Abstract
Background
The medial prefrontal cortex (mPFC) serves major executive functions. mPFC output to subcortical brain areas such as the amygdala controls emotional processing and plays an important role in fear extinction. Impaired mPFC function correlates with extinction deficits in anxiety disorders such as PTSD and with cognitive decision-making deficits in neuropsychiatric disorders and persistent pain. Controlling mPFC output is a desirable therapeutic goal in neuropsychiatric disorders but functional differences of cell types (pyramidal cells and interneurons) and regions (infralimbic and prelimbic) represent a challenge. This electrophysiological study used optogenetics for the cell- and region-specific modulation of mPFC pyramidal output in the intact anesthetized animal.
Results
Extracellular single-unit recordings were made from infralimbic (IL) pyramidal cells, IL interneurons and prelimbic (PL) pyramidal cells 2–3 weeks after intra-IL injection of a viral vector encoding channel rhodopsin 2 (ChR2) under the control of the CaMKII promoter (rAAV5/CaMKIIa-ChR2(H134R)-EYFP) or a control vector that lacked the ChR2 sequence (rAAV5/CaMKIIa-EYFP). Optical stimulation with laser-generated blue light pulses delivered through an optical fiber to the IL increased spontaneous and evoked action potential firing of ChR2 expressing IL pyramidal cells but had no effect on IL interneurons that were distinguished from pyramidal cells based on their higher firing rate and shorter spike duration. Optical activation of IL pyramidal cells also inhibited PL pyramidal cells, suggesting that IL output controls PL output. The effects were light intensity-dependent and reversible. Confocal microscopy confirmed ChR2-EYFP or control vector expression in mPFC pyramidal cells but not in GABAergic cells.
Conclusions
The novelty of our study is the analysis of optogenetic effects on background and evoked activity of defined cell types in different mPFC regions. The electrophysiological in vivo results directly demonstrate the optogenetic modulation of mPFC activity in a region- and cell type-specific manner, which is significant in conditions of impaired mPFC output.
Keywords: Optogenetics, Medial prefrontal cortex, Infralimbic, Prelimbic, Pyramidal cells, Single-unit recording, Electrophysiology, Cognitive, Emotion
Background
The medial prefrontal cortex (mPFC) serves executive functions that are essential for selecting appropriate and inhibiting inappropriate actions. Prefrontal cortex dysfunction has been identified as a key neurobiological correlate of cognitive inflexibility and behavioral disinhibition associated with neuropsychiatric disorders such as drug addiction, obsessive-compulsive disorder, anxiety disorders and schizophrenia [1-8]. The important role of the mPFC in top-down cognitive control mechanisms is particularly well documented in experimental models of behavioral “extinction” of negative emotions [9-12].
The infralimbic region of the mPFC inhibits amygdala output to suppress (“extinguish”) aversive behaviors [10,13-18]. Increased thickness and activity of the mPFC correlate with successful extinction of negative emotions [19-22] whereas decreased activity has been implicated in cognitive control deficits in models of extinction [23-26] and behavioral disinhibition [2]. The concept of behavioral extinction forms the neurobiological basis for certain cognitive behavioral therapies in emotional-affective disorders [11,27] and chronic pain [28]. Extinction deficits have been proposed as a mechanism of the persistence of pain and its negative affective dimension [29]. Abnormalities in the mPFC are found in human pain patients and in animal pain models [30,31]. Our studies showed that amygdala-driven abnormal inhibition and decreased output of mPFC pyramidal cells contribute to pain-related impaired decision-making [32]. The underlying mechanism is feedforward inhibition of mPFC pyramidal cell output [32-34].
Therefore, increasing mPFC output to engage cognitive control systems is a desirable goal in conditions and disorders that are associated with decreased mPFC activity, such as anxiety [35-37], depression [38,39] and pain [32]. Differential roles of infralimbic and prelimbic mPFC regions [23], diversity of excitatory and inhibitory neurons [40], and complex pharmacology and neurochemistry [41] present a challenge for the selective control of mPFC activity. Recently developed optogenetic tools allow the activation or inhibition of distinct neuronal cell types within defined brain regions [42,43] but few studies have analyzed the effects of optogenetic manipulation of mPFC function. Optogenetic activation, but not inhibition, of excitatory mPFC cells inhibited unconditioned social exploratory behavior and fear conditioning [44] and had antidepressant-like effects [38]. Activation of GABAergic mPFC interneurons impaired operant delayed alternation performance [45] but rescued impaired social behavior due to abnormal mPFC excitation [44]. The neuronal effects of optical activation were confirmed using patch-clamp recordings in brain slices [44,45] and activity markers [38]. Electrophysiological effects of optogenetic manipulations in the mPFC on neuronal activity in vivo were addressed only recently in studies that recorded multiunit activity [44,46].
The novelty of the present study is the use of single-unit recordings in the intact animal to determine the effect of optical activation of infralimbic pyramidal output on spontaneous and evoked responses of infralimbic pyramidal cells, infralimbic interneurons and prelimbic pyramidal cells. This work is significant because infralimbic output to subcortical brain areas such as the amygdala plays a key role in fear extinction that might be utilized in the treatment of anxiety disorders such as PTSD [17,23,47].
Methods
Animals
Adult male Sprague Dawley rats (250–350 g) were housed in standard plastic cages (40 × 20 cm) in a temperature-controlled room and maintained on a 12-h day/night cycle. Water and food were available without restriction. All experimental procedures were approved by the Institutional Animal Care and Use Committee at The University of Texas Medical Branch and conform to the guidelines of the International Association for the Study of Pain and of the National Institutes of Health.
Optogenetics
rAAV5/CaMKIIa-ChR2(H134R)-EYFP and a control vector that lacked the ChR2 sequence (rAAV5/CaMKIIa-EYFP) from the Karl Deisseroth laboratory [48-50] were produced by the Vector Core Facility at The University of North Carolina, Chapel Hill. Rats were anesthetized with 2–4% isoflurane, and a small unilateral craniotomy was performed. Using a 10 μl Hamilton syringe, 1 μl virus suspension was delivered at a rate of 0.1 μl/min into the infralimbic cortex (stereotaxic coordinates: 3.2 mm anterior to bregma; 0.8 mm lateral to midline; depth, 5.0-5.5).
Electrophysiology
Anesthesia and surgery
2–3 weeks after virus injection the animal was prepared for electrophysiological recordings as described in detail in our previous studies [32,51]. The animal was anesthetized with pentobarbital sodium throughout the experiment (induction, 50 mg/kg, ip; maintenance, 15 mg· kg-1·h-1, iv), paralyzed with pancuronium (0.3 mg/h, iv) and artificially ventilated (3–3.5 ml; 55–65 strokes/min). End-tidal CO2 levels (kept at 4.0%), heart rate, and ECG pattern were monitored continuously. Core body temperature was maintained at 37°C by means of a homeothermic blanket system. These measures ensured a constant internal state of body functions. The animal was mounted in a stereotaxic frame (David Kopf Instruments), and a small unilateral craniotomy was performed to allow the insertion of the recording electrode and optical fiber.
Single-unit recording
Extracellular recordings were made from single neurons in the prelimbic and infralimbic part of the mPFC with glass-insulated carbon filament electrodes (4–6 MΩ) using the following stereotaxic coordinates: 3.0-3.2 mm anterior to bregma; 0.5–1.0 mm lateral to midline; depth, 3.0–4.6 mm (prelimbic) and 4.6-5.6 mm (infralimbic). Recorded signals were amplified, band-pass filtered (300 Hz – 3 kHz), displayed on analog and digital storage oscilloscopes, fed into a window discriminator (World Precision Instruments), digitized (1401 plus interface, Cambridge Electronic Design), and recorded on a Pentium 4 personal computer (PC) using Spike2 software (version 4; Cambridge Electronic Design) to analyze single-unit activity.
Identification of mPFC neurons
An individual neuron was identified by the configuration, shape, and height of the recorded action potentials (spikes) that occurred spontaneously (background activity) or in response to mechanical (tissue compression) search stimuli (evoked responses). mPFC pyramidal neurons can be distinguished from interneurons based on their broader action potential waveform (peak-to-valley >500 μs) and lower baseline discharge rate (<10 Hz) as described before [32,51]. Spikes were detected and recorded based on the waveform signal that crossed a trigger level and matched a pre-set shape or template, which was created for the individual neuron at the beginning of the recording period using Spike2 software. Only those neurons were included in this study whose spike configuration remained constant (matching the template) and could be clearly discriminated from activity in the background throughout the experiment, indicating that the activity of one and the same one neuron was measured.
Experimental protocol
Background and evoked activity
Spontaneous activity (in the absence of intentional stimulation) and responses evoked by brief (10 s) mechanical test stimuli of innocuous (300 g/30 mm2) and noxious (2000 g/30 mm2) intensities were recorded. Mechanical stimuli (compression) were applied to the knee joint by means of a calibrated forceps equipped with a force transducer, whose output signal was amplified, displayed in grams on a liquid-crystal display screen, digitized by the CED interface, and recorded on the Pentium PC for on- and offline analysis as described before [32,51]. For the analysis of net evoked activity, background activity in the 15-s time period preceding the 15-s stimulus was subtracted from the total activity during stimulation.
Optical stimulation
After a control period of 20–30 min the effect of optical stimulation of ChR2-expressing cells on background and evoked activity of an individual neuron was recorded. Laser-generated blue light pulses (15 ms; frequency, 10 Hz; intensities, 1, 5, 10 mW) were delivered to the infralimbic mPFC through an optical fiber (200 μm diameter) coupled to a 473-nm laser (ThorLabs). Stimulus parameters of the laser were controlled with a pulse generator (Grass Instruments). The fiber was attached to a stereotaxic cannula holder (Kopf Instruments). Several hours before the start of the electrophysiological recordings, the optical fiber was lowered vertically into the infralimbic mPFC at the site of the virus vector injection (see above). Duration of optical stimulation was 40–60 s to determine effects on background activity in the absence of peripheral mechanical stimuli, and 1–2 min while mechanical stimuli were applied to measure the effect on evoked activity.
Verification of recording and stimulation sites and virus expression
At the end of each experiment, the recording site in the mPFC was marked by injecting direct current (250 μA for 3 min) through the carbon-filament glass electrode. Brains were removed and fixed in 4% paraformaldehyde for 6–12 h; then they were placed in 30% sucrose for 48–72 h before they were frozen-sectioned at 50 μm. For verification of virus expression, brain slices were washed in PBS and mounted on gelatin-coated slides treated with fluorescent-mounting media. To verify absence of virus expression in GABAergic cells, brain slices were rinsed in PBS and placed in 10% normal goat serum (NGS) for 1 hour. Tissues were rinsed in PBS several times and incubated in primary antibody (mouse monoclonal antibody, anti-GAD67, 1G10.2, Millipore, dilution 1:500) for 48 hours at 4°C. Sections were washed again in PBS and transferred to TRITC conjugated goat anti-mouse IgG (AP503R, Millipore, dilution 1:100) for 1 h at 37°C. After three rinses in PBS, sections were mounted on slides treated with fluorescent-mounting media. Expression of ChR2-eYFP and GABA was then examined using a Zeiss laser-scanning confocal microscope. For verification of the position of the optical fiber tip and the recording/lesion site, sections were mounted on gel-coated slides, stained with hematoxylin and eosin, and cover slipped.
Data analysis and statistics
Single-unit action potentials were analyzed offline from peristimulus rate histograms using Spike2 software (version 4; Cambridge Electronic Design). Background and evoked activity was expressed as spikes/s (Hz). Background activity preceding each stimulus was subtracted from the total activity during stimulation to calculate net evoked activity. All averaged values are given as the mean ± SE. Statistical analysis was performed on the raw data (spikes/s). For multiple comparisons, one-way ANOVA was used with appropriate posttests. Statistical significance was accepted at the level P < 0.05. GraphPad Prism 3.0 software was used for all statistical analyses.
Results
Channel rhodopsin 2 (ChR2) expression in infralimbic mPFC pyramidal cells
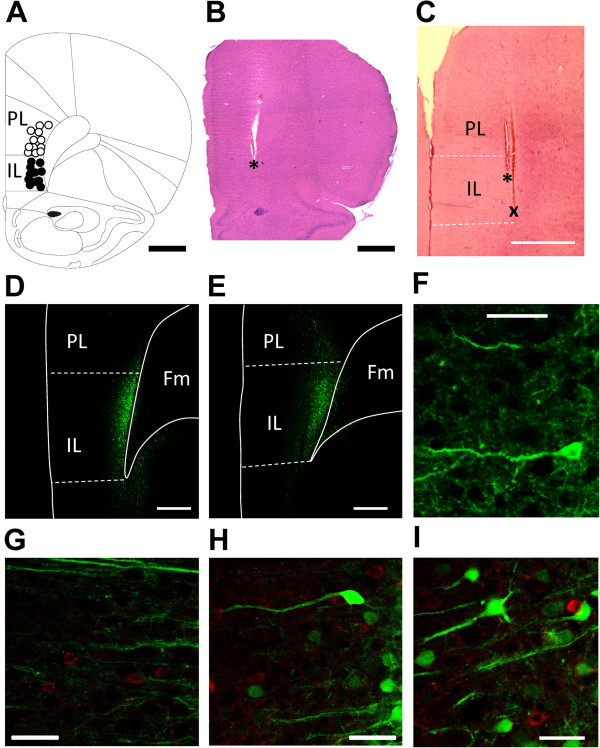
Stereotaxic injection of a viral vector encoding channel rhodopsin 2 (ChR2) and yellow fluorescent protein (YFP) under the control of the CaMKII promoter (rAAV5/CaMKIIa-ChR2(H134R)-EYFP) led to the expression of ChR2 in infralimbic pyramidal cells (Figure 1D,F) but not in GABAergic interneurons (Figure 1G-I). The spread of rAAV5/CaMKIIa-ChR2(H134R)-EYFP (Figure 1D) and of a control vector lacking the ChR2 sequence (rAAV5/CaMKIIa-EYFP; Figure 1E) was similar and largely restricted to the deep layers of the infralimbic mPFC.
Figure 1.
Recording and stimulation sites and viral vector expression in the mPFC. (A) Recording sites of pyramidal cells in the infralimbic (IL, n = 11) and prelimbic (PL, n = 12) cortex. (B) Position of the optic fiber in the IL (* indicates tip). (C) Positions of the optic fiber (*) and the recording site (x) in the IL. (A-C) Scale bars, 1 mm. (D) Yellow fluorescent protein (YFP) labeling indicates ChR2 expression restricted largely to IL (deep layers) following rAAV5/CaMKIIa-ChR2(H134R)-EYFP injection into IL. (E) Expression of control vector that lacked the ChR2 sequence (rAAV5/CaMKIIa-EYFP). (D, E) Scale bars, 500 μm. Fm, forceps minor. (F) Example of a ChR2 expressing pyramidal cell in IL. Scale bar, 50 μm. (G-I) Lack of ChR2/viral vector co-expression (green) in GAD positive (GABAergic) interneurons (red). Scale bars, 50 μm. All sections are taken at the level of 3.2 anterior to bregma.
Effect of optical stimulation on mPFC neurons
Extracellular single-unit recordings were made from 23 pyramidal cells (see recording sites in Figure 1A; 11 infralimbic, IL; 12 prelimbic, PL) and from 5 infralimbic interneurons in rats injected with rAAV5/CaMKIIa-ChR2(H134R)-EYFP. For optical stimulation the fiber was positioned in the IL (Figure 1B and C).
Background activity
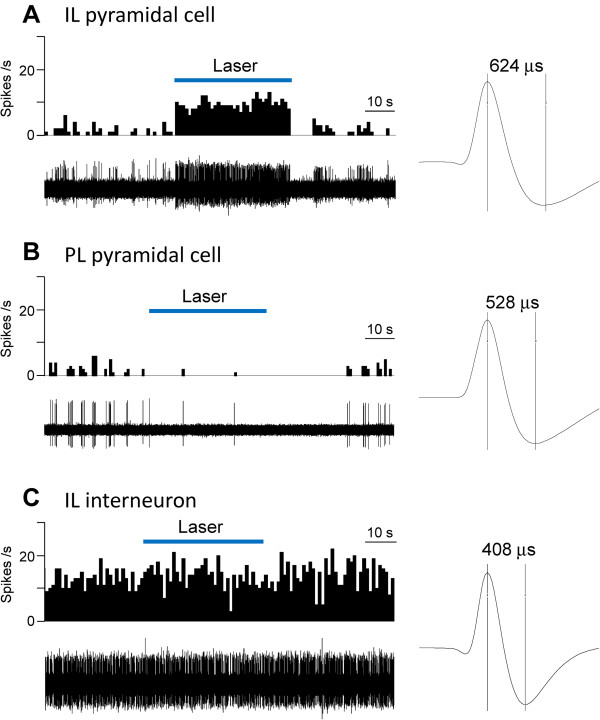
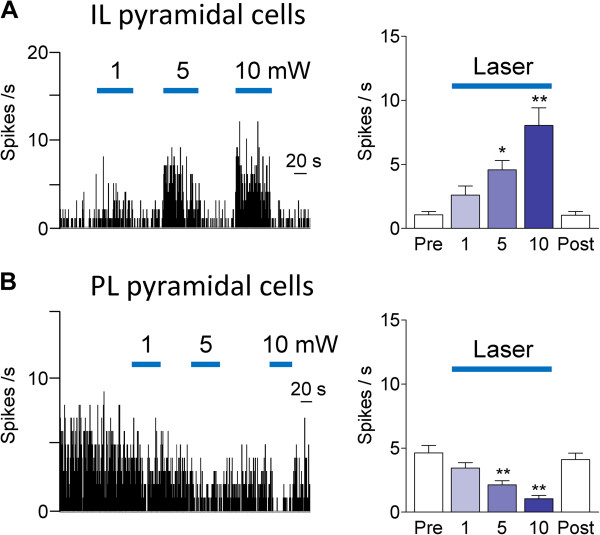
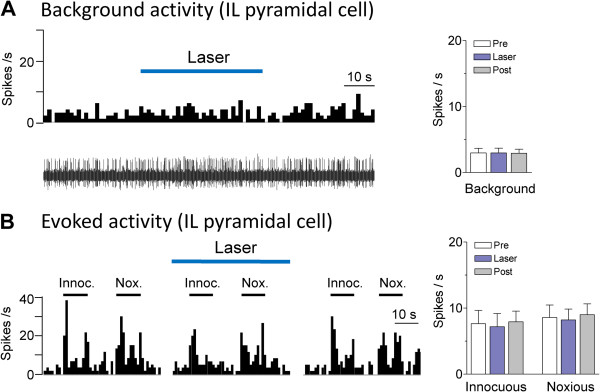
The effect of optical stimulation (5 mW, 10 Hz, for 40–60 s) on background activity in the absence of peripheral mechanical stimuli (see Methods) in individual mPFC neurons is shown in Figure 2. Optical stimulation of ChR2 expressing neurons in IL increased activity in an IL pyramidal cell (Figure 2A) but inhibited background activity in a PL pyramidal cell (Figure 2B) and had no effect on an IL interneuron (Figure 2C). Compared to pyramidal cells, the interneuron showed higher levels of background activity and had a narrower spike width, which are distinguishing characteristics as described in our previous studies [32,51]. The differential effects of optical activation (laser pulses of 1–10 mW, 10 Hz, for 40–60 s) of ChR2 expressing neurons in IL on pyramidal cells in IL and PL are summarized in Figure 3. 9 of 11 IL pyramidal cells were excited (Figure 3A) whereas 2 IL pyramidal cells were inhibited (not shown). Background activity of all 12 PL pyramidal cells was inhibited (Figure 3B). The excitatory and inhibitory effects of optical stimulation were intensity-dependent and reversible. Laser intensities of 5 and 10 mW produced significant effects (P < 0.05 and 0.01, Dunnett’s multiple comparison tests, Figure 3). Fast-spiking interneurons (n = 5) in IL were not affected by optical stimulation.
Figure 2.
Effects of optical activation on background activity of individual mPFC neurons. Extracellular single-unit recordings in anesthetized rats. (A) Laser stimulation in IL activated an IL pyramidal cell. (B) Optical stimulation in IL inhibited a PL pyramidal cell. (C) Laser stimulation had no effect on an IL interneuron. Peristimulus time histograms (PSTHs, bin width, 1 s) show number of action potentials (spikes/s) before, during and after laser stimulation (5 mW, 10 Hz). Original recordings of action potentials are shown below PSTHs. Spike 2 software was used to isolate and count single-unit activity based on waveform signal that crossed a trigger level and matched a pre-set shape or template (see Methods). Traces on the right show individual spikes; numbers indicate peak-to-valley spike width.
Figure 3.
Effects of optical activation on background activity. Effect of optical stimulation with different light intensities (1, 5, 10 mW; 10 Hz) for 40–60 s on background activity of mPFC pyramidal cells. (A) Intensity-dependent activation of IL pyramidal cells by optical stimulation in IL. Left, Peristimulus time histograms (bin width, 1 s) show background activity of an individual neuron. Right, Bar histograms show summary data (means ± SE) for the sample of IL pyramidal cells (n = 9). Background activity was recorded before (Pre), during (1, 5 and 10 mW) and after (Post) optical stimulation. (B) Intensity-dependent inhibition of PL pyramidal cells by optical stimulation in IL. Left, PSTHs show background activity of an individual neuron. Right, Bar histograms show summary data (means ± SE) for the sample of PL pyramidal cells (n = 12). *, ** P < 0.05, 0.01 (compared to control before stimulation “Pre”, Dunnett’s multiple comparison tests).
Evoked activity
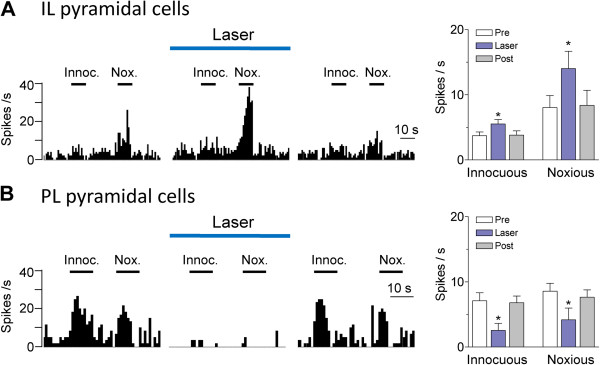
The mPFC receives multisensory including nociceptive information, is particularly concerned with the affective value of a stimulus, and forms strong reciprocal connections with limbic forebrain structures such as the amygdala that provides value-based emotional information [32,33,52]. However the optogenetic modulation of sensory-evoked responses of mPFC neurons remains to be determined. Laser stimulation (1 mW, 10 Hz, 1–2 min) of Chr2 expressing IL neurons led to a significant (P < 0.05, Bonferroni posttests) increase of brief (10 s) evoked responses of IL pyramidal cells (n = 8 neurons tested; Figure 4A). The facilitatory effect was particularly pronounced for responses to high-intensity stimuli. The lower intensity of 1 mW was selected to avoid confounding strong increases in background activity (see Figure 3A). In contrast, optical stimulation (5 mW, 10 Hz, 1–2 min) of ChR2 expressing IL neurons decreased the responses of PL neurons to innocuous and noxious stimuli significantly (n = 5 neurons tested, P < 0.05, Bonferroni posttests; Figure 4B).
Figure 4.
Effects of optical activation on evoked activity. Effect of optical stimulation on the responses of mPFC pyramidal cells to brief (10 s) innocuous (300 g/30 mm2) and noxious (2000 g/30 mm2) somatosensory stimuli (mechanical compression of the knee, see Methods). (A) Responses of IL pyramidal cells increased during optical stimulation (1 mW, 10 Hz) in IL. Left, Peristimulus time histograms (bin width, 1 s) show responses of an individual neuron to innocuous (Innoc.) and noxious (Nox.) stimuli (horizontal bars) before, during and after laser stimulation. Right, Bar histograms show summary data (means ± SE) for the sample of IL pyramidal cells tested (n = 8). Pre, before; Post, after laser stimulation. Background activity preceding each stimulus has been subtracted from the total activity during stimulation to obtain the net evoked response. (B) Responses of PL pyramidal cells decreased during optical stimulation (5 mW, 10 Hz) in IL. Same display as in (A). Bar histograms show summary data for the sample of PL pyramidal cells tested (n = 5). * P < 0.05 (compared to control before stimulation “Pre”, Bonferroni posttests).
Controls
Optical stimulation (5 mW, 10 Hz, 2 min) in the IL of animals injected with a control virus that lacked the ChR2 sequence (rAAV5/CaMKIIa-EYFP) had no significant effect on IL pyramidal cells (n = 5 neurons; Figure 5).
Figure 5.
Lack of effect of optical activation in animals treated with control virus. Optical stimulation (5 mW, 10 Hz) in the IL of animals injected with a control virus that lacked the ChR2 sequence (rAAV5/CaMKIIa-EYFP) had no effect on background (A) and evoked (B) activity of IL pyramidal cells (n = 5 neurons). (A) Left, Peristimulus time histograms (PSTHs, bin width, 1 s) show number of action potentials (spikes/s) before, during and after laser stimulation in an individual neuron. Right, Bar histograms show summary data (means ± SE) for the sample of neurons tested (n = 5). (B) Left, PSTHs show responses of an individual neuron to innocuous (Innoc.) and noxious (Nox.) stimuli (horizontal bars) before, during and after laser stimulation. Right, Bar histograms show summary data (means ± SE) for the sample of neurons tested (n = 5). Background activity preceding each stimulus has been subtracted from the total activity during stimulation to obtain the net evoked response.
Discussion
The mPFC serves major executive functions and plays an important role in the modulation of emotional processing in subcortical centers such as the amygdala [53-58]. The present study advances our knowledge about function and manipulation of mPFC neurons in several ways.
First, optogenetic stimulation of ChR2-expressing excitatory neurons in the IL produced not only excitation of IL pyramidal cells but also increased their responsiveness to excitatory inputs driven by peripheral mechanical stimuli. mPFC neurons receive multisensory including somatosensory and nociceptive information [52]. Physiological nociceptive signals that normally activate mPFC output cells [32] likely serve protective functions such as attention, awareness and appraisal [59-61]. mPFC responses are believed to be related to the affective value of the stimulus which is consistent with the close reciprocal connections between mPFC and limbic forebrain structures such as the amygdala that provide emotion- and value-based information [32,33,51,52]. Our data suggest that optical stimulation provides a tool to increase pyramidal output not only through direct excitation but also by facilitating afferent input to drive pyramidal output.
Second, our results directly demonstrate an inverse interaction between infra- and prelimbic mPFC regions. Activation of IL output inhibited PL pyramidal cells, which likely involved feedforward inhibition [62]. Differential roles of infralimbic and prelimbic mPFC regions have been proposed with regard to their modulation of emotional processing associated with conditioned fear [15,23]. Specifically, IL plays a critical role in fear extinction likely through direct excitatory projections to a cluster of inhibitory neurons (intercalated cells) interposed between input and output regions of the amygdala [16]. Stimulation of IL facilitates extinction [63] and causes inhibition of amygdala output neurons [64]. Increased IL activity correlates with successful fear extinction [19,22] and decreased IL activity with extinction deficits [23-26]. In contrast, PL is involved in expression and renewal of fear [15,23]. Stimulation of the PL results in freezing behavior (Vidal-Gonzalez et al., 2006) and increased activation of amygdala input neurons [14]. Evoked responses of PL neurons correlate with fear conditioning and persistent activity with extinction deficits [65] whereas PL inactivation impairs fear expression [23]. Our data show that IL activation can inhibit PL output, suggesting that IL-mediated extinction mechanisms may not only involve direct interactions with the amygdala but also control of PL-driven facilitatory influences on fear expression.
The results of this study are significant because impaired mPFC function is associated with several neuropsychiatric disorders [1-8]. Modulating mPFC output may be utilized in the treatment of anxiety disorders such as PTSD [17,23,47] and optogenetic strategies to increase excitatory or inhibitory processes in the mPFC have been suggested as novel treatment strategies in neuropsychiatric disorders such as depression [66] and schizophrenia [67].
In a previous study we used electrical stimulation of labeled afferent fibers from the amygdala in the IL to show feedforward inhibition of PL pyramidal cells [32-34]. While these data are not fully comparable with the results of optical stimulation in the IL in the present study, they do agree on the presence of a circuit involving the IL that leads to inhibition of PL pyramidal cells. There are likely different cortical and extracortical sources that can engage feedforward inhibition of PL neurons (and IL neurons for that matter); these cannot easily be distinguished using electrical stimulation which may activate interneurons, pyramidal cells or fibers of passage. In contrast, optogenetics-based stimulation allows the activation of a defined population of neurons (IL pyramidal cells in the present study), which is one of the key advantages of this technology that we used here to show IL-induced inhibition of PL pyramidal cells.
As a technical consideration, the differential effects of optical stimulation on different neuronal populations and the lack of effect in animals injected with control virus argue against nonspecific confounding factors, including heating as the result of high light power, toxicity at high expression levels or long-term expression, and transient changes in ion balance [43]. The stimulus intensities used in this study (1, 5 and 10 mW) are well within the suitable range for optogenetic control despite the stimulation of a small volume of tissue [38]. We used the channelrhodopsin variant ChR2(H134R) that is widely used and can drive precise low-frequency spike trains [50].
Conclusion
The electrophysiological in vivo results directly demonstrate the optogenetic modulation of mPFC activity in a region- and cell type-specific manner, which is significant because optogenetic tools may be useful in neuropsychiatric disorders associated with impaired mPFC function [43,68]. The selective, controlled, cell type-specific intervention can provide important insights into neurobiological mechanisms of complex brain functions and disorders.
Our results show an inverse relationship between IL and PL, suggesting that some IL functions may not only involve direct projections to subcortical limbic structures but also engage inhibitory control over PL output. The effects on background and evoked activity suggest that optical stimulation provides a tool to increase pyramidal output not only through direct excitation but also by facilitating the effectiveness of afferent drives.
Abbreviations
ChR2: Channel rhodopsin 2; IL: Infralimbic; mPFC: Medial prefrontal cortex; PL: Prelimbic.
Competing interests
The authors declare that they have no competing financial interests.
Authors’ contributions
G.J. performed the experiments, analyzed data, provided figures and wrote the first draft of the manuscript. V.N. conceptualized the hypothesis, designed and supervised the experiments, directed the data analysis, and finalized the manuscript. All authors read and approved the manuscript.
Contributor Information
Guangchen Ji, Email: guji@utmb.edu.
Volker Neugebauer, Email: voneugeb@utmb.edu.
Acknowledgements
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS-38261 and NS-11255.
References
- Bowie CR, Harvey PD. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr Dis Treat. 2006;2:531–536. doi: 10.2147/nedt.2006.2.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Chudasama Y. Animal models of prefrontal-executive function. Behav Neurosci. 2011;125:327–343. doi: 10.1037/a0023766. [DOI] [PubMed] [Google Scholar]
- Gu BM, Park JY, Kang DH, Lee SJ, Yoo SY, Jo HJ, Choi CH, Lee JM, Kwon JS. Neural correlates of cognitive inflexibility during task-switching in obsessive-compulsive disorder. Brain. 2008;131:155–164. doi: 10.1093/brain/awm277. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Takahashi Y, Roesch MR, Schoenbaum G. Neural substrates of cognitive inflexibility after chronic cocaine exposure. Neuropharmacology. 2009;56(Suppl 1):63–72. doi: 10.1016/j.neuropharm.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Yang CR, Otani S. Functional and dysfunctional synaptic plasticity in prefrontal cortex: roles in psychiatric disorders. Biol Psychiatry. 2010;67:199–207. doi: 10.1016/j.biopsych.2009.08.026. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51:68–80. doi: 10.1016/S0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A. Neuronal circuits of fear extinction. Eur J Neurosci. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- Hartley CA, Phelps EA. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35:136–146. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Pare D, Richardson R, Herry C, Monfils MH, Schiller D, Vicentic A. Erasing fear memories with extinction training. J Neurosci. 2010;30:14993–14997. doi: 10.1523/JNEUROSCI.4268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Richardson R. New findings on extinction of conditioned fear early in development: theoretical and clinical implications. Biol Psychiatry. 2010;67:297–303. doi: 10.1016/j.biopsych.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, Pare D. Prefrontal control of the amygdala. J Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Westbrook RF. Behavioural neuroscience: The circuit of fear. Nature. 2008;454:589–590. doi: 10.1038/454589a. [DOI] [PubMed] [Google Scholar]
- Chang CH, Berke JD, Maren S. Single-unit activity in the medial prefrontal cortex during immediate and delayed extinction of fear in rats. PLoS One. 2010;5:e11971. doi: 10.1371/journal.pone.0011971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci U S A. 2005;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickley GA, Kenmuir CL, Yocom AM, Wellman JA, Biada JM. A role for prefrontal cortex in the extinction of a conditioned taste aversion. Brain Res. 2005;1051:176–182. doi: 10.1016/j.brainres.2005.05.033. [DOI] [PubMed] [Google Scholar]
- Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem. 2009;16:486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SC, Jo YS, Kim IH, Kim H, Choi JS. Lack of medial prefrontal cortex activation underlies the immediate extinction deficit. J Neurosci. 2010;30:832–837. doi: 10.1523/JNEUROSCI.4145-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Maren S. Strain difference in the effect of infralimbic cortex lesions on fear extinction in rats. Behav Neurosci. 2010;124:391–397. doi: 10.1037/a0019479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, Bussey TJ, Singewald N, Holmes A. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GB, Heinrichs SC, Carey RJ. Treatment of addiction and anxiety using extinction approaches: neural mechanisms and their treatment implications. Pharmacol Biochem Behav. 2011;97:619–625. doi: 10.1016/j.pbb.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Vlaeyen JW, Linton SJ. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain. 2012;153:1144–1147. doi: 10.1016/j.pain.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz AE, Yau HJ, Centeno MV, Apkarian AV, Martina M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci U S A. 2009;106:2423–2428. doi: 10.1073/pnas.0809897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Sun H, Fu Y, Li Z, Pais-Vieira M, Galhardo V, Neugebauer V. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J Neurosci. 2010;30:5451–5464. doi: 10.1523/JNEUROSCI.0225-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Galhardo V, Maione S, Mackey SC. Forebrain pain mechanisms. Brain Res Rev. 2009;60:226–242. doi: 10.1016/j.brainresrev.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Neugebauer V. mGluR1, but not mGluR5, activates feed-forward inhibition in the medial prefrontal cortex to impair decision making. J Neurophysiol. 2011;106:960–973. doi: 10.1152/jn.00762.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- de Visser L, Baars AM, van’t Klooster J, van den Bos R. Transient inactivation of the medial prefrontal cortex affects both anxiety and decision-making in male wistar rats. Front Neurosci. 2011;5:102. doi: 10.3389/fnins.2011.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Covington HE III, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, LaPlant Q, Mouzon E, Ghose S, Tamminga CA. et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci. 2010;30:16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. 2010;167:1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- DeFelipe J. Neocortical neuronal diversity: chemical heterogeneity revealed by colocalization studies of classic neurotransmitters, neuropeptides, calcium-binding proteins, and cell surface molecules. Cereb Cortex. 1993;3:273–289. doi: 10.1093/cercor/3.4.273. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Feng G, Majewska AK, Miesenbock G, Ting A, Schnitzer MJ. Next-generation optical technologies for illuminating genetically targeted brain circuits. J Neurosci. 2006;26:10380–10386. doi: 10.1523/JNEUROSCI.3863-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci. 2012;13:251–266. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT. et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi MA, Hayrapetyan VY, Maimon B, Mak K, Je HS, Yin HH. Prefrontal cortical mechanisms underlying delayed alternation in mice. J Neurophysiol. 2012;108:1211–1222. doi: 10.1152/jn.01060.2011. [DOI] [PubMed] [Google Scholar]
- Anikeeva P, Andalman AS, Witten I, Warden M, Goshen I, Grosenick L, Gunaydin LA, Frank LM, Deisseroth K. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nat Neurosci. 2012;15:163–170. doi: 10.1038/nn.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanez-Nuin DE, Santini E, Quirk GJ, Porter JT. Memory for fear extinction requires mGluR5-mediated activation of infralimbic neurons. Cereb Cortex. 2011;21:727–735. doi: 10.1093/cercor/bhq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Durand R, Gradinaru V, Zhang F, Goshen I, Kim DS, Fenno LE, Ramakrishnan C, Deisseroth K. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature. 2010;465:788–792. doi: 10.1038/nature09108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Hamanaka H, Monfils MH, Behnia R, Deisseroth K, Blair HT, LeDoux JE. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proc Natl Acad Sci U S A. 2010;107:12692–12697. doi: 10.1073/pnas.1002418107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Pain-related deactivation of medial prefrontal cortical neurons involves mGluR1 and GABAA receptors. J Neurophysiol. 2011;106:2642–2652. doi: 10.1152/jn.00461.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Franz TM, Singh T, Schoenbaum G. Basolateral amygdala lesions abolish orbitofrontal-dependent reversal impairments. Neuron. 2007;54:51–58. doi: 10.1016/j.neuron.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Curr Opin Neurobiol. 2004;14:148–155. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. Cannabinoids potentiate emotional learning plasticity in neurons of the medial prefrontal cortex through basolateral amygdala inputs. J Neurosci. 2006;26:6458–6468. doi: 10.1523/JNEUROSCI.0707-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Ohara S, Crone NE, Weiss N, Lenz FA. Analysis of synchrony demonstrates 'pain networks' defined by rapidly switching, task-specific, functional connectivity between pain-related cortical structures. Pain. 2006;123:244–253. doi: 10.1016/j.pain.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Lorenz J, Casey KL. Imaging of acute versus pathological pain in humans. Eur J Pain. 2005;9:163–165. doi: 10.1016/j.ejpain.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Ferrante M, Migliore M, Ascoli GA. Feed-forward inhibition as a buffer of the neuronal input–output relation. Proc Natl Acad Sci U S A. 2009;106:18004–18009. doi: 10.1073/pnas.0904784106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Nestler EJ, Covington HE III. Potential utility of optogenetics in the study of depression. Biol Psychiatry. 2012;71:1068–1074. doi: 10.1016/j.biopsych.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled A. Optogenetic neuronal control in schizophrenia. Med Hypotheses. 2011;76:914–921. doi: 10.1016/j.mehy.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Deisseroth K. Optogenetics and psychiatry: applications, challenges, and opportunities. Biol Psychiatry. 2012;71:1030–1032. doi: 10.1016/j.biopsych.2011.12.021. [DOI] [PubMed] [Google Scholar]