10.1128/jvi.1.5.980-987.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Berns K. I., Hoggan M. D., Koczot F. J. Evidence for a single-stranded adenovirus-associated virus genome: formation of a DNA density hybrid on release of viral DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):863–869. doi: 10.1073/pnas.64.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Hoggan M. D., Shatkin A. J. Nucleic acid from an adeno-associated virus: chemical and physical studies. Proc Natl Acad Sci U S A. 1966 Jul;56(1):86–92. doi: 10.1073/pnas.56.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Koczot F. Adenovirus-associated virus multiplication. VI. Base compostion of the deoxyribonucleic acid strand species and strand-specific in vivo transcription. J Virol. 1971 Nov;8(5):771–777. doi: 10.1128/jvi.8.5.771-777.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Koczot F. Adenovirus-associated virus multiplication. VII. Helper requirement for viral deoxyribonucleic acid and ribonucleic acid synthesis. J Virol. 1972 Jul;10(1):1–8. doi: 10.1128/jvi.10.1.1-8.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton W. D. A crude nuclease preparation suitable for use in DNA reassociation experiments. Biochim Biophys Acta. 1971 Jul 29;240(4):522–531. doi: 10.1016/0005-2787(71)90709-x. [DOI] [PubMed] [Google Scholar]
- Warmaar S. O., Cohen J. A. A quantitative assay for DNA-DNA hybrids using membrane filters. Biochem Biophys Res Commun. 1966 Aug 23;24(4):554–558. doi: 10.1016/0006-291x(66)90356-1. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]



