Abstract
Angiotensin-converting enzyme (ACE) is a zinc-dependent peptidase responsible for converting angiotensin I into the vasoconstrictor angiotensin II. However, ACE is a relatively nonspecific peptidase that is capable of cleaving a wide range of substrates. Because of this, ACE and its peptide substrates and products affect many physiologic processes, including blood pressure control, hematopoiesis, reproduction, renal development, renal function, and the immune response. The defining feature of ACE is that it is composed of two homologous and independently catalytic domains, the result of an ancient gene duplication, and ACE-like genes are widely distributed in nature. The two ACE catalytic domains contribute to the wide substrate diversity of ACE and, by extension, the physiologic impact of the enzyme. Several studies suggest that the two catalytic domains have different biologic functions. Recently, the X-ray crystal structure of ACE has elucidated some of the structural differences between the two ACE domains. This is important now that ACE domain-specific inhibitors have been synthesized and characterized. Once widely available, these reagents will undoubtedly be powerful tools for probing the physiologic actions of each ACE domain. In turn, this knowledge should allow clinicians to envision new therapies for diseases not currently treated with ACE inhibitors.
I. Introduction
Angiotensin converting-enzyme (ACE) is known as peptidyl-dipeptidase A. It is also known as kininase II, CD143, or EC 3.5.15.1 (http://www.chem.qmul.ac.uk/iubmb/enzyme/EC3/4/15/1.html; http://www.uniprot.org/uniprot/P12821). Mostly, however, it is known as the enzyme that produces the vasoconstrictor angiotensin II. Although the cleavage of angiotensin I to the eight–amino acid peptide angiotensin II is certainly a central action of ACE, to go no farther in understanding this enzyme is to miss something essential: ACE and its peptide substrates and products affect many physiologic processes in addition to blood pressure control. These include hematopoiesis, reproduction, renal development, renal function, and the immune response. The diversity of ACE function is the central theme of this review.
A literature search for “angiotensin-converting enzyme” returns more than 46,000 articles, with more than 10,000 classified as reviews. Several are excellent (Acharya et al., 2003; Riordan, 2003; Corvol et al., 2004). Even an analysis of the most recent 100 PubMed articles reveals the enormous diversity of areas in which ACE, angiotensin II, and their inhibitors are being studied. Many of these articles concern hypertension, heart failure, or nephropathy (Ahmed et al., 2012; Pedrinelli et al., 2012). Other articles study hepatitis C, Alzheimer’s disease, tumor growth, or even erectile dysfunction (Kehoe and Passmore, 2012; Purnak et al., 2012; Santos et al., 2012; Wang et al., 2012). Given the many in vivo roles of ACE, both classic ACE inhibitors and newer compounds inhibiting only one ACE domain may evolve into important treatments for diseases outside the cardiovascular system.
II. Renin Versus Angiotensin-Converting Enzyme
At first glance, the renin-angiotensin system (RAS) is simple. Two enzymes, renin and ACE, act sequentially to produce angiotensin II. However, these enzymes are very different, akin to the dimorphism among peacocks. Renin is the more colorful enzyme. An aspartyl protease, renin cleaves only the single-substrate angiotensinogen at only a single position, releasing the amino-terminal peptide angiotensin I (Inagami, 1981). Renin specialization is further evidenced by its extremely limited tissue distribution: active renin is made in the kidney by granular cells in the wall of the afferent arteriole at the base of the glomerulus in the juxtaglomerular apparatus (Kurtz, 2011). This location is perfectly positioned to sense and respond to changes in renal arteriolar blood pressure and fluid flow within the nephron. Simply put, the business of renin is blood pressure regulation. The expression of active renin is tightly regulated, quite variable, and inversely related to blood pressure. In addition, the physiology of its regulated expression demonstrates complex biochemical feedback systems working to maintain blood pressure homeostasis. As beautiful as is the biology of renin, its highly specialized catalytic activity, localization, and physiologic regulation affect blood pressure—and really nothing else.
Compare this with ACE, a zinc-dependent dicarboxypeptidase that is expressed in high amounts by the vascular endothelium and the lung, renal proximal tubular epithelium, ciliated intestinal epithelium, and developing male germ cells (Ng and Vane, 1967; Cushman and Cheung, 1971; Bruneval et al., 1986; Skidgel and Erdös, 1993). It is expressed when monocytes differentiate into macrophages, and when dendritic cells become immunologically activated (Friedland et al., 1978; Shen et al., 2011). It is made by the choroid plexus and in several areas of the brain (Defendini et al., 1982; Defendini et al., 1983; Strittmatter et al., 1984). In fact, polymerase chain reaction analysis identified significant ACE mRNA expression in all 72 human tissues studied (Harmer et al., 2002).
ACE is much more promiscuous in substrate specificity than renin. Although this is discussed in detail later, reported ACE substrates are as small as tripeptides and as large as 42 amino acids (Skidgel and Erdös, 1987). Some substrates, such as angiotensin I and bradykinin, directly affect blood pressure, whereas others substrates, such as the peptide acetyl Ser-Asp-Lys-Pro (AcSDKP), do not (Liao et al., 2010). Although renin regulation is physiologically critical, the significance of regulated ACE expression in vivo is much less clear and certainly less physiologically important. ACE expression by the vascular endothelium is affected by a wide variety of stimuli, particularly when studied in vitro using cultured endothelial cells. Such cells increase ACE expression at confluence, in response to steroids, thyroid hormone, intracellular calcium, intracellular cAMP, ACE inhibitors, and several other stimuli (Del Vecchio and Smith, 1981; Forslund et al., 1982; Fyhrquist et al., 1983; Krulewitz et al., 1984; Krulewitz and Fanburg, 1986; Shai et al., 1992). Serum ACE levels are elevated in hyperthyroidism, but this does not appear to affect blood pressure (Nakamura et al., 1982). In fact, genetic experiments in both mice and computer modeling suggest that ACE regulation in vivo has very little effect on resting blood pressure. Targeted recombination has been used to create mice with one, two, three, or four copies of the ACE gene (Krege et al., 1997). Plasma ACE levels in mice varied from 62% of normal (one ACE gene) to 213% of normal (four ACE genes). Yet this study showed that systemic blood pressure was not significantly affected by ACE gene copy number and the related changes in ACE expression. Other mouse genetic models in which ACE was aberrantly expressed by hepatocytes and not by the vascular endothelium also showed normal basal blood pressures (Cole et al., 2002, 2003). Finally, a complex computer simulation was used to model the blood pressure effects of changes in the concentration of RAS components, including ACE (Smithies et al., 2000). Similar to the mouse models, the authors concluded that changes in ACE expression have little effect on blood pressure due to renin-mediated compensation of angiotensin I levels. Only when ACE inhibition is nearly complete (more than 90%) is maximum renin/angiotensin I compensation reached. Then, further inhibition of ACE results in a decrease in angiotensin II levels and reduced blood pressure.
In summarizing a comparison between renin and ACE, it is best to consider renin as highly specialized for the regulated control of blood pressure. ACE contributes to blood pressure; the effect of ACE inhibitors, for which more than 168 million prescriptions were written in the United States in 2010, provides proof of this truth (report by the IMS Institute for Healthcare Informatics - The Use of Medicines in the United States: Review of 2010). However, the concept of ACE as only participating in hemodynamic processes is incorrect, and impedes expanding the use of available ACE inhibitors or developing new ACE domain-specific inhibitors to treat disease.
III. The Discovery and Physical Properties of Angiotensin-Converting Enzyme
A. Discovery
“I have long believed that most important scientific discoveries are in large part accidental.” Leonard T. Skeggs, Jr. (Skeggs, 1993).
ACE was discovered in the mid-1950s, and was the last of the traditional components of the RAS to be identified. In fact, ACE was found more than 50 years after the identification of renin by physiologist Robert Tigerstedt working in Stockholm with a medical student, Per Gustav Bergman (Tigerstedt and Bergman, 1898). Understanding how these proteins were discovered gives insight into why ACE and the RAS are associated with blood pressure control. In November 1896, Tigerstedt began a series of experiments in which a cold-water extract of rabbit kidney was injected into the jugular vein of a recipient rabbit. Within little more than a minute, the blood pressure of the recipient increased more than 40 mm Hg (Phillips and Schmidt-Ott, 1999; Hall, 2003). Over the next year, Tigerstedt showed that the active agent in the renal extract was both water- and alcohol-soluble, nondialyzable, and heat sensitive. It was found in the renal cortex and renal venous blood, but not in the renal arterial blood. The pressor effect was not mediated by a change in heart rate and was not prevented by destruction of the spinal cord or other nerves. Tigerstedt named his discovery renin.
Tigerstedt’s experiments reflected the science of this period. In 1889, the French physician/physiologist Charles-Édouard Brown-Séquard achieved notoriety by studying organ extracts, including injecting himself with an extract of testis (Marks and Maxwell, 1979). In Tigerstedt’s case, his experiments reflected his belief in an “intimate connection between some renal and cardiac diseases” (Tigerstedt and Bergman, 1898). At the end of Tigerstedt’s 1898 paper, the authors write that they did not want to propose a new hypothesis, but wanted “to draw attention to the possible importance of a blood pressure-raising substance formed in the kidneys” (Marks and Maxwell, 1979). This was not to be, as no one, including Tigerstedt, recognized the significance of his discovery. Other diseases besides heart disease were killing people. In 1900, tuberculosis killed 61,888 Americans, as compared with the 45,279 killed by circulatory system diseases (http://www.cdc.gov/nchs/data/vsushistorical/mortstatsh_1900-1904.pdf); in these official death statistics, hypertension was not even recognized. In fact, it was not common in the United States to measure blood pressure in a medical examination until the early years of the 1900s (Kotchen, 2011). After October 1897, Tigerstedt stopped his investigation of renin. Although confirmatory work was published in 1909 by Bingel and Strauss (1909), the role of the kidney in blood pressure control was not recognized as physiologically important until 1934, when Goldblatt et al. (1934) published that clamping the renal arteries produced chronic hypertension. By then, the measurement of blood pressure was common in a medical setting, and there was some appreciation of the detrimental effects of hypertension (Kotchen, 2011).
The discovery by Goldblatt et al. (1934) that a humoral product raised blood pressure led to investigation into the nature of the pressor substance. Scientists, led by Irvine Page in Indianapolis and Eduardo Braun-Menéndez in Buenos Aires, identified renin as an enzyme and called the product of renin catalysis hypertensin (Braun-Menéndez) or angiotonin (Page) (Kohlstaedt et al., 1938; Muñoz et al., 1939; Braun-Menéndez et al., 1940; Page and Helmer, 1940). In 1958, both groups agreed to the name angiotensin (Braun-Menéndez and Page, 1958).
ACE was discovered in the mid-1950s by Leonard T. Skeggs, Jr. (Skeggs et al., 1956a; Skeggs, 1993). Skeggs and his colleagues Joseph Kahn and Norman Shumway were competing with research groups at the Cleveland Clinic and in London to purify and determine the structure of angiotensin. Skeggs was working with hog kidney as a source of renin and horse blood to provide the renin substrate. Both preparations were dialyzed exhaustively against distilled water before reaction and purification of the product (what we now call angiotensin I) by countercurrent distribution. The presence of the product in the individual countercurrent distribution fractions was then tested by a bioassay, the ability to raise the blood pressure of rats. This allowed Skeggs to purify angiotensin. However, following a move to a new laboratory space, the protocol suddenly resulted in a product with a new position in the countercurrent distribution, which was traced to the simple substitution of normal saline for water during the dialysis steps. The two products (the first produced in the absence of salt, the second in the presence of salt) were termed hypertensin I and hypertensin II, or in modern terms, angiotensin I and angiotensin II (Skeggs et al., 1954). Both peptide products were active in the rat bioassay, but further study revealed fundamental differences between the peptides (Skeggs et al., 1956b). It was immediately realized that angiotensin I was formed due to the action of renin. The second product was due to a plasma enzyme, initially termed hypertensin-converting enzyme (now ACE), that required chloride or other halides or nitrate for activation (Skeggs et al., 1954; Skeggs et al., 1956a). By 1957, the use of three separate models—isolated perfused rat kidney, rabbit aortic strips, and an isolated uterine preparation—showed that angiotensin II was a potent vascular and smooth muscle constrictor, whereas its precursor, angiotensin I, was not (Bumpus et al., 1956; Skeggs et al., 1956a; Helmer, 1957). ACE was found to release the carboxyl-terminal histidyl-leucine of angiotensin I in producing angiotensin II (Lentz et al., 1956). This burst of biochemical investigation led to the amino acid sequence of angiotensin II, its laboratory synthesis, and ultimately the wide availability of this peptide, which facilitated enormous progress in understanding the pharmacology and physiology of the RAS (Elliott and Peart, 1956; Skeggs et al., 1956c; Bumpus et al., 1957; Rittel et al., 1957).
Importantly, what we now know as ACE was discovered for a second time in 1966 when Erdös and Yang characterized a bradykinin-degrading enzyme from hog kidney (Erdös and Yang, 1967; Erdös, 2006). These researchers rapidly identified a similar activity in human plasma, and they named the enzyme kininase II to distinguish it from the previously characterized enzyme kininase I (Yang and Erdös, 1967). This discovery played a major role in the development of ACE inhibitors and is discussed in detail later.
B. Purification of Angiotensin-Converting Enzyme
When ACE was initially identified, the bioassays used to measure ACE activity were poorly suited for extensive biochemical purification and analysis of the enzyme. This served as a major impediment to detailed characterization, and was finally solved when model tripeptide substrates such as hippuryl-His-Leu, hippuryl-Gly-Gly, and benzyloxycarbonyl (Z)-Phe-His-Leu were developed (Cushman and Cheung, 1969; Piquilloud et al., 1970; Yang et al., 1970). These and similar assays were used to purify ACE first from pig lung (Dorer et al., 1972; Nakajima et al., 1973). By 1980, ACE had been purified from the lung of pig, rabbit, dog, and cow, and from the sera of rabbit and humans (Soffer, 1981). These studies indicated that ACE composed approximately 0.1% of the total protein of lung and 0.0017% of total serum protein. Although ACE was initially purified using columns exploiting several different physicochemical properties, the development of affinity columns, in which ACE inhibitors were used as the affinity ligand, greatly simplified purification protocols. Such columns provided up to 100,000-fold single-pass purification (Pantoliano et al., 1984; Bull et al., 1985a; Lanzillo et al., 1985; Bernstein et al., 1988b). Purified ACE is a single polypeptide of around 150–180 kDa (Corvol et al., 2004). Recent purification of porcine-lung ACE reported a molecular mass of 170 kDa by SDS-PAGE and 175 kDa by matrix-assisted laser desorption ionization mass spectrometry (Chen et al., 2010). Using this last figure and the known amino acid sequence of porcine ACE, approximately 16% of the mass spectrometry–determined molecular weight is due to glycosylation (http://www.ncbi.nlm.nih.gov/protein/NP_001077410.1). Other estimates of glycosylation indicate approximately 22% of the molecular weight (Baudin et al., 1997). ACE isolated from the brain is slightly smaller in size, probably due to differences in glycosylation (Hooper and Turner, 1987; Williams et al., 1991). A smaller isozyme of ACE is found in testis and is discussed later.
The preparation of anti-ACE antibodies, the eventual availability of radiolabeled ACE inhibitors, and the simplified ACE assay facilitated the study of ACE and led to several important conclusions (Oshima et al., 1974; Das and Soffer; 1976). ACE was identified on the luminal surface of endothelial cells and in organs such as the lung and retina that have heavy vascularization (Ryan et al., 1975; Caldwell et al., 1976; Skidgel and Erdös, 1993). The enzyme was found bound to cell membranes by its C-terminal amino acid sequence (Hooper et al., 1987). ACE expression is reported to be higher in the cultured endothelium from arterial sources than from cultured venous endothelial cells (Johnson and Erdös, 1977). ACE is present in high concentrations in epithelial cells with brush borders, including proximal tubules of the kidney, gut, choroid plexus, and placenta (Skidgel and Erdös, 1993). Although most ACE is associated with tissues, soluble ACE is also found in virtually all body fluids, including serum, urine, cerebrospinal fluid, seminal fluid, and amniotic fluid (Das et al., 1977; El-Dorry et al., 1983; Schweisfurth and Schiöberg-Schiegnitz, 1984; Yasui et al., 1984; Hooper, 1991). As is discussed later, soluble ACE is derived by the enzymatic cleavage of tissue-bound ACE. There are also anomalies of expression. For example, human and other species of kidney are described as having high levels of ACE, whereas rat kidney seems to have particularly low renal ACE (Cushman and Cheung, 1971; Skidgel and Erdös, 1993). Similarly, guinea pig plasma is described as quite high in ACE, whereas dog plasma is very low (Yang et al., 1971; Erdös, 1975).
In humans, plasma ACE levels are described as stable in any individual (Dux et al., 1984). Levels are higher in men than in women (Bénéteau-Burnat et al., 1990; Tiret et al., 1992). Children 4–18 years old have higher levels than adults (Bénéteau-Burnat et al., 1990).
C. Angiotensin-Converting Enzyme Substrates
Since its discovery, ACE was thought to be a metal-dependent peptidase because enzymatic activity was virtually totally inhibited by EDTA (Skeggs et al., 1956a; Bünning and Riordan, 1983). The purified enzyme was shown to contain zinc by atomic absorption spectroscopy, and it was estimated (incorrectly) that each molecule of ACE contained only one metal atom (Das and Soffer, 1975; Bünning and Riordan, 1985). It is now known that each molecule of ACE contains two zinc atoms (Ehlers and Riordan, 1991).
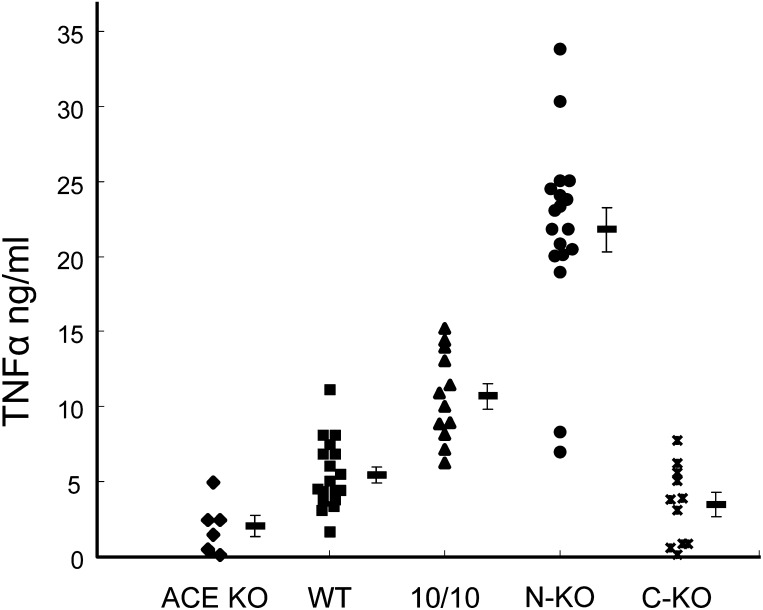
ACE plays a role in many different physiological processes, in part because the enzyme is promiscuous. At least in vitro, it can hydrolyze a wide range of substrates (Skidgel et al., 1984; Skidgel and Erdös, 1985; Skidgel and Erdös, 1987). ACE acts as a C-terminal dipeptidase for angiotensin I, bradykinin, and other small peptide hormones, including neurotensin, substance P, enkephalins, N-formyl-Met-Leu-Phe, acetyl Ser-Asp-Lys-Pro (AcSDKP) and angiotensin 1-7. ACE can cleave a C-terminal tripeptide from des-Arg9-bradykinin, and it can cleave substrates in which the carboxyl terminus is amidated, including the release of either a dipeptide or a tripeptide from the C-terminus of substance P and a tripeptide from GnRH (previously referred to as LH-RH). In vitro, ACE cleaves a C-terminal dipeptide from the amidated peptide cholecystokinin-8 (Dubreuil et al., 1989). ACE can even cleave an N-terminal tripeptide from GnRH, perhaps because the amino-terminal pyroglutamic acid of GnRH is not charged. When an GnRH derivative containing a free N-terminal glutamic acid was tested as a substrate, N-terminal hydrolysis was much reduced (Skidgel and Erdös, 1987). We emphasize that the studies referenced here are the result of in vitro analysis of ACE catalytic activity. At present, in vivo evidence for ACE activity has been established for angiotensin I, bradykinin, and the tetrapeptide AcSDKP (Azizi et al., 1996, 1997). For example, studies in mice treated with an ACE inhibitor or in mice genetically lacking ACE expression show that ACE is responsible for at least 90% of angiotensin I conversion to angiotensin II in the blood, kidney, heart, lung, and brain (Campbell et al., 2004). The studies also showed that blood bradykinin (1–9) levels were increased 6.4- to 8.4-fold in these animals, with the authors concluding that ACE plays a significant role in bradykinin metabolism in blood and, to a lesser extent, in the kidney and heart. Other studies have suggested that increased levels of bradykinin or substance P are responsible for the dry cough associated with ACE inhibitors (Dicpinigaitis, 2006). In mice, increased bradykinin has been shown to induce cardiac mast cell degranulation, releasing chymase (mast cell protease 4), which can produce angiotensin II locally (Wei et al., 2010).
Although ACE is best known for cleaving small peptides, there have been reports of ACE cleavage of the insulin β chain (Igic et al., 1972). Also, several studies have now identified ACE as capable of cleaving the amyloid peptides (Aβ1-40 and Aβ1-42) implicated in the pathogenesis of Alzheimer’s disease (Hu et al., 2001; Hemming and Selkoe, 2005; Oba et al., 2005). ACE was found to have both carboxypeptidic and endopeptidic activity against Aβ peptides, as determined by mass spectrometry (Sun et al., 2008; Zou et al., 2009). Although these publications leave little doubt that, under in vitro conditions, ACE cleaves large Aβ peptides, smaller amyloid peptide fragments, such as Aβ4-15, are hydrolyzed far more rapidly (Sun et al., 2008). In fact, ACE-mediated cleavage of full-length Aβ1-40 was slow and required long incubations of ACE with the Aβ peptide. Further, analysis of the brains of ACE knockout mice found that the lack of ACE does not significantly raise steady-state Aβ levels (Eckman et al., 2006). Although all of these findings indicate that ACE is not the major peptidase responsible for Aβ cleavage in vivo, there is some genetic evidence, discussed later, suggesting that ACE levels may influence the risk of Alzheimer’s disease.
D. Discovery of Testis Angiotensin-Converting Enzyme
In 1971, a study of ACE enzyme activity in rat tissues demonstrated that epididymis and testes had the highest specific activity (units per milligram of protein) of all the tissues measured (Cushman and Cheung, 1971). ACE activity in the testes increased between 40 and 50 days after birth, when mature sperm are first present. This increase in ACE activity was not observed in hypophysectomized rats. The ACE in the testes was found to be immunologically related to the enzyme made by the lung and other somatic tissues, but the testis enzyme, with a molecular mass of 90–110 kDa, was approximately half the size of pulmonary ACE (El-Dorry et al., 1982a). Because of this, the testis ACE isozyme is often termed testis ACE (or germinal ACE), in distinction from the isozyme made by all other tissues, which is called somatic ACE (Howard et al., 1990; Nadaud et al., 1992). Testis ACE is expressed by postmeiotic male germ cells; high-level expression of the protein is found in round and elongated spermatids at step 10 and beyond (Langford et al., 1993).
E. Structure of Angiotensin-Converting Enzyme
ACE was first cloned by Corvol and colleagues in 1988 from human endothelial cells and by Bernstein and colleagues in 1989 from mouse kidney (Bernstein et al., 1988a; Soubrier et al., 1988; Bernstein et al., 1989). Both groups isolated cDNA based on probes derived from a partial amino acid sequence. Human ACE is synthesized as a 1306–amino acid polypeptide; the mature enzyme contains 1277 residues after cleavage of a hydrophobic amino acid leader sequence. ACE has a predicted molecular mass of 146.6 kDa. Mouse ACE is synthesized as a 1312–amino acid peptide and then processed to contain 1278 amino acids (predicted molecular mass of 147.4 kDa). Human and mouse ACE are highly homologous, with 1088 of the 1312 amino acids being identical (83%), whereas another 60 are conserved substitutions.
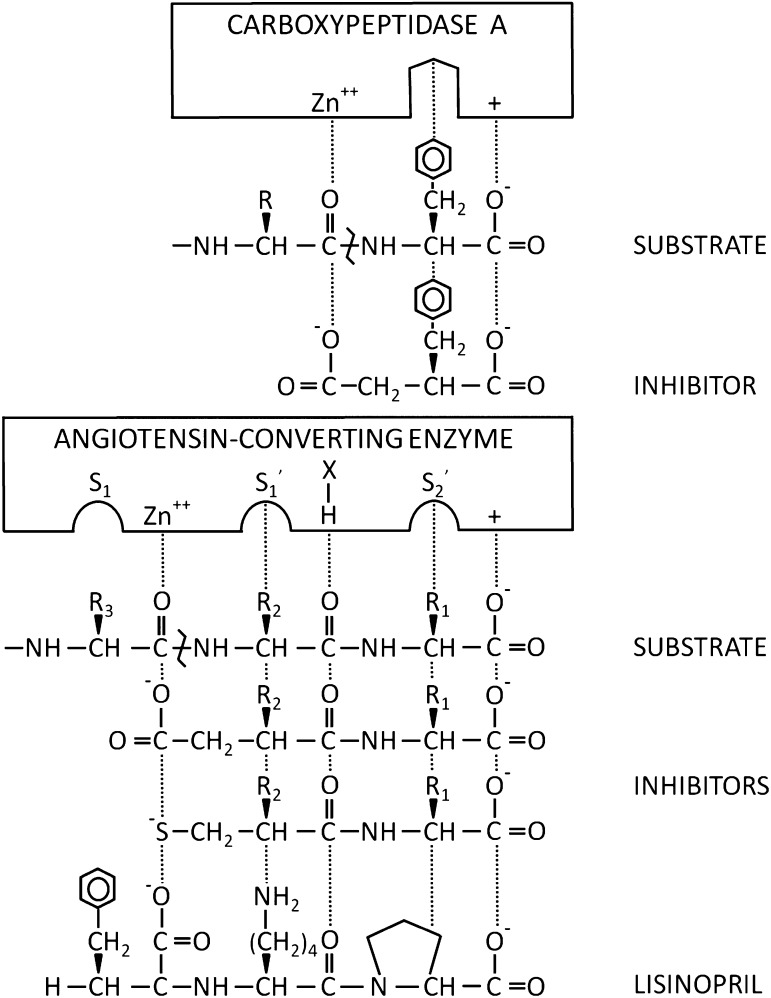
A surprise derived from cloning and from a subsequent study was that ACE has two internal areas of amino acid homology, each about 612 amino acids. These are now termed the ACE N- and C-domains. For human ACE, the two domains are about 60% homologous in both DNA and amino acid sequences. Higher homology (89%) is observed in a 40–amino acid “core” region containing residues comprising part of the catalytic site of the enzyme (Soubrier et al., 1988). For mouse ACE, the structure is similar, with predicted amino acids 47–610 (the N-domain) aligning with amino acids 650–1208 (the C-domain). In each domain, 300 amino acids are identical and an additional 47 are conserved substitutions (Bernstein et al., 1989). Each of the two catalytic domains contains the zinc-coordinating amino acid motif His-Glu-XX-His, which is a structural feature of many zinc peptidases (Spyroulias et al., 2004). The exact motif in ACE is His-Glu-Met-Gly-His (HEMGH), with the two histidines coordinating zinc. A third zinc-coordinating amino acid residue is glutamate, which is present on the C-terminal side of the HEMGH motif, and is found in its own characteristic motif EXIXD. Originally, the importance of these residues for binding zinc was deduced by comparison with the sequence of thermolysin, but their functional roles have been substantiated by site-directed mutagenesis and ultimately by X-ray crystallography (Wei et al., 1991a; Anthony et al., 2012). As a function of its amino acid sequence, ACE is classified as a member of the gluzincin family (i.e., thermolysin-like peptidases) (Hooper, 1994). The MEROPS database classification is clan MA, subclan MA(E), family M2, and peptidase XM02-001(Rawlings et al., 2008). Expression of each ACE domain in Chinese hamster ovary cells and the creation of mice with ACE genetic mutations has shown that each domain binds zinc and is independently catalytic (Wei et al., 1991a; Fuchs et al., 2004, 2008).
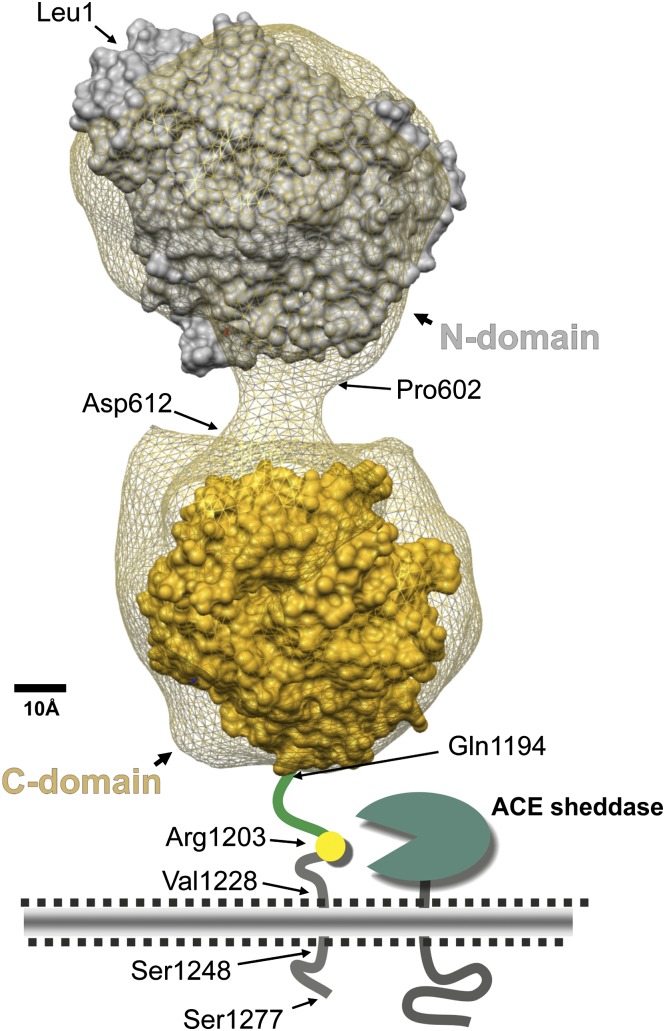
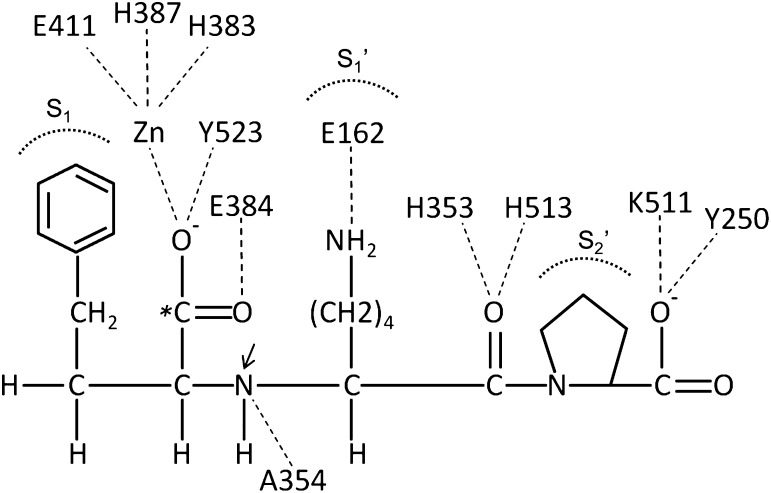
Our understanding of ACE structure is further enhanced by 1) the availability of ACE sequence from several species; 2) the three-dimensional crystal structure of the N-domain of ACE, the C-domain, and the enzyme ACE2, which is a C-domain homolog of ACE; and 3) the three-dimensional electron microscopic reconstruction of porcine ACE followed by the fitting of these data to the human atomic models (Natesh et al., 2003; Towler et al., 2004; Corradi et al., 2006; Chen et al., 2010). These studies allow the representation of mature human ACE, as shown in Fig. 1 (Danilov et al., 2011). The N-domain of the molecule begins with Leu1 and extends to Pro601 (Acharya et al., 2003). Within this region, H361EMGH365 and Glu389 coordinate zinc binding. The interdomain linker is about 11 amino acids from Pro602 to Asp612, followed by the C-terminal domain, which comprises Leu613 to about Pro1193 (the last amino acid seen in the crystal structure of the C domain) (Natesh et al., 2003; Corradi et al., 2006). In this domain, H959EMGH963 and Glu987 coordinate zinc. The C-domain is followed by a stalk from approximately Gln1194 to Arg1227, a hydrophobic transmembrane domain from Val1228 to Ser1248, and an intracellular C-terminal tail from Gln1249 to Ser1277 at the end of the molecule. Limited proteolysis of ACE with endoproteinase Asp-N will cleave between the Thr615–Asp616 and the Leu1219–Asp1220 peptide bonds to generate the two catalytic domains in active form that can be separated by a lisinopril affinity column (Sturrock et al., 1997; Chen et al., 2010). Several groups have suggested that in somatic ACE, the catalytic activity of the C-domain is negatively regulated by the presence of the N-domain (Binevski et al., 2003; Woodman et al., 2005).
Fig. 1.
Model of human somatic ACE. This model is a three-dimensional reconstruction of the electron microscopic appearance of porcine somatic ACE (the net) combined with available human X-ray crystal structures (Chen et al., 2010; Danilov et al., 2011). It shows the ACE N-domain (Leu1–Pro601), linker region (Pro602–Asp612), C-domain (Leu613–Pro1193), stalk region (Gln1194–Arg1227), transmembrane segment (Val1228–Ser1248), and intracellular domain (Gln1249–Ser1277) (Acharya et al., 2003; Corradi et al., 2006). We also indicate the membrane-bound ACE sheddase and Arg1203, the C-terminal residue of soluble ACE.
X-ray and electron microscopic studies provide a detailed picture of the deep substrate-binding clefts suspected by earlier biochemical studies (Pantoliano et al., 1984; Bernstein et al., 1990). In this model of ACE, the interdomain linker keeps the two domains separate by about 2.0–2.5 nm and is somewhat different from other models positing contact between the N- and C-domains (Corradi et al., 2006; Naperova et al., 2008). Recent analyses, using both X-ray crystallographic data and monoclonal antibody–mediated epitope mapping and blocking studies, have indicated that membrane-bound ACE may form dimers by association of two N-terminal domains (Kost OA et al., 2003; Danilov et al., 2011).
F. Shedding of Angiotensin-Converting Enzyme
Although ACE activity was detected in plasma as early as 1954, the purification of serum ACE led to the suggestion that it was derived from sloughing of endothelial ACE into the bloodstream (Skeggs et al., 1954; Das et al., 1977). Biochemical solubilization of ACE fragments from membranes and subsequent amino acid sequencing led to the conclusion that ACE is anchored to the cell membrane at its C terminus, and is released by a hydrolase inhibitable by EDTA (Hooper et al., 1987). Insight into this process came from a DNA sequence derived from the cloning of somatic and testis ACE, which suggested that both isozymes were bound to cells by a C-terminal hydrophobic transmembrane region followed by a small intracellular C-terminal tail. Confirmation of this was provided by Wei et al. (1991a) and Ehlers et al. (1991), who introduced a stop codon before the putative transmembrane domain, and demonstrated that this resulted in secreted enzyme. Further work again implicated a proteolytic enzyme in the natural release of ACE from cell membranes (Wei et al., 1991b).
Several groups have focused on the enzymatic process that releases ACE from the cell membrane. The cleavage site was first identified in a study of rabbit testis ACE, in which the amino acid sequence of the purified membrane “tail” remaining after ACE shedding was determined. This, combined with the C-terminal amino acid sequence of shed ACE, established a cleavage site between Arg663 and Ser664 of the testis isoform (Ramchandran et al., 1994). A variety of biochemical studies, including mass spectroscopic analysis, established that human and porcine ACE were cleaved between Arg1203 and Ser1204, essentially identical to the cleavage site determined in rabbit (Fig. 1) (Woodman et al., 2000). Amino acid substitutions or even small deletions at the cleavage site did not markedly affect the cleavage rate, indicating that the precise sequences in the stalk region are not critical for secretion (Sadhukhan et al., 1998). Ehlers et al. (1996) have further characterized the minimal structural requirements of the stalk region as requiring about 11 amino acids. Corvol et al. (2004) have reviewed the structural requirements of the stalk region.
Although the work of Sadhukhan et al. (1998) discussed earlier indicates that the amino acid sequence of the stalk at the cleavage site is not critical, the identification of families with genetic changes in ACE leading to increased ACE shedding does indicate that stalk sequence can affect the rate of shedding. Specifically, the identification of eight Dutch families with serum ACE levels averaging 5 times normal appeared to be the result of autosomal dominant transmission (Eyries et al., 2001; Kramers et al., 2001). Genetic sequencing in three individuals showed a C to T point mutation in the ACE gene resulting in a P1199L amino acid substitution. In vitro analysis confirmed enhanced ACE shedding. Hydrophobic cluster analysis was used to argue that this point mutation changed the configuration of the stalk region, increasing the accessibility of this region to enzymatic cleavage. Despite the increased serum ACE, renin levels and blood pressures in the eight families were normal.
Recently, another family with high ACE levels was reported to have a mutation in ACE exon 8, causing a Y465D substitution (Danilov et al., 2011). This mutation resulted in serum ACE levels that were 5–7 times the normal level. Several family members with the Y465D mutation presented with clinical malaise, including nausea, vomiting, and depression. It was unclear if the abnormal serum levels of ACE played a role in the clinical symptoms.
Although the Y465D mutation is in the ACE N-terminal domain, there was markedly increased shedding when an ACE cDNA bearing the mutation was expressed in cultured cells. Detailed biochemical analysis of the mutant protein using a panel of monoclonal antibodies showed significant changes in ACE conformation in both the N- and C-domains, particularly in the region of the stalk (Danilov et al., 2011). The authors speculated on a link between ACE dimer formation via the N-domain and the rate of shedding. These data are consistent with earlier studies showing that the secretion rate of somatic ACE (containing both the N- and C-domains) was about 10-fold less than that of constructs containing only the ACE C-domain, suggesting that in normal somatic ACE, the ACE N-domain has an effect on the rate of shedding (Beldent et al., 1995; Woodman et al., 2000). Interaction between the sheddase enzyme and structural motifs on the C-domain of ACE have been hypothesized (Woodman et al., 2006). In contrast, the N-domain may lack such recognition sequences. Consistent with this is the finding that an ACE construct, composed of a tandem repeat of two ACE C-domains, is shed from cells with kinetics similar to the testis ACE protein, which contains only a single C-domain (Woodman et al., 2005). Not only was the repeated C-domain construct shed, the sheddase enzyme cut both within the ACE stalk region and in the interdomain protein bridge connecting the two C-domains. Thus, it seems that as yet, unknown structural motifs on the C-domain interact with the membrane sheddase enzyme facilitating cleavage, and that the presence of the N-domain in somatic ACE hinders the recognition and cleavage process.
Several studies have examined the nature of the ACE sheddase enzyme, although its identity has not been established with certainty. Oppong and Hooper (1993) identified the enzyme as associated with membranes and inhibited with EDTA. The sheddase is also inhibitable by hydroxamic acid–based compounds, such as batimastat, but not by several other classes of metalloprotease inhibitors (Parvathy et al., 1997). The enzyme has been suggested to be a member of the ADAM family of proteins, although probably not ADAM10 or ADAM17 (Allinson et al., 2004; Parkin et al., 2004). ADAM9 has been implicated in lipopolysaccharide-stimulated shedding of ACE from endothelial cells (English et al., 2012). There may be more than one enzyme capable of releasing ACE, as it has been suggested that testis ACE release from sperm in the caput epididymis is due to a serine protease (Thimon et al., 2005).
G. Other Causes of Increased Serum Angiotensin-Converting Enzyme
In 1975, it was reported that patients with active sarcoidosis have elevated serum ACE levels, as compared with treated sarcoidosis patients or patients with resolved disease in which serum ACE was normal (Lieberman, 1975, 1976). Serial measurements of serum ACE were found to be a sensitive means of following the clinical course of sarcoidosis and predicting clinical relapse or improvement (Lieberman et al., 1983). Subsequent analysis described elevated ACE levels in a variety of granulomatous pulmonary diseases, including histoplasmosis, silicosis, and miliary tuberculosis (Ryder et al., 1983; Brice et al., 1995). This is due to increased ACE expression by the epithelioid cells present in sarcoid and other granuloma (Silverstein et al., 1979). These cells are monocytic in origin (Williams and Williams, 1983). ACE levels are also elevated in Gaucher’s disease, and are again thought to be a product of macrophage-like cells (Silverstein and Friedland, 1977). Although the precise biochemistry of ACE elevation is not yet known, it is thought that CD4+ T cells and a Th1 immune response are important etiologic components initiating granuloma formation in sarcoid (Grunewald and Eklund, 2007). Recently, apoE-deficient mice fed a cholate-containing high-fat diet developed lung granuloma similar to those present in sarcoidosis, including high levels of ACE expression by epithelioid cells (Samokhin et al., 2010). It is hoped that such a model may facilitate elucidation of precisely what stimulates ACE expression.
IV. Function of Angiotensin-Converting Enzyme
A. Production and Function of Angiotensin II
Whereas the pioneering studies of ACE were oriented toward understanding blood pressure, a more modern view recognizes many other physiologic roles for this enzyme. In part, this is due to the diversity of ACE substrates and products. However, even if we only consider a single product—angiotensin II—the physiologic effects of ACE are extraordinarily diverse. This review cannot discuss all the known actions of angiotensin II, but many of these are summarized in Table 1. Angiotensin II has effects on the kidney, the vasculature, the heart, the nervous system, metabolism, cell proliferation, and many other processes. Thus, the actions of just one ACE product demonstrate many effects of ACE.
TABLE 1.
Actions of angiotensin II
| Effect | Target | Mechanism: Intracellular Pathways Elicited |
|---|---|---|
| Actions in the kidney | ||
| Sodium and water retention | Renal microvasculature | Reduces GFR and renal plasma flow through the following: Afferent and efferent vasoconstriction via AT1 receptor activation (Navar et al., 1996; Arendshorst et al., 1999). Filtration coefficient reduction, probably due to constriction of mesangial cells (Blantz et al., 1976; Baylis and Brenner, 1978). Increased sensitivity of tubule-glomerular feedback mechanism via AT1 receptor activation in mesangial cells (Schnermann and Briggs, 1986; Mitchell and Navar, 1988) and increased activity of Na/H exchanger (Peti-Peterdi and Bell, 1998) and Na+/K+/2Cl- transporter (Kovács et al., 2002). |
| Proximal tubule | Increases sodium reabsorption at physiologic concentrations (Harris and Young, 1977; Schuster et al., 1984) via activation of Na+/H+ exchanger, basolateral Na+/K+ ATPase and H+-ATPase (Wang and Giebisch, 1996). AT1 receptor activation in proximal tubule cells leads to activation of multiple signaling pathways (including phospholipase C, D, and A2; Src-MAPK, and tyrosine kinases), increase in intracellular calcium, and inhibition of adynlyl cyclase (Zhuo and Li, 2007). | |
| Thick ascending limb | Stimulates Na+-K+-2Cl-transporter activity (Kovács et al., 2002). AT1 receptor activation leads to increases in Gq–PKCα and NADPH oxidase and superoxide production(Herrera et al., 2010). | |
| Distal tubule | Stimulates Na+/H+ exchanger activity (Barreto-Chaves and Mello-Aires, 1996). | |
| Collecting duct | Activates ENaC through stimulation of aldosterone secretion by adrenal glands (Navar et al., 1996), and directly via AT1 receptor (Peti-Peterdi et al., 2002). Increases urea transport (Kato et al., 2000). | |
| Cell hypertrophy, fibrosis, and matrix remodeling | Whole kidney | Most actions are due to induction of TGF-β. Other factors include endothelin-1, MMP-2, and hypoxia (Rüster and Wolf, 2011). |
| Oxidative stress | Whole kidney | Induces assembly and activation of the NADPH oxidase complex (Sachse and Wolf, 2007). ROS activates several pathways, including MAP kinases, NF-κB, tyrosine kinases, metalloproteinases, and AP-1 (Sachse and Wolf, 2007). |
| Inflammation | Whole kidney | AT1 receptor–mediated upregulation of proinflammatory genes, such as VCAM-1, ICAM-1, IL-6, TNFα, and MCP-1. Activation of multiple pathways, including NF-κB, MAPK cascade, Rho proteins, and ROS (Ruiz-Ortega et al., 2006a, b). |
| Actions in the vasculaturea | ||
| Vasoconstriction | VSMC | AT1 receptor–mediated activation of G proteins, including Gq/11, G12, and G13, leads to increases in several second messengers, including intracellular calcium, IP3, ROS, and Rho kinase and ARHGEF1 factor (Guilluy et al., 2010). |
| Growth, inflammation/ fibrosis | VSMC | AT1 receptor–mediated activation of MAPKs (including P38 and ERK), JNK, and tyrosine kinases (SRC, JAK, FAK, PYK2, P130Cas), intracellular calcium increases and ROS (Touyz et al., 1999; Savoia et al., 2011). Transactivation of receptor tyrosine kinases such as EGF, PDGF, and IGF1 (Saito and Berk, 2001). Increased production of endothelin-1, TGF-β, bFGF, and IGF1. |
| Vasodilation | VSMC | AT2 receptor–mediated activation of NO-GMP pathway (Savoia et al., 2011) or through bradykinin (Savoia et al., 2011). AT2 receptor activation is also associated with other antiproliferative and anti-inflammatory effects. |
| Actions in the heart | ||
| Increased inotropism | Ventricular myocardium | Increases inotropism indirectly by stimulating the sympathetic nervous system (Koch-Weser, 1965) and directly by intracellular calcium influx and changes of the plateau phase of the cardiac action potential (Dempsey et al., 1971). |
| Papillary muscles | Induces release of endothelin, which activates the Na+/H+ exchanger, increases [Na+]i, and promotes the influx of Ca2+ that leads to a positive inotropic effect (Perez et al., 2003). Stretch of papillary muscles induces the release of angiotensin II (Cingolani et al., 2005). | |
| Hypertrophy | Whole heart | Several reports indicate that local AT1 receptor stimulation induces cardiac hypertrophy (Dostal and Baker, 1998). However, others have shown that local increase of angiotensin II production in the heart does not produce cardiac hypertrophy (Xiao et al., 2008), and that AT1 receptor exclusively in the kidneys is sufficient to induce hypertension and cardiac hypertrophy (Crowley et al., 2006). |
| Left ventricle | Leads to diastolic dysfunction as a consequence of impaired diastolic sarcoplasmic reticulum calcium pump (SERCA2) activity via AT1 receptor (Rothermund et al., 2001). Promotes myocardial distensibility through AT1 receptor. May be an important adaptive mechanism in an acute overload context (Castro-Chaves et al., 2009). | |
| Cultured cardiomyocytes | Mediates myocyte hypertrophy through AT1 receptor (van Kesteren et al., 1997) and release of endothelin-1 and TGF-β by cardiac fibroblasts (Gray et al., 1998). Stimulates cardiac growth via AT1 receptor–induced JAK-STAT signaling activation (McWhinney et al., 1997). Mediates stretch-induced hypertrophy via AT1 receptor (Sadoshima et al., 1993). Induces hypertrophy through AT1 receptor independently of blood pressure elevation (Ainscough et al., 2009). Aldosterone receptor activation boosts angiotensin II–induced expansion of extracellular matrix proteins, fibrosis, and oxidative stress (Di Zhang et al., 2008). | |
| Remodeling and dysfunction | Whole heart | Produces multifocal antimyosin labeling of cardiac myocytes and myocytolysis (Tan et al., 1991). Contributes to arrhythmogenic atrial structural remodeling by MAPK activation (Li et al., 2001). Leads to cardiac dysfunction in absence of hemodynamic overload (Domenighetti et al., 2005). |
| Fibroblasts | Induces fibrosis at least partially mediated through TGF-β production via AT1 receptor activation (Pinto et al., 2000). Generates proliferative stimuli for the fibroblast portion of cardiac cell population (Schelling et al., 1991). Increases DNA synthesis rate and proliferation of fibroblast (Tan et al., 1991). | |
| Cultured cardiomyocytes | Increases mRNA and protein levels of osteopontin through AT1 receptor (Ashizawa et al., 1996). | |
| Induces periostin expression through the activation of the Ras/p38 MAPK/CREB and the ERK1/2/TGF-β pathways (Li et al., 2011). | ||
| Induces apoptosis through AT receptor (Cigola et al., 1997). | ||
| Leads to a proinflammatory/profibrogenic phenotype and enhances reactive oxygen species production (Zhao et al., 2006). | ||
| Apoptosis and Inflammation | Aortic endothelial cells | Induces mitochondrial dysfunction via a protein kinase C-dependent pathway by activating the endothelial cell NADPH oxidase and formation of peroxynitrite (Doughan et al., 2008). |
| Actions in the central nervous system | ||
| Blood pressure regulation | NTS | Baroreceptor reflex suppression through: AT receptor activation (Lucius et al., 1998; Paul et al., 2006) and inhibition of ACE2 activity (Xia et al., 2009). Increases expression of GABAb receptor that could contribute to baroreceptor reflex suppression (Yao et al., 2008). |
| RVLM, PVN, SFO | Increased sensitivity of cardiac sympathetic afferents via AT1 receptor (Epstein et al., 1970; Xia et al., 2009). | |
| Supraoptic nuclei, PVN | Increases vasopressin released via AT1 receptor (Qadri et al., 1993). | |
| MnPO, OVLT, SFO, CVOs, and limbic structures | Increases water intake and NaCl intake via AT1 receptor activation (Mathai et al., 1997; Weisinger et al., 1997). The dipsogenic action involves participation of catecholamines released from neurons (Grossman, 1962) and could be mediated by NMDA receptors (Xu et al., 1997). | |
| Induces chronic activation of renal sympathetic nerve shifting renal function to higher blood pressure levels (Osborn and Camara, 1997). | ||
| The combination of angiotensin II and high salt intake increases splanchnic SNA and decreased renal SNA, creating a hemodynamic environmnet capable of producing sustained hypertension (Osborn and Fink, 2010). | ||
| Augments fluid intake and generates polyuria and chronic hyponatremia via AT1 receptor and increased adrenal steroids (Grobe et al., 2010). | ||
| Metabolism | Hypothalamus: increases metabolism and anorexigenic effect | Regulates food intake and weight gain through release of anorexigenic neuropeptide Crh via AT1 receptor (Yamamoto et al., 2011). |
| ICV infusion: promotes negative energy balance | Augments whole-body heat production and oxygen consumption, and reduces body adipose mass through increased sympathetic activation via increased β3-adrenergic receptor expression in brown and white adipose tissue (de Kloet et al., 2011). Induces a profound reduction in both subcutaneous and visceral adiposity (Grobe et al., 2010). Leads to enhanced brown adipose tissue thermogenesis and white adipose tissue lipolysis, possibly through AT2 receptor (Watanabe et al., 1999; de Kloet et al., 2011). | |
| Nervous system development | Microexplant cultures of the cerebellum | Increases elongation of neurites and cell migration in rat neonates via AT2 receptor (Cote et al., 1999). |
| Optic nerve | Promotes differentiation and axonal regeneration, and inhibits proliferation of neuronal cells via AT2 receptor stimulation (Lucius et al., 1998). | |
| Cultured neurons | Enhances UV radiation–induced apoptosis through AT2 receptor (Shenoy et al., 1999). After focal brain injury, can prevent damage of neurons or activate neural repair systems through AT2 receptor (Mogi et al., 2006). | |
| Reproductive system | Pituitary cells | Increases prolactin release and regulates intracellular Ca2+ levels (Diaz-Torga et al., 1998). |
| Visual system | Superior colliculus | Reduces the amplitude of visual evoked potentials through AT1 receptor (Merabet et al., 1997; Coude et al., 2000). |
| Behavior | Left CA1 hippocampal area | Facilitates learning and memory of rats (Belcheva et al., 2000). Increases the pain threshold through AT2 receptor (Georgieva and Georgiev, 1999). |
| Sympathetic nervous system | Superior cervical ganglia cells, sympathetic region of the thoracic and lumbar spinal cord | Increases the excitability and facilitates the action potential-induced release of norepinephrine (Lewis and Coote, 1993; Osborn et al., 2011). |
| Parasympathetic nervous system | Preganglionic neurons | Inhibits release of acetylcholine via a presynaptic mechanism (Potter, 1982). |
| Actions in the digestive system | ||
| Digestion and water and electrolyte absorption | Small intestine | Increases bicarbonate secretion (Johansson et al., 2001) and sodium and water retention, directly or through stimulation of sympathetic nervous system (Levens et al., 1981; Garg et al., 2012). |
| Large intestine | Increases sodium and water reabsorption (de los Rios et al., 1980), modulation of colonic motility (Fishlock and Gunn, 1970). | |
| Esophagus | Increases motility (Casselbrant et al., 2007). | |
| Inflammation | Stomach | High expression of AT1 receptor in Helicobacter pylori (Hallersund et al., 2011). Greater expression of AT1 receptor in cancer cells than in normal tissue (Kinoshita et al., 2009). Angiotensin II stimulates MAPK kinase, NF-κB, and surviving activation in cancer cells in vitro (Kinoshita et al., 2009). |
| Small and large intestine | Mucosal levels of angiotensin II are elevated in patients with Crohn’s colitis (Jaszewski et al., 1990). ARBs and ACE inhibitors have beneficial effects in rodent models of intestinal inflammation and autoimmune diseases. Reviewed in Garg et al. (2012). | |
| Actions in the clotting system | ||
| Platelet aggregation | Platelet | Increases platelet aggregation by a mechanism that involves AT1 receptor, AT2 receptor, and AT4 receptor (Senchenkova et al., 2010). Stimulates platelet activating factor synthesis (Neuwirth et al., 1989). Induces platelet activation through thromboxane A2 with a resultant increase in the initiation of coagulation (Farmer, 2000). Increases plasma β-thromboglobulin levels, surface expression of P-selectin, and platelet fibrinogen binding (Larsson et al., 2000). Induces changes in the cytosolic platelet proteome suggestive of premature aging of platelets (Gebhard et al., 2011). Elicits a dose- and time-dependent increase in platelet-leukocyte-endothelial cell interactions (Ishikawa et al., 2007). |
| Cerebral endothelial cells | Causes mild activation of the coagulation cascade with increases in plasma levels of thrombin-antithrombin complex and prothrombin fragment F1 + 2 (Larsson et al., 2000). | |
| Coagulation | Coagulation cascade proteins | Accelerates thrombosis (Senchenkova et al., 2010). |
| Thrombosis and fibrinolysis | Arterioles | At physiologic levels, stimulates PAI-1 production by bovine aortic endothelial cells (Vaughan et al., 1995). |
| Endothelial cells | Possibly AT4 receptor (Vaughan, 1997). Augments tissue factor expression, thus promoting thrombosis (Nishimura et al., 1997). | |
| T lymphocytes | T lymphocytes (CD4+ and CD8+) and NADPH oxidase-derived reactive oxygen species play a major role in mediating the accelerated microvascular thrombosis associated with angiotensin II–induced hypertension (Senchenkova et al., 2011). Angiotensin II–induced PAI-1 synthesis is mediated by AT1 receptor (Goodfield et al., 1999). | |
| Actions in the liver | ||
| Hemodynamics | Hepatocytes | Stimulates angiotensinogen synthesis by inhibiting adenylyl cyclase activity and stabilizing angiotensinogen mRNA (Klett et al., 1993). |
| In vivo | Decreases hepatic blood flow (Messerli et al., 1977) and raises portal pressure (Vlachogiannakos et al., 2001). | |
| Metabolism | Hepatocytes | Degrades glycogen (Hems et al., 1978) and stimulates gluconeogenesis (Whitton et al., 1978) through a non-Ca2+-dependent mechanism. |
| In vivo | Induces hyperglycemic effects by increased hepatic glucose output (Rao, 1996). Reduces triglyceride content in the liver via an AT1 receptor–dependent mechanism (Ishizaka et al., 2011). | |
| Inflammation | In vivo | Generates infiltration of inflammatory cells, oxidative stress, increases intercellular adhesion molecule and interleukin-6 hepatic gene expression (Moreno et al., 2009), activates NF-κB through ubiquitination of IKKβ via AT1 receptor (McAllister-Lucas et al., 2007). Generates hepatic steatosis via AT1 receptor (Nabeshima et al., 2009). |
AP-1, activator protein-1; ARB, angiotensin receptor blocker; ARHGEF1, Rho guanine nucleotide exchange factor 1; AT1, angiotensin II type 1 receptor; bFGF, basic fibroblast growth factor; CA1, carbonic anhydrase 1; CREB, cAMP response element-binding protein; Crh, corticotropin-releasing hormone; CVO, circumventricular organ; EGF, epidermal growth factor; ENaC, epithelial sodium channel; ERK, extracellular signal-regulated kinase; FAK, focal adhesion kinase; GFR, glomerular filtration rate; ICAM-1, intercellular adhesion molecule-1; ICV, intracerebroventricular; IGF1, insulin-like growth factor 1; IKKβ, IκB kinase complex; IP3, inositol trisphosphate; JAK, Janus tyrosine kinase; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MCP-1, monocyte chemoattractant protein-1; MMP-2, matrix metalloproteinase-2; MnPO, median preoptic nucleus; NF-κB, nuclear factor-κB; NMDA, N-methyl-d-aspartate; NO-GMP, nitric oxide-guanosine monophosphate; NTS, nucleus tractus solitarii; OVLT, organum vasculosum of the lamina terminalis; PAI-1, plasminogen activator inhibitor type 1; P130Cas, p130 Crk-associated substrate; PKCα, protein kinase Cα; PDGF, platelet-derived growth factor; PVN, paraventricular nucleus; PYK2, proline-rich tyrosine kinase 2; ROS, reactive oxygen species; RVLM, rostral ventrolateral medulla; SERCA2, sarco(endo)plasmic reticulum Ca2+ ATPase 2; SFO, subfornical organ; SNA, sulfosuccinimidyl acetate; SRC, Src (Sarcoma) family of tyrosine kinase; STAT, signal transducer and activator of transcription; VCAM-1, vascular cell adhesion molecule-1; VSMC, vascular smooth muscle cell.
Only angiotensin II effects on microvasculature are discussed.
B. Blood Pressure
In considering ACE, it is often important to discriminate between the effects of angiotensin II and other ACE substrates and products. There are two major approaches. First, mice having null mutations in ACE can be compared with mice lacking other components of the renin-angiotensin system, such as angiotensinogen or the AT1 receptor. A second method, usable in mice and humans, is to compare the results of an ACE inhibitor to those of an angiotensin II receptor antagonist. Both of these approaches indicate that ACE-mediated production of angiotensin II is critical in blood pressure regulation. In knockout mice with a genetic mutation that eliminates angiotensinogen, renin, ACE, or all AT1 receptors (i.e., AT1A and AT1B), the blood pressure is very markedly reduced (Tanimoto et al., 1994; Kim et al., 1995; Krege et al., 1995; Niimura et al., 1995; Esther et al., 1996; Sharp et al., 1996; Tsuchida et al., 1998). For example, as measured using a tail cuff manometer, a wild-type mouse averages a systolic blood pressure of about 110 mm Hg. This is essentially unchanged in mice with only a single functional copy of the ACE gene. In contrast, an ACE-null animal has an average systolic blood pressure of approximately 73 mm Hg. An equivalent reduction in blood pressure in animals lacking angiotensinogen, renin, or ACE indicates that, at least in mice, the effect of eliminating ACE activity is a marked inability to produce angiotensin II, and that this is the key feature resulting in low blood pressure. The blood pressure in a mouse lacking both ACE and the bradykinin B2 receptor did not differ from a mouse lacking just ACE (Xiao et al., 2003). Although equivalent genetic studies in humans are not possible, a recent comparison of the benefits of ACE inhibitors versus angiotensin II receptor antagonists for treating essential hypertension showed that both classes of inhibitors had similar long-term effects on blood pressure. This meta-analysis was not able to identify consistent differential effects on death, cardiovascular events, quality of life, or other outcomes (except cough) between ACE inhibitors and angiotensin II receptor antagonists in clinical trials (Matchar et al., 2008). Thus, these human studies are grossly analogous to the animal data in suggesting that the major role of ACE affecting blood pressure is the production of angiotensin II.
C. Bradykinin
Another important substrate of ACE is bradykinin, and as discussed, bradykinin levels are elevated in the absence of ACE activity. The kinin system, similar to the RAS, is composed of multiple peptides and receptors (Leeb-Lundberg et al., 2005). The two major active kinins are bradykinin and kallidin. The latter is a decapeptide and is very rapidly converted to bradykinin by the action of aminopeptidases. Bradykinin has a short half-life of approximately 15 seconds in plasma due to the action of multiple metalloproteases (Nussberger et al., 1999; Moreau et al., 2005). Neutral endopeptidase and ACE are the two major bradykinin-degrading enzymes (Ura et al., 1994; Duncan et al., 2000). Pharmacologic inhibition of ACE increases plasma bradykinin levels in patients, but has very little or no effect on the concentration of kallidin. Kinins bind two types of receptors called B1 and B2. The B2 receptor is the predominant receptor and is constitutively expressed, whereas the B1 receptor is induced by tissue injury such as ischemia and inflammation (Madeddu et al., 2007; Maurer et al., 2011). Leeb-Lundberg et al. (2005) reviewed the pharmacology and physiology of these receptors. In blood vessels, bradykinin binds the B2 receptor and induces the production of nitric oxide (NO) and the release of prostacyclin, resulting in vasodilation and increased vascular permeability (Sharma, 2009). In the kidney, bradykinin has natriuretic effects (Sharma, 2009; Katori and Majima, 2003). Inducing bradykinin formation in hypertensive models reduces blood pressure (Martorana et al., 1990; Xiong et al., 1995; Chao et al., 1998; Spillmann et al., 2002; Wang et al., 2004). Thus, besides reducing angiotensin II production, ACE inhibitors may contribute to the control of blood pressure by increasing the concentration of bradykinin. Mice that are genetically null for the B2 receptor have an increase in blood pressure on some genetic backgrounds, but this increase is much less or absent on a C57BL/6 genetic background (Trabold et al., 2002; Maestri et al., 2003).
D. Renal Development
Angiotensin II plays an important role in renal development, as shown by the phenotype of mice null for angiotensinogen, ACE, or all AT1 receptors. Such mice cannot effectively concentrate urine due to a marked expansion of the renal pelvis with a resulting underdevelopment of the renal medulla and papilla. In extreme cases, the renal medulla is virtually absent (Kim et al., 1995; Krege et al., 1995; Niimura et al., 1995; Esther et al., 1996; Tsuchida et al., 1998). The mice also show juxtaglomerular cell hypertrophy, medial thickening of small arteries and arterioles, interstitial fibrosis, and tubular dilatation (Niimura et al., 1995). These renal lesions are not present in a newborn ACE knockout mouse but begin to be seen by 16 days after birth (Ertoy and Bernstein, 2000). Renal pathology was also observed in rats treated with either an ACE inhibitor or an AT1 receptor antagonist during the first two weeks of life. Despite limited exposure to the drug, the adult animals demonstrated widening of the renal papillary space and a reduction in the ability to concentrate urine (Friberg et al., 1994; Guron et al., 1997).
Great insight into the role of angiotensin II in renal development was provided by Ichikawa and colleagues, who showed that the expansion of the renal pelvis results from a functional hydronephrosis and elevation of intrapelvic urinary pressure (Miyazaki et al., 1998; Matsusaka et al., 2002). In the absence of the angiotensin II AT1 receptor, hypoplastic development of smooth muscle along the renal pelvis and ureter occurs. Further, wild-type mice exhibit rhythmic pulsatile pressure elevations in the renal pelvis which, roughly every 2 seconds, cycle the renal pelvic pressure from about 5 to 15 mm Hg and then back down. This rhythmic contraction and the resulting peristaltic movement from the renal hilum toward the bladder was absent in mice lacking AT1 receptors. These mice showed a constant elevation of intrapelvic pressure to just under 20 mm Hg. Although an equivalent experiment has not been performed in ACE-null mice, the fact that these animals have a virtually identical phenotype strongly suggests that similar pathophysiologic mechanisms are at play.
There are several other abnormalities in the kidneys of ACE-null mice. These animals have a significantly reduced glomerular filtration rate (GFR) and single-nephron GFR due, undoubtedly, to their low blood pressure (Hashimoto et al., 2005). Although proximal tubular fractional reabsorption was normal in the ACE-null mice, tubuloglomerular feedback was essentially absent. Interestingly, a different line of mice with one ACE-null allele and a second ACE allele targeting ACE expression to the liver also showed a marked reduction in tubuloglomerular feedback, despite a normal blood pressure, GFR, and single-nephron GFR. The authors concluded that the expression of ACE in renal tissues was an important component of tubuloglomerular feedback. Additional work has shown that ACE-mediated intrarenal generation of angiotensin II works in conjunction with adenosine to induce afferent arteriolar contraction and regulate GFR (Schnermann and Briggs, 2008).
The studies in animals have correlations in humans, where reports have shown that ACE inhibitors taken during the second and third trimesters of pregnancy are associated with intrauterine growth retardation, neonatal hypotension, renal failure, oligohydramnios, and patent ductus arteriosus (Pryde et al., 1993; Quan, 2006). The kidneys of newborns exposed to ACE inhibitors show juxtaglomerular hyperplasia, dilatation of Bowman’s space, renal tubular dilatation, and increased cortical and medullary fibrosis. Similar effects on newborns have also been observed with AT1 receptor antagonists.
E. Role of Testis Angiotensin-Converting Enzyme
As discussed, early work identified testis as having abundant ACE (Cushman and Cheung, 1971). Although the enzyme was catalytically similar to somatic ACE (the isozyme of ACE made by somatic tissues), the molecular mass of testis ACE at about 95 kDa was substantially different from that of the somatic isozyme (El-Dorry et al., 1982a). Further, different mRNAs encoded the two isozymes, and they were regulated differently by hormones (El-Dorry et al., 1982b). Using immunologic approaches, testis ACE was identified in male germ cells, whereas somatic ACE was found in the epididymal epithelium, cells of the vas deferens, and within seminal fluid (Berg et al., 1986; Brentjens et al., 1986; Danilov et al., 1987).
Part of the mystery surrounding testis ACE was solved with the cloning of this isozyme (Ehlers et al., 1989; Kumar et al., 1989; Lattion et al., 1989; Howard et al., 1990). This showed a protein of 732 amino acids. In the human, the amino-terminal 67 amino acids (66 amino acids in the mouse) are not found in somatic ACE. The remainder of the protein (665 amino acids in the human, 666 amino acids in the mouse) is completely identical to the C-terminal sequence of somatic ACE. In other words, whereas somatic ACE is composed of two catalytic domains, testis ACE, after beginning with a unique sequence, comprises only a single catalytic domain, as well as the stalk, C-terminal transmembrane domain, and C-terminal intracytoplasmic tail, which are identical to the C-terminal portion of somatic ACE.
The mystery of this unique structure was solved when it was shown, using RNase protection and primer extension techniques, that mouse testis ACE transcription begins at the 13th exon of the ACE gene (Howard et al., 1990). This is 7.2 kilobases 3′ of the translation start site of somatic ACE in mice. Somatic tissues treat exon 13 as intronic and splice from exon 12 to exon 14. It is the male germ cell specific initiation of transcription at exon 13 that endows testis ACE with 66 amino acids of unique N-terminal sequence. After that, exon 14 and the remainder of testis ACE correspond exactly to the carboxyl half of somatic ACE. That male germ cells begin transcription at a different location from somatic tissues is due to a tissue-specific promoter located immediately 5′ of the transcription start site. This was demonstrated in two studies of transgenic mice in which Escherichia coli lacZ gene expression was placed under the control of the putative testis ACE promoter region, comprising either 682 or 91 base pairs of DNA immediately upstream from the start of testis ACE transcription (Langford et al., 1991; Howard et al., 1993). Mice transgenic for these constructs expressed β-galactosidase only within elongating spermatozoa within seminiferous tubules, a histologic pattern identical to that of testis ACE. Thus, these experiments establish that a testis-specific promoter is positioned between the somatic ACE N- and C-domains.
Detailed functional analysis of the testis ACE promoter demonstrated two important protein-binding motifs: a transcription factor II D (TFIID) binding site at −32 and a cAMP response element (CRE) at position −55 relative to transcription initiation (Zhou et al., 1995). Although both of these promoter elements are necessary for high-level expression, studies in transgenic mice established that neither DNA sequence is specific for testis promoter activity (Zhou et al., 1996a; Esther et al., 1997). In contrast, an isoform of the cyclic AMP response element modifier (CREM) family of transcription factors, CREMτ (tau), does appear to play an important role in testis-specific transcription (Zhou et al., 1996b).
The testis ACE promoter has several properties that render it useful in gene targeting. Specifically, the lack of transcriptional activity of this promoter in embryonic stem cells, but its high activity in developing male germ cells, allows the promoter to be used in targeting schemes in which testis ACE promoter–mediated expression of CRE recombinase is used to drive the self excision of a neomycin cassette (Bunting et al., 1999). Apart from developing male germ cells, no other tissue has been described as making the testis ACE isozyme.
In terms of function, male mice lacking ACE are severely compromised in their ability to reproduce (Krege et al., 1995; Esther et al.,1996). In contrast, reproduction in female mice lacking ACE is normal. This defect in males was shown to be directly attributable to the lack of testis ACE when mice expressing only testis ACE were studied (Hagaman et al., 1998). Male mice with this phenotype have low blood pressure, since they lack somatic ACE, but they reproduce normally. The role of testis ACE is unique, as male fertility was not restored in ACE knockout mice made transgenic for the expression of somatic ACE in sperm (Kessler et al., 2000). In contrast, an equivalent experiment performed with testis ACE did restore normal fertility (Ramaraj et al., 1998). This nonequivalence of the two isozymes of ACE, despite the correspondence of the testis isozyme to the C-terminal domain of somatic ACE, suggests a unique functional role for the testis isozyme. In addition, analysis of several lines of transgenic mice suggests that angiotensin II is not the crucial ACE product necessary for fertility. For example, the fertility of angiotensinogen-null mice (mice unable to produce angiotensin II) was reported as 8.3 pups per litter, which is quite normal for laboratory mice (Hagaman et al., 1998). Further, mice lacking both isoforms of the angiotensin II AT1 receptor have not been reported as having male reproductive defects (Tsuchida et al., 1998).
The light microscopic histology of testes from male mice null for ACE is not different from wild-type mice (Krege et al., 1995; Esther et al., 1996). Such mice have normal numbers of sperm and normal in vitro sperm motility. When mated with female mice, ACE-null animals inseminate females with normal frequency, as indicated by the presence of vaginal plugs. Further, when sperm from male ACE-null mice were collected from the uteruses of normal females 1 hour after mating, there were no significant differences in the mean number of sperm or the viability, mobility, capacitation, or acrosome reaction as compared with a similar analysis using wild-type males (Hagaman et al., 1998). However, the sperm count in the oviducts 1 hour after mating was only about 15% the number of sperm in the oviducts when females were mated with wild-type male mice and assayed under identical conditions. Further, in vitro sperm-ovocyte binding was significantly less when using sperm from ACE-null mice as compared with sperm from wild-type mice. In contrast to ACE-null mice, mice heterozygous for testis ACE isozyme expression (i.e., mice with one wild-type allele and one ACE-null allele) produce offspring in numbers equivalent to that of wild-type mice.
A major question in understanding the role of testis ACE is whether its enzymatic activity is critical for biologic function or whether the reproductive effect is only due to the presence of the testis ACE protein, perhaps in some structural capacity within sperm. This was examined in a mouse model in which testis ACE was expressed in its normal location and quantity but in an enzymatically inactive form, due to a genetic mutation eliminating zinc-binding and catalytic activity (Fuchs et al., 2005). Despite the presence of testis ACE protein, the lack of catalytic activity severely reduced male fertility, a phenotype identical to mice lacking testis ACE protein. In terms of magnitude of effect, consider that when six wild-type males were mated with wild-type females, 19 vaginal plugs were observed that resulted in 15 litters and 153 offspring. In contrast, seven male mice lacking testis ACE catalytic activity produced 22 vaginal plugs, but only one litter and one offspring. A major defect in the in vitro binding of sperm to ovocytes was also observed in that the binding of sperm from mice lacking testis ACE activity was only 4% the number of sperm from wild-type controls. Thus, male mice lacking testis ACE activity do mate, but this is so ineffective as to be highly noncompetitive in an evolutionary sense.
Recently, there was a report describing testis ACE as having direct glycosylphosphatidylinositol-anchored protein-releasing activity (Kondoh et al., 2005). However, this claim has been disputed by other research groups (Fuchs et al., 2005; Leisle et al., 2005).
As discussed by Muro and Okabe (2011), sperm from six different gene-disrupted mouse lines (calmegin, calsperin, ADAM1A, ADAM2, ADAM3, and testis ACE) all share the same phenotype of defective sperm oviduct-migrating ability and reduced binding to the zona pellucida (Yamaguchi et al., 2009). It was suggested that this may be due to aberrant ADAM3 function.
Given the evidence of a critical role for testis ACE in male fertility, we note that there have been no reports of human patients on ACE inhibitors having male reproductive defects (Manolis and Doumas, 2008). This may be due to an inability to completely and consistently inhibit testis ACE function with the administered dose of ACE inhibitor. Thus, testis ACE remains a mystery, particularly in terms of its exact role in enabling sperm function. What is intriguing is the possibility that pharmaceutical agents specific for the C-terminal domain of ACE, the domain present in testis ACE, could conceivably be used to induce male infertility without fully inhibiting somatic ACE (which also bears an N-terminal domain) and probably without reducing normal blood pressure.
F. Early Hematopoiesis
Several papers have now established that ACE and the renin-angiotensin system play a significant role in hematopoietic cell development (Hubert et al., 2006). In the chicken egg, ACE is found at 24 hours of development (stage HH6) in the vicinity of the blood islands in the yolk sac (Savary et al., 2005). During human embryonic development, hematopoietic cells are first found in yolk sac blood islands during the third week of development (Tavian et al., 1999). In the embryo proper, lymphomyeloid hematopoietic progenitors originate as clusters of CD34+CD45+ cells appearing on the ventral wall of the aorta-gonad-mesonephros region in the fourth week of human embryonic development (Tavian et al., 1996). However, even before that, on days 23–26, early CD34—CD45— hematopoietic progenitors are present in the para-aortic splanchnopleura underlying the aorta-gonad-mesonephros region (Cumano et al., 2001; Tavian et al., 2001). Remarkably, ACE is a marker identifying these early progenitors (Jokubaitis et al., 2008; Tavian et al., 2010). ACE+ cells sorted from the splanchnopleura generated colonies of hematopoietic cells over 40 times more frequently than ACE— cells (Sinka et al., 2012). ACE therefore appears as the earliest marker of prehematopoietic mesoderm inside the human embryo, and these ACE+CD34—CD45— mesodermal precursors migrate from the splanchnopleura toward the ventral aorta, to give rise to early intra-aortic hematopoietic clusters.
In mice and human fetuses, what are finally termed hematopoietic stem cells arise from bipotential hemangioblasts, which give rise to both hematopoietic and endothelial cells. Study of human embryonic stem-cell cultures showed that an ACE+CD34+/— CD45— phenotype mark these hemangioblasts (Zambidis et al., 2008). In fact, ACE surface expression was more reliable in identifying hematopoietic progenitors than CD34 expression. Once developed from hemangioblasts, hematopoietic stem cells continuously express ACE in human embryonic, fetal, and adult hematopoietic tissues and in all stages of hematopoietic ontogeny, including within the umbilical cord blood and fetal liver (Jokubaitis et al., 2008; Zambidis et al., 2008). NOD/SCID mice transplanted with CD34+ACE+ umbilical cord blood demonstrated higher levels of engraftment than those receiving CD34+ACE— cells, which exhibited low to undetectable levels of engraftment (Jokubaitis et al., 2008). A similar experiment using fetal liver and bone marrow–derived CD34+ACE+ cells showed that these cells, but not CD34+ACE— cells, are endowed with “long-term culture-initiating cell” potential and sustain multilineage hematopoietic cell engraftment when transplanted into NOD/SCID mice (Sinka et al., 2012). Thus, the presence of ACE is a hallmark of virtually all developing blood-forming tissues of the human embryo and fetus: para-aortic splanchnopleura, yolk sac, aorta-gonad-mesonephros, liver, and bone marrow.
In addition to marking progenitors, ACE has an important functional role in the normal development of early hematopoietic progenitors. For example, ACE inhibition reduced the ability of embryoid bodies to generate hematopoietic colony-forming cells by blocking hemangioblast expansion and differentiation toward either the endothelium or multipotent hematopoietic progenitors (Zambidis et al., 2008). Acute ACE inhibitor administration in mice prevented potential colony-forming cells from entering the cell cycle, and thus protected these cells from the lethal effects of chemotherapy or irradiation (Chisi et al., 2000). Further in vivo analysis of an irradiation model showed that ACE inhibitors preserved stem cells and bone marrow multilineage hematopoietic progenitors, including colony-forming unit (CFU) granulocyte/macrophage, burst-forming unit erythroid, and CFU megakaryocyte (Charrier et al., 2004). The protective effect appeared due to the loss of angiotensin II–mediated AT1 signaling. In another study, subcutaneous infusion of angiotensin II after sublethal irradiation or chemotherapy increased recovery in the number of bone marrow and blood hematopoietic progenitors, and accelerated the rebound in peripheral leukocyte number (Rodgers et al., 2002, 2003). Angiotensin II treatment also reduced the drop in platelets observed after irradiation and increased the number of megakaryocytes in the bone marrow. A similar effect was observed with angiotensin 1–7.
The pan-lineage proliferative effect of angiotensin II led to a more detailed investigation of mechanism. Administration of angiotensin II to bone marrow cultures stimulated colony formation of the relatively immature CFU granulocyte/macrophage and CFU granulocyte/erythrocyte/monocyte/megakaryocyte colonies under pan-myeloid culture condition [i.e., cell culture with stem-cell factor, granulocyte/monocyte colony-stimulating factor (CSF), interleukin 3 (IL-3), and erythropoietin] (Rodgers et al., 2000). However, neither angiotensin II nor losartan affected bone marrow formation of the more mature CFU granulocyte and CFU macrophage colonies during culture with “instructive” myeloid growth factors, such as granulocyte CSF, macrophage CSF, and granulocyte/macrophage CSF (Lin et al., 2011). Also, neither angiotensin II nor losartan affected CFU megakaryocyte colony formation in a defined megakaryocyte lineage assay. These data suggest that angiotensin II facilitates hematopoietic proliferation at an early differentiation point when the progenitors have not terminally committed to a particular lineage. Supporting this, the bone marrow from ACE knockout mice contains a 2-fold expansion in the number of Lin−Sca1+c-kit+ cells, a fraction of which are enriched for hematopoietic stem cells (i.e., early cells), but the numbers of more committed common myeloid progenitors and granulocyte/macrophage progenitors are normal (Lin et al., 2011).
If anything, the most recent studies indicate that the effects of ACE on hematopoiesis are quite complex. In normal mice, ACE inhibition shows a biphasic modulation of bone marrow progenitor proliferation. Short-term ACE inhibition (2 days) suppressed the G0 to G1 transition of Lin− cells (i.e., bone marrow progenitor cells) (Davis et al., 2010). This effect of short-term ACE inhibition reduces the potential short-term injury to stem cells, and is what was seen in the irradiation experiments described earlier that resulted in enhanced stem-cell survival (Charrier et al., 2004). In contrast, longer-term treatment with ACE inhibitors (greater than 7 days) appeared to increase the proliferation of bone marrow progenitors (Davis et al., 2010). The paradoxical effects between acute versus chronic ACE inhibition imply that ACE regulates hematopoiesis through multiple peptides and/or pathways.
Finally, we note that one of the physiologic substrates of ACE is the 4–amino acid peptide AcSDKP; when normal volunteers were administered, ACE inhibitors, plasma, and urine levels of AcSDKP increased 5-fold, showing that ACE is the major enzyme responsible for the degradation of this peptide (Azizi et al., 1996). AcSDKP is the N-terminal sequence of its precursor thymosin β4, from which it is released by proteolytic cleavage. Initial investigations of AcSDKP indicated that this peptide inhibited the recruitment of primitive hematopoietic progenitors into active proliferation (Bonnet et al., 1993; Waeckel et al., 2006). Thus, by degrading AcSDKP, ACE may help recruit stem cells into the S phase.
G. Erythropoiesis
An early study of the ACE inhibitor enalapril in both hypertensive patients and normal volunteers found a small reduction in hematocrit levels (Griffing and Melby, 1982). Others have reported anemia as a side effect of treatment with ACE inhibitors (Pratt et al., 1992). ACE-null mice have a mild normocytic anemia, with the hematocrit consistently lowered by approximately 20% as compared with control mice (Esther et al., 1996). However, ACE knockout mice also have other abnormalities, including reduced renal function, which could induce a secondary anemia.
To understand the anemia, Cole et al. (2000) studied a mouse line expressing a truncated form of ACE in which the enzyme was secreted from tissues. In these mice, plasma ACE activity was 35% the normal level, and there was no evidence of renal insufficiency, but the mice did have anemia similar to ACE-null mice. The anemia was due to a reduction of red blood cell mass, and not volume expansion. In this model, a 2-week infusion of angiotensin II corrected the hematocrit to near wild-type levels, strongly implicating a direct role of angiotensin II in erythropoiesis. Consistent with this finding, angiotensin II receptor antagonists reduce hemoglobin, hematocrit, and erythrocyte counts in rats (Naeshiro et al., 1998).
The role of ACE and angiotensin II in erythropoiesis is complex. First, in vitro culture suggests that angiotensin II is associated with the production of endogenous erythropoietin from peritubular fibroblasts of the kidney (Vlahakos et al., 1995). However, there is still uncertainty about the exact relationship of ACE inhibition and erythropoietin levels in vivo. Some studies suggest that the administration of ACE inhibitors reduces plasma erythropoietin levels or induces resistance to this hormone (Walter, 1993; Gossmann et al., 1996; Albitar et al., 1998). Angiotensin II modulation of erythropoietin production is hypothesized to occur via activation of AT1 receptors and/or altered renal hemodynamics (Gossmann et al., 2001; Benohr et al., 2004). However, other studies found no causative link between erythropoietin and anemia induced with ACE inhibitors or AT1 receptor antagonists (Perazella et al., 1995; Charytan et al., 1998; Chew et al., 1999). Second, angiotensin II is a mitogen for erythroid progenitors. In vitro culture of either human peripheral blood mononuclear cells or murine bone marrow cells showed that burst-forming unit erythroid and CFU erythroid were elevated by the addition of angiotensin II in the culture medium (Mrug et al., 1997; Lin et al., 2011). This effect was inhibited by losartan. Further, losartan alone retarded the formation of CFU erythroid. It should be noted that a variety of clinical reports have observed an association between activation of the renin-angiotensin system and increased erythropoiesis (Jensen et al., 1993; Julian et al., 1994; Vlahakos et al., 1999; Cole et al., 2000). These studies come from analyses of patients with a variety of chronic diseases, including chronic obstructive pulmonary disease, heart failure, and renal transplantation.
H. Myelopoiesis
Besides erythropoiesis, ACE appears to affect myelopoiesis. This is best appreciated in ACE knockout mice that have increased numbers of immature myelocytic cells (myeloblasts and myelocytes) in their bone marrow (Lin et al., 2011). These mice also have diminished numbers of mature segmented neutrophils in the bone marrow. Extensive extramedullary hematopoiesis is present in ACE knockout mice, with collections of CD11b+Gr.1low/— immature myeloid cells in the subcapsular portion of the spleen. Moreover, ACE knockout mice have an increased susceptibility to Staphylococcus aureus infection (Okwan-Duodu et al., 2010).
In vitro analysis of bone marrow proliferation and differentiation showed that ACE inhibition expanded the number of colony-forming units (i.e., increased proliferation) but slowed the formation of mature myeloid cells (decreased maturation), which is consistent with the bone marrow phenotype of ACE knockout mice (Lin et al., 2011). Substance P (SP) is a peptide that affects bone marrow development by stimulating production of several myeloid growth factors, such as IL-1, IL-3, stem-cell factor, and granulocyte/monocyte CSF by bone marrow stromal cells (Rameshwar et al., 1993; Rameshwar and Gascon, 1995). ACE is a major peptidase responsible for hydrolyzing (and inactivating) SP, and elevated SP is found in the bone marrow of ACE knockout mice (Lin et al., 2011). In fact, ACE appears to regulate myeloid proliferation through SP. ACE inhibitor treatment increases the number of colonies seen in an in vitro myeloid colony-forming assay. However, when the ACE inhibitor–treated cultures were coincubated with either an anti–SP-neutralizing antibody or an SP receptor antagonist, the increase in colony numbers was completely eliminated. ACE inhibitor–mediated myeloproliferation was not observed using purified hematopoietic stem cells, but was found when these cells were cocultured with a stromal cell line, implying an important role for bone marrow stromal cells in modulating myeloproliferation, possibly by SP production.
In addition to destroying SP, ACE also produces angiotensin II, and it is the lack of angiotensin II in the ACE knockout bone marrow that retards myeloid maturation. During culture of bone marrow under conditions promoting myeloid differentiation, wild-type bone marrow Lin−c-kit+ progenitors showed a slower upregulation of the myeloid maturation markers FcγR, Gr.1 and F4/80 when treated with either an ACE inhibitor or an AT1 receptor antagonist (Lin et al., 2011). These data suggest that AT1 receptor signaling is required for terminal myeloid differentiation. In studies using AT1 receptor knockout hematopoietic cells, Tsubakimoto et al. (2009) also concluded that AT1 signaling was required for normal macrophage development.
Although blockade of the AT1 receptor slowed myeloid differentiation, macrophage production was ultimately not blocked since, in steady state, both AT1 receptor knockout mice and ACE knockout mice have normal numbers of monocytes and macrophages in the bone marrow and in peripheral blood (Tsubakimoto et al., 2009; Lin et al., 2011). When wild-type bone marrow cultures were treated with losartan, differentiated F4/80high macrophages developed with normal morphology, but these cells showed functional immaturity, as indicated by decreased tumor necrosis factor α (TNFα) secretion, decreased CD86 upregulation, and a decreased oxidative burst (Lin et al., 2011). Macrophages from ACE knockout mice had similar functional defects, but angiotensin II administration in the mice partially or completely reverted macrophage function to that of cells from wild-type mice. On a transcriptional level, CCAAT/enhancer-binding protein α upregulation, a key transcriptional event stimulating myelopoiesis, was suppressed in wild-type bone marrow cultures treated with losartan. Macrophages derived from ACE knockout mice also had depressed CCAAT/enhancer-binding protein α expression that was rescued by angiotensin II administration.
I. Immune Response
1. Major Histocompatibility Complex Class I
The surface presentation of peptides by proteins comprising the major histocompatibility complex (MHC) class I molecules is a critical part of almost all CD8+ T cell–adaptive immune responses (Jensen, 2007). MHC class I molecules are on the surface of all nucleated cells, and thus all cells bear the biochemical machinery necessary to process proteins into peptides and load these peptides onto MHC class I molecules for cell-surface display. A major function of MHC class I molecules is to display viral proteins on a virally infected cell, leading to activation of T cells and destruction of the cell. That ACE may play a role in the processing of such peptides was first suggested in 1992, when serum ACE activity was found capable of trimming a synthetic influenza antigen into a form capable of being bound and presented by MHC class I molecules (Sherman et al., 1992). Using a cell-free system, a second group described serum ACE activity as necessary to trim the C terminus of a peptide epitope of human immunodeficiency virus 1 into the final presented 10-mer peptide (Kozlowski et al., 1992; Nakagawa et al., 2000). Although these reports of extracellular processing are interesting, MHC class I peptides are typically generated within antigen-presenting cells. The first evidence that ACE is capable of affecting intracellular class I peptide processing was presented by Yewdell and colleagues (1992). This group used a vaccinia virus expression system to overexpress ACE in a fibrosarcoma cell line and convincingly demonstrate that ACE was capable of processing the influenza nucleoprotein peptide M147-158/R− into the final MHC class I epitope M147-155 via C-terminal dipeptide release. Further analysis showed that the M147-158/R− peptide was transported to the endoplasmic reticulum, where it was cleaved by ACE. The authors speculated that ACE acted on the peptide prior to antigen binding to MHC class I molecules. This study used ACE overexpression as an artificial tool to investigate the biochemistry of MHC class I peptide transport and trimming. The authors concluded that peptidases do play a role in final antigen processing. However, the authors indicated that they were not suggesting that ACE normally plays a role in antigen processing.
Little follow-up work was performed on the role of ACE and MHC class I. In fact, the detailed study of antigen processing led to the belief that carboxypeptidases do not play a natural role in MHC class I peptide trimming (Craiu et al., 1997; Kunisawa and Shastri, 2003). In 2008, this question was re-examined using genetically modified macrophages and other cells designed to overexpress ACE (Shen et al., 2008). Several different antigens were studied, and antigens were presented to cells in a variety of fashions. All studies suggested that the overexpression of ACE was able to cleave MHC class I peptide precursors within the endoplasmic reticulum. Additional in vivo experiments using an adoptive transfer strategy with wild-type macrophages or macrophages overexpressing ACE demonstrated enhanced generation of antigen-specific CD8+ T cells in mice receiving the macrophages overexpressing ACE. Thus, this work was similar to that of Eisenlohr et al. (1992) in that it showed that ACE overexpression had significant effects on antigen presentation.
Understanding the physiologic role of ACE in MHC class I antigen processing began with experiments showing that macrophages and dendritic cells (antigen-presenting cells) increase their expression of ACE following immune activation, either in vitro by interferon γ (IFNγ) or in vivo by infection of a mouse with Listeria monocytogenes (Shen et al., 2011). Such data suggested a role of ACE in response to immune challenge. However, the most revealing experiments were obtained using a cross-immunization approach. If a mouse is immunized with completely syngeneic cells, the recipient will be tolerant of the immunizing cells and there will be no CD8+ T cell activation, as measured by the absence of IFNγ production by these cells. However, if female mice are immunized with male syngeneic cells, some CD8+ T cells from the female mouse will react to the male-specific H-Y peptide epitopes Smcy and Uty presented by MHC class I molecules (Simpson and Roopenian, 1997). Such an experiment showed that when wild-type female mice were immunized with male cells from ACE knockout mice, the female recipients’ response to the H-Y peptides was only approximately 50% that of female ACE wild-type mice immunized with cells from a male ACE wild-type mouse. When this type of protocol was used to test the response not just to the H-Y peptides but to all of the different MHC class I peptides present on cells from ACE knockout or wild-type mice, there was a substantial difference in the peptide epitopes presented by the two populations of cells. The difference was so large that, when immunized, wild-type mice recognized the cells of an ACE knockout mouse as foreign, even if the two animals were the same sex and matched for genetic background. Perhaps the most convincing experiment was one in which wild-type recipient mice were immunized with cells from sex- and background-matched ACE knockout mice. Splenocytes from the recipients were then restimulated with wild-type cells for ACE (i.e., syngeneic to the original recipient mice). Not surprisingly, the challenge of wild-type splenocytes for ACE with identical cells resulted in essentially no CD8+ T cell activation. However, when the same experiment was performed, but the restimulating cells were genetically wild-type for ACE but from a wild-type mouse treated for several days with an ACE inhibitor, they now induced a significant CD8+ T cell response. In other words, syngeneic cells from an animal treated with an ACE inhibitor were no longer recognized as being immunologically identical; presumably, the treatment with the ACE inhibitor changed the peptides presented on the cell surface.
An analysis of the role of ACE in MHC class I peptide processing is significant in that it demonstrates that carboxypeptidases play an important role in the natural processing of the MHC class I repertoire. But more than just a theoretical understanding of MHC class I processing, the differences in response suggest that there may be differences in how animals lacking ACE respond to viral challenge, since the immune response is so dependent on MHC class I–mediated activation of T cells. In fact, analysis of how wild-type and ACE knockout mice respond to viral epitopes did show differences in response to known polyoma viral epitopes (Shen et al., 2011). Although further work is necessary to investigate the role of ACE in immunogenic peptide processing, several conclusions are appropriate. The finding that ACE affects presentation of immunologic peptides, and that ACE is upregulated by macrophages and dendritic cells after infection with listeria, raises the question of whether this ACE upregulation diversifies the presented MHC class I peptides and enhances the immune response. It is also important to recognize that, in humans, the natural immune response consists of many different overlapping responses, and we know of no credible evidence that medical treatment with ACE inhibitors significantly reduces immune function.
2. Role of Angiotensin II
It has become increasingly clear that ACE and angiotensin II play an important role in the immune response. The following section presents a summary of some of the known effects of angiotensin II on basic immune processes. In a separate section, we present immune effects of ACE that are independent of angiotensin II generation.
a. Chemotaxis
As measured in a Transwell culture chamber, angiotensin II induces the migration of human peripheral blood monocytes and THP1 cells, a human monocytic leukemia cell line (Kintscher et al., 2001). The angiotensin II–induced migration was inhibited by losartan in a dose-dependent manner, implicating AT1 receptor activation. It was found that angiotensin II induced its effects, in part, through the phosphorylation of Pyk2 and paxillin 2, cytoskeleton-associated proteins involved in cell movement. Angiotensin II also induced c-src activation.
Recently, studies by Swirski et al. (2009) showed that the spleen is a reservoir for monocytes, and that, after acute myocardial ischemic injury, approximately 40% of monocytes recruited to the area of injury originate from this splenic pool. In contrast, AT1A receptor knockout mice do not effectively mobilize splenic monocytes following cardiac injury. An additional study showed that splenic monocytes increase their motility and egress from the spleen in response to angiotensin II (Leuschner et al., 2010). Injury-induced splenic release of monocytes was inhibited by the ACE inhibitor enalapril. This was not due to the vasodilatory action of blocking angiotensin II production, as the nonspecific vasodilator hydralazine did not have an equivalent effect. The authors concluded that one of the benefits of ACE inhibition after myocardial infarction was the antagonism of the angiotensin II–mediated chemotactic mobilization of the splenic monocyte reservoir and, as a result, a reduction in the inflammatory infiltrate at the site of myocardial injury (Leuschner et al., 2010).
b. Stromal attachment
Angiotensin II increases monocyte adhesion to endothelial cells, an important early step in the inflammatory process (AbdAlla et al., 2004). Furthermore, endogenous production of angiotensin II seems required for optimal T-cell function early in inflammation (Silva-Filho et al., 2011). Specifically, the use of an ACE inhibitor or an AT1 receptor antagonist in an in vitro assessment of T-cell activation had several effects, including reducing the expression of the activation markers CD25+ and CD69+, reducing the in vitro adhesion of activated T cells to fibronectin or laminin, and reducing the transmigration of these cells, as measured in an in vitro Transwell migration assay.
c. Activation
As indicated previously, ACE-mediated generation of angiotensin II affects the expression of T cell activation markers, including CD44 (Guzik et al., 2007; Silva-Filho et al., 2011). Additional experiments have shown that intrinsically generated angiotensin II is also important for T-cell expression of the important cytokine TNFα (Hoch et al., 2009). Perhaps even more important is the concept that ACE and angiotensin II can induce or suppress specific classes of T cells. This is particularly important in autoimmunity, and has recently been investigated in murine experimental autoimmune encephalomyelitis (EAE), a model mimicking multiple sclerosis (Platten et al., 2009). In this study, proinflammatory Th1 and Th17 cells play a critical role in the inflammatory response. Induction of these cells was associated with an increase in angiotensin II in both CD4+ T cells and CD11b+ monocytes. The pretreatment of mice before the induction of autoimmunity with either an ACE inhibitor or an AT1 receptor antagonist suppressed the number of Th1 and Th17 T cells and increased the number of FoxP3+ T-regulatory cells. Even more striking, the administration of the ACE inhibitor lisinopril after the establishment of EAE actually reversed the severity of animal paralysis. Both in their original study and in a follow-up investigation, this group identified angiotensin II–induced expression of transforming growth factor-β as being important in the pathogenesis of EAE (Lanz et al., 2010). Several other studies have demonstrated a role of ACE and angiotensin II in models of autoimmunity, including rheumatoid arthritis and experimental autoimmune uveoretinitis (Dalbeth et al., 2005; Sagawa et al., 2005; Okunuki et al., 2009). Other studies have found the renin-angiotensin system as pivotal in inducing central nervous system autoimmune inflammation (Stegbauer et al., 2009). Recently, a review by Lühder et al. (2009) summarized some of the effects of ACE and angiotensin II on inflammatory cells, and additional studies have demonstrated the role of the RAS in autoimmune demyelinating diseases.
d. Production of reactive oxygen species
It is well known that angiotensin II can induce an oxidative burst in the endothelium, smooth muscle, and macrophages (Lassègue et al., 2012). This is also true in T cells. Angiotensin II exposure increases T cell expression of NADPH oxidase subunits P47phox, P22phox, and Nox2, resulting in a marked increase in the ability of T cells to produce reactive oxygen species, which in turn influences parameters of T cell activation (Guzik et al., 2007).
3. Immune Responses of Angiotensin-Converting Enzyme Not Associated with Angiotensin II
a. ACE-mediated anti-tumor response
Evidence of an important role of ACE independent of angiotensin II comes from the analysis of a mouse model called ACE 10/10. In this model, gene-targeting approaches were used to inactivate the intrinsic ACE promoter and place ACE expression under the control of the c-fms promoter (Shen et al., 2007). c-fms is expressed in high levels by myelomonocytic lineage cells as it encodes the receptor for macrophage colony-stimulating factor (Himes et al., 2001; Sasmono et al., 2003). The result is that mice homozygous for the modified ACE allele (i.e., ACE 10/10) substantially overexpress ACE in monocytes, macrophages, Kupfer cells, and other myelomonocytic lineage cells. However, these animals lack ACE expression by endothelial cells, renal epithelial cells, and other tissues that do not recognize the c-fms promoter. Care must be taken in extrapolating results from this ACE overexpression model to the “normal” function of ACE, due to both the high ACE expression level in monocytes and the unusual tissue pattern of ACE expression in these mice. In other words, this model is akin to using ACE in a pharmacologic fashion.
Despite the marked change in tissue patterns of ACE expression, the basal physiology of the model is equivalent to wild-type mice. Serum ACE levels are in the normal range, and the mice have a normal blood pressure, renal function, and histologic appearance of both bone marrow and peripheral blood (Shen et al., 2012). Further, the model has normal testis ACE expression and thus normal reproductive behavior. However, several studies now indicate that the ACE 10/10 mice have a marked enhancement of both their innate and adaptive immune responses that becomes evident when the mice are immunologically challenged. This was first observed when these animals were challenged with the B16 melanoma, an aggressive mouse neoplasm that is often used to study mouse tumor immunology (Becker et al., 2010). Two weeks after a challenge with an intradermal implantation of B16 cells, tumor size averaged 540 mm3 in wild-type mice and 252 mm3 in heterozygous mice, but only 90 mm3 in ACE 10/10 mice (Fig. 2). This enhanced immune response was associated with a marked increase in the number of inflammatory cells, particularly monocytes and macrophages adherent to tumor vessels and infiltrating the tumor tissue itself. Analysis of the immune response using a B16 tumor line expressing ovalbumin indicated that the number of CD8+ T cells reactive against either the major MHC class I epitope of ovalbumin or the major MHC class I epitope of tyrosinase-related protein-2 was increased. Finally, in vitro studies suggested that, in response to immune challenge, macrophages from ACE 10/10 mice produced higher quantities of the proinflammatory cytokines IL-12 and nitric oxide. In contrast, macrophage production of the anti-inflammatory cytokine IL-10 appeared reduced.
Fig. 2.
Response of ACE 10/10 mice to challenge with melanoma. B16-F10 melanoma cells were injected into the skin of ACE 10/10, ACE 10/ACE wild-type heterozygous (HZ), and ACE wild-type (WT) mice. Two weeks later, the mice were sacrificed and the tumor volume was measured. Data points from individual mice are shown, as well as group means and S.E.M. ACE 10/10 mice consistently showed much smaller tumors than wild-type mice (Shen et al., 2007).
There are three important points to emphasize in the ACE 10/10 model. First, the enhanced immune response is due to the enzyme activity of ACE expressed by inflammatory cells and not the absence of ACE expression by the endothelium and other tissues. This was demonstrated through bone marrow transplantation: when wild-type mice, animals having endothelial ACE, were transplanted with bone marrow from either wild-type or ACE 10/10 mice, the recipients of ACE 10/10 bone marrow resisted B16 tumor growth far better than the animals receiving wild-type bone marrow. In addition, treatment of ACE 10/10 mice with an ACE inhibitor eliminated the increased adaptive immune response such that ACE 10/10 mice demonstrated tumor growth equivalent to similarly treated wild-type mice.
The second major point is that the enhanced immune response of the ACE 10/10 mice is almost certainly due to a response other than increased production of angiotensin II. This was demonstrated in two ways (Shen et al., 2007). First, whereas ACE 10/10 mice treated with an ACE inhibitor demonstrated tumor growth equivalent to that of wild-type mice, a similar experiment performed with an AT1 receptor antagonist had no effect on tumor growth, i.e., tumor size in the ACE 10/10 animals treated with an AT1 receptor antagonist was significantly smaller than equivalently treated wild-type controls. Second, when double-mutant ACE 10/10 angiotensinogen knockout mice were compared with angiotensinogen knockout mice with wild-type ACE, the animals with the ACE 10/10 phenotype had significantly smaller tumor growth. Thus, in the absence of all ability to produce angiotensin II, the ACE 10/10 mutation still led to a marked enhancement of the immune response. These data provide strong evidence that, whatever the precise mechanism endowing the ACE 10/10 model with an increased antitumor immune response, it is due to a catalytic effect of ACE not dependent on the conversion of angiotensin I to angiotensin II.
Although the precise substrate created or destroyed by myelomonocytic expression of ACE is not yet known, there is some insight into the phenotypic means of achieving an increased immune response. Investigations into the developmental phenotype of macrophages have demonstrated distinct subsets of macrophages with very different biologic responses. Classically activated (or M1) macrophages are highly inflammatory cells that are thought to play a major role in defending against acute infection and tumor growth (Mantovani et al., 2004; Gordon and Taylor, 2005). In contrast, alternatively activated (or M2) macrophages appear later in the inflammatory response. Such cells are angiogenic, profibrotic, and are thought responsible for the suppression of the immune response, including allowing tumor growth. In characterizing the behavior of macrophages in the ACE 10/10 mice, the cells appear shifted toward an M1 phenotype in that their cytokine profile of elevated IL-12, TNFα, nitric oxide, and a reduced production of IL-10 is consistent with what has been described for M1 macrophages. Thus, in response to immune challenge, the overexpression of ACE by myelomonocytic cells may affect their phenotypic differentiation. This hypothesis is consistent with previously discussed studies defining a role for ACE in myelopoiesis.
b. Infection
The increased immune response of the ACE 10/10 mice is not restricted to just the B16 melanoma. A similar enhanced immune response was also observed with skin implantation of EL4 lymphoma cells (Shen et al., 2007). Further, ACE 10/10 mice show a markedly enhanced innate immune response after challenge with either L. monocytogenes (listeria) or methicillin-resistant S. aureus (MRSA) (Okwan-Duodu et al., 2010). After a challenge with either bacteria, ACE 10/10 mice showed a substantially enhanced ability to limit infection, as detected by both the number of viable bacteria and, in the case of MRSA, the ability to limit and ultimately heal the skin ulcerations associated with acute dermal infection. For example, four days after ACE 10/10 or wild-type control mice were injected with intradermal MRSA, the quantity of bacteria in the skin of the wild-type mice was 50-fold greater than that present in the ACE 10/10 mice. As with the tumor studies, the treatment of ACE 10/10 mice with an ACE inhibitor (but not an AT1 receptor antagonist) caused a response to infection equivalent to similarly treated wild-type mice.
A critical part of the increased innate immune response present in the ACE 10/10 model is the increase in inducible nitric oxide synthase (iNOS) and NO production by ACE 10/10 macrophages. This is known to be a prime mechanism used by phagocytic cells to kill bacteria, and appears crucial in the enhanced response of the ACE 10/10 mice (MacMicking et al., 1997; Shiloh et al., 1999). When cells from these animals were challenged with listeria or lipopolysaccharide, the cells showed a marked increase in both iNOS and NO production as compared with wild-type cells (Okwan-Duodu et al., 2010). Further, treatment of the ACE 10/10 mice with the iNOS inhibitor 1400W [N-(3-(aminomethyl)benzyl)acetamidine] rendered these animals equivalent to wild-type mice in their response to bacterial challenge.
Study of the in vitro ability of the ACE 10/10 macrophages to kill listeria suggested that the ACE 10/10 phenotype changed the pattern of monocytic differentiation (Okwan-Duodu et al., 2010). In the absence of immune stimulation, the cells from ACE 10/10 and wild-type mice were equivalent in function. Only when the ACE 10/10 cells were stimulated with an immune activator, such as IFNγ, was a marked enhancement of in vitro killing observed, as compared with similarly treated wild-type cells. The increased killing exhibited by ACE 10/10 cells could be inhibited by an ACE inhibitor, but not when the inhibitor was acutely administered; only when ACE 10/10 mice were treated with an ACE inhibitor for several days were their cells equivalent to wild-type cells in terms of ability to kill bacteria.
J. Angiotensin-Converting Enzyme Signaling
Fleming (2006) has suggested a novel role for ACE as a signaling molecule. This study used coprecipitation to show that various intracellular signaling molecules physically associate with the intracellular C-terminal tail of ACE. These include casein kinase 2, mitogen-activated protein kinase kinase 7, and c-Jun N-terminal kinase (Kohlstedt et al., 2002, 2004). In response to ACE inhibitor administration, phosphorylation of ACE residue Ser1270 initiates a signaling cascade with nuclear translocation of phosphorylated c-Jun, leading to changes in gene expression, including cyclooxygenase-2 and ACE itself (Kohlstedt et al., 2004, 2005). This group has also presented data for ACE inhibitor-induced ACE signaling in adipocytes (Kohlstedt et al., 2009). While the work from Fleming found ACE signaling in response to ACE inhibitors, Guimarães and colleagues have published data indicating that the binding of angiotensin II to ACE can trigger intracellular calcium signaling (Guimarães et al., 2012). Hopefully, additional studies will determine the exact physiologic significance of ACE mediated signaling.
V. Evolutionary Implications of Two Angiotensin-Converting Enzyme Catalytic Domains
A. Introduction
A surprise determined from the cloning of ACE cDNA was that somatic ACE is composed of two separate catalytic domains. This was because previous analyses of both the zinc content of ACE and the kinetics of catalysis had indicated only a single catalytic site (Bull et al., 1985b; Strittmatter and Snyder, 1986). When ACE was first cloned, it was unclear whether both domains of somatic ACE in vivo were in fact catalytic (Cumin et al., 1989). However, these doubts were removed when each domain was separately expressed and shown to be independently catalytic (Wei et al., 1991a).
In humans, ACE maps to chromosome 17 (chromosome 11 in mice) (Jeunemaitre et al., 1992; http://www.informatics.jax.org/marker/MGI:87874). The gene is encoded by 26 exons encompassing 21 kilobases of DNA (Hubert et al., 1991). Somatic ACE comprises exons 1–12 and14–26, whereas testis ACE is composed of exons 13–26. There is very strong evidence that the modern structure of ACE is the result of an ancient gene duplication. First, both catalytic domains of ACE are homologous in amino acid sequence. Further, exons 4–11 and 17–24 are identical in size and codon phases at the exon-intron boundaries (Hubert et al., 1991). In many ways, the two domains of ACE are the central feature of this enzyme. Genetic studies have indicated that, as measured on an evolutionary time frame, genetic duplication is not an extraordinary event. However, most genes undergoing genetic reduplication will not confer sufficient advantage to be selected and fixed into the genome (Hurles, 2004). For example, a genetic change that confers a 10% fitness advantage (a very large advantage) has only about a 20% chance of becoming widely incorporated into a species’ genome (Cochran and Harpending, 2009). Nonetheless, approximately 1% of genes are duplicated and fixed every million years (Lynch and Conery, 2000). Even in those instances in which genetic change does become fixed in the genome, the long-term outcome of having two copies of a gene is variable. As evolution progresses, both copies of the gene undergo mutation. Such a process often leads to one copy of the gene becoming inactivated (i.e., a pseudogene). For example, it has been estimated that in the human and in the mouse, about half of new gene copies become inactive within 7.3 million years (Pennisi, 2000). Importantly, another outcome is that over time, the two genes diverge in their biologic function. Concerning ACE, evidence suggests that the two catalytic domains have different biologic functions.
B. Distribution in Nature
ACE-related proteins have been biochemically characterized in mammals, insects (fruit flies, domestic flies, moths, and mosquitos), invertebrates (oysters, mussels, and crayfish), protozoa (Leishmania), and bacteria (Rivière, 2009). In addition, the increasing use of genome-wide DNA sequencing has resulted in many species in which sequences of substantial regions of genomic DNA are available. We have used in silico approaches to search available databases and compile ACE orthologs. Using this approach, the number of species in which ACE-related proteins can be found is very large (Fig. 3). The search looked for a sequence comparison with the sequence of the human ACE gene, especially in the protein domains surrounding the ACE zinc-binding site HEXXH. By definition, such an approach is not a biochemical characterization of whether these proteins cleave angiotensin I or are inhibited by a classic ACE inhibitor. However, the presence of ACE-like genes in organisms as distant as prokaryotes indicates that zinc-containing ACE-like enzymes emerged early during evolution and at a time point well before blood pressure was physiologically relevant (Rivière, 2009). To our knowledge, ACE-like enzymes are absent from all plant genomes currently available.
Fig. 3.
Phylogenetic tree of ACE. (A) Human ACE amino acid sequence was used to search several publically available databases. Retrieved sequences were aligned for homology and plotted in tree form using JACOP software (http://myhits.isb-sib.ch/cgi-bin/jacop). The degree of homology is reflected in the position of each sequence, with more homologous sequences clustered together. The retrieved ACE homologs consist of proteins with two (blue), one (green), or no putative zinc binding-sites (black). Human ACE is indicated with an arrow. (B) The top panels show a diagonal dot plot that was prepared using Dotlet software (http://myhits.isb-sib.ch/cgi-bin/dotlet). The amino acid sequence of human ACE is positioned along the vertical axis, and is compared with the amino acid sequence indicated along the horizontal axis. The program used a sliding window of 39 amino acids with a grayscale dot that varies from 100% (white dot) to 0% (black dot). Comparing human ACE against itself produces a 45° diagonal of identity, as well as two other parallel segments confirming the amino acid sequence homology between the N- and C-domains. Comparison of the human sequence to sequences from five species from diverse phyla demonstrates the wide prevalence of an ACE-like protein containing two homologous domains. The bottom part of the figure shows, in schematic form, the evolutionary relationship between the six species. The DNA sequences used in this figure are as follows: no active domain: Brugia malayi (lymphatic filariasis roundworm): XP_001897661.1, Caenorhabditis briggsae: XP_002644637.1, and C. elegans: NP_001024453.1; one active domain: Acromyrmex echinatior (Panamanian leafcutter ant): EGI64832.1, Aedes aegypti (yellow fever mosquito): XP_001659916.1, Ornithorhynchus anatinus (platypus): XP_001515597.1, Pongo abelii (Sumatran orangutan): NP_001124604.1, Monodelphis domestica (gray short-tailed opossum): XP_001376640.2, Ailuropoda melanoleuca (giant panda): XP_002922087.1, Sorangium cellulosum So ce 56 (bacteria): YP_001615731.1, Anaeromyxobacter dehalogenans 2CP-C (bacteria): YP_465231.1, Stigmatella aurantiaca DW4/3-1 (bacteria): YP_003953730.1; Myxococcus fulvus HW-1 (bacteria): YP_004668148.1, Myxococcus xanthus DK 1622 (bacteria): YP_631771.1, Gloeobacter violaceus PCC 7421 (cyanobacteria): NP_926089.1, Shewanella baltica OS678 (bacteria): YP_005273382.1; Candidatus solibacter usitatus Ellin6076: YP_826088.1, Saccoglossus kowalevskii (acorn worm): XP_002734849.1, Crassostrea gigas (Pacific oyster): AEV53960.1, Hydra magnipapillata (hydra): XM_002162385.1, Strongylocentrotus purpuratus (purple sea urchin): XP_001183772.1, Ixodes scapularis (black-legged tick): XP_002435481.1, Pediculus humanus corporis (human body louse): XP_002431342.1, Tribolium castaneum (red flour beetle): NP_001164243.1, Pontastacus leptodactylus (narrow-clawed crayfish): CAX48990.1, Drosophila melanogaster (fruit fly): NP_477046.1, Acyrthosiphon pisum (pea aphid): NP_001129384.1, S. littoralis (African cotton leafworm): ABW34729.1, Apis mellifera (honey bee): XP_393561.3, Locusta migratoria (migratory locust): AAR85358.1, T. tessulatum (duck leech): AAS57725.1, and Trichinella spiralis (trichinosis nematode parasite): XP_003379675.1; two active domains: A. darlingi (American mosquito): EFR22959.1, C. quinquefasciatus (southern house mosquito): XP_001845716.1, A. gambiae str. PEST: XP_313865.3, Heterocephalus glaber (naked mole-rat): EHB07361.1, Mesocricetus auratus (golden hamster): BAD98304.1, Macaca mulatta (rhesus monkey): XP_002800616.1, Homo sapiens (human): NP_000780.1, Pan troglodytes (chimpanzee): NP_001008995.1, Mus musculus (house mouse): NP_997507.1, Rattus norvegicus (Norway rat): NP_036676.1, E. caballus (horse): XP_001495639.3, Oryctolagus cuniculus (rabbit): NP_001075864.1, Cavia porcellus (domestic guinea pig): XP_003465969.1, Canis lupus familiaris (dog): XP_003639297.1, Loxodonta africana (African savanna elephant): XP_003414368.1, Bos taurus (cattle): 449408, Sus scrofa (pig): ABL73884.1, Tetraodon nigroviridis (green spotted pufferfish): CAG04404.1, Danio rerio (zebrafish): XP_694336.5, Dicentrarchus labrax (European seabass): CBN81253.1, Oreochromis niloticus (Nile tilapia): XP_003438793.1, Xenopus (Silurana) tropicalis (Western clawed frog): NP_001116882.1, Anolis carolinensis (green anole): XP_003222396.1, Taeniopygia guttata (zebra finch): XP_002191587.1, Gallus gallus (chicken): NP_001161204.1, Meleagris gallopavo (turkey): XP_003213080.1, C. intestinalis (sea squirt): XP_002123029.1, Branchiostoma floridae (Florida lancelet): XM_002594635.1, N. vectensis (starlet sea anemone): XP_001627759.1, T. adhaerens (Placozoa): XP_002111333.1, and D. pulex (common water flea): EFX86779.1.
Until recently, it was believed that an ACE-like protein containing two homologous catalytic domains was only found in vertebrates (Riordan, 2003). This would suggest that the duplication of the ACE gene occurred approximately 350–550 million years ago (Cornell et al., 1995). This early time point would have occurred in the Paleozoic era, when widespread diversification of life took place on Earth (Valentine et al., 1999). However, searching the presently available gene sequences identified a two-domain ACE-like gene in distant Metazoans, including Insecta (mosquito), Crustacea (common water flea), Cnidaria (starlet sea anemone), and even Placozoa (Fig. 3, A and B) [accession numbers: Culex quinquefasciatus (southern house mosquito): XP_001845716.1; Anopheles gambiae (mosquito): XP_313865.3; Anopheles darlingi (American mosquito): EFR22959.1; Daphnia pulex (common water flea): EFX86779.1; Nematostella vectensis (starlet sea anemone): XP_001627759.1; Trichoplax adhaerens: XP_002111333.1] (Putnam et al., 2007; Colbourne et al., 2011). Although the complete characterization of these enzymes is not available, the potential protein identified in the mosquito genome contains, in both domains, the canonical HEMGH zinc-binding sequence present in mammalian ACE. In the common water flea and starlet sea anemone, this sequence is present in the N-domain but is mutated in the C-domain (water flea: HEQGH; sea anemone: HELGH). Nonetheless, these sequences are probably compatible with zinc-binding and enzymatic activity. The finding of ACE-like genes containing two domains in such diverse species suggests that the duplication event may have occurred earlier than previously thought; the common ancestor of Arthropoda, Cnidaria, and Chordata existed approximately 700 million years ago (Peterson et al., 2008). It is possible that a gene duplication occurred more than once in an evolutionary time frame. Nonetheless, the presence of a two-catalytic domain ACE in such remarkably divergent species, as is shown in Fig. 3, indicates that the duplication ACE took place a long time ago.
In all of the ACE sequences identified to date in vertebrates (including mammals, fish, birds, reptiles, and amphibians), only the enzyme found in Equus caballus (horse) does not contain two HEMGH zinc-binding consensus sequences. In horses, the C-domain sequence is HEIGH (accession number: XM_001495589.3). Despite this, the equine testis isoform of ACE (C-domain of the somatic isozyme) is enzymatically active (Ball et al., 2003). Thus, during hundreds of millions of years of evolution, the zinc-binding and enzymatic activity within both domains of ACE was maintained. Or, put differently, the inactivation of one of the catalytic sites of ACE must result in a significant reduction of evolutionary fitness. This is probably not due to a critical need of both catalytic domains for control of blood pressure, since in genetically manipulated strains of mice, an ACE protein with only one ACE catalytic domain maintains normal blood pressure (Fuchs et al., 2004, 2008). Further, the presence of two domain ACE orthologs in species with no real blood pressure to control [such as the sea squirt (Ciona intestinalis: XP_002123029.1), the starlet sea anemone, or the common water flea) also supports the concept that maintenance of cardiovascular function is not the critical evolutionary feature maintaining two active ACE catalytic domains.
C. Fertility
As discussed previously, testis ACE (corresponding to the ACE C-domain) is very important in maintaining normal male fertility. Thus, it is possible that conservation of ACE C-domain function was due to the catastrophic consequences following loss of testis ACE activity. It is the C-domain that is also the major domain responsible for the conversion of angiotensin I to angiotensin II in vivo (Fuchs et al., 2008). However, as previously discussed, angiotensin II does not appear to be the critical product of testis ACE (Hagaman et al., 1998; Ball et al., 2003). Also, chicken (Gallus gallus) lacks testis ACE expression so that the physiologic requirement for testis ACE does not appear absolute (Esther et al., 1994). ACE, however, has been implicated in playing an important role in the fertility of insects and oysters (Ekbote et al., 2003; Hurst et al., 2003; Riviere et al., 2011). Thus, the role of ACE in reproduction may have played an important role in maintaining the structure of at least one of the two domains.
D. Digestion
As discussed, ACE is present in many different tissues, including the gastrointestinal tract, where some epithelia express high levels of somatic ACE (Yoshioka et al., 1987). Although the precise function of ACE in this location is not fully understood, the nonspecificity of ACE peptide cleavage suggests the hypothesis that it may play a role in the digestion of peptides (Rivière et al., 2004). Analysis has demonstrated that single-catalytic domain ACE-like enzymes are expressed in the gut of other species such as the moth (Spodoptera littoralis: ABW34729.1) or the leech (Theromyzon tessulatum: AAS57725.1) (Wijffels et al., 1996; Rivière et al., 2004; Lemeire et al., 2008). In the leech, the expression of ACE is restricted to intestinal epithelial cells. Thus, the localization of ACE in the gut has been found in animals ranging from the leech to humans; a role in gut function or nutrition may be another factor maintaining the structure of ACE.
E. Development
One species that does not appear to have an enzymatically active ACE-like enzyme is Caenorhabditis elegans (Brooks et al., 2003). Nonetheless, regulation of the C. elegans inactive ACE homolog ACE-like non-metallopeptidase-1 (ACN-1) is important in development, since its downregulation causes the arrest of larval development. C. elegans is unusual in the sense of not having catalytically active ACE, but studies of other species do suggest a role for orthologs of this enzyme in development. In the silkworm (Bombyx mori), ACE is present throughout developmental stages, with a peak in the feeding and late pupal stage (Yan et al., 2007). In Drosophila, a mutation in an ACE ortholog termed Ance can result in larval and pupal death (Tatei et al., 1995). In the mosquito (Anopheles stephensi), an ACE-like ortholog accumulates in the ovaries where it is transferred to mosquito eggs, possibly regulating titers of peptides important for oocyte development and embryogenesis (Ekbote et al., 1999). In this species, feeding the ACE inhibitors captopril or lisinopril reduced, in a dose-dependent manner, the size of the batch of eggs laid by females (Ekbote et al., 2003). It should be noted that many insects’ ACE-like enzymes are not membrane-bound but secrete proteins (Macours and Hens, 2004; Yan et al., 2007).
In summary, an evolutionary analysis of ACE leads to the conclusion that the mutation resulting in a two-catalytic domain enzyme is very ancient. Further, nature appears to have taken advantage of the nonspecific catalytic specificity of ACE. Almost certainly, the important role of testis ACE led to evolutionary pressure to maintain C-domain catalytic functionality. However, the fact that we cannot find species with a duplicated ACE enzyme in which the N-domain has been enzymatically inactivated also suggests a very important functional role for this domain. Whatever the precise selective pressures, an important point is that the role of ACE in blood pressure regulation apparently does not demand an enzyme with two functional catalytic domains, at least under the conditions present in laboratory mice (Bernstein et al., 2011).
VI. Differences between the N- and C-domains of Angiotensin-Converting Enzyme
A. Thermal Stability
It is important to understand differences between the N- and C-domains of ACE as a means of understanding why both domains were preserved. Information about such differences is available from a variety of sources, including the X-ray crystallographic analyses of both domains and a variety of pharmacologic and physicochemical studies. Both the N- and the C-domains are thought to be glycosylated, with the N-domain containing 10 and the C-domain containing seven potential N-glycosylation sites (Yu et al., 1997; O’Neill et al., 2008). It appears that glycosylation plays a critical role in maintaining the stability of ACE, since the expression of this protein in bacterial cells, which are unable to perform eukaryotic glycosylation, or the tissue culture expression of ACE protein in the presence of tunicamycin, an inhibitor of glycosylation, results in protein that is catalytically inactive and subject to rapid degradation (Sadhukhan and Sen, 1996). One difference between the two domains is in their thermostability, with the N-domain being more stable than the C-domain. Specifically, the N-domain has a melting point of 70°C, which is 15° higher than the 55°C melting point of the C-domain (Voronov et al., 2002; O’Neill et al., 2008). The increased thermostability of the N-domain may be due to the structure of amino acids 29–133, as this sequence appears to have a greater number of α helices, perhaps more glycosylation, and an increased proline content.
Detailed analysis has shown that there is a difference in the precise role of glycosylation between the two domains. Although glycosylation undoubtedly contributes to the stability of both domains, it was found that the elimination of the three most C-terminal sites of glycosylation in the N-domain resulted in the complete loss of enzymatic activity, in contrast to results from a similar manipulation of the C-domain, where the most C-terminal sites of glycosylation are not required for the correct folding of the active catalytic domain (Anthony et al., 2010).
B. Chloride Dependency
There is a significant difference in the chloride dependency of the ACE N- and C-domains. This is evident in catalytic activity, the binding of inhibitors, and in the difference in the number of bound chloride ions between the two catalytic domains, as determined by X-ray crystallography. For example, the concentration of NaCl required for maximal catalysis of angiotensin I is 10 mM for the N-domain and 30 mM for the C-domain (Wei et al., 1992). An even more pronounced difference is seen using the artificial substrate Hip-His-Leu, in which the optimal Cl− concentration for activity was 10 mM for the N-domain and 800 mM for the C-domain. In the absence of chloride, the N-domain has approximately 13%–17% maximal activity, whereas the C-domain has less than 0.2%. This effect of Cl− is also observed in the study of inhibitor binding, where chloride stabilizes the enzyme-inhibitor complex by predominantly slowing its disassociation rate and thus decreasing the Ki of inhibitors (Wei et al., 1992). For example, in the presence of 20 and 300 mM Cl−, trandolaprilat has Ki values of 33 and 31 nM for the N-domain. In contrast, for the C-domain, the Ki at 20 and 300 mM Cl− are 22 and 2.9 nM. This same effect is noted for other inhibitors, such as captopril or lisinopril. Crystallographic data have provided a molecular explanation for the effect of chloride. In the ACE C-domain, two chloride ions were visualized in the crystal structure (Natesh et al., 2003; Acharya et al., 2003). Both ions were outside the active site, located approximately 20.7 and 10.4 A from the bound catalytic zinc ion. In the C-domain, the first chloride was bound to Arg762, Arg1065, and Trp1061 (amino acid numbers are those in somatic ACE), whereas the second was bound to Tyr800 and Arg1098. In contrast, X-ray crystallographic study of the ACE N-domain showed only a single chloride bound by Tyr202 and Arg500, in positions equivalent to that of the second chloride present in the C-domain (Corradi et al., 2006).
C. Substrate Specificity
1. Angiotensin I
One of the most interesting aspects of the two domains of ACE is that there are differences in substrate specificity. For example, in vitro studies have shown that, whereas the affinity of the N- and C- domains for angiotensin I are equivalent, the catalytic efficiency (κcat) of the C-domain is approximately 3 times that of the N-domain (Wei et al., 1991a). It is also highly informative to examine mice, created using targeted homologous recombination, that lack catalytic activity in either the ACE N- or C-domain (Fuchs et al., 2004, 2008). Such mice were created by mutating DNA within the 8th or 20th exons that encode the N- or C-domain zinc-binding sequence HEMGH into sequence encoding KEMGK, which is unable to bind zinc (Wei et al., 1991a). In the absence of zinc, an ACE domain is catalytically inactive. To assist in the selection of targeted stem cells, a neomycin cassette was placed in the ACE gene, but strategies were used to ultimately delete the neomycin cassette so that the final mice would have only a single LoxP site within intronic DNA plus the mutation of HEMGH to KEMGH. Such mice are called N-KO and C-KO, indicating which ACE catalytic domain was functionally eliminated. These mice have no change in their tissue pattern of ACE expression, and both N-KO and C-KO mice have blood and kidney angiotensin II levels that are equivalent to wild-type mice. Bradykinin levels in these mice are also indistinguishable from wild-type mice. The N-KO and C-KO mouse models have normal basal blood pressures, but how they achieve homeostasis of blood pressure is very different between the two strains. This is best indicated by the angiotensin I concentrations within the blood. N-KO mice (animals with a functional ACE C-domain) have angiotensin I levels that are indistinguishable from wild-type mice. In contrast, the C-KO mice (animals with an active N-domain) achieve normal blood pressure only by upregulating renin and increasing blood angiotensin I levels to 7-fold normal levels. In other words, the ACE C-domain is the major locus in vivo for the conversion of angiotensin I to angiotensin II. In the absence of this domain, the ACE N-domain is capable of relatively inefficient conversion of angiotensin I to angiotensin II, and to maintain homeostatic levels of angiotensin II, angiotensin I levels must be significantly elevated.
2. Bradykinin, GnRH, and Angiotensin 1–7
In vitro, bradykinin is hydrolyzed with near equal efficiency by both catalytic domains (Jaspard et al., 1993). However, there are several substrates that have been described as being cleaved predominantly by the N-domain. In vitro study of ACE mediated cleavage of GnRH (previously called LH-RH, sequence pyroEHWSYGLRPG) at the bond between Trp3 and Ser4 indicated that the κcat was at least 30 times higher for the N-domain as compared with the C-domain (Jaspard et al., 1993).
Another reported substrate of the ACE N-domain is angiotensin 1-7 (Deddish et al., 1998). This peptide was hydrolyzed by the N-domain at approximately half the rate of bradykinin but was reported hydrolyzed very poorly by the C-domain. Because the angiotensin 1-7 binding constant is approximately equivalent for both domains, this allowed angiotensin 1-7 to serve as an antagonist of angiotensin I for C-domain mediated cleavage. Thus, as measured by the hydrolysis of angiotensin I, angiotensin 1-7 inhibited the C-domain with an IC50 of greater than 20 times that measured for inhibition of the N-domain
The assignment of angiotensin 1-7 as an N-domain specific substrate has been challenged by Rice (Rice et al., 2004). Using recombinant forms of the ACE N- and C-domains, this group concluded that angiotensin 1-7 was cleaved nearly equally by the two domains, with the N-domains having a lower affinity but a higher catalytic efficiency (κcat ) than the ACE C-domain.
While angiotensin 1-7 was suggested as a possible ACE N-domain specific substrate by in vitro analysis, examination of the N-KO and C-KO mice showed no difference in blood or kidney levels of this peptide under basal conditions (Ong et al., 2011). Thus, these data are more consistent with the findings of Rice than with those of Deddish. Angiotensin 1-7 plasma levels are reported as elevated in rats treated with the ACE inhibitor ramipril which inhibits both ACE domains (Campbell et al., 1993).
3. β-Amyloid
A third substrate in which a specific degradative activity is noted for the ACE N-domain is the β amyloid peptide 1-42 (Aβ1-42). Although endopeptidic activity has been demonstrated for both the C- and N-domains of ACE, only the ACE N-domain was documented as capable of converting Aβ1-42 to the less neurotoxic peptide Aβ1-40 (Zou et al., 2009). However, mass spectrometry analysis showed that both domains of ACE, in vitro, are able to generate other degradation products from both Aβ1-42 and Aβ1-40 (Sun et al., 2008). ACE-mediated hydrolysis of such large peptides as Aβ1-42 and Aβ1-40 is substantially slower than the rate of hydrolysis of typical peptides 13 amino acids and shorter. For example, measurement of degradation products of Aβ peptides was carried out after overnight incubation of the peptides with ACE.
The in vivo role of ACE in degrading amyloidogenic peptides is not established with certainty. Eckman et al. (2006) examined the brains of mice lacking neprilysin, endothelin-converting enzyme, or ACE. Whereas loss of these first two enzymes resulted in increased steady-state levels of β amyloid, neither the deficiency of ACE nor the treatment of mice with ACE inhibitors had such a pathologic effect. These data are consistent with a clinical analysis of a large population of elderly patients in the United States taking a variety of drugs for hypertension or cardiovascular disease. This study found that the incidence of Alzheimer’s disease in patients administered the ACE inhibitor captopril was not higher than in the general population (Wolozin et al., 2000). A similar study in Japan came to the same conclusion (Ohrui et al., 2004). On the other hand, the overexpression of ACE in cultured cells was shown to promote the degradation of Aβ1-40 and Aβ1-42 (Hemming and Selkoe, 2005). Thus, it is possible that higher ACE levels may be advantageous in degrading amyloidogenic peptides.
4. Acetyl Ser-Asp-Lys-Pro and Other Substrates
Perhaps the most interesting N-specific substrate is the four-amino-acid peptide acetyl Ser-Asp-Lys-Pro (AcSDKP)(Rousseau et al., 1995). As with many of the N-specific peptides discussed earlier, the Km for binding of AcSDKP is nearly equivalent between the two ACE catalytic domains. However, the κcat for the ACE N-domain was 40 times that of the C-domain.
There is substantial evidence indicating that the ACE N-domain is the primary in vivo enzyme responsible for the degradation of AcSDKP. When normal human volunteers were administered an ACE inhibitor acutely, the plasma and urine levels of AcSDKP increased 5-fold (Azizi et al., 1996). A similar study after continuous administration of ACE inhibitors also demonstrated elevation of AcSDKP in humans (Azizi et al., 1999). Studies in rats and mice are similar to the human studies (Junot et al., 1999; Bernstein et al., 2011). For example, the plasma AcSDKP concentration in N-KO mice was 7.3-fold that measured in wild-type mice. The N-KO mice also have a markedly elevated concentration of AcSDKP in their urine.
AcSDKP is released from its precursor protein thymosin β4 by the enzyme prolyl-oligopeptidase (Cavasin et al., 2004). The initial investigations of AcSDKP suggested that it inhibited the recruitment of primitive hematopoietic progenitors into active proliferation (Lenfant et al., 1989; Bonnet et al., 1993). Thus, it has been suggested that, by degrading AcSDKP, ACE may recruit stem cells into the S phase. As discussed, ACE does have effects on erythropoiesis, and ACE-null mice are anemic. However, studies of mice with selective inactivation of the ACE N-domain throw doubt on the conclusion that these effects are mediated by elevated AcSDKP. Although this mouse model does have elevated AcSDKP, these animals have normal hematocrit and bone marrow morphology. Further, when these mice were made anemic by the administration of phenylhydrazine, they recovered in a temporal fashion indistinguishable from wild-type mice (Fuchs et al., 2004).
In addition to effects on hematopoiesis, AcSDKP has been implicated in other physiologic processes, including promoting angiogenesis (Sosne et al., 2010; Myöhänen et al., 2011). Several studies have demonstrated that AcSDKP inhibits fibroblast proliferation in the myocardium, aorta, and kidney following injury (Peng et al., 2001; Lin et al., 2008; Liao et al., 2010). For example, Peng et al. (2003) reported that in two-kidney, one-clip hypertensive rats, administration of AcSDKP by osmotic minipump increased plasma AcSDKP to a similar level as present in patients treated with an ACE inhibitor (Yang et al., 2004). Although this had no effect on blood pressure, the peptide prevented the development of fibrosis in models of heart injury. Recently, bleomycin-induced lung injury was studied in N-KO mice, animals with elevated AcSDKP levels due to the absence of a functional ACE N-domain (Li et al., 2010). In this model, the direct administration of bleomycin into the lung typically induces significant lung injury, resulting in both focal inflammation and fibrosis. Lung injury in C-KO mice, animals lacking ACE C-domain activity, was indistinguishable from the results of wild-type mice. In contrast, bleomycin injury in the N-KO animals showed significant reduction in the amount of both inflammation and fibrosis. When the N-KO mice were treated with an inhibitor of prolyl-oligopeptidase to reduce AcSDKP formation, these mice were then susceptible to bleomycin-induced lung fibrosis equivalent to that in wild-type mice. In summary, the ACE N-terminal substrate AcSDKP is, in addition to angiotensin I and bradykinin, an in vivo substrate of ACE. As seen in the N-KO mouse, loss of ACE N-domain activity does not reduce blood pressure, but it does elevate in vivo levels of AcSDKP.
Other substrates show asymmetric hydrolysis by the two ACE domains. For example, cleavage of the heptopeptide Met5 -enkephalin-Arg6-Phe7 is about 5-fold faster by the ACE N-domain than by the C-domain at a physiologic pH (Deddish et al., 1997). In addition, domain-specific fluorogenic substrates have been developed, including the C-domain substrate O-aminobenzoic acid-Phe-Arg-Lys(2,4-dinitrophenyl)-Pro-hydroxide and the murine N-domain–specific substrate (7-methoxycoumarin-4-yl)acetyl-Ala-Ser-Asp-Lys-N3-(2,4-dinitrophenyl)-l-2,3-diaminopropionyl (Araujo et al., 2000; Bersanetti et al., 2004; Jullien et al., 2006).
D. Cytokine Production
Macrophages derived from genetically modified N-KO or C-KO mice revealed a surprising effect of ACE domain function on cytokine production (Ong et al., 2012). As measured in an in vitro assay in which peritoneal macrophages produced TNFα in response to lipopolysaccharide, cells derived from wild-type and C-KO mice made roughly equivalent amounts of TNFα (Fig. 4). Somewhat lesser amounts were made by macrophages from ACE-null mice. Macrophages from ACE 10/10 mice had about a 2-fold increase in TNFα production. Unexpectedly, the peritoneal macrophages derived from N-KO mice showed greater than a 4-fold increase in TNFα compared with wild-type mice. In this carefully controlled, in vitro experiment, the behavior of the N-KO macrophages, as compared with identically treated wild-type or C-KO cells, indicates a fundamental difference in the developmental phenotype of these cells.
Fig. 4.
TNFα production by peritoneal macrophages. Peritoneal macrophages were isolated from ACE knockout (ACE KO), wild-type (WT), ACE 10/10 (10/10), N-KO, and C-KO mice. The cells were cultured in vitro overnight with lipopolysaccharide, and the level of TNFα within the culture media was measured. Data points from individual mice are shown, as well as group means and S.E.M. Large amounts of TNFα were made by N-KO macrophages, implying fundamental biochemical differences between these macrophages and equivalent cells from wild-type mice (Ong et al., 2012).
E. Chimeric Angiotensin-Converting Enzyme Domains
The different physical and enzymatic properties of the two ACE domains led to studies of precisely which structural parts of each domain confer domain specificity. To investigate this, chimeric single ACE domains were created and assessed for whether they more closely resembled the natural N- or C-domain. An early study focused on the amino acid sequence surrounding the HEMGH motif responsible for zinc coordination. ACE N-domain amino acids 331–391 were exchanged for amino acids 929–989 in a C-domain construct (Williams et al., 1996). The equivalent portion of the C-domain was also placed into an N-domain construct. Each chimeric domain was then individually expressed and tested for catalytic activity. The ACE N-domain chimera containing the zinc-coordinating amino acids from the C-domain showed catalytic activity similar to the parent N-domain. Similarly, the C-domain chimera, containing N-domain sequences, was catalytically similar to the natural C-domain. The authors concluded that this highly homologous portion of each ACE domain, in which only 13 of 60 amino acids were different, was not responsible for the distinct enzymatic properties of each domain.
Another study examined a human ACE chimera in which a single ACE domain was composed of the first 141 amino acids of the N-domain coupled to the C-domain, including the C-domain zinc-coordinating sequences, the stalk, the transmembrane domain, and the cytoplasmic tail (Marcic et al., 2000). The authors evaluated several catalytic parameters, including substrate specificity and chloride ion activation. The chimeric domain displayed properties that were unique to the chimera. The authors concluded that their work was consistent with the observations of Williams et al. (1996) since, once again, it was not the core zinc-coordinating sequences that established domain specificity, but rather sequences outside the core.
Perhaps the most informative paper to examine chimeric ACE molecules studied five human testis ACE chimeric molecules in which testis ACE amino acids Ser1–Pro163, Asp164–Val416 (the portion of testis ACE containing the core zinc-binding residues), Arg191–Val214, Leu417–Gln579, and Gly583–Pro623 were replaced with the corresponding portions of the somatic N-domain (Leu1–Pro141, Asp142–Val394, Ile169–Thr192, Leu395–Lys557, and Gly561–Pro601) (Woodman et al., 2006). Testis ACE is functionally equivalent to the somatic ACE C-domain. For example, the testis ACE chimera containing amino acids 164–416 from the N-domain is equivalent to a C-domain construct in which amino acids Asp740–Val992 of somatic ACE are substituted with the N-domain sequence. Thus, each of the five chimeric molecules can be viewed as having selected portions of the C-domain replaced with the N-domain sequence. This study examined the expression of the chimeric protein, its catalytic specificity, and its shedding from the cell membrane. The three chimeric enzymes containing the N-domain sequence in place of testis ACE amino acids 1–163, 417–579, or 583–623 were expressed but showed catalytic properties, as assessed by domain-specific substrates, characteristic of the N-domain. Nonetheless, each of these proteins was cleaved from the cell membrane at precisely the amino acid sequence used in shedding the parent testis ACE protein. The two chimeric proteins in which residues 164–416 and 191–214 were from the N-domain were neither catalytically active nor were they processed by the cells in which they were expressed.
The X-ray characterization of the ACE C-domain identified the first three helices in the molecule as forming a lid-like structure that restricted access to the catalytic site (Natesh et al., 2003). This lid was thought to participate in ACE C-domain catalytic specificity (Acharya et al., 2003). In the chimeric molecule in which testis ACE residues 1–163 were substituted with the N-domain sequence, the helices comprising the lid would be derived from the N-domain. This N-domain sequence contained additional N-glycosylation sites not normally present in the C-domain, which could conceivably alter the three-dimensional configuration of the molecule. Despite this, the chimeric protein had the enzymatic characteristics of the N-domain. These data support speculations derived from X-ray analysis suggesting that the lid-like structure present in each of the two ACE catalytic domains influences substrate specificity, and that the amino-terminal sequence of testis ACE and, by extension, the C-domain of somatic ACE are critical players in determining the catalytic specificity of this domain. In addition, these chimeric studies indicate that a relatively small region corresponding to Arg191–Val214 in testis ACE (Arg767–Val790 in somatic ACE) plays a critical role in stabilizing the three-dimensional structure of the protein allowing for ACE enzyme processing and cell-surface targeting.
VII. Angiotensin-Converting Enzyme Inhibitors
A. Development of First-Generation Inhibitors
There are several accounts of the development of captopril and enalapril (Ondetti and Cushmen, 1981; Patchett and Cordes, 1985; Menard and Patchett, 2001; Erdös, 2006). Acharya et al. (2003) have remarked that the design of ACE inhibitors was based on brilliant insights sprinkled with serendipity, or what they termed “rational intuition.” They identified three major conceptual insights that were critical in the development of ACE inhibitors. The first was the realization that ACE and kininase II, an enzyme that degraded bradykinin, were the same enzyme. In 1966, while studying the degradation of bradykinin, Erdös and Yang characterized an endopeptidase from hog kidney with dipeptidyl carboxypeptidase activity (Erdös and Yang, 1967; Erdös, 2006). They rapidly identified a similar activity in human plasma and named the enzyme kininase II to distinguish it from the previously characterized enzyme kininase I that inactivated bradykinin with monopeptidyl carboxypeptidase activity (Yang and Erdös, 1967). The investigation of ACE-mediated catalysis of angiotensin I and the degradation of bradykinin began to converge with studies by Ng and Vane (1967) showing that the ACE activity in blood could not produce angiotensin II in sufficient amounts to account for the activity of the vasoconstrictor. They identified the lung as the major locus of where ACE converts angiotensin I to angiotensin II, and they showed that virtually all angiotensin I was converted to angiotensin II in a single pass through this organ. These were seminal observations (Soffer, 1981). First, this was an early suggestion that the lung functions in other physiologic processes other than gas exchange. Also, the work recognized the pre-eminent role of tissue-bound ACE, as opposed to ACE activity in the plasma. Finally, Ng and Vane drew a parallel between the pulmonary production of angiotensin I and the inactivation of bradykinin, which also was rapidly destroyed in the lung (Ferreira and Vane, 1967; Ng and Vane, 1968). Although the researchers attributed this activity to kininase I (now called carboxypeptidase N), later studies rapidly established that it was the dicarboxypeptidase ACE that was capable of both angiotensin II generation and the destruction of bradykinin (Yang et al., 1970, 1971; Igic et al., 1972).
The observation that ACE was the enzyme responsible for bradykinin degradation was critical to the development of ACE inhibitors because Sërgio Ferreira, a Brazilian physician and pharmacologist, discovered a substance in the venom of the South American pit viper Bothrops jararaca that, in small amounts, potentiated the activity of bradykinin on the guinea pig ileum (contraction) and the hypotension induced by systemic administration of bradykinin (Ferreira, 1965). This substance was called bradykinin potentiating factor. In 1968, bradykinin potentiating factor was shown to inhibit ACE, setting the stage for the remarkably innovative science that led to the development of ACE inhibitors (Bakhle 1968; Bakhle et al., 1969).
Research continued along two independent and simultaneous tracks. Ferreira et al. (1970) determined the sequence of the bradykinin potentiating factor peptides, while Ondetti and colleagues (1971) performed a similar analysis of the Bothrops venom for peptides that inhibited ACE (Ondetti and Cushmen, 1981). As noted by Ondetti and Cushmen (1981), it was not then self evident that bradykinin potentiation and ACE inhibition would result from the same compounds. Ultimately, the most potent bradykinin potentiating factor peptide isolated by Ferreira et al. (1970) and the peptide most effective at inhibiting ACE were found to be identical. The sequence of this peptide (often referred to as SQ 20,881 or BBP9a) is GluTrpProArgProGlnIleProPro. The very first clinical trials in humans were carried out by intravenous infusion of SQ 20,881 into normotensive volunteers, which resulted in the inhibition of the pressor response observed after the infusion of angiotensin I (Collier et al., 1973).
Inhibitors such as SQ 20,881 were not potential pharmaceuticals because they were not orally active. However, the sequence of the ACE inhibitors present in B. jararaca, Agkistrodon halys blomhoffii, and other venoms stimulated a systematic study of peptide derivatives to inhibit ACE (Ondetti and Cushmen, 1981). The structure-activity analysis of these synthetic peptides provided insight into the catalytic site of ACE, and ultimately indicated that the optimal carboxyl-terminal amino acid sequence of ACE inhibitors was Phe-Ala-Pro (Cushman and Ondetti, 1999). Even more important than any particular structure, these studies led to an understanding of certain basics necessary for a successful ACE inhibitor. These included the observation that a free C-terminal carboxyl group was important in binding to the enzyme, the positioning of aromatic amino acids in the antipenultimate position enhanced inhibitory activity, and the N terminus of any inhibitor had to provide a critical interaction with the enzyme.
In addition to understanding the structure of ACE peptide inhibitors, Cushman and Ondetti (1999) made two other important insights that eventually led to the development of captopril (SQ 14,225), the first orally active ACE inhibitor approved for human use. One was to understand the significance of the early work by Skeggs et al. (1956a) describing the inhibition of ACE by EDTA and subsequent additional data strongly suggesting that ACE was a metallopeptidase (Bakhle, 1974). This was formally demonstrated when Das and Soffer (1975) verified that ACE was a zinc-containing metallopeptidase. At the time that captopril was being developed, little was known about the detailed catalytic site of ACE. In contrast, detailed studies of the zinc peptidase carboxypeptidase A, including X-ray crystallographic studies at 2 Å, had been performed by Lipscomb and colleagues (1968). Although carboxypeptidase A is a zinc peptidase, it has little to do with ACE, being a monocarboxypeptidase made by the pancreas and used in the digestion of peptides. The enzyme has only 307 amino acids and little sequence similarity to ACE (Patchett and Cordes, 1985). Further, the zinc-coordinating domain of this enzyme is HXXE, not the HEXXH sequence found in ACE (Acharya et al., 2003). And yet, the modeling of ACE as a carboxypeptidase A–like enzyme that cleaves a carboxyl dipeptide provided a critical tool in developing an ACE inhibitor.
A third insight that proved crucial was derived from work by Byers and Wolfenden (1973) on the development of carboxypeptidase A inhibitors, based on the structure of benzylsuccinic acid. This led Ondetti and Cushmen (1981) to the model shown in Fig. 5. Similar to carboxypeptidase A, it was thought that a C-terminal carboxyl was important. Further, it was thought that the N-terminal carboxyl would bind to the catalytic zinc in ACE. This led to d-2-methylsuccinyl-Pro (SQ 13,297), an inhibitor with an IC50 of 22 μM. The real breakthrough came with the substitution of a sulfhydryl group for the N-terminal carboxyl, giving rise to captopril with an IC50 of 0.02 μM., an agent that, when orally administered to rats, inhibited the pressor effects of angiotensin I, augmented the actions of bradykinin, and “lower(ed) blood pressure in a model of renovascular hypertension” (Ondetti et al., 1977). It should be noted that sulfhydryl compounds had been found to inhibit ACE before the development of captopril; both Ferreira and Rocha e Silva (1962) and Erdoes and Wohler (1963) had used sulfhydryl compounds to potentiate bradykinin in the 1960s. Nonetheless, the development of captopril was a seminal event. The drug was first approved for use in human hypertensive patients with difficult-to-control disease in 1981; in 1985, it received the US Food and Drug Administration approval for general use in hypertension (Menard and Patchett, 2001). In recognition of the development of captopril, Cushman and Ondetti received the 1999 Lasker Prize (http://www.laskerfoundation.org/awards/1999_c_description.htm). Additional inhibitors of ACE followed, and in particular, the work of Patchett et al. (1980) led to the development of enalaprilat and lisinopril, which resemble the tripeptides Phe-Ala-Pro and Phe-Lys-Pro. Menard and Patchett (2001) have reviewed the detailed development of several ACE inhibitors.
Fig. 5.
Model comparing carboxypeptidase A and ACE. In this schematic, the active sites of carboxypeptidase A and ACE are compared. The pockets in the enzyme represent side chain binding sites within the active sites of the enzymes. In ACE, these pockets are often labeled S1, S1′, and S2′ based on their position relative to the zinc molecule. The + indicate positive charges and X-H represents a potential hydrogen bond. Interactions stabilizing substrates or inhibitors are indicated with dots. The substrate amino bond hydrolyzed by the enzymes is indicated with a bent line. Initial thinking about ACE inhibitors was derived from work showing that benzylsuccinic acid was an inhibitor of carboxypeptidase A (Byers and Wolfenden, 1973). A major advance was the use of a sulfhydryl group to bind to the zinc molecule (Ondetti and Cushmen, 1981). The figure also shows the structure of the ACE inhibitor lisinopril, which has a high affinity for ACE due to interaction with the S1 pocket, in addition to the zinc atom and other structural features of ACE. Adapted with permission from Cushman et al. (1977). Copyright (1978) American Chemical Society.
B. Crystal Structure of Angiotensin-Converting Enzyme
Although the development of ACE inhibitors had tremendous significance for understanding the biologic function of ACE, the pharmacologic development of these drugs also gave insight into the mechanism of ACE substrate binding and catalysis. A major advance in this area was the successful determination of the crystal structure of human testis ACE. These studies were made possible by work from Riordan, Sturrock, and others who systematically investigated the role of glycosylation in the activity and stability of testis ACE (Yu et al., 1997; Gordon et al., 2003). With the exception of the N-terminal 36 amino acids, mature human testis ACE is identical to the C terminus of somatic ACE, and it was thought to be easier to crystallize than the larger somatic isozyme. Toward this end, the testis isozyme was modified by removing these amino-terminal 36 amino acids, which, although heavily O-glycosylated, are unnecessary for either the activity or the stability of the enzyme (Ehlers et al., 1992). The protein construct was also modified by truncation at Ser625 (corresponding to Ser1201 of somatic ACE), which eliminated part of the stalk region, the hydrophobic transmembrane domain, and the C-terminal intracellular domain. When this shortened protein was expressed in the presence of N-butyldeoxynojirimycin, an α-glycosidase I inhibitor, and treated with endoglycosidase-H, it yielded crystals by vapor-diffusion hanging drop that were suitable for X-ray diffraction studies at a resolution of 2.0 A (Gordon et al., 2003; Natesh et al., 2003).
X-ray diffraction analysis showed testis ACE to be an ellipsoid protein divided into two halves by a deep central groove containing the catalytic zinc ion and the other components of the catalytic site. The first three amino-terminal helices form a lid-like structure that appeared to block access to the active-site groove and to restrict the groove opening to approximately 3 A in diameter (Acharya et al., 2003; Natesh et al., 2003). This functionally blocks large or complexly folded substrates from entering the active site, a finding consistent with earlier biochemical characterization of ACE as having a deeply buried active site (Pantoliano et al., 1984; Bernstein et al., 1990). X-ray analysis confirmed the coordination of the zinc ion by His383, His387, and Glu411 (His959, His963, and Glu987 in somatic ACE). Additional zinc coordination is provided by an acetate ion from the crystallization medium that presumably would be a different ion in a physiologic solution. Thus, zinc coordination in ACE is similar to that of thermolysin.
X-ray crystal analysis identified two chloride ions in the testis ACE molecule. These do not directly interact with substrate but may influence the position of other residues important in substrate binding (Natesh et al., 2003). Surprisingly, when the three-dimensional structure of testis ACE was compared with the structure of other available proteins, it had little similarity to the three-dimensional structure of carboxypeptidase A. Rather, it had the most homology with neurolysin, a zinc-containing peptidase best known for cleaving neurotensin, and a carboxypeptidase isolated from the hyperthermophilic archaeon Pyrococcus furiosus. Both of these enzymes have a substrate-binding groove with the active site residues, including the metal coordinating sequence HEXXH, set deep within the groove and away from the surface (Brown et al., 2001; Arndt et al., 2002). Neurolysin is similar to ACE in that it contains structural elements blocking access to the groove and thus limits catalytic access to small substrates. The carboxypeptidase from P. furiosus is even more interesting. The enzyme has optimal catalytic activity at temperatures higher than 90°C. Although the enzyme has the classic HEXXH zinc-binding structure, it appears to be inactivated by zinc but active with either Co2+ or Mn2+. The enzyme is composed of only 499 amino acids and has little amino acid sequence homology to ACE. At this point, it is impossible to know whether the three-dimensional similarity between ACE, neurolysin, and the P. furiosus carboxypeptidase resulted from divergent evolution of a very ancient common ancestor or some form of convergent evolution (Arndt et al., 2002). At the very least, these findings strengthen the supposition that modern ACE originated from an ancestral enzyme present early in the formation of life on Earth.
Perhaps the most informative part of the ACE crystal structure came about because the protein was crystalized both with and without the ACE inhibitor lisinopril. This allowed precise analysis of inhibitor binding and gave great insight into the structural specificity that ACE uses in binding substrates and inhibitors (Natesh et al., 2003). The major interactions of lisinopril with specific amino acid residues in testis ACE are summarized in Fig. 6. The lisinopril molecule was found deep within the active site groove. The binding of lisinopril does not markedly change the physical structure of the enzyme or the location of the chloride ions. Lisinopril resembles the tripeptide Phe-Lys-Pro. The side chains of these amino acids (the phenyl, the propylamine, and the pyrrolidine) are in the S1-, S1′-, and S2′-binding pockets of ACE. The carboxyalkyl carboxylate of lisinopril (indicated in Fig. 6 with an asterisk) substitutes for the amide carbonyl that is positioned at the site of amino acid cleavage in an ACE substrate. This coordinates with the active-site zinc atom, and forms a hydrogen bond with Tyr523 (Tyr1099 in somatic ACE). The second oxygen atom of this carboxylate makes a hydrogen bond with Glu384 (Glu960 in somatic ACE). What would be the scissile amide nitrogen (indicated by an arrow in Fig. 6) in an ACE substrate makes a hydrogen bond with Ala354 (Ala930 in somatic ACE). The C-terminal carboxylate, which was found to be one of the critical features required in designing an ACE inhibitor, formed hydrogen bonds with Lys511 and Tyr520 (Lys1087 and Tyr1096 in somatic ACE).
Fig. 6.
Binding of lisinopril to testis ACE. As compared with the schematic in Fig. 5, the crystal structure of human testis ACE–binding lisinopril shows the detailed interactions of individual ACE amino acids with the inhibitor (Natesh et al., 2003). Amino acid numbers are those of testis ACE. Stabilizing interactions are indicated by dashes. The S1-, S1′-, and S2′-binding pockets of ACE are indicated. The carboxyalkyl carboxylate of lisinopril (indicated with an asterisk) substitutes for the amide carbonyl in an ACE substrate. What would be the scissile amide nitrogen in an ACE substrate is indicated by an arrow. Adapted with permission from Macmillan Publishers Ltd: Natesh et al. (2003).
X-ray analysis of testis ACE (equivalent to the ACE C-domain) immediately provided a framework into which to model the known amino acid sequence of the somatic ACE N-domain and estimate the differences between the two domains (Acharya et al., 2003). Eventually, X-ray diffraction data for this domain also became available (Corradi et al., 2006). Both domains use their first three amino-terminal α helices to form a lid-like structure that hinders entry to the catalytic site, particularly to large substrates. What remains unresolved is whether any degree of flexing or movement of this lid-like structure plays a role in substrate entry and catalysis, as is postulated for the related protein ACE2 (Corradi et al., 2006). Initial empirical evidence supporting the potential of the helices comprising the lid-like structure to shift away from the opening of the catalytic channel was found in crystallographic studies of the ACE N-domain (Anthony et al., 2010). Further, the differences in hydrophobicity and charge in the lid-like structures between the ACE N- and C-domains do appear to play a role in substrate specificity, as indicated by substitution experiments previously discussed (Woodman et al., 2006). As noted, there is a difference in chloride activation between the two ACE domains, and this is reflected by a single chloride binding within the N-domain rather than the two chlorides found in the C-domain. However, the precise kinetic mechanism by which chloride influences catalysis is not yet totally clear. Despite these remaining questions, the increased understanding of ACE structure provides additional impetus toward the development of newer drugs designed to inhibit only one of the two ACE domains.
C. Domain-Specific Inhibitors
As each of the two ACE domains has a somewhat different biologic function, it was thought important to develop ACE domain–specific inhibitors (Anthony et al., 2012). Such inhibitors will undoubtedly provide important insight into the in vivo functions of ACE. They may be safer for humans than current medications, and they may also be useful in treating patients when blockade of a single ACE domain is advantageous. As an example, the substrate AcSDKP is only hydrolyzed by the ACE N-domain. A selective inhibitor of this domain raises the concentration of this peptide without markedly changing the conversion of angiotensin I to angiotensin II (Junot et al., 2001). Natural peptides had already indicated that it is possible to develop domain-specific inhibitors. For example, the snake venom bradykinin potentiating peptide BPPb has the sequence Glu-Gly-Leu-Pro-Pro-Arg-Pro-Lys-Ile-Pro-Pro, whereas the peptide BPPc has the sequence Glu-Gly-Leu-Pro-Pro-Gly-Pro-Pro-Ile-Pro-Pro. Despite this small difference, BPPb exhibits Ki values of 10,000 and 30 nM for the ACE N- and C-domains, respectively, whereas BPPc has a Ki of 80 nM for both the N- and C-domains (Cotton et al., 2002). Thus, relatively small changes can make an enormous difference in domain selectivity.
Several excellent reviews have discussed the approach toward constructing domain-specific ACE inhibitors (Dive et al., 2004; Redelinghuys et al., 2005). A critical feature of all ACE inhibitors is the chemical nature of the zinc-binding group. Although all ACE inhibitors are peptide analogs, they use different chemical moieties to bind with the ACE zinc atom. A sulfhydryl is used in captopril, a carboxyl is used in lisinopril, and a ketone is used in keto-ACE, a derivative of Phe-Gly-Pro that is approximately 40 times more selective for the ACE C-domain (Deddish et al., 1998; Anthony et al., 2012). Chemical structures placed on the left- and right-hand side of the zinc-binding domain are then able to determine the relative potency and domain selectivity of individual inhibitors.
Recently, phosphinic peptides have been developed as zinc metalloproteinase inhibitors. Replacement of the scissile peptide bond (-CO-NH) with a phosphinic acid moiety (-PO2-CH2) has several advantages (Dive et al., 2004). First, there appears to be a weaker coordination of the phosphate with the ACE zinc ion that permits other chemical determinants within the ACE inhibitor to have greater discrimination in relative binding with the ACE N- or C-domain (Corradi et al., 2007). Further, it is thought that phosphinic peptides mimic the transition state formed during enzyme hydrolysis of a substrate (Dive et al., 2004). Two phosphinic peptides have been characterized that have very pronounced ACE domain specificity: the N-domain–specific inhibitor RXP 407 has a Ki of 7 nM for the ACE N-domain and 7500 nM for the C-domain, whereas RXP A380 has a Ki of 10,000 nM for the ACE N-domain and 3 nM for the C-domain (Dive et al., 2004). The availability of inhibitors with three orders of magnitude difference between N- and C-domain specificity suggests that drugs suitable for human use should someday be available. The in vivo administration of RXP 407 in mice blocks degradation of the ACE N-domain–specific substrate AcSDKP, resulting in an approximately 5-fold increase in basal plasma concentration of this peptide (Junot et al., 2001). Further, the drug appeared to have no effect upon C-domain–mediated angiotensin I hydrolysis in vitro, and it did not modify the pressor response observed after an intravenous bolus injection of angiotensin I. In terms of how domain specificity is achieved, data from X-ray crystal analysis, the study of chimeric ACE domains, and the results of other RXP-like compounds suggest a key feature of specificity is the ACE S2 pocket, the portion of ACE that binds the amino part of an inhibitor. In particular, Tyr369 and Arg381 have been implicated in N-domain selectivity for RXP 407 (Kröger et al., 2009; Anthony et al., 2010, 2012). These two S2 pocket residues appear critical in domain specificity since the equivalent amino acids in the C-domain, Phe967 and Glu979, have been determined by X-ray crystal analysis as being critical for the specificity of RXP A380 for the ACE C-domain. Other residues important for C-domain selectivity of the RXP A380 compound include Val955 and Val956, which in the N-domain are the polar residues Ser357 and Thr358 (Corradi et al., 2007).
D. Natural Angiotensin-Converting Enzyme Inhibitors
ACE inhibitors are not only the creations of humans but are also found naturally. ACE inhibitors in venoms were discussed. Another inhibitor is K-26, which is produced by the soil-dwelling actinomycete K-26. When hippuryl-His-Leu was used as an ACE substrate, this inhibitor showed an IC50 of 6.7 ng/ml, and it demonstrated hypotensive activity in vivo (Yamato et al., 1986). Other natural products that inhibit ACE have also been identified (Koguchi et al., 1986). Although the precise ligands for these agents in vivo have not been determined, the presence of natural ACE inhibitors in such primitive organisms again underlines the wide distribution of ACE and ACE-like enzymes among diverse organisms.
VIII. Angiotensin-Converting Enzyme Insertion/Deletion Polymorphism
In 1990, Rigat and colleagues (1990) identified a 287 base pair Alu repeat-DNA fragment within the 16th intron of the ACE gene in some humans (17q23). This insertion/deletion polymorphism in ACE was used as a marker genotype in a study involving healthy subjects (n = 80), where it was found that the allele frequency was 0.4 for the insertion (I) and 0.6 for the deletion (D). The I and D genotypes correlated with serum ACE concentration, where individuals with the DD genotype had the highest average serum ACE levels (average of 494.1 ± 88.3 μg/l), those with the II genotype averaged the lowest (299.3 ± 49 μg/l), and heterozygous individuals (ID) had an intermediate value (392.6 ± 66.8 μg/l). It was concluded that the I/D polymorphism accounted for 47% of the total phenotypic variance of serum ACE (Rigat et al., 1990). Two years later, a further analysis showed that the major genetic effect regulating serum ACE was not the Alu repeat itself, but was due to an ACE gene variant in strong linkage disequilibrium with the I/D polymorphism (Tiret et al., 1992). The effect of the I/D gene polymorphism was found not only in people of European ancestry, but also among Japanese and Pima Indians, where it was associated with serum ACE activity (Nakai et al., 1994; Foy et al., 1996). In contrast, there was no such association in African Americans (Bloem et al., 1996). The I/D polymorphism is not the only polymorphism in the human ACE gene; in 1999, ACE gene sequencing in 11 individuals (a total of 22 copies of the ACE gene) identified 78 different polymorphisms, of which 17 were in absolute linkage disequilibrium with the intron 16 I/D Alu repeat (Rieder et al., 1999). Thirteen distinct haplotypes were identified.
Early studies examined the association of the ACE I/D polymorphism with coronary artery disease. Cambien et al. (1992) reported that the D/D genotype, associated with high levels of circulating ACE, was found more frequently in patients with myocardial infarction than in control subjects. Leatham et al. (1994) corroborated this finding in Caucasian patients with myocardial infarction, but not in patients with unstable angina. Nakai et al. (1994) also found an association of the D/D genotype with coronary artery disease in Japanese patients. However, clinical studies eventually began to show conflicting results. For example, whereas Schunkert et al. (1994) reported that, among subjects with left ventricular hypertrophy (n = 141 females, 149 males), there was an excess of the D/D genotype, and that this association was especially strong in males 45— 59 years of age, an analysis of echocardiographic data from the Framingham Heart Study (n = 2439) found no association of left ventricular mass with the ACE I/D polymorphism, nor any increased risk of left ventricular hypertrophy (Lindpaintner et al., 1996). Kupari et al. (1994) also concluded that, in the absence of heart disease, the ACE I/D polymorphism had no major influence on left ventricular mass or function that was detectable with echocardiography.
Although the early studies implicated an association of the ACE D/D genotype with myocardial infarction, no convincing evidence was initially found associating this polymorphism with hypertension. For example, Schmidt et al. (1993) reported findings from the Dutch Hypertension and Offspring Study that the allele frequencies for both the I and D allele were similar in parents and offspring with high and low blood pressure (n = 111 parents and 75 offspring). Gu et al. (1994) also reported a lack of association between the I/D polymorphism in a hypertensive Belgian population (n = 119) compared with controls, regardless of age or sex. In Japanese patients with essential hypertension, Ishigami et al. (1995) reported that there was no association with ACE I/D polymorphism. Barley et al. (1996) suggested that there may be racial differences in the association of ACE I/D polymorphism with hypertension in that subjects of European descent showed no association between ACE genotype and blood pressure, whereas subjects of African Carribean descent did. However, the same group also did not find any association between I/D polymorphism and plasma renin or aldosterone levels in either Caucasians (n = 210) or African Caribbeans (n = 110) (Barley et al., 1996). Eventually, a very large study was conducted as part of the Framingham Heart Study (n = 3095) to examine the association between the I/D ACE polymorphism and hypertension. This study showed that there was a statistically significant association of the ACE locus with small effects on hypertension and with diastolic blood pressure, but these findings were only present in men (O'Donnell et al., 1998). Recently, the Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium studied 29,136 subjects and compared their findings with those in the Global Blood Pressure Genetics Consortium (n = 34,433) and the Women's Genome Health Study (n = 23,019) (Johnson et al., 2011). A single-base polymorphism in ACE termed rs4305, which is different from the traditional I/D polymorphism referred to in the study by Johnson et al. (2011) as rs4350-I/D, showed modest replication of an association with increased hypertension. However, the effect was very modest (β = 0.06, where β is the log odds of hypertension per allele dose). Thus, the available evidence suggests that any effect of ACE on blood pressure in humans is very small. This conclusion is consistent with the results of a recent meta-analysis (Takeuchi et al., 2012).
After the initial reports associating coronary artery disease with the ACE I/D polymorphism, larger studies were conducted. Friedl et al. (1995) examined 315 Austrian patients with coronary artery disease, and were not able to demonstrate an association of the D/D genotype with disease or with lipid parameters. In the Physicians' Health Study, a large prospective cohort study following U.S. male physicians, the presence of the ACE D allele conferred no increased risk of ischemic heart disease or myocardial infarction in study subjects (n = 1250) versus matched controls (n = 2340) (Lindpaintner et al., 1995). Another study of 697 patients undergoing coronary angiography suggested the ACE I/D polymorphism as a genetic risk factor for myocardial infarction in Caucasian males, but not as a predictor of coronary artery stenosis (Ludwig et al., 1995). The authors suggested something that is certainly true, namely, that several independent processes contribute to the risk of myocardial infarction. Winkelmann et al. (1996) confirmed that plasma ACE was significantly associated with the I/D polymorphism, but that the ACE genotype was not associated with the presence of coronary artery disease or myocardial infarction in an angiographically defined study sample (n = 209).
As study sample sizes increased with population-based cohort studies, the association of the ACE I/D gene polymorphism with coronary artery disease continued to blur. In two large studies from Copenhagen, a case-referent study (n = 10,150) and a retrospective cohort study (n = 7263), there was no evidence of an association of the ACE I/D gene polymorphism and myocardial infarction or ischemic heart disease in either males or females (Agerholm-Larsen et al., 1997). In a Japanese study of coronary artery disease in subjects who underwent coronary angiography (n = 947), there was no association between ACE genotype or serum ACE activity and disease (Fujimura et al., 1997). Arnett et al. (1998) found no evidence that the ACE I/D polymorphism was associated with carotid intima-media thickness in a sample of middle-aged adults with no history of coronary artery disease (n = 495). Girerd et al. (1998) also found no association between the I/D polymorphism and mean wall thickness of the radial artery or carotid artery intimal thickness in a cohort without evidence of coronary artery disease (n = 340). Finally, a meta-analysis conducted by Agerholm-Larsen et al. (2000) found that in 46 studies comprising 32,715 Caucasian individuals, plasma ACE was increased with D/D genotype, but blood pressure, increased risk of myocardial infarction, coronary disease, or stroke was not associated with any polymorphism in the largest studies. What then to conclude about the I/D polymorphism? First, there continue to be many studies examining the association of this polymorphism with many different diseases in many different ethnic groups. As concerns hypertension and coronary artery disease, the association with the I/D polymorphism certainly appears less clear in later studies than in the initial studies. It seems that whatever effect does exist is small and is only one of many genetic influences in these diseases.
The association of ACE with Alzheimer’s disease is also worth considering. Here, the question is 2-fold: is there an association between ACE levels and susceptibility to disease, and do ACE inhibitors impact the incidence of Alzheimer’s disease? These questions are quite complex, because hypertension itself may affect the progression of Alzheimer’s disease (Kehoe et al., 2009; Kehoe and Passmore, 2012). The consideration of whether the ACE I/D polymorphism is associated with Alzheimer’s disease is similar to the complexity of I/D and cardiovascular disease: there are many studies with conflicting results. As summarized by Kehoe and Passmore (2012), several meta-analyses have consistently supported a small contribution of ACE variation to disease risk. However, other recent studies, including genome-wide association studies, have not found this to be true. Thus, at present, it seems that whatever effect natural ACE variation may have on the incidence of Alzheimer’s disease is very small. Concerning the question of whether ACE inhibitors or AT1 receptor antagonists contribute to the onset of Alzheimer’s disease, the data suggest otherwise. As comprehensively reviewed by Kehoe and Passmore (2012), several studies suggest that AT1 receptor antagonists may reduce the incidence of Alzheimer’s disease, and, although less so, the same may be true of ACE inhibitors.
IX. Conclusions
In November 1993, the results of a clinical trial examining treatment with an ACE inhibitor for diabetic nephropathy appeared in the New England Journal of Medicine (Lewis et al., 1993). This was a randomized, controlled trial that compared captopril with placebo in insulin-dependent diabetes mellitus patients with urinary protein excretion greater than 500 mg/day and serum creatinine concentrations less than 2.5 mg/dl. The study showed that patients taking captopril had a 48% reduction in the risk of doubling their serum creatinine. An even greater reduction in risk was seen in those patients with an initial serum creatinine of 2.0 mg/dl. In many ways, this study perfectly exemplifies the trend in the intellectual and clinical understanding of the renin-angiotensin system over the last 30 years. During this period, there has been a consistent expansion in understanding the pharmacologic, physiologic, and pathologic effects of ACE, angiotensin II, and other components of the renin-angiotensin system. This has directly translated into novel and better uses of inhibitors. These trends are not only due to more scientists performing more experiments, but rather reflect a profound truth, namely, that the many parts of the renin-angiotensin system affect an enormous diversity of biologic processes.
Scientists have studied ACE since its discovery in 1956, and yet only in the last 15 years have scientists really focused on the significance of the enzyme having two independent catalytic domains. As we emphasize in this review, the genetic mutation that gave rise to both domains—a gene duplication—happened hundreds of millions of years ago. That both domains remain functionally active is the defining characteristic of ACE and the secret to understanding much of what is special about this enzyme. The two ACE catalytic domains contribute to the wide substrate diversity of ACE and, by extension, the wide physiologic impact of the enzyme. This is particularly important now that domain-specific inhibitors have been synthesized and characterized. Once widely available, these reagents will undoubtedly be powerful tools for probing the actions of each ACE domain. In turn, this knowledge should allow clinicians to envision new therapies for diseases not currently treated with ACE inhibitors. Thus, we conclude that new ways to manipulate ACE and other aspects of the renin-angiotensin system will continue to be discovered in the future. The availability of classic ACE inhibitors and perhaps these newer domain-specific inhibitors, coupled with an increased understanding of the RAS, should provide powerful weapons to treat an increasing number of human diseases.
Supplementary Material
Acknowledgments
We acknowledge Brian Taylor for enormous help in preparing this manuscript, and Cedars-Sinai Medical Center for support of our research effort. We also gratefully acknowledge the assistance of Drs. Heinrich Lünsdorf (Helmholtz-Zentrum für Infektionsfoschung) and Edward D. Sturrock (University of Cape Town) in the preparation of Fig. 1. Daniella Bernstein corrected part of the manuscript. K.E.B. and S.F. congratulate Professor Pierre Corvol for many contributions to our field.
Abbreviations
- Aβ
β-amyloid
- ACE
angiotensin-converting enzyme
- AcSDKP
acetyl Ser-Asp-Lys-Pro
- κcat
catalytic efficiency
- BBP9a
Glu-Trp-Pro-Arg-Pro-Gln-Ile-Pro-Pro
- CFU
colony-forming unit
- CSF
colony-stimulating factor
- D
deletion
- EAE
experimental autoimmune encephalomyelitis
- GFR
glomerular filtration rate
- GnRH
gonadotropin-releasing hormone
- I
insertion
- IFNγ
interferon γ
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- LH-RH
luteinizing-releasing hormone
- MHC
major histocompatibility complex
- MRSA
methicillin-resistant S. aureus
- NO
nitric oxide
- RAS
renin-angiotensin system
- SQ 13,297
d-2-methylsuccinyl-Pro
- SQ 14,225
captopril
- SQ 20,881
pGlu-Trp-Pro-Arg-Pro-Glu-Ile-Pro-Pro-OH or teprotide
- SP
substance P
- TGF-β
transforming growth factor-β
- TNFα
tumor necrosis factor α
- 1400W
N-(3-(aminomethyl)benzyl)acetamidine
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Bernstein, Ong, Blackwell, Shah, Giani, Gonzalez-Villalobos, Shen, Fuchs.
Footnotes
The authors' laboratories are supported by grants from the National Institutes of Health National Heart, Lung and Blood Institute [Grants R01HL110353 (to K.E.B.), F32H105036 (to F.S.O.)]; and the National Institutes of Health National Institute for Diabetes, Digestive and Kidney Disease [Grants T32DK007770 (to W.-L.B.B.), R00DK083455 (to R.A.G.-V.)]. F.S.O. is also supported by the Cedars-Sinai Medical Center Clinical and Translational Science Institute Clinical Scholars Award.
References
- AbdAlla S, Lother H, Langer A, el Faramawy Y, Quitterer U. (2004) Factor XIIIA transglutaminase crosslinks AT1 receptor dimers of monocytes at the onset of atherosclerosis. Cell 119:343–354 [DOI] [PubMed] [Google Scholar]
- Acharya KR, Sturrock ED, Riordan JF, Ehlers MR. (2003) Ace revisited: a new target for structure-based drug design. Nat Rev Drug Discov 2:891–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agerholm-Larsen B, Nordestgaard BG, Tybjaerg-Hansen A. (2000) ACE gene polymorphism in cardiovascular disease: meta-analyses of small and large studies in whites. Arterioscler Thromb Vasc Biol 20:484–492 [DOI] [PubMed] [Google Scholar]
- Agerholm-Larsen B, Nordestgaard BG, Steffensen R, Sørensen TI, Jensen G, Tybjaerg-Hansen A. (1997) ACE gene polymorphism: ischemic heart disease and longevity in 10,150 individuals. A case-referent and retrospective cohort study based on the Copenhagen City Heart Study. Circulation 95:2358–2367 [DOI] [PubMed] [Google Scholar]
- Ahmed A, Fonarow GC, Zhang Y, Sanders PW, Allman RM, Arnett DK, Feller MA, Love TE, Aban IB, Levesque R, et al. (2012) Renin-angiotensin inhibition in systolic heart failure and chronic kidney disease. Am J Med 125:399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainscough JF, Drinkhill MJ, Sedo A, Turner NA, Brooke DA, Balmforth AJ, Ball SG. (2009) Angiotensin II type-1 receptor activation in the adult heart causes blood pressure-independent hypertrophy and cardiac dysfunction. Cardiovasc Res 81:592–600 [DOI] [PubMed] [Google Scholar]
- Albitar S, Genin R, Fen-Chong M, Serveaux MO, Bourgeon B. (1998) High dose enalapril impairs the response to erythropoietin treatment in haemodialysis patients. Nephrol Dial Transplant 13:1206–1210 [DOI] [PubMed] [Google Scholar]
- Allinson TM, Parkin ET, Condon TP, Schwager SL, Sturrock ED, Turner AJ, Hooper NM. (2004) The role of ADAM10 and ADAM17 in the ectodomain shedding of angiotensin converting enzyme and the amyloid precursor protein. Eur J Biochem 271:2539–2547 [DOI] [PubMed] [Google Scholar]
- Anthony CS, Corradi HR, Schwager SL, Redelinghuys P, Georgiadis D, Dive V, Acharya KR, Sturrock ED. (2010) The N domain of human angiotensin-I-converting enzyme: the role of N-glycosylation and the crystal structure in complex with an N domain-specific phosphinic inhibitor, RXP407. J Biol Chem 285:35685–35693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony CS, Masuyer G, Sturrock ED, Acharya KR. (2012) Structure based drug design of angiotensin-I converting enzyme inhibitors. Curr Med Chem 19:845–855 [DOI] [PubMed] [Google Scholar]
- Araujo MC, Melo RL, Cesari MH, Juliano MA, Juliano L, Carmona AK. (2000) Peptidase specificity characterization of C- and N-terminal catalytic sites of angiotensin I-converting enzyme. Biochemistry 39:8519–8525 [DOI] [PubMed] [Google Scholar]
- Arendshorst WJ, Brännström K, Ruan X. (1999) Actions of angiotensin II on the renal microvasculature. J Am Soc Nephrol 10 (Suppl 11):S149–S161 [PubMed] [Google Scholar]
- Arndt JW, Hao B, Ramakrishnan V, Cheng T, Chan SI, Chan MK. (2002) Crystal structure of a novel carboxypeptidase from the hyperthermophilic archaeon Pyrococcus furiosus. Structure 10:215–224 [DOI] [PubMed] [Google Scholar]
- Arnett DK, Borecki IB, Ludwig EH, Pankow JS, Myers R, Evans G, Folsom AR, Heiss G, Higgins M. (1998) Angiotensinogen and angiotensin converting enzyme genotypes and carotid atherosclerosis: the atherosclerosis risk in communities and the NHLBI family heart studies. Atherosclerosis 138:111–116 [DOI] [PubMed] [Google Scholar]
- Ashizawa N, Graf K, Do YS, Nunohiro T, Giachelli CM, Meehan WP, Tuan TL, Hsueh WA. (1996) Osteopontin is produced by rat cardiac fibroblasts and mediates A(II)-induced DNA synthesis and collagen gel contraction. J Clin Invest 98:2218–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi M, Ezan E, Nicolet L, Grognet JM, Ménard J. (1997) High plasma level of N-acetyl-seryl-aspartyl-lysyl-proline: a new marker of chronic angiotensin-converting enzyme inhibition. Hypertension 30:1015–1019 [DOI] [PubMed] [Google Scholar]
- Azizi M, Ezan E, Reny JL, Wdzieczak-Bakala J, Gerineau V, Ménard J. (1999) Renal and metabolic clearance of N-acetyl-seryl-aspartyl-lysyl-proline (AcSDKP) during angiotensin-converting enzyme inhibition in humans. Hypertension 33:879–886 [DOI] [PubMed] [Google Scholar]
- Azizi M, Rousseau A, Ezan E, Guyene TT, Michelet S, Grognet JM, Lenfant M, Corvol P, Ménard J. (1996) Acute angiotensin-converting enzyme inhibition increases the plasma level of the natural stem cell regulator N-acetyl-seryl-aspartyl-lysyl-proline. J Clin Invest 97:839–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhle YS. (1968) Conversion of angiotensin I to angiotensin II by cell-free extracts of dog lung. Nature 220:919–921 [DOI] [PubMed] [Google Scholar]
- Bakhle YS. (1974) Converting enzyme in vitro measurement and properties, in Angiotensin (Handbook of Experimental Pharmacology) (Page IH, Bumpus FM. eds) pp 41–80, Springer-Verlag, New York [Google Scholar]
- Bakhle YS, Reynard AM, Vane JR. (1969) Metabolism of the angiotensins in isolated perfused tissues. Nature 222:956–959 [DOI] [PubMed] [Google Scholar]
- Ball BA, Gravance CG, Wessel MT, Sabeur K. (2003) Activity of angiotensin-converting enzyme (ACE) in reproductive tissues of the stallion and effects of angiotensin II on sperm motility. Theriogenology 59:901–914 [DOI] [PubMed] [Google Scholar]
- Barley J, Blackwood A, Miller M, Markandu ND, Carter ND, Jeffery S, Cappuccio FP, MacGregor GA, Sagnella GA. (1996) Angiotensin converting enzyme gene I/D polymorphism, blood pressure and the renin-angiotensin system in Caucasian and Afro-Caribbean peoples. J Hum Hypertens 10:31–35 [PubMed] [Google Scholar]
- Barreto-Chaves ML, Mello-Aires M. (1996) Effect of luminal angiotensin II and ANP on early and late cortical distal tubule HCO3- reabsorption. Am J Physiol 271:F977–F984 [DOI] [PubMed] [Google Scholar]
- Baylis C, Brenner BM. (1978) Modulation by prostaglandin synthesis inhibitors of the action of exogenous angiotensin II on glomerular ultrafiltration in the rat. Circ Res 43:889–898 [DOI] [PubMed] [Google Scholar]
- Baudin B, Alves N, Pilon A, Bénéteau-Burnat B, Giboudeau J. (1997) Structural and biological roles of glycosylations in pulmonary angiotensin I-converting enzyme. Glycobiology 7:565–570 [DOI] [PubMed] [Google Scholar]
- Becker JC, Houben R, Schrama D, Voigt H, Ugurel S, Reisfeld RA. (2010) Mouse models for melanoma: a personal perspective. Exp Dermatol 19:157–164 [DOI] [PubMed] [Google Scholar]
- Belcheva I, Ternianov A, Georgiev V. (2000) Lateralized learning and memory effects of angiotensin II microinjected into the rat CA1 hippocampal area. Peptides 21:407–411 [DOI] [PubMed] [Google Scholar]
- Beldent V, Michaud A, Bonnefoy C, Chauvet MT, Corvol P. (1995) Cell surface localization of proteolysis of human endothelial angiotensin I-converting enzyme. Effect of the amino-terminal domain in the solubilization process. J Biol Chem 270:28962–28969 [DOI] [PubMed] [Google Scholar]
- Bénéteau-Burnat B, Baudin B, Morgant G, Baumann FC, Giboudeau J. (1990) Serum angiotensin-converting enzyme in healthy and sarcoidotic children: comparison with the reference interval for adults. Clin Chem 36:344–346 [PubMed] [Google Scholar]
- Benöhr P, Harsch S, Proksch B, Gleiter CH. (2004) Does angiotensin II modulate erythropoietin production in HepG2 cells? Nephron, Exp Nephrol 98:e124–e131 [DOI] [PubMed] [Google Scholar]
- Berg T, Sulner J, Lai CY, Soffer RL. (1986) Immunohistochemical localization of two angiotensin I-converting isoenzymes in the reproductive tract of the male rabbit. J Histochem Cytochem 34:753–760 [DOI] [PubMed] [Google Scholar]
- Bernstein KE, Martin BM, Bernstein EA, Linton J, Striker L, Striker G. (1988a) The isolation of angiotensin-converting enzyme cDNA. J Biol Chem 263:11021–11024 [PubMed] [Google Scholar]
- Bernstein KE, Martin BM, Edwards AS, Bernstein EA. (1989) Mouse angiotensin-converting enzyme is a protein composed of two homologous domains. J Biol Chem 264:11945–11951 [PubMed] [Google Scholar]
- Bernstein KE, Martin BM, Striker L, Striker G. (1988b) Partial protein sequence of mouse and bovine kidney angiotensin converting enzyme. Kidney Int 33:652–655 [DOI] [PubMed] [Google Scholar]
- Bernstein KE, Shen XZ, Gonzalez-Villalobos RA, Billet S, Okwan-Duodu D, Ong FS, Fuchs S. (2011) Different in vivo functions of the two catalytic domains of angiotensin-converting enzyme (ACE). Curr Opin Pharmacol 11:105–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein KE, Welsh SL, Inman JK. (1990) A deeply recessed active site in angiotensin-converting enzyme is indicated from the binding characteristics of biotin-spacer-inhibitor reagents. Biochem Biophys Res Commun 167:310–316 [DOI] [PubMed] [Google Scholar]
- Bersanetti PA, Andrade MC, Casarini DE, Juliano MA, Nchinda AT, Sturrock ED, Juliano L, Carmona AK. (2004) Positional-scanning combinatorial libraries of fluorescence resonance energy transfer peptides for defining substrate specificity of the angiotensin I-converting enzyme and development of selective C-domain substrates. Biochemistry 43:15729–36 [DOI] [PubMed] [Google Scholar]
- Binevski PV, Sizova EA, Pozdnev VF, Kost OA. (2003) Evidence for the negative cooperativity of the two active sites within bovine somatic angiotensin-converting enzyme. FEBS Lett 550:84–88 [DOI] [PubMed] [Google Scholar]
- Bingel A, Strauss E. (1909) Uber die blutdrucksteigernde Substanz der Niere. Dtsch Arch Klin Med 96:476–492 [Google Scholar]
- Blantz RC, Konnen KS, Tucker BJ. (1976) Angiotensin II effects upon the glomerular microcirculation and ultrafiltration coefficient of the rat. J Clin Invest 57:419–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloem LJ, Manatunga AK, Pratt JH. (1996) Racial difference in the relationship of an angiotensin I-converting enzyme gene polymorphism to serum angiotensin I-converting enzyme activity. Hypertension 27:62–66 [DOI] [PubMed] [Google Scholar]
- Bonnet D, Lemoine FM, Pontvert-Delucq S, Baillou C, Najman A, Guigon M. (1993) Direct and reversible inhibitory effect of the tetrapeptide acetyl-N-Ser-Asp-Lys-Pro (Seraspenide) on the growth of human CD34+ subpopulations in response to growth factors. Blood 82:3307–3314 [PubMed] [Google Scholar]
- Braun-Menendez E, Fasciolo JC, Leloir LF, Muñoz JM. (1940) The substance causing renal hypertension. J Physiol 98:283–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun-Menendez E, Page IH. (1958) Suggested Revision of Nomenclature—Angiotensin. Science 127:242. [DOI] [PubMed] [Google Scholar]
- Brentjens JR, Matsuo S, Andres GA, Caldwell PR, Zamboni L. (1986) Gametes contain angiotensin converting enzyme (kininase II). Experientia 42:399–402 [DOI] [PubMed] [Google Scholar]
- Brice EA, Friedlander W, Bateman ED, Kirsch RE. (1995) Serum angiotensin-converting enzyme activity, concentration, and specific activity in granulomatous interstitial lung disease, tuberculosis, and COPD. Chest 107:706–710 [DOI] [PubMed] [Google Scholar]
- Brooks DR, Appleford PJ, Murray L, Isaac RE. (2003) An essential role in molting and morphogenesis of Caenorhabditis elegans for ACN-1, a novel member of the angiotensin-converting enzyme family that lacks a metallopeptidase active site. J Biol Chem 278:52340–52346 [DOI] [PubMed] [Google Scholar]
- Brown CK, Madauss K, Lian W, Beck MR, Tolbert WD, Rodgers DW. (2001) Structure of neurolysin reveals a deep channel that limits substrate access. Proc Natl Acad Sci USA 98:3127–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneval P, Hinglais N, Alhenc-Gelas F, Tricottet V, Corvol P, Menard J, Camilleri JP, Bariety J. (1986) Angiotensin I converting enzyme in human intestine and kidney. Ultrastructural immunohistochemical localization. Histochemistry 85:73–80 [DOI] [PubMed] [Google Scholar]
- Bull HG, Thornberry NA, Cordes EH. (1985a) Purification of angiotensin-converting enzyme from rabbit lung and human plasma by affinity chromatography. J Biol Chem 260:2963–2972 [PubMed] [Google Scholar]
- Bull HG, Thornberry NA, Cordes MH, Patchett AA, Cordes EH. (1985b) Inhibition of rabbit lung angiotensin-converting enzyme by N alpha-[(S)-1-carboxy-3-phenylpropyl]L-alanyl-L-proline and N alpha-[(S)-1-carboxy-3-phenylpropyl]L-lysyl-L-proline. J Biol Chem 260:2952–2962 [PubMed] [Google Scholar]
- Bumpus FM, Schwarz H, Page IH. (1957) Synthesis and pharmacology of the octapeptide angiotonin. Science 125:886–887 [DOI] [PubMed] [Google Scholar]
- Bumpus FM, Schwarz H, Page IH. (1956) Partial separation of an oxytocic principle from preparations of angiotonin. Circ Res 4:488–492 [DOI] [PubMed] [Google Scholar]
- Bünning P, Riordan JF. (1983) Activation of angiotensin converting enzyme by monovalent anions. Biochemistry 22:110–116 [DOI] [PubMed] [Google Scholar]
- Bünning P, Riordan JF. (1985) The functional role of zinc in angiotensin converting enzyme: implications for the enzyme mechanism. J Inorg Biochem 24:183–198 [DOI] [PubMed] [Google Scholar]
- Bunting M, Bernstein KE, Greer JM, Capecchi MR, Thomas KR. (1999) Targeting genes for self-excision in the germ line. Genes Dev 13:1524–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers LD, Wolfenden R. (1973) Binding of the by-product analog benzylsuccinic acid by carboxypeptidase A. Biochemistry 12:2070–2078 [DOI] [PubMed] [Google Scholar]
- Caldwell PR, Seegal BC, Hsu KC, Das M, Soffer RL. (1976) Angiotensin-converting enzyme: vascular endothelial localization. Science 191:1050–1051 [DOI] [PubMed] [Google Scholar]
- Cambien F, Poirier O, Lecerf L, Evans A, Cambou JP, Arveiler D, Luc G, Bard JM, Bara L, Ricard S, et al. (1992) Deletion polymorphism in the gene for angiotensin-converting enzyme is a potent risk factor for myocardial infarction. Nature 359:641–644 [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Alexiou T, Xiao HD, Fuchs S, McKinley MJ, Corvol P, Bernstein KE. (2004) Effect of reduced angiotensin-converting enzyme gene expression and angiotensin-converting enzyme inhibition on angiotensin and bradykinin peptide levels in mice. Hypertension 43:854–859 [DOI] [PubMed] [Google Scholar]
- Casselbrant A, Edebo A, Wennerblom J, Lönroth H, Helander HF, Vieth M, Lundell L, Fändriks L. (2007) Actions by angiotensin II on esophageal contractility in humans. Gastroenterology 132:249–260 [DOI] [PubMed] [Google Scholar]
- Castro-Chaves P, Fontes-Carvalho R, Pintalhao M, Pimentel-Nunes P, Leite-Moreira AF. (2009) Angiotensin II-induced increase in myocardial distensibility and its modulation by the endocardial endothelium in the rabbit heart. Exp Physiol 94:665–674 [DOI] [PubMed] [Google Scholar]
- Cavasin MA, Rhaleb NE, Yang XP, Carretero OA. (2004) Prolyl oligopeptidase is involved in release of the antifibrotic peptide Ac-SDKP. Hypertension 43:1140–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao J, Zhang JJ, Lin KF, Chao L. (1998) Human kallikrein gene delivery attenuates hypertension, cardiac hypertrophy, and renal injury in Dahl salt-sensitive rats. Hum Gene Ther 9:21–31 [DOI] [PubMed] [Google Scholar]
- Charrier S, Michaud A, Badaoui S, Giroux S, Ezan E, Sainteny F, Corvol P, Vainchenker W. (2004) Inhibition of angiotensin I-converting enzyme induces radioprotection by preserving murine hematopoietic short-term reconstituting cells. Blood 104:978–985 [DOI] [PubMed] [Google Scholar]
- Charytan C, Goldfarb-Rumyantzev A, Wang YF, Schwenk MH, Spinowitz BS. (1998) Effect of angiotensin-converting enzyme inhibitors on response to erythropoietin therapy in chronic dialysis patients. Am J Nephrol 18:498–503 [DOI] [PubMed] [Google Scholar]
- Chen HL, Lünsdorf H, Hecht HJ, Tsai H. (2010) Porcine pulmonary angiotensin I-converting enzyme—biochemical characterization and spatial arrangement of the N- and C-domains by three-dimensional electron microscopic reconstruction. Micron 41:674–685 [DOI] [PubMed] [Google Scholar]
- Chew CG, Weise MD, Disney AP. (1999) The effect of angiotensin II receptor antagonist on the exogenous erythropoietin requirement of haemodialysis patients. Nephrol Dial Transplant 14:2047–2049 [PubMed] [Google Scholar]
- Chisi JE, Briscoe CV, Ezan E, Genet R, Riches AC, Wdzieczak-Bakala J. (2000) Captopril inhibits in vitro and in vivo the proliferation of primitive haematopoietic cells induced into cell cycle by cytotoxic drug administration or irradiation but has no effect on myeloid leukaemia cell proliferation. Br J Haematol 109:563–570 [DOI] [PubMed] [Google Scholar]
- Cigola E, Kajstura J, Li B, Meggs LG, Anversa P. (1997) Angiotensin II activates programmed myocyte cell death in vitro. Exp Cell Res 231:363–371 [DOI] [PubMed] [Google Scholar]
- Cingolani HE, Pérez NG, Aiello EA, de Hurtado MC. (2005) Intracellular signaling following myocardial stretch: an autocrine/paracrine loop. Regul Pept 128:211–220 [DOI] [PubMed] [Google Scholar]
- Cochran G, Harpending H. (2009) The 10,000 Year Explosion: How Civilization Accelerated Human Evolution, p 42, Basic Books, New York [Google Scholar]
- Colbourne JK, Pfrender ME, Gilbert D, Thomas WK, Tucker A, Oakley TH, Tokishita S, Aerts A, Arnold GJ, Basu MK, et al. (2011) The ecoresponsive genome of Daphnia pulex. Science 331:555–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J, Ertoy D, Lin H, Sutliff RL, Ezan E, Guyene TT, Capecchi M, Corvol P, Bernstein KE. (2000) Lack of angiotensin II-facilitated erythropoiesis causes anemia in angiotensin-converting enzyme-deficient mice. J Clin Invest 106:1391–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JM, Khokhlova N, Sutliff RL, Adams JW, Disher KM, Zhao H, Capecchi MR, Corvol P, Bernstein KE. (2003) Mice lacking endothelial ACE: normal blood pressure with elevated angiotensin II. Hypertension 41:313–321 [DOI] [PubMed] [Google Scholar]
- Cole J, Quach DL, Sundaram K, Corvol P, Capecchi MR, Bernstein KE. (2002) Mice lacking endothelial angiotensin-converting enzyme have a normal blood pressure. Circ Res 90:87–92 [DOI] [PubMed] [Google Scholar]
- Collier JG, Robinson BF, Vane JR. (1973) Reduction of pressor effects of angiotensin I in man by synthetic nonapeptide (B.P.P. 9a or SQ 20,881) which inhibits converting enzyme. Lancet 1:72–74 [DOI] [PubMed] [Google Scholar]
- Cornell MJ, Williams TA, Lamango NS, Coates D, Corvol P, Soubrier F, Hoheisel J, Lehrach H, Isaac RE. (1995) Cloning and expression of an evolutionary conserved single-domain angiotensin converting enzyme from Drosophila melanogaster. J Biol Chem 270:13613–13619 [DOI] [PubMed] [Google Scholar]
- Corradi HR, Chitapi I, Sewell BT, Georgiadis D, Dive V, Sturrock ED, Acharya KR. (2007) The structure of testis angiotensin-converting enzyme in complex with the C domain-specific inhibitor RXPA380. Biochemistry 46:5473–5478 [DOI] [PubMed] [Google Scholar]
- Corradi HR, Schwager SL, Nchinda AT, Sturrock ED, Acharya KR. (2006) Crystal structure of the N domain of human somatic angiotensin I-converting enzyme provides a structural basis for domain-specific inhibitor design. J Mol Biol 357:964–974 [DOI] [PubMed] [Google Scholar]
- Corvol P, Eyries M, Soubrier F. (2004) Peptidyl-dipeptidase A/Angiotensin I-converting enzyme, in Handbook of Proteolytic Enzymes (Barret A, Rawlings N, Woessner J. eds) pp 332–349, Elsevier Academic Press, New York [Google Scholar]
- Côté F, Do TH, Laflamme L, Gallo JM, Gallo-Payet N. (1999) Activation of the AT(2) receptor of angiotensin II induces neurite outgrowth and cell migration in microexplant cultures of the cerebellum. J Biol Chem 274:31686–31692 [DOI] [PubMed] [Google Scholar]
- Cotton J, Hayashi MA, Cuniasse P, Vazeux G, Ianzer D, De Camargo AC, Dive V. (2002) Selective inhibition of the C-domain of angiotensin I converting enzyme by bradykinin potentiating peptides. Biochemistry 41:6065–6071 [DOI] [PubMed] [Google Scholar]
- Coudé G, Marois A, Casanova C. (2000) Effects of angiotensin II on visual evoked potentials in the superior colliculus of juvenile rats. Neuropeptides 34:203–210 [DOI] [PubMed] [Google Scholar]
- Craiu A, Akopian T, Goldberg A, Rock KL. (1997) Two distinct proteolytic processes in the generation of a major histocompatibility complex class I-presented peptide. Proc Natl Acad Sci USA 94:10850–10855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. (2006) Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA 103:17985–17990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumano A, Ferraz JC, Klaine M, Di Santo JP, Godin I. (2001) Intraembryonic, but not yolk sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity 15:477–485 [DOI] [PubMed] [Google Scholar]
- Cumin F, Vellaud V, Corvol P, Alhenc-Gelas F. (1989) Evidence for a single active site in the human angiotensin I-converting enzyme from inhibitor binding studies with [3H] RU 44 403: role of chloride. Biochem Biophys Res Commun 163:718–725 [DOI] [PubMed] [Google Scholar]
- Cushman DW, Cheung HS. (1969) A simple substrate for the assay of dog lung angiotensin converting enzyme. Fed Proc 28:799 [Google Scholar]
- Cushman DW, Cheung HS. (1971) Concentrations of angiotensin-converting enzyme in tissues of the rat. Biochim Biophys Acta 250:261–265 [DOI] [PubMed] [Google Scholar]
- Cushman DW, Cheung HS, Sabo EF, Ondetti MA. (1977) Design of potent competitive inhibitors of angiotensin-converting enzyme. Carboxyalkanoyl and mercaptoalkanoyl amino acids. Biochemistry 16:5484–5491 [DOI] [PubMed] [Google Scholar]
- Cushman DW, Ondetti MA. (1999) Design of angiotensin converting enzyme inhibitors. Nat Med 5:1110–1113 [DOI] [PubMed] [Google Scholar]
- Dalbeth N, Edwards J, Fairchild S, Callan M, Hall FC. (2005) The non-thiol angiotensin-converting enzyme inhibitor quinapril suppresses inflammatory arthritis. Rheumatology (Oxford) 44:24–31 [DOI] [PubMed] [Google Scholar]
- Danilov SM, Faerman AI, Printseva OYu, Martynov AV, Sakharov IYu, Trakht IN. (1987) Immunohistochemical study of angiotensin-converting enzyme in human tissues using monoclonal antibodies. Histochemistry 87:487–490 [DOI] [PubMed] [Google Scholar]
- Danilov SM, Gordon K, Nesterovitch AB, Lünsdorf H, Chen Z, Castellon M, Popova IA, Kalinin S, Mendonca E, Petukhov PA, Schwartz DE, Minshall RD, Sturrock ED. (2011) An angiotensin I-converting enzyme mutation (Y465D) causes a dramatic increase in blood ACE via accelerated ACE shedding. PLoS One 6:e25952.1–e25952.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Soffer RL. (1975) Pulmonary angiotensin-converting enzyme. Structural and catalytic properties. J Biol Chem 250:6762–6768 [PubMed] [Google Scholar]
- Das M, Soffer RL. (1976) Pulmonary angiotensin-converting enzyme antienzyme antibody. Biochemistry 15:5088–5094 [DOI] [PubMed] [Google Scholar]
- Das M, Hartley JL, Soffers RL. (1977) Serum angiotensin-converting enzyme. Isolation and relationship to the pulmonary enzyme. J Biol Chem 252:1316–1319 [PubMed] [Google Scholar]
- Davis TA, Landauer MR, Mog SR, Barshishat-Kupper M, Zins SR, Amare MF, Day RM. (2010) Timing of captopril administration determines radiation protection or radiation sensitization in a murine model of total body irradiation. Exp Hematol 38:270–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet AD, Krause EG, Scott KA, Foster MT, Herman JP, Sakai RR, Seeley RJ, Woods SC. (2011) Central angiotensin II has catabolic action at white and brown adipose tissue. Am J Physiol Endocrinol Metab 301:E1081–E1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de los Rios AD, Labajos M, Manteca A, Morell M, Souviron A. (1980) Stimulatory action of angiotensin II on water and electrolyte transport by the proximal colon of the rat. J Endocrinol 86:35–43 [DOI] [PubMed] [Google Scholar]
- Deddish PA, Jackman HL, Skidgel RA, Erdös EG. (1997) Differences in the hydrolysis of enkephalin congeners by the two domains of angiotensin converting enzyme. Biochem Pharmacol 53:1459–1463 [DOI] [PubMed] [Google Scholar]
- Deddish PA, Marcic B, Jackman HL, Wang HZ, Skidgel RA, Erdös EG. (1998) N-domain-specific substrate and C-domain inhibitors of angiotensin-converting enzyme: angiotensin-(1-7) and keto-ACE. Hypertension 31:912–917 [DOI] [PubMed] [Google Scholar]
- Defendini R, Zimmerman EA, Weare JA, Alhenc-Gelas F, Edros EG. (1982) Hydrolysis of enkephalins by human converting enzyme and localization of the enzyme in neuronal components of the brain. Adv Biochem Psychopharmacol 33:271–280 [PubMed] [Google Scholar]
- Defendini R, Zimmerman EA, Weare JA, Alhenc-Gelas F, Erdös EG. (1983) Angiotensin-converting enzyme in epithelial and neuroepithelial cells. Neuroendocrinology 37:32–40 [DOI] [PubMed] [Google Scholar]
- Del Vecchio PJ, Smith JR. (1981) Expression of angiotensin-converting enzyme activity in cultured pulmonary artery endothelial cells. J Cell Physiol 108:337–345 [DOI] [PubMed] [Google Scholar]
- Dempsey PJ, McCallum ZT, Kent KM, Cooper T. (1971) Direct myocardial effects of angiotensin II. Am J Physiol 220:477–481 [DOI] [PubMed] [Google Scholar]
- Di Zhang A, Nguyen Dinh Cat A, Soukaseum C, Escoubet B, Cherfa A, Messaoudi S, Delcayre C, Samuel JL, Jaisser F. (2008) Cross-talk between mineralocorticoid and angiotensin II signaling for cardiac remodeling. Hypertension 52:1060–1067 [DOI] [PubMed] [Google Scholar]
- Díaz-Torga G, González Iglesias A, Achával-Zaia R, Libertun C, Becú-Villalobos D. (1998) Angiotensin II-induced Ca2+ mobilization and prolactin release in normal and hyperplastic pituitary cells. Am J Physiol 274:E534–E540 [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV. (2006) Angiotensin-converting enzyme inhibitor-induced cough: ACCP evidence-based clinical practice guidelines. Chest 129(1, Suppl)169S–173S [DOI] [PubMed] [Google Scholar]
- Dive V, Georgiadis D, Matziari M, Makaritis A, Beau F, Cuniasse P, Yiotakis A. (2004) Phosphinic peptides as zinc metalloproteinase inhibitors. Cell Mol Life Sci 61:2010–2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenighetti AA, Wang Q, Egger M, Richards SM, Pedrazzini T, Delbridge LM. (2005) Angiotensin II-mediated phenotypic cardiomyocyte remodeling leads to age-dependent cardiac dysfunction and failure. Hypertension 46:426–432 [DOI] [PubMed] [Google Scholar]
- Dorer FE, Kahn JR, Lentz KE, Levine M, Skeggs LT. (1972) Purification and properties of angiotensin-converting enzyme from hog lung. Circ Res 31:356–366 [DOI] [PubMed] [Google Scholar]
- Dostal DE, Baker KM. (1998) Angiotensin and endothelin: messengers that couple ventricular stretch to the Na+/H+ exchanger and cardiac hypertrophy. Circ Res 83:870–873 [DOI] [PubMed] [Google Scholar]
- Doughan AK, Harrison DG, Dikalov SI. (2008) Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102:488–496 [DOI] [PubMed] [Google Scholar]
- Dubreuil P, Fulcrand P, Rodriguez M, Fulcrand H, Laur J, Martinez J. (1989) Novel activity of angiotensin-converting enzyme. Hydrolysis of cholecystokinin and gastrin analogues with release of the amidated C-terminal dipeptide. Biochem J 262:125–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AM, Kladis A, Jennings GL, Dart AM, Esler M, Campbell DJ. (2000) Kinins in humans. Am J Physiol Regul Integr Comp Physiol 278:R897–R904 [DOI] [PubMed] [Google Scholar]
- Dux S, Aron N, Boner G, Carmel A, Yaron A, Rosenfeld JB. (1984) Serum angiotensin converting enzyme activity in normal adults and patients with different types of hypertension. Isr J Med Sci 20:1138–1142 [PubMed] [Google Scholar]
- Eckman EA, Adams SK, Troendle FJ, Stodola BA, Kahn MA, Fauq AH, Xiao HD, Bernstein KE, Eckman CB. (2006) Regulation of steady-state beta-amyloid levels in the brain by neprilysin and endothelin-converting enzyme but not angiotensin-converting enzyme. J Biol Chem 281:30471–30478 [DOI] [PubMed] [Google Scholar]
- Ehlers MR, Riordan JF. (1991) Angiotensin-converting enzyme: zinc- and inhibitor-binding stoichiometries of the somatic and testis isozymes. Biochemistry 30:7118–7126 [DOI] [PubMed] [Google Scholar]
- Ehlers MR, Chen YN, Riordan JF. (1991) Spontaneous solubilization of membrane-bound human testis angiotensin-converting enzyme expressed in Chinese hamster ovary cells. Proc Natl Acad Sci USA 88:1009–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MR, Chen YN, Riordan JF. (1992) The unique N-terminal sequence of testis angiotensin-converting enzyme is heavily O-glycosylated and unessential for activity or stability. Biochem Biophys Res Commun 183:199–205 [DOI] [PubMed] [Google Scholar]
- Ehlers MR, Fox EA, Strydom DJ, Riordan JF. (1989) Molecular cloning of human testicular angiotensin-converting enzyme: the testis isozyme is identical to the C-terminal half of endothelial angiotensin-converting enzyme. Proc Natl Acad Sci USA 86:7741–7745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MR, Schwager SL, Scholle RR, Manji GA, Brandt WF, Riordan JF. (1996) Proteolytic release of membrane-bound angiotensin-converting enzyme: role of the juxtamembrane stalk sequence. Biochemistry 35:9549–9559 [DOI] [PubMed] [Google Scholar]
- Eisenlohr LC, Bacik I, Bennink JR, Bernstein K, Yewdell JW. (1992) Expression of a membrane protease enhances presentation of endogenous antigens to MHC class I-restricted T lymphocytes. Cell 71:963–972 [DOI] [PubMed] [Google Scholar]
- Ekbote U, Coates D, Isaac RE. (1999) A mosquito (Anopheles stephensi) angiotensin I-converting enzyme (ACE) is induced by a blood meal and accumulates in the developing ovary. FEBS Lett 455:219–222 [DOI] [PubMed] [Google Scholar]
- Ekbote U, Looker M, Isaac RE. (2003) ACE inhibitors reduce fecundity in the mosquito, Anopheles stephensi. Comp Biochem Physiol B Biochem Mol Biol 134:593–598 [DOI] [PubMed] [Google Scholar]
- El-Dorry HA, Bull HG, Iwata K, Thornberry NA, Cordes EH, Soffer RL. (1982a) Molecular and catalytic properties of rabbit testicular dipeptidyl carboxypeptidase. J Biol Chem 257:14128–14133 [PubMed] [Google Scholar]
- El-Dorry HA, MacGregor JS, Soffer RL. (1983) Dipeptidyl carboxypeptidase from seminal fluid resembles the pulmonary rather than the testicular isoenzyme. Biochem Biophys Res Commun 115:1096–1100 [DOI] [PubMed] [Google Scholar]
- El-Dorry HA, Pickett CB, MacGregor JS, Soffer RL. (1982b) Tissue-specific expression of mRNAs for dipeptidyl carboxypeptidase isoenzymes. Proc Natl Acad Sci USA 79:4295–4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott DF, Peart WS. (1956) Amino-acid sequence in a hypertensin. Nature 177:527–528 [DOI] [PubMed] [Google Scholar]
- English WR, Corvol P, Murphy G. (2012) LPS activates ADAM9 dependent shedding of ACE from endothelial cells. Biochem Biophys Res Commun 421:70–75 [DOI] [PubMed] [Google Scholar]
- Epstein AN, Fitzsimons JT, Rolls BJ. (1970) Drinking induced by injection of angiotensin into the rain of the rat. J Physiol 210:457–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdös EG. (1975) Angiotensin I converting enzyme. Circ Res 36:247–255 [DOI] [PubMed] [Google Scholar]
- Erdös EG. (2006) The ACE and I: how ACE inhibitors came to be. FASEB J 20:1034–1038 [DOI] [PubMed] [Google Scholar]
- Erdoes EG, Wohler JR. (1963) Inhibition in vivo of the enzymatic inactivation of bradykinin and kallidin. Biochem Pharmacol 12:1193–1199 [DOI] [PubMed] [Google Scholar]
- Erdös EG, Yang HYT. (1967) An enzyme in microsomal fraction of kidney that inactivates bradykinin. Life Sci 6:569–574 [DOI] [PubMed] [Google Scholar]
- Ertoy D, Bernstein KE. (2000) Targeted interruption of the renin-angiotensin system, in Drugs, Enzymes and Receptors of the Renin-Angiotensin System: Celebrating a Century of Discovery (Husain A, Graham R. eds) pp 205–223, Harwood Academic Publishers, The Netherlands [Google Scholar]
- Esther CR, Jr, Howard TE, Marino EM, Goddard JM, Capecchi MR, Bernstein KE. (1996) Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab Invest 74:953–965 [PubMed] [Google Scholar]
- Esther CR, Jr, Semeniuk D, Marino EM, Zhou Y, Overbeek PA, Bernstein KE. (1997) Expression of testis angiotensin-converting enzyme is mediated by a cyclic AMP responsive element. Lab Invest 77:483–488 [PubMed] [Google Scholar]
- Esther CR, Jr, Thomas KE, Bernstein KE. (1994) Chicken lacks the testis specific isozyme of angiotensin converting enzyme found in mammals. Biochem Biophys Res Commun 205:1916–1921 [DOI] [PubMed] [Google Scholar]
- Eyries M, Michaud A, Deinum J, Agrapart M, Chomilier J, Kramers C, Soubrier F. (2001) Increased shedding of angiotensin-converting enzyme by a mutation identified in the stalk region. J Biol Chem 276:5525–5532 [DOI] [PubMed] [Google Scholar]
- Farmer JA. (2000) Renin angiotensin system and ASCVD. Curr Opin Cardiol 15:141–150 [DOI] [PubMed] [Google Scholar]
- Ferreira SH. (1965) A bradykinin-potentiating factor (BPF) present in the venom of Bothrops jararaca. Br J Pharmacol Chemother 24:163–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira SH, Rocha e Silva M. (1962) Potentiation of bradykinin by dimercaptopropanol (bal) and other inhibitors of its destroying enzyme in plasma. Biochem Pharmacol 11:1123–1128 [DOI] [PubMed] [Google Scholar]
- Ferreira SH, Vane JR. (1967) The disappearance of bradykinin and eledoisin in the circulation and vascular beds of the cat. Br Pharmacol Chemother 30:417–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira SH, Bartelt DC, Greene LJ. (1970) Isolation of bradykinin-potentiating peptides from Bothrops jararaca venom. Biochemistry 9:2583–2593 [DOI] [PubMed] [Google Scholar]
- Fishlock DJ, Gunn A. (1970) The action of angiotensin on the human colon in vitro. Br J Pharmacol 39:34–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming I. (2006) Signaling by the angiotensin-converting enzyme. Circ Res 98:887–896 [DOI] [PubMed] [Google Scholar]
- Forslund T, Fyhrquist F, Grönhagen-Riska C, Tikkanen I. (1982) Induction of angiotensin-converting enzyme with the ACE inhibitory compound MK-421 in rat lung. Eur J Pharmacol 80:121–5 [DOI] [PubMed] [Google Scholar]
- Foy CA, McCormack LJ, Knowler WC, Barrett JH, Catto A, Grant PJ. (1996) The angiotensin-I converting enzyme (ACE) gene I/D polymorphism and ACE levels in Pima Indians. J Med Genet 33:336–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friberg P, Sundelin B, Bohman SO, Bobik A, Nilsson H, Wickman A, Gustafsson H, Petersen J, Adams MA. (1994) Renin-angiotensin system in neonatal rats: induction of a renal abnormality in response to ACE inhibition or angiotensin II antagonism. Kidney Int 45:485–492 [DOI] [PubMed] [Google Scholar]
- Friedl W, Krempler F, Paulweber B, Pichler M, Sandhofer F. (1995) A deletion polymorphism in the angiotensin converting enzyme gene is not associated with coronary heart disease in an Austrian population. Atherosclerosis 112:137–143 [DOI] [PubMed] [Google Scholar]
- Friedland J, Setton C, Silverstein E. (1978) Induction of angiotensin converting enzyme in human monocytes in culture. Biochem Biophys Res Commun 83:843–849 [DOI] [PubMed] [Google Scholar]
- Fuchs S, Frenzel K, Hubert C, Lyng R, Muller L, Michaud A, Xiao HD, Adams JW, Capecchi MR, Corvol P, et al. (2005) Male fertility is dependent on dipeptidase activity of testis ACE. Nat Med 11:1140–1142, author reply 1142–1143 [DOI] [PubMed] [Google Scholar]
- Fuchs S, Xiao HD, Cole JM, Adams JW, Frenzel K, Michaud A, Zhao H, Keshelava G, Capecchi MR, Corvol P, et al. (2004) Role of the N-terminal catalytic domain of angiotensin-converting enzyme investigated by targeted inactivation in mice. J Biol Chem 279:15946–15953 [DOI] [PubMed] [Google Scholar]
- Fuchs S, Xiao HD, Hubert C, Michaud A, Campbell DJ, Adams JW, Capecchi MR, Corvol P, Bernstein KE. (2008) Angiotensin-converting enzyme C-terminal catalytic domain is the main site of angiotensin I cleavage in vivo. Hypertension 51:267–274 [DOI] [PubMed] [Google Scholar]
- Fujimura T, Yokota M, Kato S, Hirayama H, Tsunekawa A, Inagaki H, Takatsu F, Nakashima N, Yamada Y. (1997) Lack of association of angiotensin converting enzyme gene polymorphism or serum enzyme activity with coronary artery disease in Japanese subjects. Am J Hypertens 10:1384–1390 [DOI] [PubMed] [Google Scholar]
- Fyhrquist F, Grönhagen-Riska C, Hortling L, Forslund T, Tikkanen I, Klockars M. (1983) The induction of angiotensin converting enzyme by its inhibitors. Clin Exp Hypertens A 5:1319–1330 [DOI] [PubMed] [Google Scholar]
- Garg M, Angus PW, Burrell LM, Herath C, Gibson PR, Lubel JS. (2012) Review article: the pathophysiological roles of the renin-angiotensin system in the gastrointestinal tract. Aliment Pharmacol Ther 35:414–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhard S, Steil L, Peters B, Gesell-Salazar M, Hammer E, Kuttler B, Clemetson KJ, Scharf C, Peters J, Völker U, et al. (2011) Angiotensin II-dependent hypertension causes reversible changes in the platelet proteome. J Hypertens 29:2126–2137 [DOI] [PubMed] [Google Scholar]
- Georgieva D, Georgiev V. (1999) The role of angiotensin II and of its receptor subtypes in the acetic acid-induced abdominal constriction test. Pharmacol Biochem Behav 62:229–232 [DOI] [PubMed] [Google Scholar]
- Girerd X, Hanon O, Mourad JJ, Boutouyrie P, Laurent S, Jeunemaitre X. (1998) Lack of association between renin-angiotensin system, gene polymorphisms, and wall thickness of the radial and carotid arteries. Hypertension 32:579–583 [DOI] [PubMed] [Google Scholar]
- Goldblatt H, Lynch J, Hanzal RF, Summerville WW. (1934) Studies on experimental hypertension: I. the production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med 59:347–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfield NE, Newby DE, Ludlam CA, Flapan AD. (1999) Effects of acute angiotensin II type 1 receptor antagonism and angiotensin converting enzyme inhibition on plasma fibrinolytic parameters in patients with heart failure. Circulation 99:2983–2985 [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. (2005) Monocyte and macrophage heterogeneity. Nat Rev Immunol 5:953–964 [DOI] [PubMed] [Google Scholar]
- Gordon K, Redelinghuys P, Schwager SL, Ehlers MR, Papageorgiou AC, Natesh R, Acharya KR, Sturrock ED. (2003) Deglycosylation, processing and crystallization of human testis angiotensin-converting enzyme. Biochem J 371:437–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossmann J, Burkhardt R, Harder S, Lenz T, Sedlmeyer A, Klinkhardt U, Geiger H, Scheuermann EH. (2001) Angiotensin II infusion increases plasma erythropoietin levels via an angiotensin II type 1 receptor-dependent pathway. Kidney Int 60:83–86 [DOI] [PubMed] [Google Scholar]
- Gossmann J, Thürmann P, Bachmann T, Weller S, Kachel HG, Schoeppe W, Scheuermann EH. (1996) Mechanism of angiotensin converting enzyme inhibitor-related anemia in renal transplant recipients. Kidney Int 50:973–978 [DOI] [PubMed] [Google Scholar]
- Gray MO, Long CS, Kalinyak JE, Li HT, Karliner JS. (1998) Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-beta 1 and endothelin-1 from fibroblasts. Cardiovasc Res 40:352–363 [DOI] [PubMed] [Google Scholar]
- Griffing GT, Melby JC. (1982) Enalapril (MK-421) and the white cell count and haematocrit. Lancet 1:1361. [DOI] [PubMed] [Google Scholar]
- Grobe JL, Grobe CL, Beltz TG, Westphal SG, Morgan DA, Xu D, de Lange WJ, Li H, Sakai K, Thedens DR, et al. (2010) The brain Renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab 12:431–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman SP. (1962) Direct adrenergic and cholinergic stimulation of hypothalamic mechanisms. Am J Physiol 202:872–882 [DOI] [PubMed] [Google Scholar]
- Grunewald J, Eklund A. (2007) Role of CD4+ T cells in sarcoidosis. Proc Am Thorac Soc 4:461–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu XX, Spaepen M, Guo C, Fagard R, Amery A, Lijnen P, Cassiman JJ. (1994) Lack of association between the I/D polymorphism of the angiotensin-converting enzyme gene and essential hypertension in a Belgian population. J Hum Hypertens 8:683–685 [PubMed] [Google Scholar]
- Guilluy C, Brégeon J, Toumaniantz G, Rolli-Derkinderen M, Retailleau K, Loufrani L, Henrion D, Scalbert E, Bril A, Torres RM, Offermann S, et al. (2010) The Rho exchange factor Arhgef1 mediates the effects of angiotensin II on vascular tone and blood pressure. Nat Med 16:183–190 [DOI] [PubMed] [Google Scholar]
- Guimarães PB, Alvarenga ÉC Siqueira PD, Paredes-Gamero EJ, Sabatini RA, Morais RL, Reis RI, Santos EL, Teixeira LG, Casarini DE, Martin RP, Shimuta SI, Carmona AK, Nakaie CR, Jasiulionis MG, Ferreira AT, Pesquero JL, Oliveira SM, Bader M, Costa-Neto CM, Pesquero JB. (2011) Angiotensin II binding to angiotensin I-converting enzyme triggers calcium signaling. Hypertension 57:965–972 [DOI] [PubMed] [Google Scholar]
- Guron G, Adams MA, Sundelin B, Friberg P. (1997) Neonatal angiotensin-converting enzyme inhibition in the rat induces persistent abnormalities in renal function and histology. Hypertension 29:91–97 [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. (2007) Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204:2449–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagaman JR, Moyer JS, Bachman ES, Sibony M, Magyar PL, Welch JE, Smithies O, Krege JH, O’Brien DA. (1998) Angiotensin-converting enzyme and male fertility. Proc Natl Acad Sci USA 95:2552–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JE. (2003) Historical perspective of the renin-angiotensin system. Mol Biotechnol 24:27–39 [DOI] [PubMed] [Google Scholar]
- Hallersund P, Elfvin A, Helander HF, Fändriks L. (2011) The expression of renin-angiotensin system components in the human gastric mucosa. J Renin Angiotensin Aldosterone Syst 12:54–64 [DOI] [PubMed] [Google Scholar]
- Harmer D, Gilbert M, Borman R, Clark KL. (2002) Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett 532:107–110 [DOI] [PubMed] [Google Scholar]
- Harris PJ, Young JA. (1977) Dose-dependent stimulation and inhibition of proximal tubular sodium reabsorption by angiotensin II in the rat kidney. Pflugers Arch 367:295–297 [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Adams JW, Bernstein KE, Schnermann J. (2005) Micropuncture determination of nephron function in mice without tissue angiotensin-converting enzyme. Am J Physiol Renal Physiol 288:F445–F452 [DOI] [PubMed] [Google Scholar]
- Helmer OM. (1957) Differentiation between two forms of angiotonin by means of spirally cut strips of rabbit aorta. Am J Physiol 188:571–577 [DOI] [PubMed] [Google Scholar]
- Hemming ML, Selkoe DJ. (2005) Amyloid beta-protein is degraded by cellular angiotensin-converting enzyme (ACE) and elevated by an ACE inhibitor. J Biol Chem 280:37644–37650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems DA, Rodrigues LM, Whitton PD. (1978) Rapid stimulation by vasopressin, oxytocin and angiotensin II of glycogen degradation in hepatocyte suspensions. Biochem J 172:311–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera M, Silva GB, Garvin JL. (2010) Angiotensin II stimulates thick ascending limb superoxide production via protein kinase C(α)-dependent NADPH oxidase activation. J Biol Chem 285:21323–21328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes SR, Tagoh H, Goonetilleke N, Sasmono T, Oceandy D, Clark R, Bonifer C, Hume DA. (2001) A highly conserved c-fms gene intronic element controls macrophage-specific and regulated expression. J Leukoc Biol 70:812–820 [PubMed] [Google Scholar]
- Hoch NE, Guzik TJ, Chen W, Deans T, Maalouf SA, Gratze P, Weyand C, Harrison DG. (2009) Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol 296:R208–R216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper NM. (1991) Angiotensin converting enzyme: implications from molecular biology for its physiological functions. Int J Biochem 23:641–647 [DOI] [PubMed] [Google Scholar]
- Hooper NM. (1994) Families of zinc metalloproteases. FEBS Lett 354:1–6 [DOI] [PubMed] [Google Scholar]
- Hooper NM, Turner AJ. (1987) Isolation of two differentially glycosylated forms of peptidyl-dipeptidase A (angiotensin converting enzyme) from pig brain: a re-evaluation of their role in neuropeptide metabolism. Biochem J 241:625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper NM, Keen J, Pappin DJ, Turner AJ. (1987) Pig kidney angiotensin converting enzyme. Purification and characterization of amphipathic and hydrophilic forms of the enzyme establishes C-terminal anchorage to the plasma membrane. Biochem J 247:85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard T, Balogh R, Overbeek P, Bernstein KE. (1993) Sperm-specific expression of angiotensin-converting enzyme (ACE) is mediated by a 91-base-pair promoter containing a CRE-like element. Mol Cell Biol 13:18–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard TE, Shai S-Y, Langford KG, Martin BM, Bernstein KE. (1990) Transcription of testicular angiotensin-converting enzyme (ACE) is initiated within the 12th intron of the somatic ACE gene. Mol Cell Biol 10:4294–4302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Igarashi A, Kamata M, Nakagawa H. (2001) Angiotensin-converting enzyme degrades Alzheimer amyloid β-peptide (A β ); retards A β aggregation, deposition, fibril formation; and inhibits cytotoxicity. J Biol Chem 276:47863–47868 [DOI] [PubMed] [Google Scholar]
- Hubert C, Houot AM, Corvol P, Soubrier F. (1991) Structure of the angiotensin I-converting enzyme gene. Two alternate promoters correspond to evolutionary steps of a duplicated gene. J Biol Chem 266:15377–15383 [PubMed] [Google Scholar]
- Hubert C, Savary K, Gasc JM, Corvol P. (2006) The hematopoietic system: a new niche for the renin-angiotensin system. Nat Clin Pract Cardiovasc Med 3:80–85 [DOI] [PubMed] [Google Scholar]
- Hurles M. (2004) Gene duplication: the genomic trade in spare parts. PLoS Biol 2:E206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst D, Rylett CM, Isaac RE, Shirras AD. (2003) The drosophila angiotensin-converting enzyme homologue Ance is required for spermiogenesis. Dev Biol 254:238–247 [DOI] [PubMed] [Google Scholar]
- Igic R, Erdös EG, Yeh HSJ, Sorrells K, Nakajima T. (1972) Angiotensin I converting enzyme of the lung. Circ Res 31:2–, 51–61. [PubMed] [Google Scholar]
- Inagami T. (1981) Renin, in Biochemical Regulation of Blood Pressure (Soffer RL. ed) pp 39–72, John Wiley and Sons, New York [Google Scholar]
- Ishigami T, Iwamoto T, Tamura K, Yamaguchi S, Iwasawa K, Uchino K, Umemura S, Ishii M. (1995) Angiotensin I converting enzyme (ACE) gene polymorphism and essential hypertension in Japan. Ethnic difference of ACE genotype. Am J Hypertens 8:95–97 [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Sekizuka E, Yamaguchi N, Nakadate H, Terao S, Granger DN, Minamitani H. (2007) Angiotensin II type 1 receptor signaling contributes to platelet-leukocyte-endothelial cell interactions in the cerebral microvasculature. Am J Physiol Heart Circ Physiol 292:H2306–H2315 [DOI] [PubMed] [Google Scholar]
- Ishizaka N, Hongo M, Sakamoto A, Saito K, Furuta K, Koike K. (2011) Liver lipid content is reduced in rat given 7-day administration of angiotensin II. J Renin Angiotensin Aldosterone Syst 12:462–468 [DOI] [PubMed] [Google Scholar]
- Jaspard E, Wei L, Alhenc-Gelas F. (1993) Differences in the properties and enzymatic specificities of the two active sites of angiotensin I-converting enzyme (kininase II). Studies with bradykinin and other natural peptides. J Biol Chem 268:9496–9503 [PubMed] [Google Scholar]
- Jaszewski R, Tolia V, Ehrinpreis MN, Bodzin JH, Peleman RR, Korlipara R, Weinstock JV. (1990) Increased colonic mucosal angiotensin I and II concentrations in Crohn’s colitis. Gastroenterology 98:1543–1548 [DOI] [PubMed] [Google Scholar]
- Jensen JD, Eiskjaer H, Bagger JP, Pedersen EB. (1993) Elevated serum level of erythropoietin in congestive heart failure related to renal function. J Intern Med 233:125–130 [DOI] [PubMed] [Google Scholar]
- Jensen PE. (2007) Recent advances in antigen processing and presentation. Nat Immunol 8:1041–1048 [DOI] [PubMed] [Google Scholar]
- Jeunemaitre X, Lifton RP, Hunt SC, Williams RR, Lalouel JM. (1992) Absence of linkage between the angiotensin converting enzyme locus and human essential hypertension. Nat Genet 1:72–75 [DOI] [PubMed] [Google Scholar]
- Johansson B, Holm M, Ewert S, Casselbrant A, Pettersson A, Fändriks L. (2001) Angiotensin II type 2 receptor-mediated duodenal mucosal alkaline secretion in the rat. Am J Physiol Gastrointest Liver Physiol 280:G1254–G1260 [DOI] [PubMed] [Google Scholar]
- Johnson AD, Newton-Cheh C, Chasman DI, Ehret GB, Johnson T, Rose L, Rice K, Verwoert GC, Launer LJ, Gudnason V, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium. Global BPgen Consortium. Women’s Genome Health Study (2011) Association of hypertension drug target genes with blood pressure and hypertension in 86,588 individuals. Hypertension 57:903–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AR, Erdös EG. (1977) Metabolism of vasoactive peptides by human endothelial cells in culture. Angiotensin I converting enzyme (kininase II) and angiotensinase. J Clin Invest 59:684–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokubaitis VJ, Sinka L, Driessen R, Whitty G, Haylock DN, Bertoncello I, Smith I, Péault B, Tavian M, Simmons PJ. (2008) Angiotensin-converting enzyme (CD143) marks hematopoietic stem cells in human embryonic, fetal, and adult hematopoietic tissues. Blood 111:4055–4063 [DOI] [PubMed] [Google Scholar]
- Julian BA, Gaston RS, Barker CV, Krystal G, Diethelm AG, Curtis JJ. (1994) Erythropoiesis after withdrawal of enalapril in post-transplant erythrocytosis. Kidney Int 46:1397–1403 [DOI] [PubMed] [Google Scholar]
- Jullien ND, Cuniasse P, Georgiadis D, Yiotakis A, Dive V. (2006) Combined use of selective inhibitors and fluorogenic substrates to study the specificity of somatic wild-type angiotensin-converting enzyme. FEBS J 273:1772–1781 [DOI] [PubMed] [Google Scholar]
- Junot C, Gonzales MF, Ezan E, Cotton J, Vazeux G, Michaud A, Azizi M, Vassiliou S, Yiotakis A, Corvol P, et al. (2001) RXP 407, a selective inhibitor of the N-domain of angiotensin I-converting enzyme, blocks in vivo the degradation of hemoregulatory peptide acetyl-Ser-Asp-Lys-Pro with no effect on angiotensin I hydrolysis. J Pharmacol Exp Ther 297:606–611 [PubMed] [Google Scholar]
- Junot C, Nicolet L, Ezan E, Gonzales MF, Menard J, Azizi M. (1999) Effect of angiotensin-converting enzyme inhibition on plasma, urine, and tissue concentrations of hemoregulatory peptide acetyl-Ser-Asp-Lys-Pro in rats. J Pharmacol Exp Ther 291:982–987 [PubMed] [Google Scholar]
- Kato A, Klein JD, Zhang C, Sands JM. (2000) Angiotensin II increases vasopressin-stimulated facilitated urea permeability in rat terminal IMCDs. Am J Physiol Renal Physiol 279:F835–F840 [DOI] [PubMed] [Google Scholar]
- Katori M, Majima M. (2003) The renal kallikrein-kinin system: its role as a safety valve for excess sodium intake, and its attenuation as a possible etiologic factor in salt-sensitive hypertension. Crit Rev Clin Lab Sci 40:43–115 [DOI] [PubMed] [Google Scholar]
- Kehoe PG, Miners S, Love S. (2009) Angiotensins in Alzheimer’s disease - friend or foe? Trends Neurosci 32:619–628 [DOI] [PubMed] [Google Scholar]
- Kehoe PG, Passmore PA. (2012) The renin-angiotensin system and antihypertensive drugs in Alzheimer’s disease: current standing of the angiotensin hypothesis? J Alzheimers Dis 30 (Suppl 2):S251–S268 [DOI] [PubMed] [Google Scholar]
- Kessler SP, Rowe TM, Gomos JB, Kessler PM, Sen GC. (2000) Physiological non-equivalence of the two isoforms of angiotensin-converting enzyme. J Biol Chem 275:26259–26264 [DOI] [PubMed] [Google Scholar]
- Kim HS, Krege JH, Kluckman KD, Hagaman JR, Hodgin JB, Best CF, Jennette JC, Coffman TM, Maeda N, Smithies O. (1995) Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci USA 92:2735–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita J, Fushida S, Harada S, Yagi Y, Fujita H, Kinami S, Ninomiya I, Fujimura T, Kayahara M, Yashiro M, et al. (2009) Local angiotensin II-generation in human gastric cancer: correlation with tumor progression through the activation of ERK1/2, NF-kappaB and survivin. Int J Oncol 34:1573–1582 [DOI] [PubMed] [Google Scholar]
- Kintscher U, Wakino S, Kim S, Fleck E, Hsueh WA, Law RE. (2001) Angiotensin II induces migration and Pyk2/paxillin phosphorylation of human monocytes. Hypertension 37:587–593 [DOI] [PubMed] [Google Scholar]
- Klett C, Nobiling R, Gierschik P, Hackenthal E. (1993) Angiotensin II stimulates the synthesis of angiotensinogen in hepatocytes by inhibiting adenylylcyclase activity and stabilizing angiotensinogen mRNA. J Biol Chem 268:25095–25107 [PubMed] [Google Scholar]
- Koch-Weser J. (1965) Nature of the Inotropic Action of Angiotensin on Ventricular Myocardium. Circ Res 16:230–237 [DOI] [PubMed] [Google Scholar]
- Koguchi T, Yamada K, Yamato M, Okachi R, Nakayama K, Kase H. (1986) K-4, a novel inhibitor of angiotensin I converting enzyme produced by Actinomadura spiculosospora. J Antibiot (Tokyo) 39:364–371 [DOI] [PubMed] [Google Scholar]
- Kohlstaedt KG, Helmer O, Page IH. (1938) Activation of renin by blood colloids. Proc Soc Exp Biol Med 39:214–215 [Google Scholar]
- Kohlstedt K, Brandes RP, Müller-Esterl W, Busse R, Fleming I. (2004) Angiotensin-converting enzyme is involved in outside-in signaling in endothelial cells. Circ Res 94:60–67 [DOI] [PubMed] [Google Scholar]
- Kohlstedt K, Busse R, Fleming I. (2005) Signaling via the angiotensin-converting enzyme enhances the expression of cyclooxygenase-2 in endothelial cells. Hypertension 45:126–132 [DOI] [PubMed] [Google Scholar]
- Kohlstedt K, Gershome C, Trouvain C, Hofmann WK, Fichtlscherer S, Fleming I. (2009) Angiotensin-converting enzyme (ACE) inhibitors modulate cellular retinol-binding protein 1 and adiponectin expression in adipocytes via the ACE-dependent signaling cascade. Mol Pharmacol 75:685–692 [DOI] [PubMed] [Google Scholar]
- Kohlstedt K, Shoghi F, Müller-Esterl W, Busse R, Fleming I. (2002) CK2 phosphorylates the angiotensin-converting enzyme and regulates its retention in the endothelial cell plasma membrane. Circ Res 91:749–756 [DOI] [PubMed] [Google Scholar]
- Kondoh G, Tojo H, Nakatani Y, Komazawa N, Murata C, Yamagata K, Maeda Y, Kinoshita T, Okabe M, Taguchi R, et al. (2005) Angiotensin-converting enzyme is a GPI-anchored protein releasing factor crucial for fertilization. Nat Med 11:160–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost OA, Balyasnikova IV, Chemodanova EE, Nikolskaya II, Albrecht RF, 2nd, Danilov SM. (2003) Epitope-dependent blocking of the angiotensin-converting enzyme dimerization by monoclonal antibodies to the N-terminal domain of ACE: possible link of ACE dimerization and shedding from the cell surface. Biochemistry 42:6965–6976 [DOI] [PubMed] [Google Scholar]
- Kotchen TA. (2011) Historical trends and milestones in hypertension research: a model of the process of translational research. Hypertension 58:522–538 [DOI] [PubMed] [Google Scholar]
- Kovács G, Peti-Peterdi J, Rosivall L, Bell PD. (2002) Angiotensin II directly stimulates macula densa Na-2Cl-K cotransport via apical AT(1) receptors. Am J Physiol Renal Physiol 282:F301–F306 [DOI] [PubMed] [Google Scholar]
- Kozlowski S, Corr M, Takeshita T, Boyd LF, Pendleton CD, Germain RN, Berzofsky JA, Margulies DH. (1992) Serum angiotensin-1 converting enzyme activity processes a human immunodeficiency virus 1 gp160 peptide for presentation by major histocompatibility complex class I molecules. J Exp Med 175:1417–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramers C, Danilov SM, Deinum J, Balyasnikova IV, Scharenborg N, Looman M, Boomsma F, de Keijzer MH, van Duijn C, Martin S, et al. (2001) Point mutation in the stalk of angiotensin-converting enzyme causes a dramatic increase in serum angiotensin-converting enzyme but no cardiovascular disease. Circulation 104:1236–1240 [DOI] [PubMed] [Google Scholar]
- Krege JH, John SW, Langenbach LL, Hodgin JB, Hagaman JR, Bachman ES, Jennette JC, O’Brien DA, Smithies O. (1995) Male-female differences in fertility and blood pressure in ACE-deficient mice. Nature 375:146–148 [DOI] [PubMed] [Google Scholar]
- Krege JH, Kim HS, Moyer JS, Jennette JC, Peng L, Hiller SK, Smithies O. (1997) Angiotensin-converting enzyme gene mutations, blood pressures, and cardiovascular homeostasis. Hypertension 29:150–157 [DOI] [PubMed] [Google Scholar]
- Kröger WL, Douglas RG, O’Neill HG, Dive V, Sturrock ED. (2009) Investigating the domain specificity of phosphinic inhibitors RXPA380 and RXP407 in angiotensin-converting enzyme. Biochemistry 48:8405–8412 [DOI] [PubMed] [Google Scholar]
- Krulewitz AH, Fanburg BL. (1986) Stimulation of bovine endothelial cell angiotensin-I-converting enzyme activity by cyclic AMP-related agents. J Cell Physiol 129:147–150 [DOI] [PubMed] [Google Scholar]
- Krulewitz AH, Baur WE, Fanburg BL. (1984) Hormonal influence on endothelial cell angiotensin-converting enzyme activity. Am J Physiol 247:C163–C168 [DOI] [PubMed] [Google Scholar]
- Kumar RS, Kusari J, Roy SN, Soffer RL, Sen GC. (1989) Structure of testicular angiotensin-converting enzyme. A segmental mosaic isozyme. J Biol Chem 264:16754–16758 [PubMed] [Google Scholar]
- Kunisawa J, Shastri N. (2003) The group II chaperonin TRiC protects proteolytic intermediates from degradation in the MHC class I antigen processing pathway. Mol Cell 12:565–576 [DOI] [PubMed] [Google Scholar]
- Kupari M, Perola M, Koskinen P, Virolainen J, Karhunen PJ. (1994) Left ventricular size, mass, and function in relation to angiotensin-converting enzyme gene polymorphism in humans. Am J Physiol 267:H1107–H1111 [DOI] [PubMed] [Google Scholar]
- Kurtz A. (2011) Renin release: sites, mechanisms, and control. Annu Rev Physiol 73:377–399 [DOI] [PubMed] [Google Scholar]
- Langford KG, Shai S-Y, Howard TE, Kovac MJ, Overbeek PA, Bernstein KE. (1991) Transgenic mice demonstrate a testis-specific promoter for angiotensin-converting enzyme. J Biol Chem 266:15559–15562 [PubMed] [Google Scholar]
- Langford KG, Zhou Y, Russell LD, Wilcox JN, Bernstein KE. (1993) Regulated expression of testis angiotensin-converting enzyme during spermatogenesis in mice. Biol Reprod 48:1210–1218 [DOI] [PubMed] [Google Scholar]
- Lanz TV, Ding Z, Ho PP, Luo J, Agrawal AN, Srinagesh H, Axtell R, Zhang H, Plattern M, Wyss-Coray T, et al. (2010) Angiotensin II sustains brain inflammation in mice via TGF-beta. J Clin Invest 120:2782–2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzillo JJ, Stevens J, Dasarathy Y, Yotsumoto H, Fanburg BL. (1985) Angiotensin-converting enzyme from human tissues. Physicochemical, catalytic, and immunological properties. J Biol Chem 260:14938–14944 [PubMed] [Google Scholar]
- Larsson PT, Schwieler JH, Wallén NH. (2000) Platelet activation during angiotensin II infusion in healthy volunteers. Blood Coagul Fibrinolysis 11:61–69 [PubMed] [Google Scholar]
- Lassègue B, San Martín A, Griendling KK. (2012) Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res 110:1364–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattion AL, Soubrier F, Allegrini J, Hubert C, Corvol P, Alhenc-Gelas F. (1989) The testicular transcript of the angiotensin I-converting enzyme encodes for the ancestral, non-duplicated form of the enzyme. FEBS Lett 252:99–104 [DOI] [PubMed] [Google Scholar]
- Leatham E, Barley J, Redwood S, Hussein W, Carter N, Jeffery S, Bath PM, Camm A. (1994) Angiotensin-1 converting enzyme (ACE) polymorphism in patients presenting with myocardial infarction or unstable angina. J Hum Hypertens 8:635–638 [PubMed] [Google Scholar]
- Leeb-Lundberg LM, Marceau F, Müller-Esterl W, Pettibone DJ, Zuraw BL. (2005) International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev 57:27–77 [DOI] [PubMed] [Google Scholar]
- Leisle L, Parkin ET, Turner AJ, Hooper NM. (2005) Angiotensin-converting enzyme as a GPIase: a critical reevaluation. Nat Med 11:1139–1140 [DOI] [PubMed] [Google Scholar]
- Lemeire E, Vanholme B, Van Leeuwen T, Van Camp J, Smagghe G. (2008) Angiotensin-converting enzyme in Spodoptera littoralis: molecular characterization, expression and activity profile during development. Insect Biochem Mol Biol 38:166–175 [DOI] [PubMed] [Google Scholar]
- Lenfant M, Wdzieczak-Bakala J, Guittet E, Prome JC, Sotty D, Frindel E. (1989) Inhibitor of hematopoietic pluripotent stem cell proliferation: purification and determination of its structure. Proc Natl Acad Sci USA 86:779–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz KE, Skeggs LT, Jr, Woods KR, Kahn JR, Shumway NP. (1956) The amino acid composition of hypertensin II and its biochemical relationship to hypertensin I. J Exp Med 104:183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuschner F, Panizzi P, Chico-Calero I, Lee WW, Ueno T, Cortez-Retamozo V, Waterman P, Gorbatov R, Marinelli B, Iwamoto Y, et al. (2010) Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res 107:1364–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens NR, Peach MJ, Carey RM. (1981) Interactions between angiotensin peptides and the sympathetic nervous system mediating intestinal sodium and water absorption in the rat. J Clin Invest 67:1197–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DI, Coote JH. (1993) Angiotensin II in the spinal cord of the rat and its sympatho-excitatory effects. Brain Res 614:1–9 [DOI] [PubMed] [Google Scholar]
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD, The Collaborative Study Group (1993) The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 329:1456–1462 [DOI] [PubMed] [Google Scholar]
- Li D, Shinagawa K, Pang L, Leung TK, Cardin S, Wang Z, Nattel S. (2001) Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation 104:2608–2614 [DOI] [PubMed] [Google Scholar]
- Li L, Fan D, Wang C, Wang JY, Cui XB, Wu D, Zhou Y, Wu LL. (2011) Angiotensin II increases periostin expression via Ras/p38 MAPK/CREB and ERK1/2/TGF-β1 pathways in cardiac fibroblasts. Cardiovasc Res 91:80–89 [DOI] [PubMed] [Google Scholar]
- Li P, Xiao HD, Xu J, Ong FS, Kwon M, Roman J, Gal A, Bernstein KE, Fuchs S. (2010) Angiotensin-converting enzyme N-terminal inactivation alleviates bleomycin-induced lung injury. Am J Pathol 177:1113–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao TD, Yang XP, D’Ambrosio M, Zhang Y, Rhaleb NE, Carretero OA. (2010) N-acetyl-seryl-aspartyl-lysyl-proline attenuates renal injury and dysfunction in hypertensive rats with reduced renal mass: council for high blood pressure research. Hypertension 55:459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman J. (1975) Elevation of serum angiotensin-converting-enzyme (ACE) level in sarcoidosis. Am J Med 59:365–372 [DOI] [PubMed] [Google Scholar]
- Lieberman J. (1976) The specificity and nature of serum-angiotensin-converting enzyme (serum ACE) elevations in sarcoidosis. Ann N Y Acad Sci 278:488–497 [DOI] [PubMed] [Google Scholar]
- Lieberman J, Schleissner LA, Nosal A, Sastre A, Mishkin FS. (1983) Clinical correlations of serum angiotensin-converting enzyme (ACE) in sarcoidosis. A longitudinal study of serum ACE, 67gallium scans, chest roentgenograms, and pulmonary function. Chest 84:522–528 [DOI] [PubMed] [Google Scholar]
- Lin C, Datta V, Okwan-Duodu D, Chen X, Fuchs S, Alsabeh R, Billet S, Bernstein KE, Shen XZ. (2011) Angiotensin-converting enzyme is required for normal myelopoiesis. FASEB J 25:1145–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CX, Rhaleb NE, Yang XP, Liao TD, D’Ambrosio MA, Carretero OA. (2008) Prevention of aortic fibrosis by N-acetyl-seryl-aspartyl-lysyl-proline in angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol 295:H1253–H1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindpaintner K, Lee M, Larson MG, Rao VS, Pfeffer MA, Ordovas JM, Schaefer EJ, Wilson AF, Wilson PW, Vasan RS, et al. (1996) Absence of association or genetic linkage between the angiotensin-converting-enzyme gene and left ventricular mass. N Engl J Med 334:1023–1028 [DOI] [PubMed] [Google Scholar]
- Lindpaintner K, Pfeffer MA, Kreutz R, Stampfer MJ, Grodstein F, LaMotte F, Buring J, Hennekens CH. (1995) A prospective evaluation of an angiotensin-converting-enzyme gene polymorphism and the risk of ischemic heart disease. N Engl J Med 332:706–711 [DOI] [PubMed] [Google Scholar]
- Lipscomb WN, Hartsuck JA, Reeke GN, Jr, Quiocho FA, Bethge PH, Ludwig ML, Steitz TA, Muirhead H, Coppola JC. (1968) The structure of carboxypeptidase A. VII. The 2.0-angstrom resolution studies of the enzyme and of its complex with glycyltyrosine, and mechanistic deductions. Brookhaven Symp Biol 21:24–90 [PubMed] [Google Scholar]
- Lucius R, Gallinat S, Rosenstiel P, Herdegen T, Sievers J, Unger T. (1998) The angiotensin II type 2 (AT2) receptor promotes axonal regeneration in the optic nerve of adult rats. J Exp Med 188:661–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lühder F, Lee DH, Gold R, Stegbauer J, Linker RA. (2009) Small but powerful: short peptide hormones and their role in autoimmune inflammation. J Neuroimmunol 217:1–7 [DOI] [PubMed] [Google Scholar]
- Ludwig E, Corneli PS, Anderson JL, Marshall HW, Lalouel JM, Ward RH. (1995) Angiotensin-converting enzyme gene polymorphism is associated with myocardial infarction but not with development of coronary stenosis. Circulation 91:2120–2124 [DOI] [PubMed] [Google Scholar]
- Lynch M, Conery JS. (2000) The evolutionary fate and consequences of duplicate genes. Science 290:1151–1155 [DOI] [PubMed] [Google Scholar]
- MacMicking J, Xie QW, Nathan C. (1997) Nitric oxide and macrophage function. Annu Rev Immunol 15:323–350 [DOI] [PubMed] [Google Scholar]
- Macours N, Hens K. (2004) Zinc-metalloproteases in insects: ACE and ECE. Insect Biochem Mol Biol 34:501–510 [DOI] [PubMed] [Google Scholar]
- Madeddu P, Emanueli C, El-Dahr S. (2007) Mechanisms of disease: the tissue kallikrein-kinin system in hypertension and vascular remodeling. Nat Clin Pract Nephrol 3:208–221 [DOI] [PubMed] [Google Scholar]
- Maestri R, Milia AF, Salis MB, Graiani G, Lagrasta C, Monica M, Corradi D, Emanueli C, Madeddu P. (2003) Cardiac hypertrophy and microvascular deficit in kinin B2 receptor knockout mice. Hypertension 41:1151–1155 [DOI] [PubMed] [Google Scholar]
- Manolis A, Doumas M. (2008) Sexual dysfunction: the ‘prima ballerina’ of hypertension-related quality-of-life complications. J Hypertens 26:2074–2084 [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25:677–686 [DOI] [PubMed] [Google Scholar]
- Marcic B, Deddish PA, Jackman HL, Erdös EG, Tan F. (2000) Effects of the N-terminal sequence of ACE on the properties of its C-domain. Hypertension 36:116–121 [DOI] [PubMed] [Google Scholar]
- Marks LS, Maxwell MH. (1979) Tigerstedt and the discovery of renin. An historical note. Hypertension 1:384–388 [DOI] [PubMed] [Google Scholar]
- Martorana PA, Kettenbach B, Breipohl G, Linz W, Schölkens BA. (1990) Reduction of infarct size by local angiotensin-converting enzyme inhibition is abolished by a bradykinin antagonist. Eur J Pharmacol 182:395–396 [DOI] [PubMed] [Google Scholar]
- Matchar DB, McCrory DC, Orlando LA, Patel MR, Patel UD, Patwardhan MB, Powers B, Samsa GP, Gray RN. (2008) Systematic review: comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann Intern Med 148:16–29 [DOI] [PubMed] [Google Scholar]
- Mathai M, Evered MD, McKinley MJ. (1997) Intracerebroventricular losartan inhibits postprandial drinking in sheep. Am J Physiol 272:R1055–R1059 [DOI] [PubMed] [Google Scholar]
- Matsusaka T, Miyazaki Y, Ichikawa I. (2002) The renin angiotensin system and kidney development. Annu Rev Physiol 64:551–561 [DOI] [PubMed] [Google Scholar]
- Maurer M, Bader M, Bas M, Bossi FCicardi M, Cugno M, Howarth P, Kaplan A, Kojda G, Leeb-Lundberg F, et al. (2011) New topics in bradykinin research. Allergy 66:1397–1406 [DOI] [PubMed] [Google Scholar]
- McAllister-Lucas LM, Ruland J, Siu K, Jin X, Gu S, Kim DS, Kuffa P, Kohrt D, Mak TW, Nuñez G, et al. (2007) CARMA3/Bcl10/MALT1-dependent NF-kappaB activation mediates angiotensin II-responsive inflammatory signaling in nonimmune cells. Proc Natl Acad Sci USA 104:139–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhinney CD, Hunt RA, Conrad KM, Dostal DE, Baker KM. (1997) The type I angiotensin II receptor couples to Stat1 and Stat3 activation through Jak2 kinase in neonatal rat cardiac myocytes. J Mol Cell Cardiol 29:2513–2524 [DOI] [PubMed] [Google Scholar]
- Menard J, Patchett AA. (2001) Angiotensin-converting enzyme inhibitors. Adv Protein Chem 56:13–75 [DOI] [PubMed] [Google Scholar]
- Merabet L, de Gasparo M, Casanova C. (1997) Dose-dependent inhibitory effects of angiotensin II on visual responses of the rat superior colliculus: AT1 and AT2 receptor contributions. Neuropeptides 31:469–481 [DOI] [PubMed] [Google Scholar]
- Messerli FH, Nowaczynski W, Honda M, Genest J, Boucher R, Kuchel O, Rojo-Ortega JM. (1977) Effects of angiotensin II on steroid metabolism and hepatic blood flow in man. Circ Res 40:204–207 [DOI] [PubMed] [Google Scholar]
- Mitchell KD, Navar LG. (1988) Enhanced tubuloglomerular feedback during peritubular infusions of angiotensins I and II. Am J Physiol 255:F383–F390 [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Tsuchida S, Nishimura H, Pope JC, 4th, Harris RC, McKanna JM, Inagami T, Hogan BL, Fogo A, Ichikawa I. (1998) Angiotensin induces the urinary peristaltic machinery during the perinatal period. J Clin Invest 102:1489–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M, Li JM, Iwanami J, Min LJ, Tsukuda K, Iwai M, Horiuchi M. (2006) Angiotensin II type-2 receptor stimulation prevents neural damage by transcriptional activation of methyl methanesulfonate sensitive 2. Hypertension 48:141–148 [DOI] [PubMed] [Google Scholar]
- Moreau ME, Garbacki N, Molinaro G, Brown NJ, Marceau F, Adam A. (2005) The kallikrein-kinin system: current and future pharmacological targets. J Pharmacol Sci 99:6–38 [DOI] [PubMed] [Google Scholar]
- Moreno M, Ramalho LN, Sancho-Bru P, Ruiz-Ortega M, Ramalho F, Abraldes JG, Colmenero J, Dominguez M, Egido J, Arroyo V, et al. (2009) Atorvastatin attenuates angiotensin II-induced inflammatory actions in the liver. Am J Physiol Gastrointest Liver Physiol 296:G147–G156 [DOI] [PubMed] [Google Scholar]
- Mrug M, Stopka T, Julian BA, Prchal JF, Prchal JT. (1997) Angiotensin II stimulates proliferation of normal early erythroid progenitors. J Clin Invest 100:2310–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz JM, Braun-Menéndez E, Fasciolo JC, Leloir LF. (1939) Hypertensin: the substance causing renal hypertension. Nature 144:980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro Y, Okabe M. (2011) Mechanisms of fertilization—a view from the study of gene-manipulated mice. J Androl 32:218–225 [DOI] [PubMed] [Google Scholar]
- Myöhänen TT, Tenorio-Laranga J, Jokinen B, Vázquez-Sánchez R, Moreno-Baylach MJ, García-Horsman JA, Männistö PT. (2011) Prolyl oligopeptidase induces angiogenesis both in vitro and in vivo in a novel regulatory manner. Br J Pharmacol 163:1666–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima Y, Tazuma S, Kanno K, Hyogo H, Chayama K. (2009) Deletion of angiotensin II type I receptor reduces hepatic steatosis. J Hepatol 50:1226–1235 [DOI] [PubMed] [Google Scholar]
- Nadaud S, Houot AM, Hubert C, Corvol P, Soubrier F. (1992) Functional study of the germinal angiotensin I-converting enzyme promoter. Biochem Biophys Res Commun 189:134–140 [DOI] [PubMed] [Google Scholar]
- Naeshiro I, Sato K, Chatani F, Sato S. (1998) Possible mechanism for the anemia induced by candesartan cilexetil (TCV-116), an angiotensin II receptor antagonist, in rats. Eur J Pharmacol 354:179–187 [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Takeshita T, Berzofsky JA, Takahashi H. (2000) Analysis of the mechanism for extracellular processing in the presentation of human immunodeficiency virus-1 envelope protein-derived peptide to epitope-specific cytotoxic T lymphocytes. Immunology 101:76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai K, Itoh C, Miura Y, Hotta K, Musha T, Itoh T, Miyakawa T, Iwasaki R, Hiramori K. (1994) Deletion polymorphism of the angiotensin I-converting enzyme gene is associated with serum ACE concentration and increased risk for CAD in the Japanese. Circulation 90:2199–2202 [DOI] [PubMed] [Google Scholar]
- Nakajima T, Oshima G, Yeh HSJ, Igic R, Erdös EG. (1973) Purification of the angiotensin I-converting enzyme of the lung. Biochimica et Biophysica Acta (BBA) - Enzymology 315:430–438 [Google Scholar]
- Nakamura Y, Takeda T, Ishii M, Nishiyama K, Yamakada M, Hirata Y, Kimura K, Murao S. (1982) Elevation of serum angiotensin-converting enzyme activity in patients with hyperthyroidism. J Clin Endocrinol Metab 55:931–934 [DOI] [PubMed] [Google Scholar]
- Naperova IA, Balyasnikova IV, Schwartz DE, Watermeyer J, Sturrock ED, Kost OA, Danilov SM. (2008) Mapping of conformational mAb epitopes to the C domain of human angiotensin I-converting enzyme. J Proteome Res 7:3396–3411 [DOI] [PubMed] [Google Scholar]
- Natesh R, Schwager SL, Sturrock ED, Acharya KR. (2003) Crystal structure of the human angiotensin-converting enzyme-lisinopril complex. Nature 421:551–554 [DOI] [PubMed] [Google Scholar]
- Navar LG, Inscho EW, Majid SA, Imig JD, Harrison-Bernard LM, Mitchell KD. (1996) Paracrine regulation of the renal microcirculation. Physiol Rev 76:425–536 [DOI] [PubMed] [Google Scholar]
- Neuwirth R, Satriano JA, DeCandido S, Clay K, Schlondorff D. (1989) Angiotensin II causes formation of platelet activating factor in cultured rat mesangial cells. Circ Res 64:1224–1229 [DOI] [PubMed] [Google Scholar]
- Ng KKF, Vane JR. (1967) Conversion of angiotensin I to angiotensin II. Nature 216:762–766 [DOI] [PubMed] [Google Scholar]
- Ng KKF, Vane JR. (1968) Fate of angiotensin I in the circulation. Nature 218:144–150 [DOI] [PubMed] [Google Scholar]
- Niimura F, Labosky PA, Kakuchi J, Okubo S, Yoshida H, Oikawa T, Ichiki T, Naftilan AJ, Fogo A, Inagami T, et al. (1995) Gene targeting in mice reveals a requirement for angiotensin in the development and maintenance of kidney morphology and growth factor regulation. J Clin Invest 96:2947–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura H, Tsuji H, Masuda H, Nakagawa K, Nakahara Y, Kitamura H, Kasahara T, Sugano T, Yoshizumi M, Sawada S, et al. (1997) Angiotensin II increases plasminogen activator inhibitor-1 and tissue factor mRNA expression without changing that of tissue type plasminogen activator or tissue factor pathway inhibitor in cultured rat aortic endothelial cells. Thromb Haemost 77:1189–1195 [PubMed] [Google Scholar]
- Nussberger J, Cugno M, Cicardi M, Agostoni A. (1999) Local bradykinin generation in hereditary angioedema. J Allergy Clin Immunol 104:1321–1322 [DOI] [PubMed] [Google Scholar]
- O’Donnell CJ, Lindpaintner K, Larson MG, Rao VS, Ordovas JM, Schaefer EJ, Myers RH, Levy D. (1998) Evidence for association and genetic linkage of the angiotensin-converting enzyme locus with hypertension and blood pressure in men but not women in the Framingham Heart Study. Circulation 97:1766–1772 [DOI] [PubMed] [Google Scholar]
- O’Neill HG, Redelinghuys P, Schwager SL, Sturrock ED. (2008) The role of glycosylation and domain interactions in the thermal stability of human angiotensin-converting enzyme. Biol Chem 389:1153–1161 [DOI] [PubMed] [Google Scholar]
- Oba R, Igarashi A, Kamata M, Nagata K, Takano S, Nakagawa H. (2005) The N-terminal active centre of human angiotensin-converting enzyme degrades Alzheimer amyloid β-peptide. Eur J Neurosci 21:733–740 [DOI] [PubMed] [Google Scholar]
- Ohrui T, Matsui T, Yamaya M, Arai H, Ebihara S, Maruyama M, Sasaki H. (2004) Angiotensin-converting enzyme inhibitors and incidence of Alzheimer’s disease in Japan. J Am Geriatr Soc 52:649–650 [DOI] [PubMed] [Google Scholar]
- Okunuki Y, Usui Y, Nagai N, Kezuka T, Ishida S, Takeuchi M, Goto H. (2009) Suppression of experimental autoimmune uveitis by angiotensin II type 1 receptor blocker telmisartan. Invest Ophthalmol Vis Sci 50:2255–2261 [DOI] [PubMed] [Google Scholar]
- Okwan-Duodu D, Datta V, Shen XZ, Goodridge HS, Bernstein EA, Fuchs S, Liu GY, Bernstein KE. (2010) Angiotensin-converting enzyme overexpression in mouse myelomonocytic cells augments resistance to Listeria and methicillin-resistant Staphylococcus aureus. J Biol Chem 285:39051–39060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondetti MA, Cushmen DW. (1981) Inhibitors of angiotensin-converting enzyme, in Biochemical Regulation of Blood Pressure (Soffer RL. ed) pp 165–204, John Wiley and Sons, New York [Google Scholar]
- Ondetti MA, Rubin B, Cushman DW. (1977) Design of specific inhibitors of angiotensin-converting enzyme: new class of orally active antihypertensive agents. Science 196:441–444 [DOI] [PubMed] [Google Scholar]
- Ondetti MA, Williams NJ, Sabo EF, Pluscec J, Weaver ER, Kocy O. (1971) Angiotensin-converting enzyme inhibitors from the venom of Bothrops jararaca. Isolation, elucidation of structure, and synthesis. Biochemistry 10:4033–4039 [DOI] [PubMed] [Google Scholar]
- Ong FS, Lin CX, Campbell DJ, Okwan-Duodu D, Chen X, Blackwell WL, Shah KH, Gonzalez-Villalobos RA, Shen XZ, Fuchs S, et al. (2012) Increased angiotensin II-induced hypertension and inflammatory cytokines in mice lacking angiotensin-converting enzyme N domain activity. Hypertension 59:283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppong SY, Hooper NM. (1993) Characterization of a secretase activity which releases angiotensin-converting enzyme from the membrane. Biochem J 292:597–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn JL, Camara AK. (1997) Renal neurogenic mediation of intracerebroventricular angiotensin II hypertension in rats raised on high sodium chloride diet. Hypertension 30:331–336 [DOI] [PubMed] [Google Scholar]
- Osborn JW, Fink GD. (2010) Region-specific changes in sympathetic nerve activity in angiotensin II-salt hypertension in the rat. Exp Physiol 95:61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn JW, Fink GD, Kuroki MT. (2011) Neural mechanisms of angiotensin II-salt hypertension: implications for therapies targeting neural control of the splanchnic circulation. Curr Hypertens Rep 13:221–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima G, Gecse A, Erdös EG. (1974) Angiotensin I-converting enzyme of the kidney cortex. Biochim Biophys Acta 350:26–37 [DOI] [PubMed] [Google Scholar]
- Page IH, Helmer OM. (1940) A crystalline pressor substance (angiotonin) resulting from the interaction between renin and renin-activator. J Exp Med 71:29–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoliano MW, Holmquist B, Riordan JF. (1984) Affinity chromatographic purification of angiotensin converting enzyme. Biochemistry 23:1037–1042 [DOI] [PubMed] [Google Scholar]
- Parkin ET, Turner AJ, Hooper NM. (2004) Secretase-mediated cell surface shedding of the angiotensin-converting enzyme. Protein Pept Lett 11:423–432 [DOI] [PubMed] [Google Scholar]
- Parvathy S, Oppong SY, Karran EH, Buckle DR, Turner AJ, Hooper NM. (1997) Angiotensin-converting enzyme secretase is inhibited by zinc metalloprotease inhibitors and requires its substrate to be inserted in a lipid bilayer. Biochem J 327:37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchett AA, Cordes EH. (1985) The design and properties of N-carboxyalkyldipeptide inhibitors of angiotensin-converting enzyme. Adv Enzymol Relat Areas Mol Biol 57:1–84 [DOI] [PubMed] [Google Scholar]
- Patchett AA, Harris E, Tristram EW, Wyvratt MJ, Wu MT, Taub D, Peterson ER, Ikeler TJ, ten Broeke J, Payne LG, et al. (1980) A new class of angiotensin-converting enzyme inhibitors. Nature 288:280–283 [DOI] [PubMed] [Google Scholar]
- Paul M, Poyan Mehr A, Kreutz R. (2006) Physiology of local renin-angiotensin systems. Physiol Rev 86:747–803 [DOI] [PubMed] [Google Scholar]
- Pedrinelli R, Ballo P, Fiorentini C, Denti S, Galderisi M, Ganau A, Germanò G, Innelli P, Paini A, Perlini S, et al. Gruppo di Studio Ipertensione e Cuore, Societa’ Italiana di Cardiologia (2012) Hypertension and acute myocardial infarction: an overview. J Cardiovasc Med (Hagerstown) 13:194–202 [DOI] [PubMed] [Google Scholar]
- Peng H, Carretero OA, Brigstock DR, Oja-Tebbe N, Rhaleb NE. (2003) Ac-SDKP reverses cardiac fibrosis in rats with renovascular hypertension. Hypertension 42:1164–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Carretero OA, Raij L, Yang F, Kapke A, Rhaleb NE. (2001) Antifibrotic effects of N-acetyl-seryl-aspartyl-Lysyl-proline on the heart and kidney in aldosterone-salt hypertensive rats. Hypertension 37:794–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi E. (2000) Evolutionary biology. Twinned genes live life in the fast lane. Science 290:1065–1066 [DOI] [PubMed] [Google Scholar]
- Perazella M, McPhedran P, Kliger A, Lorber M, Levy E, Bia MJ. (1995) Enalapril treatment of posttransplant erythrocytosis: efficacy independent of circulating erythropoietin levels. Am J Kidney Dis 26:495–500 [DOI] [PubMed] [Google Scholar]
- Pérez NG, Villa-Abrille MC, Aiello EA, Dulce RA, Cingolani HE, Camilión de Hurtado MC. (2003) A low dose of angiotensin II increases inotropism through activation of reverse Na(+)/Ca(2+) exchange by endothelin release. Cardiovasc Res 60:589–597 [DOI] [PubMed] [Google Scholar]
- Peterson KJ, Cotton JA, Gehling JG, Pisani D. (2008) The Ediacaran emergence of bilaterians: congruence between the genetic and the geological fossil records. Philos Trans R Soc Lond B Biol Sci 363:1435–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peti-Peterdi J, Bell PD. (1998) Regulation of macula densa Na:H exchange by angiotensin II. Kidney Int 54:2021–2028 [DOI] [PubMed] [Google Scholar]
- Peti-Peterdi J, Warnock DG, Bell PD. (2002) Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol 13:1131–1135 [DOI] [PubMed] [Google Scholar]
- Phillips MI, Schmidt-Ott KM. (1999) The Discovery of Renin 100 Years Ago. News Physiol Sci 14:271–274 [DOI] [PubMed] [Google Scholar]
- Pinto YM, Pinto-Sietsma SJ, Philipp T, Engler S, Kossamehl P, Hocher B, Marquardt H, Sethmann S, Lauster R, Merker HJ, et al. (2000) Reduction in left ventricular messenger RNA for transforming growth factor beta(1) attenuates left ventricular fibrosis and improves survival without lowering blood pressure in the hypertensive TGR(mRen2)27 Rat. Hypertension 36:747–754 [DOI] [PubMed] [Google Scholar]
- Piquilloud Y, Reinharz A, Roth M. (1970) Studies on the angiotensin converting enzyme with different substrates. Biochim Biophys Acta 206:136–142 [DOI] [PubMed] [Google Scholar]
- Platten M, Youssef S, Hur EM, Ho PP, Han MH, Lanz TV, Phillips LK, Goldstein MJ, Bhat R, Raine CS, et al. (2009) Blocking angiotensin-converting enzyme induces potent regulatory T cells and modulates TH1- and TH17-mediated autoimmunity. Proc Natl Acad Sci USA 106:14948–14953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter EK. (1982) Angiotensin inhibits action of vagus nerve at the heart. Br J Pharmacol 75:9–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt MC, Lewis-Barned NJ, Walker RJ, Bailey RR, Shand BI, Livesey J. (1992) Effect of angiotensin converting enzyme inhibitors on erythropoietin concentrations in healthy volunteers. Br J Clin Pharmacol 34:363–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryde PG, Sedman AB, Nugent CE, Barr M., Jr (1993) Angiotensin-converting enzyme inhibitor fetopathy. J Am Soc Nephrol 3:1575–1582 [DOI] [PubMed] [Google Scholar]
- Purnak T, Beyazit Y, Oztas E, Yesil Y, Efe C, Torun S, Celik T, Tenlik I, Kurt M, Ozaslan E. (2012) Serum angiotensin-converting enzyme level as a marker of fibrosis in patients with chronic hepatitis B. J Renin Angiotensin Aldosterone Syst 13:244–249 [DOI] [PubMed] [Google Scholar]
- Putnam NH, Srivastava M, Hellsten U, Dirks B, Chapman J, Salamov A, Terry A, Shapiro H, Lindquist E, Kapitonov VV, et al. (2007) Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 317:86–94 [DOI] [PubMed] [Google Scholar]
- Qadri F, Culman J, Veltmar A, Maas K, Rascher W, Unger T. (1993) Angiotensin II-induced vasopressin release is mediated through alpha-1 adrenoceptors and angiotensin II AT1 receptors in the supraoptic nucleus. J Pharmacol Exp Ther 267:567–574 [PubMed] [Google Scholar]
- Quan A. (2006) Fetopathy associated with exposure to angiotensin converting enzyme inhibitors and angiotensin receptor antagonists. Early Hum Dev 82:23–28 [DOI] [PubMed] [Google Scholar]
- Ramaraj P, Kessler SP, Colmenares C, Sen GC. (1998) Selective restoration of male fertility in mice lacking angiotensin-converting enzymes by sperm-specific expression of the testicular isozyme. J Clin Invest 102:371–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandran R, Sen GC, Misono K, Sen I. (1994) Regulated cleavage-secretion of the membrane-bound angiotensin-converting enzyme. J Biol Chem 269:2125–2130 [PubMed] [Google Scholar]
- Rameshwar P, Ganea D, Gascón P. (1993) In vitro stimulatory effect of substance P on hematopoiesis. Blood 81:391–398 [PubMed] [Google Scholar]
- Rameshwar P, Gascón P. (1995) Substance P (SP) mediates production of stem cell factor and interleukin-1 in bone marrow stroma: potential autoregulatory role for these cytokines in SP receptor expression and induction. Blood 86:482–490 [PubMed] [Google Scholar]
- Rao RH. (1996) Pressor doses of angiotensin II increase hepatic glucose output and decrease insulin sensitivity in rats. J Endocrinol 148:311–318 [DOI] [PubMed] [Google Scholar]
- Rawlings ND, Morton FR, Kok CY, Kong J, Barrett AJ. (2008) MEROPS: the peptidase database. Nucleic Acids Res 36 (Database issue):D320–D325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redelinghuys P, Nchinda AT, Sturrock ED. (2005) Development of domain-selective angiotensin I-converting enzyme inhibitors. Ann N Y Acad Sci 1056:160–175 [DOI] [PubMed] [Google Scholar]
- Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. (2004) Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Hypertension 383:45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder MJ, Taylor SL, Clark AG, Nickerson DA. (1999) Sequence variation in the human angiotensin converting enzyme. Nat Genet 22:59–62 [DOI] [PubMed] [Google Scholar]
- Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. (1990) An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest 86:1343–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan JF. (2003) Angiotensin-I-converting enzyme and its relatives. Genome Biol 4:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittel WB, Iselin B, Kappeler H, Riniker B, Schwyzer R. (1957) Synthese eines hochwirksamen hypertensin II-amids (L-asparagynl-L-arginyl-L-valyl-L-tyrosyl-L-isoleucyl-L-histidyl-L-prolyl-L-phenylalanin). Helv Chim Acta 40:614–624 [Google Scholar]
- Rivière G. (2009) [Angiotensin-converting enzyme: a protein conserved during evolution]. J Soc Biol 203:281–293 [DOI] [PubMed] [Google Scholar]
- Riviere G, Fellous A, Franco A, Bernay B, Favrel PA. (2011) A crucial role in fertility for the oyster angiotensin-converting enzyme orthologue CgACE. PLoS One 6:e27833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivière G, Michaud A, Deloffre L, Vandenbulcke F, Levoye A, Breton C, Corvol P, Salzet M, Vieau D. (2004) Characterization of the first non-insect invertebrate functional angiotensin-converting enzyme (ACE): leech TtACE resembles the N-domain of mammalian ACE. Biochem J 382:565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers KE, Xiong S, diZerega GS. (2002) Accelerated recovery from irradiation injury by angiotensin peptides. Cancer Chemother Pharmacol 49:403–411 [DOI] [PubMed] [Google Scholar]
- Rodgers KE, Xiong S, Steer R, diZerega GS. (2000) Effect of angiotensin II on hematopoietic progenitor cell proliferation. Stem Cells 18:287–294 [DOI] [PubMed] [Google Scholar]
- Rodgers K, Xiong S, DiZerega GS. (2003) Effect of angiotensin II and angiotensin(1-7) on hematopoietic recovery after intravenous chemotherapy. Cancer Chemother Pharmacol 51:97–106 [DOI] [PubMed] [Google Scholar]
- Rothermund L, Pinto YM, Vetter R, Herfort N, Kossmehl P, Neumayer HH, Paul M, Kreutz R. (2001) Effects of angiotensin II subtype 1 receptor blockade on cardiac fibrosis and sarcoplasmic reticulum Ca2+ handling in hypertensive transgenic rats overexpressing the Ren2 gene. J Hypertens 19:1465–1472 [DOI] [PubMed] [Google Scholar]
- Rousseau A, Michaud A, Chauvet MT, Lenfant M, Corvol P. (1995) The hemoregulatory peptide N-acetyl-Ser-Asp-Lys-Pro is a natural and specific substrate of the N-terminal active site of human angiotensin-converting enzyme. J Biol Chem 270:3656–3661 [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Esteban V, Rupérez M, Sánchez-López E, Rodríguez-Vita J, Carvajal G, Egido J. (2006a) Renal and vascular hypertension-induced inflammation: role of angiotensin II. Curr Opin Nephrol Hypertens 15:159–166 [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Rupérez M, Esteban V, Rodríguez-Vita J, Sánchez-López E, Carvajal G, Egido J. (2006b) Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol Dial Transplant 21:16–20 [DOI] [PubMed] [Google Scholar]
- Rüster C, Wolf G. (2011) Angiotensin II as a morphogenic cytokine stimulating renal fibrogenesis. J Am Soc Nephrol 22:1189–1199 [DOI] [PubMed] [Google Scholar]
- Ryan JW, Ryan US, Schultz DR, Whitaker C, Chung A. (1975) Subcellular localization of pulmonary antiotensin-converting enzyme (kininase II). Biochem J 146:497–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder KW, Jay SJ, Kiblawi SO, Hull MT. (1983) Serum angiotensin converting enzyme activity in patients with histoplasmosis. JAMA 249:1888–1889 [PubMed] [Google Scholar]
- Sachse A, Wolf G. (2007) Angiotensin II-induced reactive oxygen species and the kidney. J Am Soc Nephrol 18:2439–2446 [DOI] [PubMed] [Google Scholar]
- Sadhukhan R, Sen GC, Ramchandran R, Sen I. (1998) The distal ectodomain of angiotensin-converting enzyme regulates its cleavage-secretion from the cell surface. Proc Natl Acad Sci USA 95:138–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadhukhan R, Sen I. (1996) Different glycosylation requirements for the synthesis of enzymatically active angiotensin-converting enzyme in mammalian cells and yeast. J Biol Chem 271:6429–6434 [DOI] [PubMed] [Google Scholar]
- Sadoshima J, Xu Y, Slayter HS, Izumo S. (1993) Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell 75:977–984 [DOI] [PubMed] [Google Scholar]
- Sagawa K, Nagatani K, Komagata Y, Yamamoto K. (2005) Angiotensin receptor blockers suppress antigen-specific T cell responses and ameliorate collagen-induced arthritis in mice. Arthritis Rheum 52:1920–1928 [DOI] [PubMed] [Google Scholar]
- Saito Y, Berk BC. (2001) Transactivation: a novel signaling pathway from angiotensin II to tyrosine kinase receptors. J Mol Cell Cardiol 33:3–7 [DOI] [PubMed] [Google Scholar]
- Samokhin AO, Bühling F, Theissig F, Brömme D. (2010) ApoE-deficient mice on cholate-containing high-fat diet reveal a pathology similar to lung sarcoidosis. Am J Pathol 176:1148–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos T, Drummond M, Botelho F. (2012) Erectile dysfunction in obstructive sleep apnea syndrome—prevalence and determinants. Rev Port Pneumol 18:64–71 [DOI] [PubMed] [Google Scholar]
- Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, Wainwright BJ, Ostrowski MC, Himes SR, Hume DA. (2003) A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood 101:1155–1163 [DOI] [PubMed] [Google Scholar]
- Savary K, Michaud A, Favier J, Larger E, Corvol P, Gasc JM. (2005) Role of the renin-angiotensin system in primitive erythropoiesis in the chick embryo. Blood 105:103–110 [DOI] [PubMed] [Google Scholar]
- Savoia C, Burger D, Nishigaki N, Montezano A, Touyz RM. (2011) Angiotensin II and the vascular phenotype in hypertension. Expert Rev Mol Med 13:e11. [DOI] [PubMed] [Google Scholar]
- Schelling P, Fischer H, Ganten D. (1991) Angiotensin and cell growth: a link to cardiovascular hypertrophy? J Hypertens 9:3–15 [PubMed] [Google Scholar]
- Schmidt S, van Hooft IM, Grobbee DE, Ganten D, Ritz E. (1993) Polymorphism of the angiotensin I converting enzyme gene is apparently not related to high blood pressure: Dutch Hypertension and Offspring Study. J Hypertens 11:345–348 [DOI] [PubMed] [Google Scholar]
- Schnermann J, Briggs J. (1986) Role of the renin-angiotensin system in tubuloglomerular feedback. Fed Proc 45:1426–1430 [PubMed] [Google Scholar]
- Schnermann J, Briggs JP. (2008) Tubuloglomerular feedback: mechanistic insights from gene-manipulated mice. Kidney Int 74:418–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schunkert H, Hense HW, Holmer SR, Stender M, Perz S, Keil U, Lorell BH, Riegger GA. (1994) Association between a deletion polymorphism of the angiotensin-converting-enzyme gene and left ventricular hypertrophy. N Engl J Med 330:1634–1638 [DOI] [PubMed] [Google Scholar]
- Schuster VL, Kokko JP, Jacobson HR. (1984) Angiotensin II directly stimulates sodium transport in rabbit proximal convoluted tubules. J Clin Invest 73:507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweisfurth H, Schiöberg-Schiegnitz S. (1984) Assay and biochemical characterization of angiotensin-I-converting enzyme in cerebrospinal fluid. Enzyme 32:12–19 [DOI] [PubMed] [Google Scholar]
- Senchenkova EY, Russell J, Almeida-Paula LD, Harding JW, Granger DN. (2010) Angiotensin II-mediated microvascular thrombosis. Hypertension 56:1089–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senchenkova EY, Russell J, Kurmaeva E, Ostanin D, Granger DN. (2011) Role of T lymphocytes in angiotensin II-mediated microvascular thrombosis. Hypertension 58:959–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shai SY, Fishel RS, Martin BM, Berk BC, Bernstein KE. (1992) Bovine angiotensin converting enzyme cDNA cloning and regulation. Increased expression during endothelial cell growth arrest. Circ Res 70:1274–1281 [DOI] [PubMed] [Google Scholar]
- Sharma JN. (2009) Hypertension and the bradykinin system. Curr Hypertens Rep 11:178–181 [DOI] [PubMed] [Google Scholar]
- Sharp MG, Fettes D, Brooker G, Clark AF, Peters J, Fleming S, Mullins JJ. (1996) Targeted inactivation of the Ren-2 gene in mice. Hypertension 28:1126–1131 [DOI] [PubMed] [Google Scholar]
- Shen XZ, Billet S, Lin C, Okwan-Duodu D, Chen X, Lukacher AE, Bernstein KE. (2011) The carboxypeptidase ACE shapes the MHC class I peptide repertoire. Nat Immunol 12:1078–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen XZ, Li P, Weiss D, Fuchs S, Xiao HD, Adams JA, Williams IR, Capecchi MR, Taylor WR, Bernstein KE. (2007) Mice with enhanced macrophage angiotensin-converting enzyme are resistant to melanoma. Am J Pathol 170:2122–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen XZ, Lukacher AE, Billet S, Williams IR, Bernstein KE. (2008) Expression of angiotensin-converting enzyme changes major histocompatibility complex class I peptide presentation by modifying C termini of peptide precursors. J Biol Chem 283:9957–9965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen XZ, Ong FS, Bernstein EA, Janjulia T, Blackwell WL, Shah KH, Taylor BL, Gonzalez-Villalobos RA, Fuchs S, Bernstein KE. (2012) Nontraditional roles of angiotensin-converting enzyme. Hypertension 59:763–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy UV, Richards EM, Huang XC, Sumners C. (1999) Angiotensin II type 2 receptor-mediated apoptosis of cultured neurons from newborn rat brain. Endocrinology 140:500–509 [DOI] [PubMed] [Google Scholar]
- Sherman LA, Burke TA, Biggs JA. (1992) Extracellular processing of peptide antigens that bind class I major histocompatibility molecules. J Exp Med 175:1221–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiloh MU, MacMicking JD, Nicholson S, Brause JE, Potter S, Marino M, Fang F, Dinauer M, Nathan C. (1999) Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity 10:29–38 [DOI] [PubMed] [Google Scholar]
- Silva-Filho JL, Souza MC, Henriques MG, Morrot A, Savino W, Nunes MP, Caruso-Neves C, Pinheiro AA. (2011) AT1 receptor-mediated angiotensin II activation and chemotaxis of T lymphocytes. Mol Immunol 48:1835–1843 [DOI] [PubMed] [Google Scholar]
- Silverstein E, Friedland J. (1977) Elevated serum and spleen angiotensin converting enzyme and serum lysozyme in Gaucher’s disease. Clin Chim Acta 74:21–25 [DOI] [PubMed] [Google Scholar]
- Silverstein E, Pertschuk LP, Friedland J. (1979) Immunofluorescent localization of angiotensin converting enzyme in epithelioid and giant cells of sarcoidosis granulomas. Proc Natl Acad Sci USA 76:6646–6648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E, Roopenian D. (1997) Minor histocompatibility antigens. Curr Opin Immunol 9:655–661 [DOI] [PubMed] [Google Scholar]
- Sinka L, Biasch K, Khazaal I, Péault B, Tavian M. (2012) Angiotensin-converting enzyme (CD143) specifies emerging lympho-hematopoietic progenitors in the human embryo. Blood 119:3712–3723 [DOI] [PubMed] [Google Scholar]
- Skeggs LT., Jr (1993) Discovery of the two angiotensin peptides and the angiotensin converting enzyme. Hypertension 21:259–260 [DOI] [PubMed] [Google Scholar]
- Skeggs LT, Jr, Kahn JR, Shumway NP. (1956a) The preparation and function of the hypertensin-converting enzyme. J Exp Med 103:295–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeggs LT, Jr, Kahn JR, Shumway NP. (1956b) The purification of hypertensin II. J Exp Med 103:301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeggs LT, Jr, Lentz KE, Kahn JR, Shumway NP, Woods KR. (1956c) The amino acid sequence of hypertensin. II. J Exp Med 104:193–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeggs LT, Jr, Marsh WH, Kahn JR, Shumway NP. (1954) The existence of two forms of hypertensin. J Exp Med 99:275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidgel RA, Erdös EG. (1985) Novel activity of human angiotensin I converting enzyme: release of the NH2- and COOH-terminal tripeptides from the luteinizing hormone-releasing hormone. Proc Natl Acad Sci USA 82:1025–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidgel RA, Erdös EG. (1987) The broad substrate specificity of human angiotensin I converting enzyme. Clin Exp Hypertens A 9:243–259 [DOI] [PubMed] [Google Scholar]
- Skidgel RA, Erdös EG. (1993) Biochemistry of angiotensin converting enzyme, in The Renin-Angiotensin System, Vol. 1 (Robertson JIS, Nicholls MG. eds) pp 10.1–10.10, Gower Medical Publishers, London [Google Scholar]
- Skidgel RA, Engelbrecht S, Johnson AR, Erdös EG. (1984) Hydrolysis of substance p and neurotensin by converting enzyme and neutral endopeptidase. Peptides 5:769–776 [DOI] [PubMed] [Google Scholar]
- Smithies O, Kim HS, Takahashi N, Edgell MH. (2000) Importance of quantitative genetic variations in the etiology of hypertension. Kidney Int 58:2265–2280 [DOI] [PubMed] [Google Scholar]
- Soffer RL. (1981) Angiotensin-converting enzyme, in Biochemical Regulation of Blood Pressure (Soffer RL. ed) pp 123–164, John Wiley and Sons, New York [Google Scholar]
- Sosne G, Qiu P, Goldstein AL, Wheater M. (2010) Biological activities of thymosin beta4 defined by active sites in short peptide sequences. FASEB J 24:2144–2151 [DOI] [PubMed] [Google Scholar]
- Soubrier F, Alhenc-Gelas F, Hubert C, Allegrini J, John M, Tregear G, Corvol P. (1988) Two putative active centers in human angiotensin I-converting enzyme revealed by molecular cloning. Proc Natl Acad Sci USA 85:9386–9390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillmann F, Altmann C, Scheeler M, Barbosa M, Westermann D, Schultheiss HP, Walther T, Tschöpe C. (2002) Regulation of cardiac bradykinin B1- and B2-receptor mRNA in experimental ischemic, diabetic, and pressure-overload-induced cardiomyopathy. Int Immunopharmacol 2:1823–1832 [DOI] [PubMed] [Google Scholar]
- Spyroulias GA, Galanis AS, Pairas G, Manessi-Zoupa E, Cordopatis P. (2004) Structural features of angiotensin-I converting enzyme catalytic sites: conformational studies in solution, homology models and comparison with other zinc metallopeptidases. Curr Top Med Chem 4:403–429 [DOI] [PubMed] [Google Scholar]
- Stegbauer J, Lee DH, Seubert S, Ellrichmann G, Manzel A, Kvakan H, Muller DN, Gaupp S, Rump LC, Gold R, et al. (2009) Role of the renin-angiotensin system in autoimmune inflammation of the central nervous system. Proc Natl Acad Sci USA 106:14942–14947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter SM, Snyder SH. (1986) Characterization of angiotensin converting enzyme by [3H]captopril binding. Mol Pharmacol 29:142–148 [PubMed] [Google Scholar]
- Strittmatter SM, Lo MM, Javitch JA, Snyder SH. (1984) Autoradiographic visualization of angiotensin-converting enzyme in rat brain with [3H]captopril: localization to a striatonigral pathway. Proc Natl Acad Sci USA 81:1599–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturrock ED, Danilov SM, Riordan JF. (1997) Limited proteolysis of human kidney angiotensin-converting enzyme and generation of catalytically active N- and C-terminal domains. Biochem Biophys Res Commun 236:16–19 [DOI] [PubMed] [Google Scholar]
- Sun X, Becker M, Pankow K, Krause E, Ringling M, Beyermann M, Maul B, Walther T, Siems WE. (2008) Catabolic attacks of membrane-bound angiotensin-converting enzyme on the N-terminal part of species-specific amyloid-beta peptides. Eur J Pharmacol 588:18–25 [DOI] [PubMed] [Google Scholar]
- Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, et al. (2009) Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325:612–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi F, Yamamoto K, Katsuya T, Sugiyama T, Nabika T, Ohnaka K, Yamaguchi S, Takayanagi R, Ogihara T, Kato N. (2012) Reevaluation of the association of seven candidate genes with blood pressure and hypertension: a replication study and meta-analysis with a larger sample size. Hypertens Res 35:825–831 [DOI] [PubMed] [Google Scholar]
- Tan LB, Jalil JE, Pick R, Janicki JS, Weber KT. (1991) Cardiac myocyte necrosis induced by angiotensin II. Circ Res 69:1185–1195 [DOI] [PubMed] [Google Scholar]
- Tanimoto K, Sugiyama F, Goto Y, Ishida J, Takimoto E, Yagami K, Fukamizu A, Murakami K. (1994) Angiotensinogen-deficient mice with hypotension. J Biol Chem 269:31334–31337 [PubMed] [Google Scholar]
- Tatei K, Cai H, Ip YT, Levine M. (1995) Race: a Drosophila homologue of the angiotensin converting enzyme. Mech Dev 51:157–168 [DOI] [PubMed] [Google Scholar]
- Tavian M, Biasch K, Sinka L, Vallet J, Péault B. (2010) Embryonic origin of human hematopoiesis. Int J Dev Biol 54:1061–1065 [DOI] [PubMed] [Google Scholar]
- Tavian M, Coulombel L, Luton D, Clemente HS, Dieterlen-Lièvre F, Péault B. (1996) Aorta-associated CD34+ hematopoietic cells in the early human embryo. Blood 87:67–72 [PubMed] [Google Scholar]
- Tavian M, Hallais MF, Péault B. (1999) Emergence of intraembryonic hematopoietic precursors in the pre-liver human embryo. Development 126:793–803 [DOI] [PubMed] [Google Scholar]
- Tavian M, Robin C, Coulombel L, Péault B. (2001) The human embryo, but not its yolk sac, generates lympho-myeloid stem cells: mapping multipotent hematopoietic cell fate in intraembryonic mesoderm. Immunity 15:487–495 [DOI] [PubMed] [Google Scholar]
- Thimon V, Métayer S, Belghazi M, Dacheux F, Dacheux JL, Gatti JL. (2005) Shedding of the germinal angiotensin I-converting enzyme (gACE) involves a serine protease and is activated by epididymal fluid. Biol Reprod 73:881–890 [DOI] [PubMed] [Google Scholar]
- Tigerstedt R, Bergman PG. (1898) Niere und kreislauf. Skand Arch Physiol 8:223–271 [Google Scholar]
- Tiret L, Rigat B, Visvikis S, Breda C, Corvol P, Cambien F, Soubrier F. (1992) Evidence, from combined segregation and linkage analysis, that a variant of the angiotensin I-converting enzyme (ACE) gene controls plasma ACE levels. Am J Hum Genet 51:197–205 [PMC free article] [PubMed] [Google Scholar]
- Touyz RM, He G, Deng LY, Schiffrin EL. (1999) Role of extracellular signal-regulated kinases in angiotensin II-stimulated contraction of smooth muscle cells from human resistance arteries. Circulation 99:392–399 [DOI] [PubMed] [Google Scholar]
- Towler P, Staker B, Prasad SG, Menon S, Tang J, Parsons T, Ryan D, Fisher M, Williams D, Dales NA, et al. (2004) ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J Biol Chem 279:17996–18007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabold F, Pons S, Hagege AA, Bloch-Faure M, Alhenc-Gelas F, Giudicelli JF, Richer-Giudicelli C, Meneton P. (2002) Cardiovascular phenotypes of kinin B2 receptor- and tissue kallikrein-deficient mice. Hypertension 40:90–95 [DOI] [PubMed] [Google Scholar]
- Tsubakimoto Y, Yamada H, Yokoi H, Kishida S, Takata H, Kawahito H, Matsui A, Urao N, Nozawa Y, Hirai H, et al. (2009) Bone marrow angiotensin AT1 receptor regulates differentiation of monocyte lineage progenitors from hematopoietic stem cells. Arterioscler Thromb Vasc Biol 29:1529–1536 [DOI] [PubMed] [Google Scholar]
- Tsuchida S, Matsusaka T, Chen X, Okubo S, Niimura F, Nishimura H, Fogo A, Utsunomiya H, Inagami T, Ichikawa I. (1998) Murine double nullizygotes of the angiotensin type 1A and 1B receptor genes duplicate severe abnormal phenotypes of angiotensinogen nullizygotes. J Clin Invest 101:755–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ura N, Shimamoto K, Kuroda S, Nomura N, Iwata M, Aoyama T, Iimura O. (1994) The role of kinins and atrial natriuretic peptide on the renal effects of neutral endopeptidase inhibitor in rats. Clin Exp Hypertens 16:799–808 [DOI] [PubMed] [Google Scholar]
- Valentine JW, Jablonski D, Erwin DH. (1999) Fossils, molecules and embryos: new perspectives on the Cambrian explosion. Development 126:851–859 [DOI] [PubMed] [Google Scholar]
- van Kesteren CA, van Heugten HA, Lamers JM, Saxena PR, Schalekamp MA, Danser AH. (1997) Angiotensin II-mediated growth and antigrowth effects in cultured neonatal rat cardiac myocytes and fibroblasts. J Mol Cell Cardiol 29:2147–2157 [DOI] [PubMed] [Google Scholar]
- Vaughan DE. (1997) The renin-angiotensin system and fibrinolysis. Am J Cardiol 79 (5A):12–16 [DOI] [PubMed] [Google Scholar]
- Vaughan DE, Lazos SA, Tong K. (1995) Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells. A potential link between the renin-angiotensin system and thrombosis. J Clin Invest 95:995–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachogiannakos J, Tang AK, Patch D, Burroughs AK. (2001) Angiotensin converting enzyme inhibitors and angiotensin II antagonists as therapy in chronic liver disease. Gut 49:303–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahakos DV, Balodimos C, Papachristopoulos V, Vassilakos P, Hinari E, Vlachojannis JG. (1995) Renin-angiotensin system stimulates erythropoietin secretion in chronic hemodialysis patients. Clin Nephrol 43:53–59 [PubMed] [Google Scholar]
- Vlahakos DV, Kosmas EN, Dimopoulou I, Ikonomou E, Jullien G, Vassilakos P, Marathias KP. (1999) Association between activation of the renin-angiotensin system and secondary erythrocytosis in patients with chronic obstructive pulmonary disease. Am J Med 106:158–164 [DOI] [PubMed] [Google Scholar]
- Voronov S, Zueva N, Orlov V, Arutyunyan A, Kost O. (2002) Temperature-induced selective death of the C-domain within angiotensin-converting enzyme molecule. FEBS Lett 522:77–82 [DOI] [PubMed] [Google Scholar]
- Waeckel L, Bignon J, Liu JM, Markovits D, Ebrahimian TG, Vilar J, Mees B, Blanc-Brude O, Barateau V, Le Ricousse-Roussanne S, et al. (2006) Tetrapeptide AcSDKP induces postischemic neovascularization through monocyte chemoattractant protein-1 signaling. Arterioscler Thromb Vasc Biol 26:773–779 [DOI] [PubMed] [Google Scholar]
- Walter J. (1993) Does captopril decrease the effect of human recombinant erythropoietin in haemodialysis patients? Nephrol Dial Transplant 8:1428. [PubMed] [Google Scholar]
- Wang Q, Lei X, Zhu S, Zhang S. (2012) Angiotensin-I converting enzyme inhibitors suppress angiogenesis and growth of esophageal carcinoma xenografts. Dis Esophagus : 757–763 [DOI] [PubMed] [Google Scholar]
- Wang T, Giebisch G. (1996) Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am J Physiol 271:F143–F149 [DOI] [PubMed] [Google Scholar]
- Wang T, Li H, Zhao C, Chen C, Li J, Chao J, Chao L, Xiao X, Wang DW. (2004) Recombinant adeno-associated virus-mediated kallikrein gene therapy reduces hypertension and attenuates its cardiovascular injuries. Gene Ther 11:1342–1350 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Hashimoto M, Okuyama S, Inagami T, Nakamura S. (1999) Effects of targeted disruption of the mouse angiotensin II type 2 receptor gene on stress-induced hyperthermia. J Physiol 515:881–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CC, Hase N, Inoue Y, Bradley EW, Yahiro E, Li M, Naqvi N, Powell PC, Shi K, Takahashi Y, et al. (2010) Mast cell chymase limits the cardiac efficacy of Ang I-converting enzyme inhibitor therapy in rodents. J Clin Invest 120:1229–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Alhenc-Gelas F, Corvol P, Clauser E. (1991a) The two homologous domains of human angiotensin I-converting enzyme are both catalytically active. J Biol Chem 266:9002–9008 [PubMed] [Google Scholar]
- Wei L, Alhenc-Gelas F, Soubrier F, Michaud A, Corvol P, Clauser E. (1991b) Expression and characterization of recombinant human angiotensin I-converting enzyme. Evidence for a C-terminal transmembrane anchor and for a proteolytic processing of the secreted recombinant and plasma enzymes. J Biol Chem 266:5540–5546 [PubMed] [Google Scholar]
- Wei L, Clauser E, Alhenc-Gelas F, Corvol P. (1992) The two homologous domains of human angiotensin I-converting enzyme interact differently with competitive inhibitors. J Biol Chem 267:13398–13405 [PubMed] [Google Scholar]
- Weisinger RS, Blair-West JR, Burns P, Denton DA, Tarjan E. (1997) Role of brain angiotensin in thirst and sodium appetite of rats. Peptides 18:977–984 [DOI] [PubMed] [Google Scholar]
- Whitton PD, Rodrigues LM, Hems DA. (1978) Stimulation by vasopressin, angiotensin and oxytocin of gluconeogenesis in hepatocyte suspensions. Biochem J 176:893–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijffels G, Fitzgerald C, Gough J, Riding G, Elvin C, Kemp D, Willadsen P. (1996) Cloning and characterisation of angiotensin-converting enzyme from the dipteran species, Haematobia irritans exigua, and its expression in the maturing male reproductive system. Eur J Biochem 237:414–423 [DOI] [PubMed] [Google Scholar]
- Williams GT, Williams WJ. (1983) Granulomatous inflammation—a review. J Clin Pathol 36:723–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TA, Danilov S, Alhenc-Gelas F, Soubrier F. (1996) A study of chimeras constructed with the two domains of angiotensin I-converting enzyme. Biochem Pharmacol 51:11–14 [DOI] [PubMed] [Google Scholar]
- Williams TA, Hooper NM, Turner AJ. (1991) Characterization of neuronal and endothelial forms of angiotensin converting enzyme in pig brain. J Neurochem 57:193–199 [DOI] [PubMed] [Google Scholar]
- Winkelmann BR, Nauck M, Klein B, Russ AP, Böhm BO, Siekmeier R, Ihnken K, Verho M, Gross W, März W. (1996) Deletion polymorphism of the angiotensin I-converting enzyme gene is associated with increased plasma angiotensin-converting enzyme activity but not with increased risk for myocardial infarction and coronary artery disease. Ann Intern Med 125:19–25 [DOI] [PubMed] [Google Scholar]
- Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. (2000) Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol 57:1439–1443 [DOI] [PubMed] [Google Scholar]
- Woodman ZL, Oppong SY, Cook S, Hooper NM, Schwager SL, Brandt WF, Ehlers MR, Sturrock ED. (2000) Shedding of somatic angiotensin-converting enzyme (ACE) is inefficient compared with testis ACE despite cleavage at identical stalk sites. Biochem J 347:711–718 [PMC free article] [PubMed] [Google Scholar]
- Woodman ZL, Schwager SL, Redelinghuys P, Carmona AK, Ehlers MR, Sturrock ED. (2005) The N domain of somatic angiotensin-converting enzyme negatively regulates ectodomain shedding and catalytic activity. Biochem J 389:739–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman ZL, Schwager SL, Redelinghuys P, Chubb AJ, van der Merwe EL, Ehlers MR, Sturrock ED. (2006) Homologous substitution of ACE C-domain regions with N-domain sequences: effect on processing, shedding, and catalytic properties. Biol Chem 387:1043–1051 [DOI] [PubMed] [Google Scholar]
- Xia H, Feng Y, Obr TD, Hickman PJ, Lazartigues E. (2009) Angiotensin II type 1 receptor-mediated reduction of angiotensin-converting enzyme 2 activity in the brain impairs baroreflex function in hypertensive mice. Hypertension 53:210–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao HD, Fuchs S, Bernstein EA, Li P, Campbell DJ, Bernstein KE. (2008) Mice expressing ACE only in the heart show that increased cardiac angiotensin II is not associated with cardiac hypertrophy. Am J Physiol Heart Circ Physiol 294:H659–H667 [DOI] [PubMed] [Google Scholar]
- Xiao HD, Fuchs S, Cole JM, Disher KM, Sutliff RL, Bernstein KE. (2003) Role of bradykinin in angiotensin-converting enzyme knockout mice. Am J Physiol Heart Circ Physiol 284:H1969–H1977 [DOI] [PubMed] [Google Scholar]
- Xiong W, Chao J, Chao L. (1995) Muscle delivery of human kallikrein gene reduces blood pressure in hypertensive rats. Hypertension 25:715–719 [DOI] [PubMed] [Google Scholar]
- Xu Z, Lane JM, Zhu B, Herbert J. (1997) Dizocilpine maleate, an N-methyl-D-aspartate antagonist, inhibits dipsogenic responses and C-Fos expression induced by intracerebral infusion of angiotensin II. Neuroscience 78:203–214 [DOI] [PubMed] [Google Scholar]
- Yamaguchi R, Muro Y, Isotani A, Tokuhiro K, Takumi K, Adham I, Ikawa M, Okabe M. (2009) Disruption of ADAM3 impairs the migration of sperm into oviduct in mouse. Biol Reprod 81:142–146 [DOI] [PubMed] [Google Scholar]
- Yamamoto R, Akazawa H, Fujihara H, Ozasa Y, Yasuda N, Ito K, Kudo Y, Qin Y, Ueta Y, Komuro I. (2011) Angiotensin II type 1 receptor signaling regulates feeding behavior through anorexigenic corticotropin-releasing hormone in hypothalamus. J Biol Chem 286:21458–21465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamato M, Koguchi T, Okachi R, Yamada K, Nakayama K, Kase H, Karasawa A, Shuto K. (1986) K-26, a novel inhibitor of angiotensin I converting enzyme produced by an actinomycete K-26. J Antibiot (Tokyo) 39:44–52 [DOI] [PubMed] [Google Scholar]
- Yan H-Y, Iwanaga M, Kawasaki H. (2007) Angiotensin-converting enzyme activity in the hemolymph during last laval and pupal stages of the silkworm, Bombyx mori. J Insect Biotechnol Sericology 76:17–23 [Google Scholar]
- Yang F, Yang XP, Liu YH, Xu J, Cingolani O, Rhaleb NE, Carretero OA. (2004) Ac-SDKP reverses inflammation and fibrosis in rats with heart failure after myocardial infarction. Hypertension 43:229–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HYT, Erdös EG. (1967) Second kininase in human blood plasma. Nature 215:1402–1403 [DOI] [PubMed] [Google Scholar]
- Yang HYT, Erdös EG, Levin Y. (1970) A dipeptidyl carboxypeptidase that converts angiotensin I and inactivates bradykinin. Biochim Biophys Acta 214:374–376 [DOI] [PubMed] [Google Scholar]
- Yang HYT, Erdös EG, Levin Y. (1971) Characterization of a dipeptide hydrolase (kininase II: angiotensin I converting enzyme). J Pharmacol Exp Ther 177:291–300 [PubMed] [Google Scholar]
- Yao F, Sumners C, O’Rourke ST, Sun C. (2008) Angiotensin II increases GABAB receptor expression in nucleus tractus solitarii of rats. Am J Physiol Heart Circ Physiol 294:H2712–H2720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui T, Alhenc-Gelas F, Corvol P, Menard J. (1984) Angiotensin I-converting enzyme in amniotic fluid. J Lab Clin Med 104:741–751 [PubMed] [Google Scholar]
- Yoshioka M, Erickson RH, Woodley JF, Gulli R, Guan D, Kim YS. (1987) Role of rat intestinal brush-border membrane angiotensin-converting enzyme in dietary protein digestion. Am J Physiol 253:G781–G786 [DOI] [PubMed] [Google Scholar]
- Yu XC, Sturrock ED, Wu Z, Biemann K, Ehlers MR, Riordan JF. (1997) Identification of N-linked glycosylation sites in human testis angiotensin-converting enzyme and expression of an active deglycosylated form. J Biol Chem 272:3511–3519 [DOI] [PubMed] [Google Scholar]
- Zambidis ET, Park TS, Yu W, Tam A, Levine M, Yuan X, Pryzhkova M, Péault B. (2008) Expression of angiotensin-converting enzyme (CD143) identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells. Blood 112:3601–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Ahokas RA, Weber KT, Sun Y. (2006) ANG II-induced cardiac molecular and cellular events: role of aldosterone. Am J Physiol Heart Circ Physiol 291:H336–H343 [DOI] [PubMed] [Google Scholar]
- Zhuo JL, Li XC. (2007) Novel roles of intracrine angiotensin II and signalling mechanisms in kidney cells. J Renin Angiotensin Aldosterone Syst 8:23–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Delafontaine P, Martin BM, Bernstein KE. (1995) Identification of two positive transcriptional elements within the 91-base pair promoter for mouse testis angiotensin converting enzyme (testis ACE). Dev Genet 16:201–209 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Overbeek PA, Bernstein KE. (1996a) Tissue specific expression of testis angiotensin converting enzyme is not determined by the -32 nonconsensus TATA motif. Biochem Biophys Res Commun 223:48–53 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Sun Z, Means AR, Sassone-Corsi P, Bernstein KE. (1996b) cAMP-response element modulator tau is a positive regulator of testis angiotensin converting enzyme transcription. Proc Natl Acad Sci USA 93:12262–12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou K, Maeda T, Watanabe A, Liu J, Liu S, Oba R, Satoh Y, Komano H, Michikawa M. (2009) Abeta42-to-Abeta40- and angiotensin-converting activities in different domains of angiotensin-converting enzyme. J Biol Chem 284:31914–31920 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.