Abstract
Limited drug penetration is an obstacle that is often encountered in treatment of central nervous system (CNS) diseases including pain and cerebral hypoxia. Over the past several years, biochemical characteristics of the brain (i.e., tight junction protein complexes at brain barrier sites, expression of influx and efflux transporters) have been shown to be directly involved in determining CNS permeation of therapeutic agents; however, the vast majority of these studies have focused on understanding those mechanisms that prevent drugs from entering the CNS. Recently, this paradigm has shifted toward identifying and characterizing brain targets that facilitate CNS drug delivery. Such targets include the organic anion–transporting polypeptides (OATPs in humans; Oatps in rodents), a family of sodium-independent transporters that are endogenously expressed in the brain and are involved in drug uptake. OATP/Oatp substrates include drugs that are efficacious in treatment of pain and/or cerebral hypoxia (i.e., opioid analgesic peptides, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors). This clearly suggests that OATP/Oatp isoforms are viable transporter targets that can be exploited for optimization of drug delivery to the brain and, therefore, improved treatment of CNS diseases. This review summarizes recent knowledge in this area and emphasizes the potential that therapeutic targeting of OATP/Oatp isoforms may have in facilitating CNS drug delivery and distribution. Additionally, information presented in this review will point to novel strategies that can be used for treatment of pain and cerebral hypoxia.
I. Introduction
Pharmacological treatment of central nervous system (CNS) disease requires that drugs achieve effective concentrations in the brain. Over the past several years, considerable research has focused on studying biologic mechanisms that prevent drugs from permeating the CNS. To this end, several studies have described the limiting effect of blood-brain barrier (BBB) tight junction protein complexes and ATP-binding cassette efflux transporters on CNS drug uptake and distribution. The role of ATP-binding cassette transporters [i.e., P-glycoprotein (P-gp), multidrug resistance proteins (MRP in humans; Mrp in rodents), breast cancer resistance protein (Bcrp)] as determinants of CNS penetration of therapeutic compounds has been well described at the BBB as well as at the blood-cerebrospinal fluid (BCSF) barrier and in brain parenchyma cellular compartments such as astrocytes and microglia (Dallas et al., 2006; Ronaldson et al., 2008; Hartz and Bauer, 2010; Tournier et al., 2011). This intense focus on processes that keep drugs out of the brain (i.e., efflux transporters) has emphasized a critical need to re-evaluate these efforts. Current studies on CNS drug delivery are aimed toward identifying and characterizing endogenous transporter targets that can facilitate CNS drug delivery. Included among these targets are the organic anion–transporting polypeptides (OATPs in humans; Oatps in rodents), a family of sodium-independent transporters that are endogenously expressed in the brain and are involved in transport of drugs (Hagenbuch and Meier, 2004). In fact, many compounds that have shown considerable promise for treatment of acute/chronic pain (i.e., opioid analgesic peptides) and/or CNS diseases involving cerebral hypoxia [i.e., 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, opioid analgesic peptides] are known OATP/Oatp substrates. The ability to transport such drugs suggests that OATP/Oatp family members are viable transporter targets that can be exploited for optimization of CNS drug delivery and, therefore, improved pharmacotherapy for CNS diseases such as pain and cerebral hypoxia. Here, we provide a detailed review on these new and exciting developments in the field of transporter biology and CNS drug delivery by describing how OATP/Oatp isoforms can be directly targeted for efficient and effective brain delivery of drugs for treatment of pain and cerebral hypoxia. Additionally, we provide an overview of the molecular biology of OATP/Oatp isoforms, signaling pathways that control their expression, and describe interactions between OATPs/Oatps and other endogenously expressed CNS transporters.
II. Diseases of the Central Nervous System and Therapeutic Opportunities
A. Pain
Pain results from activation of sensory receptors that have evolved in detecting actual or impending tissue injury and/or damage (Ossipov et al., 2010). In this context, tissue injury is often associated with inflammation, a pathophysiological process that directly leads to sensation of pain. Additionally, inflammation localized to the site of damaged and/or affected nerves is a common underlying mechanism of neuropathic (i.e., chronic) pain (Vallejo et al., 2010). The prevalence of chronic pain in the United States is approximately 30.7% (Johannes et al., 2010). Pain affects a greater proportion of women than men and increases in prevalence with advancing age (Johannes et al., 2010). Pain has a complex pathophysiology that involves immunologic mediators, neurologic inputs from the CNS, and endocrine signaling via the hypothalamic-pituitary-adrenal (HPA) axis (Wolka et al., 2003). The immunologic response to inflammatory pain is characterized by rapid production and secretion of various mediators such as cytokines, chemokines, cellular adhesion molecules, matrix metalloproteinases, kinins, and prostaglandins at the site of tissue injury. The inflammatory component of pain is also characterized by increased vascular permeability, localized edema formation and redness, and increased leukocyte migration. The CNS utilizes neuronal pathways to signal the immune system. Neuronal responses to noxious stimulation involve both excitatory and inhibitory neurotransmission within sensory areas of the spinal cord, and the balance of these CNS responses determines the level of transmission of nociceptive signals to the brain (Dickinson et al., 1999). Furthermore, proinflammatory mediators produced and released in the CNS following peripheral injury or inflammation are involved in the centrally mediated pain response. Stress responses due to physical, emotional, and environmental stimuli result in activation of the HPA axis and initiation of a “stress cascade” (Miller and O’Callaghan, 2002). Activation of the HPA axis leads to release of corticotropin-releasing hormone from the hypothalamus, and circulating corticotropin-releasing hormone and vasopressin stimulate expression and release of adrenocorticotropin from the anterior pituitary gland. Adrenocorticotropin circulates to the adrenal glands, where it induces production and secretion of glucocorticoids, which are responsible for downregulation of the immune response (Miller and O’Callaghan, 2002). Additionally, adrenal glucocorticoids can “prime” glial cells in the spinal cord, thereby potentiating pain responses to subsequent noxious stimuli (Loram et al., 2011).
In addition to inflammatory, neuronal, and endocrine mechanisms, oxidative stress demarcated by production of highly potent reactive oxygen species (ROS) (e.g., superoxide anion) is a key component of the pathophysiological pain response (Wang et al., 2004; Khattab, 2006; Salvemini et al., 2006; Lochhead et al., 2012). Biologic activity of superoxide anion, a by-product of normal physiologic processes, is under tight control by superoxide dismutase (SOD) enzymes. During acute inflammation, superoxide is produced at such high levels that the ability of SODs to metabolize these ROS may become overwhelmed (Salvemini et al., 2006). This enables conjugation of excess superoxide with nitric oxide to form peroxynitrite, a potent cytotoxic and proinflammatory molecule. Peroxynitrite induces cellular damage by its ability to nitrosylate tyrosine residues, leading to functional modifications of critical proteins (Salvemini et al., 2006). Using the well established and highly reproducible λ-carrageenan model of peripheral inflammatory pain, our laboratory demonstrated increased protein nitrosylation both at the site of injury (i.e., λ-carrageenan injection site) and in the CNS (Lochhead et al., 2012). Interestingly, CNS expression of nitrosylated proteins and other oxidative stress markers (i.e., 4-hydroxynoneal) were attenuated following treatment with tempol, a pharmacological ROS scavenger and SOD mimetic (Lochhead et al., 2012). Additionally, tempol prevented paw edema and thermal allodynia elicited by λ-carrageenan-induced inflammatory pain (Lochhead et al., 2012), further emphasizing that oxidative stress is a critical component of the pathophysiological response to pain/inflammation.
There are many drugs with analgesic properties that are currently available for clinical use. Of these therapeutic compounds, opioids are the most widely used and the most effective analgesics used in pain management regimens (Chou, 2009; Manchikanti et al., 2010; Gloth, 2011). In fact, use of opioid analgesics for pain management has dramatically increased, as evidenced by a 149% increase in overall opioid prescriptions in the United States from 1997 to 2007 (Manchikanti et al., 2010). Opioids exert their analgesic effect by binding to specific receptors (i.e., μ-, κ-, and δ-opioid receptors) that are localized to neural tissue both in the CNS and in the periphery. Although opioids can provide some analgesia by binding to peripheral opioid receptors (Vadivelu et al., 2011), optimal pharmacotherapy with these drugs requires the capability to access central opioid receptors (Hamabe et al., 2007; Labuz et al., 2007). Opioid receptors are G-protein-coupled receptors and, therefore, have profound effects on ion gating, intracellular Ca2+ disposition, and protein phosphorylation. As there are multiple subtypes of opioid receptors, a possibility for opioids to exert different pharmacological effects at different receptors exists. For example, morphine is a full agonist at the μ-opioid receptor but a weak agonist at κ and δ receptors. In contrast, codeine is a weak agonist at μ and δ receptors. Opioid analgesics have multiple actions in the CNS, not all of which are beneficial. They are known to cause euphoria, which in part accounts for their abuse potential. Pharmacotherapy with opioids is associated with several adverse effects (e.g., respiratory depression, constipation, nausea, vomiting, rapid development of tolerance) (Haas, 2002). This property may limit the maximal opioid dose that can be administered as well as the level of analgesia that can be attained. Additionally, adverse events are enhanced by opioid-induced glial activation, which occurs via nonstereoselective activation of Toll-like receptors (TLRs) at the glial cell surface (Watkins et al., 2009). TLRs are widely expressed in the CNS, particularly by astrocytes and microglia (Bsibsi et al., 2002; Kielian, 2006). Recent work by Watkins et al. (2009) has shown expression of TLR4 in astrocytes, direct involvement of astrocytic TLR4 in the pathogenesis of neuropathic pain, and counteraction of opioid analgesic efficacy by TLR4 signaling. Although opioid-mediated activation of glial TLR4 signaling can be blocked by coadministration of (+)-naloxone or (+)-naltrexone (Hutchinson et al., 2008), it remains an essential objective of pharmacotherapy that opioid concentrations in the brain be maintained precisely to ensure efficacious management of pain and to limit adverse drug reactions. This therapeutic objective emphasizes the importance of understanding biologic mechanisms involved in determining CNS delivery of opioid analgesics and how these mechanisms can be targeted to optimally deliver drugs to the brain for treatment of pain associated with peripheral inflammatory tissue injury.
Adverse events associated with opioid pharmacotherapy underscore the necessity for development of novel analgesic drugs. As a result, much interest has focused on peptides, which are key regulators of cellular and intracellular physiologic processes and have shown promise in treatment of pathophysiological conditions. Despite this advantage, treatment of pain with opioid peptides is hindered by difficulties in their effective CNS delivery. Pharmacotherapy with peptides is also limited by poor oral bioavailability, a direct result of low metabolic stability and high hepatic clearance. Opioid peptides are hydrophilic compounds, a property that restricts their ability to traverse brain barriers and achieve effective concentrations in the CNS. Other physicochemical determinants of a peptide’s ability to accumulate in the brain include molecular size, flexibility, conformation, biochemical properties of constituent amino acids, and amino acid arrangement. Peptide composition is a critical determinant of pharmacokinetic properties such as plasma protein binding, cellular sequestration, clearance, uptake into nontarget tissues, and affinity for transport proteins. Such factors are mandatory considerations for rational design of novel opioid peptide drugs (Witt et al., 2001; Wolka et al., 2003; Egleton and Davis, 2005; Witt and Davis, 2006).
To maximize peptide accessibility to the CNS, chemical modifications may be required. Peptides may be altered by addition of hydrophobic functional groups (i.e., methyl groups) that reduce hydrogen bonding potential of the molecule and enhance the ability of the peptide to diffuse across biologic membranes. For example, placement of three methyl groups on the phenylalanine residue of [d-penicillamine(2,5)]-enkephalin (DPDPE) increased brain delivery of this peptide (Witt et al., 2000). CNS peptide bioavailability can also be increased by structural modifications to reduce enzymatic cleavage. For example, replacement of d-Gly2 for d-Ala2 on opioid peptides was shown to reduce aminopeptidase activity (Roemer and Pless, 1979). Reduction of enzymatic degradation and improvement of pharmacological half-life can be accomplished by C-terminal amidation, an approach that has been applied to Met-enkephalin (Roemer and Pless, 1979). Other molecular modifications known to enhance CNS peptide delivery include halogenation (Abbruscato et al., 1996) and glycosylation (Jakas and Horvat, 2004). It is significant to consider that extensive modifications at the N terminus of an opioid peptide may result in reduced analgesia because the N-terminal region is critical for opioid receptor binding. Therefore, rational design of novel therapeutic opioid peptides seeks to achieve an appropriate balance between CNS permeability and pharmacological efficacy.
Although chemical modification has shown some success in improving CNS delivery, brain uptake and distribution of some peptides is also governed by transport systems that are endogenously expressed at the microvascular endothelium. Of these transport systems, some are unidirectional (i.e., facilitate either blood-to-brain or brain-to-blood peptide transport) and others are bidirectional. For example, BBB transport of tyrosinylated analogs of melanocyte-inhibiting factor-1, an endogenous neuropeptide with opiate and antiopiate activity, is determined by peptide transport system-1 (PTS-1) (Banks et al., 1993a, 1996a). Additionally, analogs of pituitary adenylate cyclase–activating polypeptide (PACAP), a regulatory peptide with neuroprotective, endocrine, and vasodilatory properties (Vaudry et al., 2000), are transported across the brain microvascular endothelium by PTS-6 (Banks et al., 1993b; Dogrukol-Ak et al., 2009). Specifically, blood-to-brain delivery of a 38-amino-acid-residue PACAP analog is facilitated by a saturable, carrier-mediated uptake component of PTS-6 (Banks et al., 1993b), while CNS uptake of a 27-amino-acid PACAP analog is determined by passive diffusion and a PTS-6 efflux component (Dogrukol-Ak et al., 2009). In these studies, preservation of CNS concentrations of pharmacologically active peptides and/or enhancement of blood-to-brain transport of peptide therapeutics was achieved by blocking efflux transport components of PTS (Banks et al., 1993a; Dogrukol-Ak et al., 2009). We propose an alternative to these strategies by targeting specific transporters that can directly mediate delivery of peptide therapeutics to the CNS, such as OATP/Oatp family members. For example, OATP/Oatp isoforms are known to be involved in blood-to-brain transport of opioid analgesic peptides such as deltorphin II and DPDPE (Gao et al., 2000; Ose et al., 2010; Ronaldson et al., 2011). A thorough understanding of transport mechanisms involved in CNS delivery of peptides will undoubtedly aid their development as potential therapeutics.
B. Cerebral Hypoxia
Cerebral hypoxia and subsequent reoxygenation (H/R) is a central component of several diseases, such as traumatic brain injury (Bouma and Muizelaar, 1992; Maloney-Wilensky et al., 2009), acute respiratory distress syndrome (Hopkins et al., 2006), obstructive sleep apnea (El-Ad and Lavie, 2005), high-altitude cerebral edema and acute mountain sickness (Hackett, 1999), cardiac arrest (Lim et al., 2004), and ischemic stroke (Kalaria and Ballard, 2001). H/R is directly associated with neuronal apoptosis, which is characterized by cytochrome c release, caspase-3 activation, and internucleosomal DNA fragmentation (Lobysheva et al., 2009). Oxidative stress secondary to increased production of ROS is a critical mechanism known to induce neuronal cell death during H/R (Lobysheva et al., 2009). ROS contribute to brain injury by interacting with proteins, lipids, and nucleic acids as well as via activation of redox-sensitive signaling pathways. In the CNS, ROS-associated oxidative stress has been demonstrated in both in vitro and in vivo models of H/R in several laboratories, including our own. Such responses are characterized by increased CNS production of hydrogen peroxide (Schild and Reiser, 2005), upregulation of the cellular stress marker heat shock protein-70 (Lochhead et al., 2010), and increased nuclear expression of hypoxia-sensitive transcription factors such as hypoxia-inducible factor-1 and nuclear factor-κB (Witt et al., 2005; Lochhead et al., 2010). H/R is also associated with decreased brain concentrations of the endogenous antioxidant glutathione (GSH) (Schild and Reiser, 2005), an effect that is further indicative of oxidative stress.
The critical need for novel therapeutics that can be delivered to the CNS for treatment of diseases with a cerebral hypoxia component is best illustrated by ischemic stroke. Stroke is the third-most-common cause of death in the United States and is the number one cause of long-term morbidity (Baldwin et al., 2010). Of all strokes, 86% are ischemic (Roger et al., 2011). Ischemic stroke occurs when there is a restriction of blood flow and oxygen to part of the brain. Acute ischemic stroke causes an irreversibly damaged ischemic core and salvageable surrounding tissue, which is known as the penumbra (Astrup et al., 1981; Liu et al., 2010). Physiologically, energy requirements of the CNS are met by brain uptake of both glucose and oxygen, which are metabolized to enable phosphorylation of ADP to ATP. Most ATP generated within the brain is used for maintenance of intracellular homeostasis and transmembrane gradients for monovalent and divalent ions (i.e., sodium, potassium, calcium) (Adibhatla and Hatcher, 2008). When blood flow to the brain is interrupted during a stroke, the ischemic core is rapidly deprived of oxygen and glucose. Inability to provide sufficient ATP causes collapse of ion gradients and subsequent release of excitatory neurotransmitters (i.e., dopamine, glutamate). This uncontrolled increase in extracellular dopamine and glutamate concentrations is extremely neurotoxic and leads to neuronal cell death and development of an infarction (Adibhatla and Hatcher, 2008). Excitotoxicity associated with excess release of glutamate is particularly deleterious to the CNS due to overstimulation of glutamate receptors, activation of phospholipases and sphingomyelinases, phospholipid hydrolysis, release of arachidonic acid and ceramide, and disruption of CNS calcium homeostasis (Adibhatla et al., 2006a,b; Adibhatla and Hatcher, 2008; Arai et al., 2011). Oxidative stress is also observed in the CNS at early time points following ischemic injury and is well known to contribute to cell death in the ischemic core (Candelario-Jalil, 2009). As neuronal cell damage extends to the ischemic penumbra, neuroinflammation and apoptosis become more prevalent and dramatically affect viability of salvageable brain tissue within the penumbra (Candelario-Jalil, 2009).
Cell death processes in the ischemic core occur extremely rapidly (i.e., within minutes), thereby rendering this region difficult to protect using pharmacological approaches (Arai et al., 2011). In contrast, cells within the penumbra die more slowly by active cell death mechanisms, thus rendering therapeutic interventions theoretically possible (Arai et al., 2011). The primary goal of drug therapy for acute ischemic stroke is to salvage the penumbra as much as possible and as early as possible (Liu et al., 2010). Currently, there is only one therapeutic agent that has been approved by the US Food and Drug Administration for acute ischemic stroke treatment, recombinant tissue plasminogen activator (Jahan and Vinuela, 2009). The objective of tissue plasminogen activator therapy is to restore blood flow and oxygen supply to ischemic brain tissue; however, most brain cellular damage is thought to occur when cerebral perfusion is re-established (i.e., reoxygenation). Therefore, there is a critical need in stroke therapy for neuroprotective and/or antioxidant drugs that can be effectively delivered to the brain for “rescue” of salvageable tissue from further damage.
Currently, there is considerable interest in the neuroprotective/antioxidant properties of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (i.e., statins). Recent evidence suggests that statins can act as free-radical scavengers independent of their well documented effects on cholesterol biosynthesis (Kassan et al., 2010; Barone et al., 2011; Butterfield et al., 2012). For example, studies in dogs demonstrated that high-dose atorvastatin reduced markers of oxidative and nitrosative stress (i.e., protein carbonyls, 4-hydroxy-2-nonenal, 3-nitrotyrosine) and increased the ratio of GSH to reduced GSH in the brain but not in the periphery, suggesting that this drug can act as an efficacious neuroprotectant and CNS antioxidant (Barone et al., 2011). It has been suggested that the neuroprotective effects of atorvastatin in the CNS may be due to targeting and subsequent upregulation of biliverdin reductase-A, a pleiotropic enzyme known to be involved in cellular stress responses (Barone et al., 2012). Of particular note, Barone et al. (2012) reported that increased activity of biliverdin reductase-A induced by atorvastatin was inversely correlated with several indices of oxidative stress, implying that biliverdin reductase-A may be involved in antioxidant effects of HMG-CoA reductase inhibitors in the brain. Although statins have been associated with neurotoxic effects, these drugs generally do not compromise neuronal cell viability at concentrations below 1 μM (Wood et al., 2010). Additionally, there is evidence that opioid receptor agonists such as opioid peptides may have efficacy in treatment of ischemic stroke. For example, opioid peptides that selectively bind to the μ-opioid receptor (e.g., [Tyr-d-Ala, N-CH, -Phe4, Glyol]-enkephalin [DAMGO]), δ-opioid receptor (e.g., DPDPE), and κ-opioid receptor (e.g., U50,488) all reduced water uptake in rat hippocampal slices in situ (Yang et al., 2011a,b). Because cerebral edema is a leading cause of death in stroke patients, these data suggest that opioid peptides may be effective as stroke therapeutics. Additionally, PACAP is an extremely potent neuroprotectant that is pharmacologically active at concentrations within the femtomolar range (Banks, 2008). In fact, such low concentrations have been shown to be sufficient to reverse ischemic stroke, even when administered 24 hours following onset of ischemia (Banks et al., 1996b; Banks, 2008). However, use of statins and/or peptides as stroke therapeutics requires efficient CNS delivery. This therapeutic objective emphasizes the absolute importance of understanding specific mechanisms involved in statin and/or peptide CNS delivery.
III. Overview of the OATP/Oatp Family of Solute Carrier Transporters
Although peptides and HMG-CoA reductase inhibitors have shown considerable promise as efficacious drugs for treatment of acute and chronic inflammatory pain and/or cerebral hypoxia, their therapeutic utility requires efficient and effective CNS delivery. One intriguing approach for delivering these drugs to the brain is to target endogenous CNS influx transporters such as the OATPs/Oatps. OATP/Oatp family members are sodium-independent transporters that are classified within the larger solute carrier (SLC) superfamily (Hagenbuch and Meier, 2004). Although capable of bidirectional transport, SLC transporters are generally considered to favor cellular uptake of drugs (Roth et al., 2012). To date, 319 SLC genes subdivided into 43 families (i.e., the SLC1 to SLC43 families) have been identified in humans (Sugiura et al., 2006). Of the 43 known families of SLC transporters, only members of SLC21/Slc21 and SLC22 are expressed in the brain and, therefore, may play a critical role in determining CNS drug permeation and distribution (Kusuhara and Sugiyama, 2005). Genes in the SLC21/Slc21 superfamily, which is now known as SLCO/Slco, encode OATPs/Oatps. The nomenclature was revised and standardized in 2004 based on phylogenetic relationships and the superfamily was renamed SLCO, the SLC family of the OATPs (Hagenbuch and Meier, 2004). In humans, 11 OATP isoforms have been identified and are classified into 6 families based on amino acid identity. The different proteins are named "OATP" followed by the family number (OATP1, OATP2, etc.), the subfamily letter (OATP1A, OATP2B, etc.), and then a number that identifies an individual member within the family (Oatp1a1, OATP1A2, Oatp1a3, etc.). The nomenclature for rodent Oatp transporters follows the same conventions as for human orthologs except that these proteins are designated “Oatp” instead of “OATP” to demarcate species differences. The corresponding gene symbols are SLCO followed by the same number-letter-number combination (e.g., Slco1a4 gene for Oatp1a4 protein). An overview of the human and rodent isoforms, tissue expression, and substrate specificity of the known OATP families is presented below. For the most part, human OATP isoforms and their rodent orthologs share similar tissue localization (Hagenbuch and Meier, 2004). Although consistency in substrate profiles has been reported for human OATPs and rodent Oatps, overall transport function may vary considerably. For example, studies in plated cryopreserved human hepatocytes demonstrated that unbound affinity constant and active uptake clearance for seven known OATP/Oatp substrate drugs (bosentan, pitavastatin, pravastatin, repaglinide, rosuvastatin, telmisarten, valsartan) were on average 4.3- and 7.1-fold lower than in rat hepatocytes (Menochet et al., 2012). Similarly, uptake of pravastatin by OATP1B1 and OATP1B3 was significantly lower than that observed for Oatp1b2-mediated pravastatin transport in rodent tissue (Li et al., 2012). A summary of OATP/Oatp gene and protein information as well as known tissue localization is presented in Table 1. Known substrates for various OATP isoforms are presented in Table 2.
TABLE 1.
Gene, protein, and tissue localization of human and rodent organic anion–transporting polypeptide (OATP/Oatp) isoforms
| Family | Human Gene | Human Protein | Tissue Expression | Rodent Gene | Rodent Protein | Tissue Expression |
|---|---|---|---|---|---|---|
| OATP1 | SLCO1A2 | OATP1A2 | Brain, kidney, liver, lung, testis, placenta | Slco1a1 | Oatp1a1 | Brain, liver, kidney |
| SLCO1B1 | OATP1B1 | Liver | Slco1a3 (rat only) | Oatp1a3 | Kidney | |
| SLCO1B3 | OATP1B3 | Liver, retina | Slco1a4 | Oatp1a4 | Brain, eye | |
| SLCO1C1 | OATP1C1 | Brain, testis | Slco1a5 | Oatp1a5 | Brain, eye, small intestine | |
| Slco1a6 | Oatp1a6 | Liver, eye | ||||
| Slco1b2 | Oatp1b2 | Brain | ||||
| Slco1c1 | Oatp1c1 | Brain, eye, testis | ||||
| OATP2 | SLCO2A1 | OATP2A1 | Ubiquitous | Slco2a1 | Oatp2a1 | Ubiquitous |
| SLCO2B1 | OATP2B1 | Liver, intestine, brain, heart, breast, placenta | Slco2b1 | Oatp2b1 | Brain, eye, liver | |
| OATP3 | SLCO3A1 | OATP3A1 | Brain, testis, heart, lung, spleen, peripheral blood, leukocytes, thyroid, breast | Slco3a1 | Oatp3a1 | Ubiquitous |
| OATP4 | SLCO4A1 | OATP4A1 | Ubiquitous | Slco4a1 | Oatp4a1 | Ubiquitous |
| SLCO4C1 | OATP4C1 | Kidney | Slco4c1 | Oatp4c1 | Kidney | |
| OATP5 | SLCO5A1 | OATP5A1 | Breast | |||
| OATP6 | SLCO6A1 | OATP6A1 | Brain, testis, spleen, placenta | Slco6b1 | Oatp6b1 | Testis, ovary, adrenal gland |
| Slco6c1 | Oatp6c1 | Testis | ||||
| Slco6d1 | Oatp6d1 | Unknown |
TABLE 2.
Selected transport substrates for human OATP family members
| Transporter | Substrates | Reference(s) |
|---|---|---|
| OATP1A2 | Antibiotics (e.g., ciprofloxacin, enoxacin, erythromycin, gatifloxacin, levofloxacin, lomefloxacin, norfloxacin); antihistamines (e.g., fexofenadine); antineoplastic drugs (e.g., imatinib, methotrexate); β-blockers (e.g., acebutolol, atenolol, celiprolol, labetalol, sotalol, talinolol); cardiac glycosides (e.g., ouabain); endothelin-A receptor antagonists (e.g., atrasentan, BQ-123); HIV-1 protease inhibitors (e.g., darunavir, lopinavir, saquinavir); HMG-CoA reductase inhibitors (e.g., pitavastatin, rosuvastatin); neuromuscular blocking agents (e.g., rocuronium); opioid analgesic peptides (e.g., DPDPE); bilirubin; bromosulfophthalein; cholate; deltorphin II; estradiol-17β-glucuronide; estrone-3-sulfate; glycocholate; hydroxyurea; PGE2; reverse T3; taurocholate; taurochenodeoxycholate; tauroursodeoxycholate; T4; T3; metabolite of unoprostone | Roth et al., 2012 |
| OATP1B1 | Angiotensin-converting enzyme inhibitors (e.g., enalapril, temocapril); angiotensin II receptor antagonists (e.g., olmesartan, valsartan); antibiotics (e.g., cefazolin, cefditoren, cefoperazone, rifampicin); antifungal agents (e.g., caspofungin); anti-inflammatory drugs (e.g., mesalazine); antineoplastic agents (e.g., gimatecan, methotrexate); benzylpenicillin; endothelin-A receptor antagonists (e.g., atrasentan, bosentan, BQ-123); HIV-1 protease inhibitors (e.g., darunavir, lopinavir, saquinavir); HMG-CoA reductase inhibitors (e.g., atorvastatin, cerivastatin, fluvastatin, pitavastatin, pravastatin, rosuvastatin, simvastatin acid); loop diuretics (e.g., torasemide); opioid analgesic peptides (e.g., DPDPE); arsenic; bilirubin; bisglucuronosyl bilirubin; bromosulfophthalein; cholate; estradiol-17β-glucuronide; estrone-3-sulfate; fluorescein; glycocholate; glycoursodeoxycholate; hydroxyurea; leukotriene C4; leukotriene E4; monoglyucuronosyl bilirubin; phalloidin; PGE2; taurocholate; tauroursodeoxycholate; thromboxane B2; T4 | Roth et al., 2012 |
| OATP1B3 | Angiotensin-converting enzyme inhibitors (e.g., enalapril); angiotensin II receptor antagonists (e.g., olmestartan, telmisartan, valsartan); antibiotics (e.g., benzylpenicillin, cefadroxil, cefazolin, cefditoren, cefmetazole, cefoperazone, cephalexin, erythromycin, nafcillin, rifampicin); antihistamines (e.g., fexofenadine); antineoplastic drugs (e.g., docetaxel, imatinib, paclitaxel); cardiac glycosides (e.g., digoxin, ouabain); endothelin-A receptor antagonists (e.g., atrasentan, bosentan, BQ-123); HIV-1 protease inhibitors (e.g., saqunavir); HMG-CoA reductase inhibitors (e.g., fluvastatin, pitavastatin, rosuvastatin); nonsteroidal anti-inflammatory drugs (e.g., diclofenac); opioid analgesic peptides (e.g., DPDPE); bilirubin; bromosulfophthalein; cholate; cholecystokinin octapeptide (CCK-8); dehydroepiandrosterone-3-sulfate; deltorphin II; estradiol-17β-glucuronide; estrone-3-sulfate; fluorescein; glutathione; glycocholate; glycoursodeoxycholate; hydroxyurea; leukotriene C4; phalloidin; taurocholate; taurochenodeoxycholate; tauroursodeoxycholate; T4; T3 | Roth et al., 2012 |
| OATP1C1 | Bromosulfophthalein; estradiol-17β-glucuronide; estrone-3-sulfate; thyroid hormones | Pizzagalli et al., 2002; Westholm et al., 2009a,b |
| OATP2A1 | Prostaglandins (e.g., PGE1, PGE2, PGF2α, PGH2, thromboxane B2) | Kanai et al., 1995; Chi and Schuster, 2010 |
| OATP2B1 | Antibiotics (e.g., benzylpenicillin, tebipenem pivoxil); antihistamines (e.g., fexofenadine); anti-inflammatory drugs (e.g., mesalazine); β-blockers (e.g., talinolol); endothelin-A receptor antagonists (e.g., bosentan); HMG-CoA reductase inhibitors (e.g., atorvastatin, fluvastatin, pravastatin, pitavastatin, rosuvastatin); leukotriene receptor antagonists (e.g., monteleukast); aliskiren; bromosulfophthalein; dehydroepiandrosterone-3-sulfate; estrone-3-sulfate; ezetimibe glucuronide; glibenclamide; pregnenolone sulfate; taurocholate; metabolite of unoprostone | Tamai et al., 2001; Kobayashi et al., 2003; Nozawa et al., 2004; Gao et al., 2005; Kopplow et al., 2005; Hirano et al., 2006; Ho et al., 2006; Grube et al., 2006a,b; Noe et al., 2007; Treiber et al., 2007; Oswald et al., 2008; Vaidyanathan et al., 2008; Leuthold et al., 2009; Mougey et al., 2009; Konig et al., 2011 |
| OATP3A1_v1 | Antibiotics (e.g., benzylpenicillin); endothelin-A receptor antagonists (e.g., BQ-123); PGE1; PGE2; T4; vasopressin; estrone-3-sulfate; deltorphin II | Tamai et al., 2000; Huber et al., 2007; Roth et al., 2012 |
| OATP3A1_v2 | Endothelin-A receptor antagonists (e.g., BQ-123); PGE1; PGE2; T4; vasopressin; arachidonic acid | Huber et al., 2007; Roth et al., 2012 |
| OATP4A1 | Antibiotics (e.g., benzylpenicillin); estradiol-17β-glucuronide; estrone-3-sulfate; T4; T3; reverse T3; PGE2; taurocholate; metabolite of unoprostone | Tamai et al., 2000; Fujiwara et al., 2001; Gao et al., 2005 |
| OATP4C1 | Antifolates (e.g., methotrexate); cardiac glycosides (e.g., digoxin, ouabain); cAMP; estrone-3-sulfate; sitagliptin; T4; T3 | Mikkaichi et al., 2004; Chu et al., 2007; Yamaguchi et al., 2010 |
PGE, prostaglandin.
A. OATP1 Family
The best-characterized OATPs belong to family 1. In humans, four OATP1 family members have been identified and are designated OATP1A2, OATP1B1, OATP1B3, and OATP1C1. A significant amount of gene duplication and divergence has occurred within this family, especially in rodents (Roth et al., 2012). These genetic differences have complicated direct comparisons between human (OATP) and rodent (Oatp) studies. OATP1A2 has five rodent orthologs: Oatp1a1, Oatp1a3 (in rats only), Oatp1a4, Oatp1a5, and Oatp1a6 (Roth et al., 2012). OATP1B1 and OATP1B3 have a single rodent ortholog that is designated Oatp1b2 (Roth et al., 2012). OATP1C1 has a single ortholog that has been identified in rodents and is designated Oatp1c1.
In the OATP1 family, expression of OATP1A2 mRNA is highest in the brain, followed by the kidney, liver, lung, testis, and placenta (Kullak-Ublick et al., 1995; Steckelbroeck et al., 2004; Obaidat et al., 2012). Recently, OATP1A2 protein expression was reported in MCF10A human breast epithelial cells (Banerjee et al., 2012). OATP1B1 and OATP1B3 have been detected in the liver both at the mRNA and at the protein level (Abe et al., 1999, 2001; Hsiang et al., 1999; Konig et al., 2000a,b). Although OATP1B3 mRNA expression has been detected in the retina by reverse-transcription polymerase chain reaction analysis, its protein expression has yet to be confirmed in this tissue (Roth et al., 2012). OATP1B1 and OATP1B3 mRNA have been detected in vitro in MCF10A breast epithelial cells (Banerjee et al., 2012). Expression of OATP1C1 mRNA is highest in brain and testis (Obaidat et al., 2012). Because OATP1C1 exhibits high affinity for T4 and reverse T3 (in the nanomolar range), this transporter is thought to be critical for blood-to-tissue delivery of thyroid hormones (Pizzagalli et al., 2002; Hagenbuch, 2007; Heuer and Visser, 2009).
B. OATP2 Family
The OATP2 family contains two members (i.e., OATP2A1 and OATP2B1). Both of these human OATPs possess a single ortholog in rodents, which are designated Oatp2a1 and Oatp2b1, respectively. OATP2A1, which is also known as the prostaglandin transporter, is ubiquitously expressed. In support of this hypothesis, OATP2A1 mRNA has been detected in several tissues including brain, colon, heart, liver, kidney, ovary, lung, pancreas, prostate, skeletal muscle, spleen, small intestine, and eye (Schuster, 2002; Kraft et al., 2010; Obaidat et al., 2012). In contrast, OATP2B1 is predominantly expressed in the liver (Tamai et al., 2000; Kullak-Ublick et al., 2001) but also in intestine, brain, heart, mammary tissue, and placenta (St. Pierre et al., 2002; Kobayashi et al., 2003; Pizzagalli et al., 2003; Bronger et al., 2005; Grube et al., 2006b). Rodent Oatp2b1 has also been reported in ependymal cells lining cerebral ventricles (Roberts et al., 2008).
C. OATP3 Family
The OATP3 family consists of a single, highly conserved member (OATP3A1) with a single rodent ortholog (Oatp3a1). OATP3A1 is widely expressed at the mRNA level in several tissues including brain, testis, heart, lung, spleen, peripheral blood leukocytes, breast epithelial cells, and thyroid gland (Adachi et al., 2003; Huber et al., 2007; Banerjee et al., 2012; Obaidat et al., 2012). Recently, OATP3A1 protein expression was demonstrated in epithelial cells of the lactiferous ducts in physiologic breast tissue (Kindla et al., 2011) and in MCF10A cells (Banerjee et al., 2012). Interestingly, rat Oatp3a1 has been detected at the rat nasal mucosa, a drug delivery interface that is particularly intriguing (Genter et al., 2010). In humans, two different OATP3A1 splice variants have been reported (Huber et al., 2007). These variants, designated OATP3A1_v1 and OATP3A1_v2, show some differences with respect to substrate profile (Table 2).
D. OATP4 Family
The OATP4 family consists of two members (OATP4A1, OATP4C1), each with a single rodent ortholog (Oatp4a1, Oatp4c1). OATP4A1 is ubiquitously expressed, with highest mRNA levels detected in the heart and placenta, followed by lung, liver, skeletal muscle, kidney, breast epithelium, and pancreas (Tamai et al., 2000; Fujiwara et al., 2001; Banerjee et al., 2012). OATP4A1 protein expression has only been demonstrated at the apical membrane of syncytiotrophoblasts in the placenta (Sato et al., 2003) and in MCF10A human breast epithelial cells (Banerjee et al., 2012). The other OATP4 family member, OATP4C1, is expressed only in the kidney and thus is considered to be a renal-specific OATP (Mikkaichi et al., 2004).
E. OATP5/OATP6 Family
There is currently very little known about OATP5A1 and OATP6A1. Expression of OATP5A1 protein was recently reported at the plasma membrane of epithelial cells in lactiferous ducts isolated from normal breast tissue (Kindla et al., 2011). OATP6A1 mRNA expression has been detected in the testes, spleen, brain, and placenta (Suzuki et al., 2003; Roth et al., 2012). Although a rodent ortholog for OATP5A1 has not been detected, three rodent orthologs (Oatp6b1, Oatp6c1, Oatp6d1) have been reported for OATP6A1 (Roth et al., 2012).
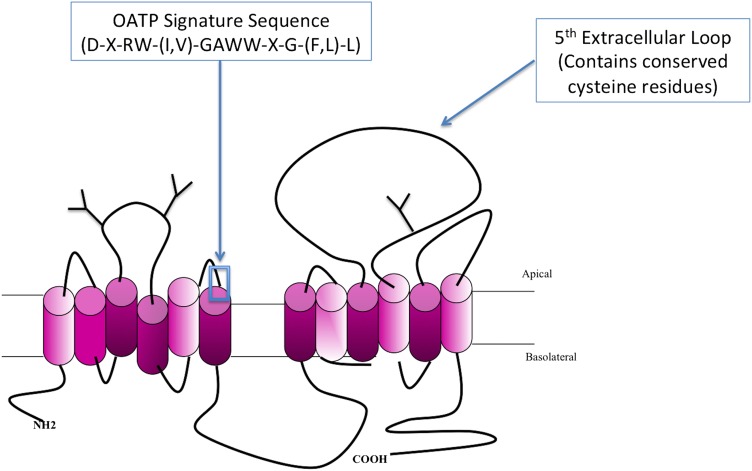
Almost all human OATPs range in size from 643 to 724 amino acids; the exception is OATP5A1, which contains 848 amino acids (Roth et al., 2012). In terms of protein structure, OATPs are integral proteins that contain 12 transmembrane helices with the amino and carboxyl termini oriented toward the cytoplasm (Wang et al., 2008). All OATP isoforms possess a conserved amino acid sequence [i.e., D-X-RW-(I,V)-GAWW-X-G-(F,L)-L], known as the OATP superfamily signature, located at the border between extracellular loop 3 and transmembrane domain 6 (Hagenbuch and Meier, 2003). Predicted and/or confirmed N-glycosylation sites are found in extracellular loops 2 and 5, and many of these sites are conserved between family members (Fig. 1). The large fifth extracellular loop, located between transmembrane domains 9 and 10, contains conserved cysteines that are involved in disulfide bond formation (Hanggi et al., 2006). In the case of OATP2B1, disulfide bond formation is required for cell-surface expression of the transporter (Hanggi et al., 2006). Similar to most other mammalian transport proteins, a crystal structure has not been obtained for any OATP/Oatp superfamily member. Therefore, putative three-dimensional models of OATPs have been constructed using homology modeling, a process that has provided great insight into the structure and function of the OATP substrate-binding pore (Meier-Abt et al., 2005; Gui and Hagenbuch, 2008, Glaeser et al., 2010; Roth et al., 2012). Based on these models, several conserved positively charged amino acids that line the substrate pore were identified as critical determinants of OATP functional expression. Amino acids Arg57, Lys361, and Arg580 in OATP1B1 (Weaver and Hagenbuch, 2010) and Lys41, Lys361, and Arg580 in OATP1B3 (Glaeser et al., 2010; Mandery et al., 2011) were shown to be absolutely required for drug transport activity. Additional experiments using chimeras between OATP1B1 and OATP1B3 identified transmembrane domains 8 and 9 for OATP1B1 (Miyagawa et al., 2009) and transmembrane domain 10 for both OATP1B1 and OATP1B3 (Gui and Hagenbuch, 2008, 2009) to be essential for both transporter expression and substrate transport.
Fig. 1.
Basic membrane topology of a prototypical OATP/Oatp transporter. OATP/Oatp family members are predicted to follow a 12-transmembrane domain topology where the OATP superfamily signature sequence is depicted at the junction between the third extracellular loop and transmembrane domain 6. Conserved cysteine residues are localized to the fifth extracellular loop. Additionally, three predicted glycosylation sites (Y) are demarcated on extracellular loops 2 and 5.
To date, the energetics of OATP/Oatp-mediated transport is poorly understood. It is generally believed that OATP/Oatp family members transport drugs in a bidirectional manner dictated by the solute gradient across the plasma membrane. That is, accumulation of an OATP/Oatp substrate drug in the cytoplasm (i.e., influx) reaches a critical threshold and induces a change in direction of the transmembrane concentration gradient, leading to solute efflux (Li et al., 2000; Ronaldson et al., 2011). When considering this possible mechanism of bidirectional flux, it is critical to appreciate that many OATP/Oatp substrates are organic anions that are predominantly charged at physiologic pH. This suggests that the chemistry of such compounds may act to hinder cytoplasmic substrate accumulation and that OATP/Oatp family members may prefer to efflux organic anions than to mediate their cellular uptake. Evidence for pH dependence of OATP/Oatp-mediated drug transport comes from extracellular acidification experiments. Several studies have shown that OATP2B1 transport is significantly increased at acidic extracellular pH (Nozawa et al., 2004; Sai et al., 2006; Varma et al., 2011). For example, studies in human small intestine epithelial cells showed an increase in Vmax for OATP2B1-mediated transport of estrone-3-sulfate when extracellular pH was decreased from 7.4 to 5.0 (Nozawa et al., 2004). In the same in vitro system, OATP2B1-mediated uptake of pravastatin was higher at pH 5.5 than at pH 7.4 (Kobayashi et al., 2003). In terms of pharmacokinetics, such a process could enhance oral bioavailability of OATP2B1 substrate drugs (e.g., atorvastatin, benzylpenicillin, fexofenadine, pravastatin, rosuvastatin) because this transporter is well expressed in the small intestine. Current evidence suggests that the effect of pH on OATP2B1 transport is substrate-dependent and can be caused by increased drug affinity and increased turnover rate (Nozawa et al., 2004; Leuthold et al., 2009). For example, substrate affinity can be increased by protonation of a conserved histidine residue at the extracellular side of transmembrane domain 3 (Leuthold et al., 2009). Similarly, studies using Xenopus laevis oocytes, Caco-2 cells, and Chinese hamster ovary cells showed that OATP1A2, OATP1B1, and OATP1B3 transport function is dependent upon an electrogenic gradient and that these transporters are strongly affected by fluctuations in external pH (Kato et al., 2010; Martinez-Becerra et al., 2011). However, pH dependence of OATP1B1- and OATP1B3-mediated transport remains controversial since Mahagita et al. (2007) showed that estrone-3-sulfate transport via either OATP1B1 or OATP1B3 was unaffected by changes in extracellular pH. Similarly, bile acid transport mediated by rat Oatp1a1 was insensitive to proton gradient changes (Marin et al., 2003).
The energetics of OATP/Oatp-mediated transport is even more confounding when the same OATP/Oatp is expressed on both sides of a tissue barrier. This is a critical consideration for drug delivery to the brain mediated by Oatp1a4, which is known to be localized at both the luminal and abluminal plasma membrane of the brain microvascular endothelium (Gao et al., 1999). In this situation, it might be expected that a drug can enter the endothelial cell via luminal Oatp1a4 and then exit via abluminal Oatp1a4 with a net effect similar to facilitated diffusion. Therefore, the rate of transendothelial transport will depend upon the relative expression of Oatp1a4 at the luminal and abluminal plasma membrane of the BBB. Relative expression levels as a determinant of transendothelial substrate transport have been demonstrated at the BBB for other transport proteins such as Glut-1 (Simpson et al., 2007).
The majority of OATP/Oatp transport substrates are organic anions, compounds that cannot easily accumulate in cells without input of metabolic energy. That is, if OATP/Oatp family members functioned as facilitative transporters, substrate equilibration and not substrate accumulation would be observed. The presence of intracellular and/or extracellular substrate drug molecules would dramatically alter the transmembrane concentration gradient and, therefore, the kinetics of transport. Such a process would promote bidirectional fluxes that are incapable of favoring blood-to-tissue drug delivery. As OATP/Oatp family members are known to function primarily as influx transporters, much emphasis has been placed on identifying and characterizing driving forces responsible for enabling OATPs/Oatps to mediate net substrate transport in a single direction across a tissue barrier. It is well established that OATP-mediated transport is both ATP-independent and sodium-independent. Similar to other SLC transporters, it is believed that OATP-mediated transport is governed by an electrochemical gradient utilizing an inorganic or organic solute as a driving force. Previous studies have suggested that the mechanism of OATP/Oatp transport may involve substrate exchange with intracellular substances such as intracellular bicarbonate (Satlin et al., 1997; Leuthold et al., 2009), GSH (Li et al., 1998; Franco and Cidlowski, 2006), or GSH conjugates (Li et al., 2000). The possible utilization of an outwardly directed GSH gradient as a driving force for OATP-mediated transport has profound implications for delivery of drugs. Reduced cellular levels of GSH are associated with oxidative stress (Price et al., 2005; Schild and Reiser, 2005; Ronaldson and Bendayan, 2008), a known component of both pain and cerebral hypoxia (Lochhead et al., 2010, 2012; Ronaldson and Davis, 2012). In fact, upregulation of Mrp transporters may contribute to cellular depletion of GSH, as this endogenous antioxidant is a known Mrp transport substrate (Hirrlinger and Dringen, 2005; Minich et al., 2006; Ronaldson and Bendayan, 2008). Therefore, increased functional expression of Mrp isoforms may lead to enhanced cellular GSH efflux and subsequent impairment of OATP influx transport. The use of GSH as a transport driving force is not a property of all OATP family members. Using Xenopus oocytes expressing either OATP1B1 or OATP1B3, Mahagita et al. (2007) demonstrated that OATP-mediated transport of taurocholate and estrone-3-sulfate was not affected by exogenous GSH, indicating that GSH is neither a substrate nor an activator of OATP1B1/OATP1B3-mediated transport activity. Indeed, a thorough understanding of OATP transport activity requires characterization of the interplay between Mrp-mediated GSH export and the ability of OATP family members to act as facilitators of drug delivery.
IV. OATP/Oatp Localization and Functional Expression in the Central Nervous System
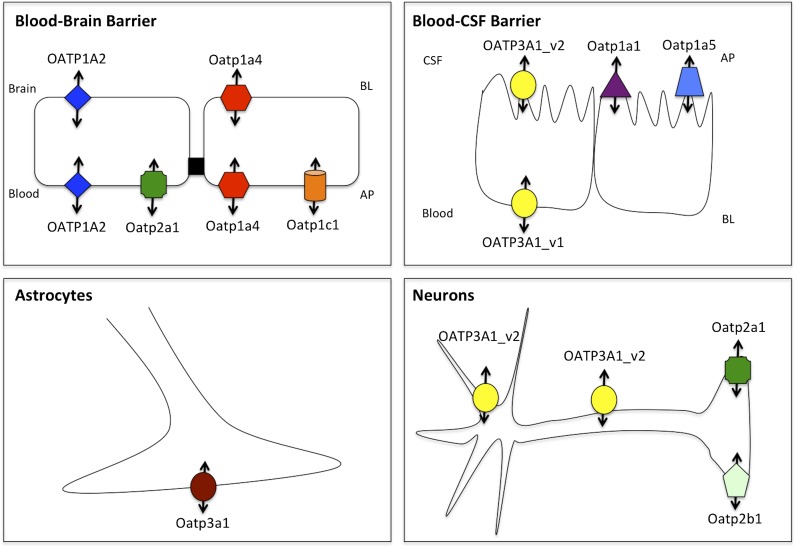
For many drugs that are efficacious in the treatment of pain and/or cerebral hypoxia, uptake into the brain and distribution throughout the CNS may be governed by OATP isoforms that are endogenously and selectively expressed at brain barriers and in brain parenchyma cellular compartments (i.e., glial cells, neurons) (Table 3). To target OATP family members for optimization of CNS drug delivery, it is essential to understand localization and functional expression of these transporters in the brain. Below, we summarize current knowledge on OATP localization and functional expression in the brain, with a particular emphasis on how these transporters can determine delivery and distribution of therapeutic agents. Localization of OATP isoforms at brain barrier sites and in glial cells is depicted in Fig. 2.
TABLE 3.
CNS expression and transport substrates for OATP/Oatp isoforms
| Human OATP Isoform | CNS Expressiona | Rodent Ortholog | CNS Expressiona | Potential Substrate Drugsb |
|---|---|---|---|---|
| OATP1A2 | BBB (ap, bl), possibly glial (detected in glioma) | Oatp1a1 | Choroid plexus (ap) | HMG CoA reductase inhibitors (e.g., atorvastatin, cerivastatin, fluvastatin, pitavastatin, pravastatin, rosuvastatin, simvastatin acid); opioid analgesic peptides (e.g., DPDPE) |
| Oatp1a4 | BBB (ap, bl), choroid plexus | |||
| Oatp1a5 | Choroid plexus (ap), neurons | |||
| Oatp1a6 | Choroid plexus | |||
| OATP1C1 | Detected in brain but localization not confirmed; possibly glial (detected in glioma) | Oatp1c1 | BBB (ap) | Substrate profile restricted to thyroid hormones and conjugated sterols |
| OATP2A1 | Detected in brain but localization not confirmed | Oatp2a1 | BBB (ap), neurons | Substrate profile restricted to prostaglandins |
| OATP2B1 | Detected in brain but localization not confirmed; possibly glial (detected in glioma) | Oatp2b1 | Neurons | HMG CoA reductase inhibitors (e.g., atorvastatin, fluvastatin, pravastatin, pitavastatin, rosuvastatin) |
| OATP3A1_v1, OATP3A2_v2 | Choroid plexus (bl), choroid plexus (ap), neurons (cell body and axon) | Oatp3a1 | Astrocytes | Endogenous opioids (e.g., deltorphin II) |
| OATP4A1 | Detected in brain but localization not confirmed; possibly glial (detected in glioma) | Oatp4a1 | Choroid plexus | Substrate profile restricted to benzylpenicillin, thyroid hormones, prostaglandins, and estrogen metabolites |
| OATP6A1 | Detected in brain but localization not confirmed | Rodent orthologs not detected in CNS tissue | N.A. | Transport activity not yet assessed |
N.A., not applicable.
Specific localization, if known, is demarcated as apical (ap) or basolateral (bl).
Drugs listed are only those with known efficacy in treatment of pain or cerebral hypoxia.
Fig. 2.
Localization of OATP/Oatp transporters at the brain barriers and in brain parenchyma cellular compartments (i.e., astrocytes, neurons). AP, apical; BL, basolateral.
A. Blood-Brain Barrier
The BBB constitutes a remarkable physical and biochemical barrier between the brain and systemic circulation. Structurally, the BBB is composed of a monolayer of nonfenestrated microvessel endothelial cells surrounded by pericytes and perivascular astrocytes. Brain microvessel endothelial cells are joined by tight junction protein complexes, which are maintained by trophic factors released from adjacent astrocytes (Janzer and Raff, 1987; Abbott, 2005). BBB tight junctions are formed by junction adhesion molecules, occludin, and claudins (i.e., claudin-1, -3, and -5), transmembrane proteins that are linked to the cytoskeleton through interactions with accessory proteins [i.e., zonula occludens (ZO)-1, -2, and -3] (Hawkins and Davis, 2005; Ronaldson and Davis, 2011, 2012). ZO proteins act as a scaffold for multiple intracellular signaling pathways and are involved in regulation of tight junction function (Haskins et al., 1998). Additionally, other protein constituents (i.e., cingulin, AF6, 7H6, EMP-1) have been localized to the tight junction, but their exact roles have yet to be elucidated. Under physiologic conditions, these tight junctions impart a high transendothelial electrical resistance of 1500–2000 Ωcm2, as compared with 3–33 Ωcm2 in other vascular tissues (Butt et al., 1990). The net result of this high transendothelial electrical resistance is very low paracellular permeability of many drugs to the brain.
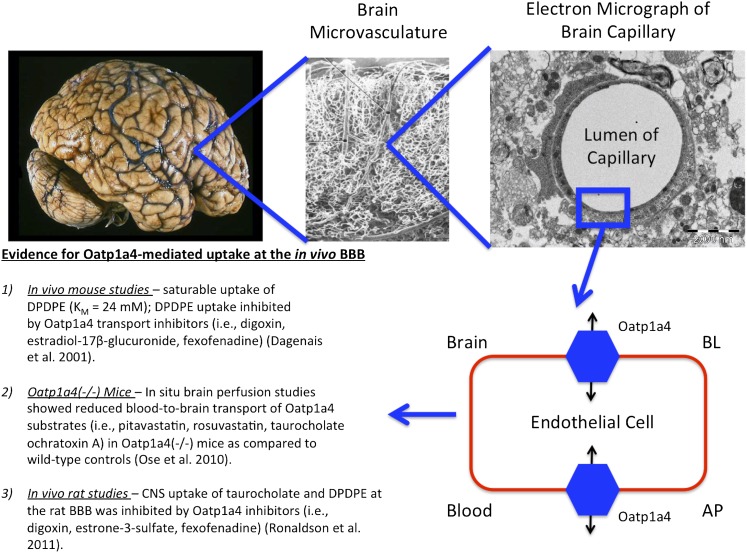
Targeting endogenous uptake transporters at the BBB endothelium provides an exceptional opportunity for efficient and effective delivery of drugs to the CNS. Several lines of evidence indicate that OATPs/Oatps are localized and functionally expressed at the mammalian BBB. In rodent brain, expression of Oatp1a4 and Oatp1c1 has been reported in capillary-enriched fractions, capillary endothelial cells, and whole-brain microvessels (Gao et al., 1999; Sugiyama et al., 2003; Taogoshi et al., 2005; Westholm et al., 2009a,b; Ronaldson et al., 2011). Immunofluorescence staining of rat brain microvessels provided further evidence for localization of Oatp1a4 at the luminal membrane of the BBB (Roberts et al., 2008). Using immunoblot analysis and immunofluorescence staining, Kis et al. (2006) detected expression of Oatp2a1 along the luminal membrane in primary cultures of rat cerebral endothelial cells. Oatp1c1 primarily transports thyroxine and conjugated sterols at the BBB (Westholm et al., 2009a,b) while Oatp2a1 is a critical mediator of CNS prostaglandin homeostasis (Kis et al., 2006). It has been proposed that Oatp1a4, a rodent ortholog of OATP1A2, is the primary drug-transporting Oatp isoform expressed at the rat BBB (Fig. 3). Oatp1a4 has been shown to mediate blood-to-brain transport of DPDPE (Gao et al., 2000; Ronaldson et al., 2011) and pravastatin (Tokui et al., 1999). More recently, studies in Oatp1a4(−/−) mice demonstrated reduced blood-to-brain transport of pitavastatin and rosuvastatin as compared with wild-type controls, which suggests that Oatp1a4 is involved in statin transport across the BBB (Ose et al., 2010).
Fig. 3.
Evidence for blood-to-brain drug transport mediated by Oatp1a4 at the BBB. Previous in vivo studies have shown that CNS uptake of drugs such as opioid peptide analgesics (e.g., DPDPE) and HMG-CoA reductase inhibitors (e.g., pitavastatin, rosuvastatin) is determined by functional expression of Oatp1a4 at the luminal and abluminal plasma membrane of the brain microvascular endothelium. AP, apical; BL, basolateral.
Although it is well established that Oatp family members are localized and functionally active at the rodent BBB, studies on OATP expression at the human BBB remain inconclusive. Immunofluorescence staining of human brain frontal cortex demonstrated OATP1A2 localization at both the apical and basolateral sides of the microvascular endothelium (Gao et al., 2000). In contrast, a recent study examined transporter expression in human brain microvessels using a quantitative targeted absolute proteomics approach and observed that expression of OATP-A (i.e., OATP1A2), OATP-B (i.e., OATP2B1), OATP-C (i.e., OATP1B1), OATP-D (i.e., OATP3A1), OATP-E (i.e., OATP4A1), OATP-F (i.e., OATP1C1), OATP-H (i.e., OATP4C1), OATP-I (i.e., OATP6A1), and OATP-J (i.e., OATP5A1) were all below the limit of detection of the assay (Uchida et al., 2011). It is critical to emphasize that all brain tissue obtained by Uchida and colleagues was collected from subjects who died from peripheral diseases, including various cancers, chronic obstructive pulmonary disease, and Guillain-Barré syndrome (Uchida et al., 2011). It is possible that mediators released during the course of these diseases (e.g., inflammatory cytokines, chemokines, ROS) may have affected BBB transporter expression in these subjects. As demonstrated by our group, presence of a pathologic stressor in the periphery can have dramatic effects on BBB transporter expression and, subsequently, CNS drug delivery (Hau et al., 2004; Seelbach et al., 2007; Ronaldson et al., 2011; Lochhead et al., 2012; McCaffrey et al., 2012). Therefore, these proteomic data cannot be interpreted to suggest that OATP family members are absent from the human BBB or that these transporters do not represent viable targets for optimization of CNS drug delivery. Rather, mechanisms of OATP regulation in both health and disease need to be studied in more detail to fully comprehend OATP localization and expression at the human BBB. Additionally, the work of Uchida et al. (2011) underscores a critical need for functional studies using in vivo imaging technologies (e.g., positron emission tomography, single photon emission computed tomography) to determine involvement of OATP isoforms in CNS drug delivery at the human BBB. Nonetheless, OATP-mediated transport at the human BBB is a particularly intriguing concept, particularly with respect to treatment of pain and cerebral hypoxia. For example, studies in X. laevis oocytes have shown OATP1A2-mediated uptake of peptides such as DPDPE but also deltorphin II (Gao et al., 2000). Transport of these substrates was inhibited by the opiate antagonists naloxone and naltrindole as well as the μ-opioid receptor agonist Tyr-d-Ala-Gly-N-methyl-Phe-glycinol and the endogenous peptide Leu-enkephalin (Gao et al., 2000). More recent studies in X. laevis oocytes showed increased pravastatin and pitavastatin uptake in OATP1A2 cDNA-injected oocytes as compared with water-injected controls (Shirasaka et al., 2010, 2011).
B. Blood-Cerebrospinal Fluid Barrier
The BCSF barrier is formed by the choroid plexus, the major interface between the systemic circulation and the CSF. The BCSF barrier is located at the outer epithelial surface of the choroid plexus, a leaf-like, highly vascular organ that protrudes into all four cerebral ventricles. It comprises fenestrated capillaries that are surrounded by a monolayer of epithelial cells joined together by tight junctions (Ghersi-Egea and Strazielle, 2001). These tight junctions form the structural basis of the BCSF barrier and seal together adjacent polarized epithelial cells (also known as ependymal cells). Thus, once a solute has crossed the capillary wall, it must also permeate these ependymal cells before accessing the CSF.
The primary function of the choroid plexus is to continuously produce CSF and to maintain its composition. The total volume of CSF (140 ml) is replaced approximately four to five times daily (Davson et al., 1970). Continuous flow of CSF through the ventricular system into the subarachnoid space and exiting into the venous system provides a “sink” that reduces the steady-state concentration of a drug penetrating into the brain and CSF (Saunders et al., 1999). The sink effect is greater for large-molecular-weight and hydrophilic compounds. The CSF also comprises approximately 0.3% of plasma proteins, totaling 15 to 40 mg/ml, depending upon the sampling site (Felgenhauer, 1974). This is in contrast to the extracellular space of the normal adult brain, which contains no detectable plasma proteins (Azzi et al., 1990).
Similar to the BBB, the choroid plexus displays polarized expression of various receptors, ion channels, and transport systems that regulate the CSF composition via secretion and reabsorption (Spector and Johanson, 1989). Additionally, several proteins involved in blood-to-CSF transport of drugs, including OATP/Oatp isoforms, have been detected at the choroid plexus epithelium. Gene and protein expression of Oatp1a1 has been observed in neonatal rat choroid plexus (Angeletti et al., 1998). Quantitative gene analysis of rat choroid plexus tissue demonstrated that Oatp1a5 mRNA was expressed at the BCSF barrier at levels much greater than observed in liver (Choudhuri et al., 2003). This same study also reported low but detectable mRNA expression of Oatp1a1, Oatp1a6, and Oatp4a1 at the rat choroid plexus (Choudhuri et al., 2003). Additionally, Oatp1c1 is highly enriched at the choroid plexus in rodent brain and is directly involved in blood-to-CSF thyroxine transport (Sugiyama et al., 2003; Mayerl et al., 2012). Immunohistochemical analysis demonstrated localization of Oatp1a5 at the brush border (i.e., apical) membrane in TR-CSFB cells, a conditionally immortalized rat choroid plexus epithelial cell line (Ohtsuki et al., 2003). Nonradioactive in situ hybridization histochemistry and immunofluorescence microscopy studies provided evidence for apical localization of Oatp1a1 in rat choroid plexus epithelial cells (Gao et al., 1999). In contrast, this same study showed basolateral localization of Oatp1a4 (Gao et al., 1999). Expression of multiple Oatp isoforms at the rodent choroid plexus suggests multiple opportunities for targeted drug delivery to the CSF and the CNS. For example, choroid plexus expression of Oatp1a4 implies existence of a transporter target that can be exploited for blood-to-CSF delivery of opioid analgesic peptides (e.g., DPDPE) (Dagenais et al., 2001). Using in situ brain perfusion, Mason et al. (2010) provided evidence for Oatp1a4-mediated transport of cortisol and corticosterone at the rat choroid plexus. Localization of OATP3A1_v1 has been reported at the basolateral surface of choroid plexus epithelial cells, while OATP3A1_v2 has been observed at the apical membrane of the BCSF barrier (Huber et al., 2007). Other OATP isoforms, such as OATP1A2 (Gao et al., 2000), OATP1C1 (Pizzagalli et al., 2002), OATP2B1 (Kullak-Ublick et al., 2001), and OATP4A1 (Fujiwara et al., 2001), have been detected in human brain tissue; however, their localization and functional expression at the BCSF barrier has yet to be determined.
C. Brain Parenchyma
1. Astrocytes
Astrocytes are the most abundant cell type in the brain. Previous studies have shown that astrocytes, localized between neuronal cell bodies and endothelial cells and ensheathing more than 99% of cerebral capillaries with their end-feet (Hawkins and Davis, 2005; Vangilder et al., 2011; Ronaldson and Davis, 2012), are critical in the development and/or maintenance of BBB characteristics (Janzer and Raff, 1987; Tao-Cheng et al., 1987; Neuhaus et al., 1991; Hayashi et al., 1997; Willis et al., 2004a,b). For example, studies using human umbilical vein endothelial cells showed that these cells could develop BBB properties when cocultured with astrocytes, which implies that astrocytes secrete trophic factors critical to maintenance of the BBB phenotype (Hayashi et al., 1997). Astrocytes may be involved in transient regulation of cerebral microvascular permeability (Ballabh et al., 2004), in particular via dynamic Ca2+ signaling between astrocytes and the endothelium via gap junctions and purinergic transmission (Goldberg et al., 2010; Pelligrino et al., 2011). Recent evidence also suggests that astrocytes may play a critical role in regulating water and ion exchange across the brain microvascular endothelium (Abbott et al., 2006; Mathiisen et al., 2010). Astrocytes possess two high-affinity transporters for uptake of glutamate, termed excitatory amino acid transporters 1 and 2 [EAAT1 (GLAST) and EAAT2 (GLT-1), respectively] (Bak et al., 2006). These transporters are critical in removal of excess glutamate from the synapse and contribute to maintenance of excitatory neurotransmitter concentrations in the brain. Additionally, astrocytes are known to express volume-regulated anion channels. These channels are involved in Ca2+-independent release of anionic amino acids (e.g., glutamate, aspartate, taurine) during conditions that cause astrocyte swelling, such as cerebral hypoxia (Kimelberg et al., 1990).
To date, there is very little information regarding localization and/or functional expression of OATP/Oatp family members in astrocytes. Gao et al. (2000) observed that Oatp1a4 immunoreactivity did not colocalize with glial fibrillary acidic protein, which implies that astrocytes do not express Oatp1a4. Immunofluorescence staining of rat brain tissue confirmed expression of Oatp3a1 in rat astrocytes, suggesting a mechanism for prostaglandin and thyroxine transport in glia (Huber et al., 2007). However, real-time polymerase chain reaction analysis of human glioma specimens from six different patients revealed mRNA expression for OATP1A2, OATP1C1, OATP2B1, and OATP4A1 (Bronger et al., 2005). The expression of these transporters in astrocytes suggests that these glial cells may act as critical determinants of CNS drug distribution. That is, the balance of transporters in astrocytes may either sequester drugs within the astrocyte cytoplasm, thereby preventing these compounds from reaching their site of action in the brain, or concentrate drugs in brain extracellular fluid. Pharmacological agents within brain extracellular space can be effluxed by active transport mechanisms at brain barrier sites or via “sink” effects of CSF (Ronaldson et al., 2008). For more detailed information on other transport mechanisms in astrocytes, readers are referred to a recent review (Ronaldson et al., 2008).
2. Microglia
The Spanish neuroanatomist del Rio-Hortega (1932) first described microglia, a cell type from the monocyte lineage that represents approximately 20% of the total glial cell population within the CNS (Raivich et al., 1999). Microglia are ubiquitously distributed within the CNS, with the basal ganglia and cerebellum possessing considerably greater numbers than the cerebral cortex (Dickson et al., 1991). Under normal physiologic conditions, microglia exist in a quiescent state lacking endocytotic and phagocytotic activity. These microglia possess a ramified morphology characterized by a small (5- to 10-μm) cell body and many radial cell processes extending from the cell body. Ramified microglia are thought to contribute to maintenance of homeostasis by participating in extracellular fluid cleansing and neurotransmitter deactivation (Ronaldson et al., 2008). During disease or trauma, microglia may become activated, and the degree of this activation is directly correlated to the type and severity of brain injury (Speth et al., 2005). Activated microglia are identified morphologically by their larger cell body and relatively short cytoplasmic processes. Biochemically, activated microglia are identified by upregulation of cell surface receptors such as CD14 and TLRs (Bsibsi et al., 2002; Rivest, 2003). The degree of microglial activation appears to be correlated to the type and severity of brain injury (Raivich et al., 1999; Speth et al., 2005). During an immune response, activated microglia may be further converted to a reactive state, which is characterized by a spheroid or rod-like morphology and presence of phagocytotic activity. Microglia activation and proliferation has been implicated in the development of various CNS pathologic states, including peripheral inflammatory pain (Roberts et al., 2009) as well as ischemic stroke and cerebral hypoxia (Deierborg et al., 2010; Faustino et al., 2011; Wei et al., 2011). When activated, microglia produce high levels of neurotoxic mediators such as nitric oxide and peroxide, as well as inflammatory cytokines (e.g., tumor necrosis factor-α), proteases, and complement components (Aloisi, 2001; Speth et al., 2005). Excessive production of these substances may further lead to cell injury in the CNS, characterized by astrocyte activation, further microglia activation, and neuronal cell death.
Although previous studies have shown that microglia express membrane proteins involved in drug transport (Lee et al., 2001; Dallas et al., 2003, 2004; Ronaldson et al., 2004), there are no published data outlining expression of OATP/Oatp isoforms in microglia. This is clearly an issue that needs to be resolved to accurately assess the ability of microglia to contribute to drug permeation and/or distribution in the CNS.
3. Neurons
Neurons form the basic structural and functional component of the CNS. The primary function of neurons is to respond to stimuli by conducting electrical signals along conductive processes (i.e., axons). The conduction of electrical impulses results in the release of neurotransmitters that further regulate (positively and negatively) nearby neuronal responses (Ludwig and Pittman, 2003). This enables the brain to maintain a highly complex communication network. A few studies have reported expression of Oatp1a5 and Oatp2b1 in neurons isolated from rat and mouse brain (Nishio et al., 2000; Feurstein et al., 2010). Neuronal expression of Oatp2b1 is particularly intriguing because many of its substrates (e.g., prostaglandins, leukotriene C4) are involved in inflammatory signaling and regulation, suggesting that this transporter may play a critical role in transporting signals to target cells during brain inflammation (Nishio et al., 2000). Additionally, Oatp1a5 and/or Oatp2b1 may be involved in neurotoxicity due to their ability to transport cytotoxic microcystins (Feurstein et al., 2010). Immunofluroescence staining of human frontal cortical tissue demonstrated expression of OATP3A1_v2 in neurons at both the cell body and the axon (Huber et al., 2007). In vivo studies in mouse brain showed that neurons express high levels of Oatp2a1 (Scafidi et al., 2007).
V. Regulation of OATPs/Oatps during Pain and Cerebral Hypoxia
The ability of a pharmacological agent to cross the BBB endothelium and achieve efficacious concentrations within the CNS is dependent on multiple mechanisms of transport. Such mechanisms include uptake into the brain via an influx transporter and/or extrusion from the CNS mediated by an efflux transporter. For many drugs, it is this discrete balance between influx and efflux that determines whether a pharmacological agent will accumulate within the brain extracellular milieu and be able to elicit a therapeutic effect. The complexity of drug transport biology at the BBB is further underscored by the observation that functional expression of such transport proteins may be dramatically altered by pathophysiological stressors (Hayashi et al., 2005, 2006; van Vliet et al., 2005; Seelbach et al., 2007; Ronaldson et al., 2010, 2011). A thorough understanding of the regulation and functional expression of endogenous BBB transporters in both health and disease is critical for optimization of pharmacotherapy. Furthermore, such information will enable efficient targeting of transporters and/or transporter regulatory mechanisms, thus allowing endogenous BBB transport systems to be specifically controlled for purposes of improving CNS drug delivery.
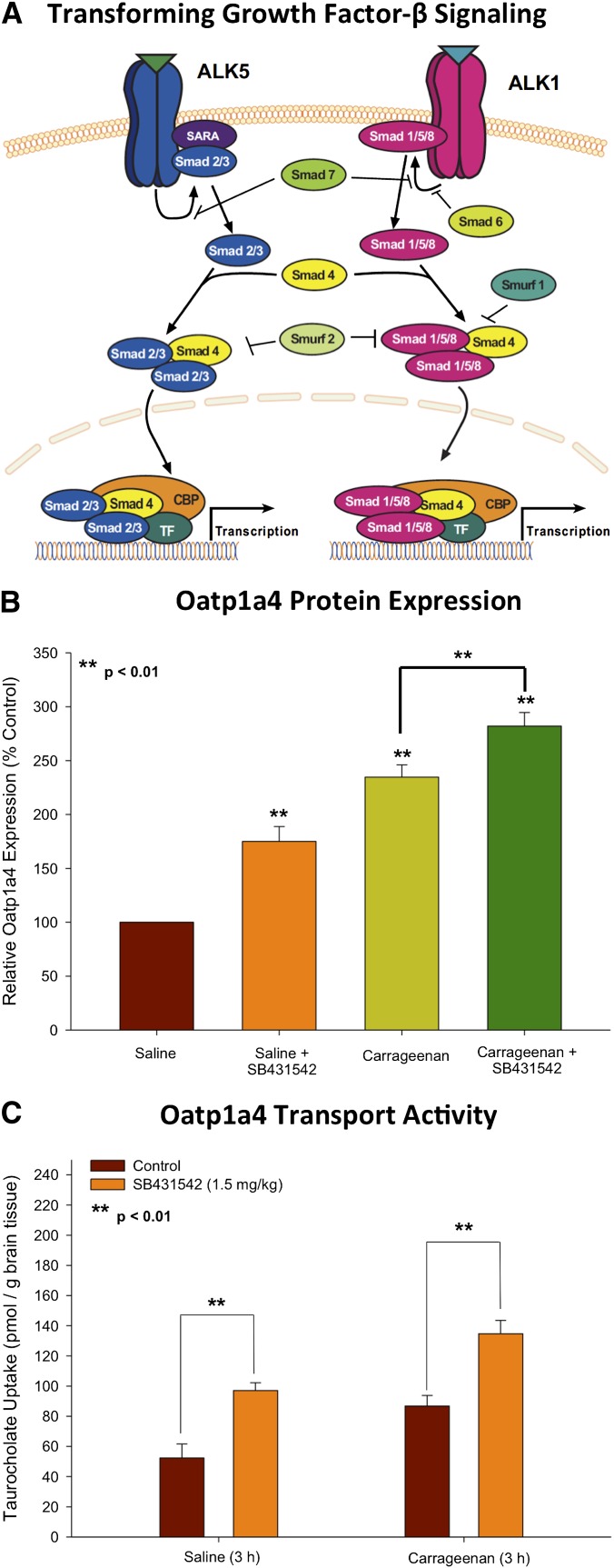
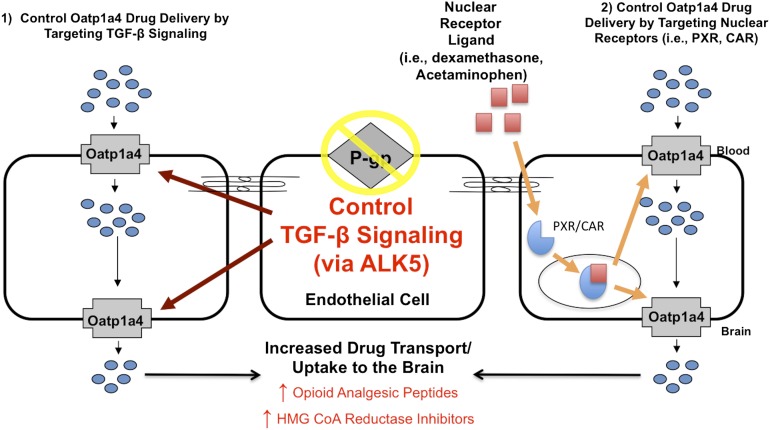
Recently, we reported for the first time increased functional expression of Oatp1a4 at the BBB in rats subjected to a pathologic stressor (i.e., peripheral inflammatory pain) (Ronaldson et al., 2011). Evidence for increased Oatp1a4 transport at the BBB included 1) increased brain accumulation of taurocholate, a selective Oatp substrate (Noe et al., 1997); 2) attenuation of taurocholate uptake by Oatp transport inhibitors (i.e., digoxin, estrone-3-sulfate, fexofenadine); 3) increased KIN for taurocholate in response to pathologic stress, which implies increased blood-to-brain transport; and 4) an increase in taurocholate accumulation within brain interstitial fluid but no change in taurocholate sequestration within the BBB endothelium itself (Ronaldson et al., 2011). To determine whether Oatp1a4 could effectively facilitate CNS drug delivery, we studied BBB transport of the opioid peptide DPDPE. Brain uptake of DPDPE is governed by multiple mechanisms in addition to Oatp1a4-mediated transport, including transcytosis (Egleton and Davis, 1999) and P-gp-mediated efflux (Dagenais et al., 2001). Although we showed increased Oatp1a4 functional expression at the BBB in animals subjected to peripheral inflammatory pain, we did not see any change in blood-to-brain DPDPE transport (Ronaldson et al., 2011). In light of our previous work with P-gp (Seelbach et al., 2007), we proposed that Oatp1a4 influx transport was negated by P-gp efflux. This implies that the relative contribution of Oatp1a4 to overall brain uptake of DPDPE could only be determined in the absence of P-gp-mediated transport activity. When we inhibited P-gp efflux transport using reversin 205, a selective P-gp-inhibitory peptide (Sharom et al., 1999), we observed that the relative contribution of Oatp1a4 to brain uptake of DPDPE increased from 56% in saline controls to 71% in animals subjected to peripheral inflammatory pain (Ronaldson et al., 2011). These data are particularly critical because they showed for the first time that Oatp1a4 can be targeted for delivering drugs such as opioid peptides to the brain.
OATP/Oatp family members are multispecific transporters capable of transporting a vast array of structurally diverse drugs, metabolites, and physiologic substrates. However, a full comprehension of how such transporters can be targeted to promote CNS delivery of therapeutics requires an appreciation that substrates transported by OATP/Oatp family members may also be transport substrates for organic anion transporters (OATs), P-gp, and MRP/Mrp isoforms. At the BBB, OAT3 (Ohtsuki et al., 2005; Miyajima et al., 2011), P-gp (Bendayan et al., 2006; Seelbach et al., 2007; Dauchy et al., 2009; Hawkins et al., 2010), and various MRPs/Mrps (Dallas et al., 2006; Hawkins et al., 2007) are all well expressed and contribute to brain-to-blood (i.e., efflux) substrate transport. In choroid plexus epithelial cells, CSF-to-blood drug transport is mediated by OAT1 and OAT3 at the apical membrane (Sykes et al., 2004; Bahn et al., 2005; Keep and Smith, 2011) and MRP1 at the basolateral membrane (Gazzin et al., 2008). In contrast, P-gp is expressed at the apical membrane of choroid plexus epithelia and functions to retain drugs in the CNS (Rao et al., 1999; Gazzin et al., 2008). MRP4/Mrp4 expression has been detected at both apical and basolateral surfaces of the choroid plexus, which implies that this transporter may act to conserve levels of some organic anions in the CSF while driving brain-to-blood efflux of others (Leggas et al., 2004; Dallas et al., 2006). Many of the drugs presented as OATP/Oatp substrates in Table 2 are also transported by at least one among OAT1, OAT3, P-gp, or MRP/Mrp isoforms. For example, DPDPE is a substrate for P-gp in addition to Oatp1a4 (Dagenais et al., 2004; Ose et al., 2010; Ronaldson et al., 2011). Rosuvastatin is also a substrate for OAT3 (Windass et al., 2007), Bcrp (Huang et al., 2006), and Mrp2 (Abe et al., 2008). Therefore, it is highly possible that drugs that enter the brain microvascular endothelium or choroid plexus epithelium via one class of transporter may exit by another. Understanding how changes in expression of a specific transporter might affect brain uptake of a given drug will depend upon an accurate assessment of all competing transporters.
A. Transforming Growth Factor-β Signaling
To successfully target a transporter system for optimization of CNS drug delivery, it is crucial to determine how a transporter of interest is regulated at the molecular level. This includes identification and characterization of biologic mechanisms that enable peripheral pain and/or cerebral hypoxia to “transmit” signals upstream and alter BBB drug transporters. Of particular interest is the transforming growth factor-β (TGF-β) signaling pathway. TGF-β signaling regulates multiple cellular processes including vascular remodeling (Pepper, 1997). The TGF-βs are a family of pleiotropic cytokines that modulate cellular function by binding to a heterotetrameric complex of type I and type II serine/threonine kinase receptors (Derynck and Zhang, 2003). The type I receptors, also known as activin receptor-like kinases (ALKs), propagate intracellular signals through phosphorylation of specific Smad proteins [i.e., receptor-regulated (R)-Smads] (Fig. 4A). Phosphorylated (R)-Smads form complexes with the common Smad (i.e., Smad4), enabling them to be translocated to the nucleus and regulate transcription of target genes (Derynck and Zhang, 2003).
Fig. 4.
TGF-β signaling modulates Oatp1a4 functional expression at the BBB. (A) The TGF-β signaling pathway. Intracellular signaling molecules associated with TGF-β signaling at the BBB. Signals elicited by the TGF-β pathway involve two cell surface receptors at the brain microvascular endothelium, which are designated ALK1 and ALK5. ALK1 transduces signals via phosphorylation of Smad proteins 1, 5, and 8, while ALK5 signals by phosphorylation of Smad2 and Smad3. Once phosphorylated, these Smad proteins bind to the common Smad (i.e., Smad4), thereby forming a protein complex that can translocate to the nucleus and affect transcription. (B) Effect of SB431542, a selective pharmacological inhibitor of TGF-β/ALK5 signaling, on Oatp1a4 protein expression. Relative levels of Oatp1a4 protein in brain microvessels isolated from rats treated with saline or λ-carrageenan in the presence and absence of SB431542. Results are expressed as mean ± S.D. of three separate experiments. **P < 0.01 vs. control. (C) Uptake of taurocholate into rat brain following 3 hours of λ-carrageenan-induced inflammatory pain in the presence and absence of SB431542. Graph shows the concentration of taurocholate detected in rat brain tissue for the four treatment groups after injection of 3% λ-carrageenan or 0.9% saline into the plantar surface of the right hind paw. SB431542 (1.5 mg/kg) was injected 30 minutes before footpad injection. Results are expressed as mean ± S.D. of six animals per treatment group. **P < 0.01 vs. control. Adapted from Ronaldson et al. (2011).
In the majority of tissues, TGF-β signaling is mediated by ALK5 (Lebrin et al., 2005); however, studies in cultured human endothelial cells (Wu et al., 2006) and in isolated arterial endothelium from ALK1-deficient mice (Seki et al., 2003) have indicated that ALK1 is also involved in vascular TGF-β signaling. TGF-β regulates the endothelial cell activation state through a precise balance between ALK1 and ALK5 signaling processes (Goumans et al., 2002; Wu et al., 2006). Whereas the ALK1 pathway leads to endothelial activation characterized by increased permeability, ALK5-mediated signaling promotes vascular resolution that is demarcated by decreased permeability (Lebrin et al., 2005; Wu et al., 2006). Such effects on vascular permeability may be due to the ability of TGF-β signaling to alter expression of tight junction constituent proteins. For example, claudin-5 expression was increased by pharmacological ALK5 inhibition in embryonic stem cells, suggesting the involvement of TGF-β/ALK5 signaling in the regulation of tight junction constituent proteins (Watabe et al., 2003). Similarly, we demonstrated that ALK5 inhibition using SB431542 increased expression of claudin-3, claudin-5, occludin monomers, and ZO-1 in vivo (Ronaldson et al., 2009). Additionally, studies using human glioblastoma cells cultured with human brain endothelial cells showed that activation of TGF-β-mediated signaling decreased endothelial expression of occludin, claudin-1, and claudin-5 (Ishihara et al., 2008).
Expression of TGF-β1 is dramatically altered both in the CNS and in the periphery in response to pain/inflammation and cerebral hypoxia (Buckwalter and Wyss-Coray, 2004; Ronaldson et al., 2009; Doyle et al., 2010). Recently, our laboratory demonstrated that pharmacological inhibition of TGF-β signaling led to increased microvascular expression (Fig. 4B) and activity (Fig. 4C) of Oatp1a4 at the BBB (Ronaldson et al., 2011). Of particular interest was the observation that this blockade of TGF-β/ALK5 signaling using SB431542 increased Oatp1a4 functional expression in saline-treated control animals (Ronaldson et al., 2011). A crucial consideration in interpretation of our data is the contribution of paracellular diffusion to brain uptake of taurocholate. Our laboratory has previously reported that pharmacological inhibition of TGF-β/ALK5 signaling also increased BBB permeability via the paracellular route for solutes such as sucrose (Ronaldson et al., 2009). The molecular weight of taurocholate (537.7 Da) is greater than that of sucrose (342 Da), suggesting a lesser degree of paracellular diffusion. As our data showed no statistical difference in taurocholate uptake in the presence and absence of Oatp1a4 inhibitors in animals subjected to a pathologic stressor known to open the BBB (i.e., pain/inflammation), we conclude that paracellular diffusion was not a significant factor in our study. This is not to say that paracellular drug transport is not a significant factor in determining blood-to-brain delivery of other drugs in the setting of a pathophysiological response. Therefore, it is critical to correct for paracellular transport in any study on the effect of targeting TGF-β signaling for optimization of CNS drug delivery. Although studies in immortalized mouse brain endothelial cells (MBE4) have shown involvement of ALK5-mediated signaling in P-gp regulation (Dohgu et al., 2004), we are the first to report TGF-β/ALK5 signaling regulation of an endogenous BBB drug uptake transporter. Our work on TGF-β/ALK5 signaling demonstrated that this pathway can regulate permeability at the BBB by increasing functional expression of an influx transporter. Furthermore, these studies highlight the potential of the TGF-β/ALK5 pathway as a pharmacological target that can be used for optimization of drug delivery to the CNS, particularly for treatment of ischemic stroke.
B. Nuclear Receptors
Nuclear receptors are ligand-activated transcription factors activated by a broad spectrum of xenobiotics (Kullak-Ublick and Becker, 2003). Some of these receptors, including pregnane X receptor (PXR) and constitutive androstane receptor (CAR), have been implicated in regulation of OATPs/Oatps (Tirona et al., 2003; Cheng et al., 2005; Urquhart et al., 2007; Svoboda et al., 2011). Expression of both PXR and CAR have been reported in cell culture models of brain microvessel endothelial cells isolated from rat (Bauer et al., 2004; Wang et al., 2010), mouse (Wang et al., 2010), pig (Ott et al., 2009), and human tissue (Dauchy et al., 2009; Chan et al., 2011). The pharmacological impact of PXR- and/or CAR-mediated upregulation of OATP/Oatp isoforms has not yet been demonstrated at brain barriers or in CNS cellular compartments; however, considerable data from studies in cancer cells and in hepatocytes point to the potential of PXR and/or CAR signaling to control OATP/Oatp-mediated drug transport. In fact, such studies provide an excellent starting point for predicting which nuclear receptors can be targeted to increase expression/activity of a specific OATP/Oatp isoform. OATP1A2 expression and activity can be induced in human breast cancer cells treated with the known PXR activator rifampin (Meyer zu Schwabedissen et al., 2008). This same study identified a PXR response element on the human OATP1A2 promoter located approximately 5.7 kb upstream of the transcription start site (Meyer zu Schwabedissen et al., 2008). Using chromatin immunoprecipitation, these researchers also confirmed the specificity of the PXR-OATP1A2 promoter interaction (Meyer zu Schwabedissen et al., 2008), data that confirm the interplay between OATP1A2 and the xenobiotic nuclear receptor PXR. Similar observations were obtained in mouse liver where murine PXR activators (i.e., pregnenolone-16α-carbonitrile, spironolactone) increased hepatic expression of Oatp1a4 mRNA (Cheng et al., 2005). Additionally, Stedman et al. (2005) showed that PXR activation induced expression of Oatp1a4 in mouse liver in vivo. These studies are particularly significant because they indicate that PXR may be a viable molecular target for control of OATP functional expression in other tissues, such as the CNS. Previous studies have also suggested that CAR may be involved in regulation of the OATP/Oatp family of transporters. For example, treatment of mice with known CAR agonists (i.e., phenobarbital, 1,4-bis-[2-(3,5-dichlorpyridyloxy)]benzene) resulted in a significant increase in hepatic expression of Oatp1a4 (Wagner et al., 2005). Taken together, results from these studies suggest that PXR/CAR activation may alter OATP/Oatp functional expression in the CNS and thereby modulate CNS delivery of drugs such as opioid analgesic peptides and HMG-CoA reductase inhibitors. A critical consideration in the targeting of PXR/CAR to induce functional expression of OATP/Oatp transporters is that these nuclear receptors can also upregulate efflux transporters at the BBB such as P-gp (Bauer et al., 2004, 2006; Wang et al., 2010), Mrp2 (Bauer et al., 2008; Wang et al., 2010), and Bcrp (Wang et al., 2010). Induction of these efflux transporters may challenge the ability of PXR and/or CAR ligands to promote OATP/Oatp-mediated drug delivery. Because the role of PXR and/or CAR in the regulation of OATP/Oatp expression in the brain remains poorly characterized, rigorous studies must be undertaken to identify and characterize the nature of the interplay between OATP/Oatp isoforms and xenobiotic nuclear receptors in the CNS.
Future work designed to study the regulation of OATPs/Oatps by PXR and/or CAR can also be directed by current knowledge on drug-transporter interactions involving nuclear receptors. It has been estimated that unwanted activation of nuclear receptors (e.g., PXR, CAR) may account for up to 60% of drug-drug interactions (Urquhart et al., 2007). Although such interactions involving PXR and/or CAR have not been directly studied in the CNS, much of this information may prove to be clinically relevant with respect to the treatment of pain. Patients afflicted by pain are often administered multiple analgesic drugs as part of their therapeutic regimen. Many of these compounds, particularly anti-inflammatory drugs, have been reported to interact with PXR and/or CAR in both in vitro and in vivo model systems. For example, dexamethasone, an anti-inflammatory glucocorticoid and established PXR activator (Matheny et al., 2004), is often used in the management of pain conditions (Stein et al., 1999; Kardash et al., 2008). There is also recent evidence that dexamethasone treatment may protect the brain from the deleterious effects of H/R stress (Feng et al., 2011). Acetaminophen is commonly prescribed for the management of acute or chronic pain and is also a known CAR activator (Zhang et al., 2002). Because OATPs/Oatps are regulated by both PXR and CAR, these data suggest that inclusion of anti-inflammatory drugs (e.g., dexamethasone, acetaminophen) in a pain treatment regimen may activate nuclear receptors and, by extension, potentially alter CNS delivery of opioids. Furthermore, knowledge that such commonly prescribed drugs can activate PXR and/or CAR may indicate that these therapeutics are pharmacological tools that can be exploited to control expression and/or function of endogenous CNS transporters such as OATPs/Oatps.
VI. Conclusions and Future Perspectives
The field of CNS biology, particularly the study of endogenous transport systems, has rapidly advanced over the past 2 decades. Although many studies have focused on biochemical mechanisms that prevent drugs from entering the brain, this paradigm has shifted toward identifying and characterizing BBB targets that can facilitate blood-to-brain drug transport. Now, it is beginning to be appreciated that endogenous CNS transporters such as OATPs/Oatps may be viable targets for optimizing CNS drug delivery. Furthermore, molecular machinery involved in regulating OATPs/Oatps (i.e., TGF-β/ALK5 signaling, nuclear receptor systems) is just now being identified and characterized. These critical discoveries have identified multiple molecular targets that can be exploited for optimization of CNS delivery of therapeutic agents. Perhaps targeting of novel drugs to OATP/Oatp transporters will lead to significant advancements in the treatment of CNS diseases such as pain and cerebral hypoxia. Identification and characterization of intracellular signaling pathways that can control functional expression of uptake transporters provides yet another approach for pharmacological control of transporter systems in an effort to deliver therapeutics to the CNS (Fig. 5). Future work will continue to provide more insight on the interplay of transporters and intracellular signaling pathways in the brain and how these systems can be effectively targeted. Ultimately, data derived from these studies will enable achievement of more precise and more effective drug concentrations within the CNS and improved treatment of pain and cerebral hypoxia.
Fig. 5.
Opportunities for targeting OATP/Oatp isoforms to optimize CNS drug delivery. Results from our recent studies demonstrate that targeting Oatp transporters during pathophysiological stress can modify CNS drug delivery. Oatp1a4 facilitates brain delivery of drugs that may exhibit efficacy in treatment of peripheral inflammatory pain or cerebral hypoxia, such as statins and opioid peptide analgesics. The TGF-β signaling pathway enables control of Oatp isoforms by targeting TGF-β receptors (i.e., ALK5) with small-molecule therapeutics such as SB431542. Additionally, nuclear receptors (e.g., PXR, CAR) offer another potential opportunity to control Oatp-mediated drug delivery by the use of a small-molecule therapeutic such as dexamethasone or acetaminophen. In this scenario, a PXR or CAR ligand binds to the nuclear receptor in the cytoplasm, triggers movement of the nuclear receptor–ligand complex to the nucleus, and thereby increases Oatp expression by enhancing transcription of an Slco gene. Although P-gp is also a critical determinant of CNS drug delivery, caution must be exercised when targeting this transporter to enable greater uptake of therapeutic agents into brain parenchyma. This warning arises from evidence obtained from several laboratories, including our own, that has shown that enhanced brain delivery of drugs can lead to CNS toxicity and unexpected adverse drug reactions.
Abbreviations
- ALK
activin receptor-like kinase
- BBB
blood-brain barrier
- Bcrp
breast cancer resistance protein
- BCSF
blood–cerebrospinal fluid
- CAR
constitutive androstane receptor
- CNS
central nervous system
- CSF
cerebrospinal fluid
- DPDPE
[d-penicillamine(2,5)]-enkephalin
- GSH
glutathione
- H/R
hypoxia-reoxygenation
- HMG-CoA
3-hydroxy-3-methylglutaryl coenzyme A
- HPA
hypothalamic-pituitary-adrenal
- Mrp
multidrug resistance protein
- OAT
organic anion transporter
- Oatp
organic anion-transporting polypeptide
- P-gp
P-glycoprotein
- PACAP
pituitary adenylate cyclase-activating polypeptide
- PTS
peptide transport system
- PXR
pregnane X receptor
- ROS
reactive oxygen species
- SLC
solute carrier
- SOD
superoxide dismutase
- TGF-β
transforming growth factor-β
- TLR
Toll-like receptor
- ZO
zonula occludens
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Ronaldson, Davis.
Footnotes
This work was supported by the National Institutes of Health [Grant R01-DA11271] (to T.P.D.); and grants from the American Heart Association [Grant 12BGIA9850007] and the Pharmaceutical Research and Manufacturers Association Foundation (to P.T.R).
References
- Abbott NJ. (2005) Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol 25:5–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ, Rönnbäck L, Hansson E. (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7:41–53 [DOI] [PubMed] [Google Scholar]
- Abbruscato TJ, Williams SA, Misicka A, Lipkowski AW, Hruby VJ, Davis TP. (1996) Blood-to-central nervous system entry and stability of biphalin, a unique double-enkephalin analog, and its halogenated derivatives. J Pharmacol Exp Ther 276:1049–1057 [PubMed] [Google Scholar]
- Abe K, Bridges AS, Yue W, Brouwer KL. (2008) In vitro biliary clearance of angiotensin II receptor blockers and 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors in sandwich-cultured rat hepatocytes: comparison with in vivo biliary clearance. J Pharmacol Exp Ther 326:983–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe T, Kakyo M, Tokui T, Nakagomi, R, Nishio, T, Nakai, D, Nomura, H, Unno, M, Suzuki, M, Naitoh, T, et al. (1999) Identification of a novel gene family encoding human liver-specific organic anion transporter LST-1. J Biol Chem 274:17159–17163 [DOI] [PubMed] [Google Scholar]
- Abe T, Unno M, Onogawa T, Tokui, T, Kondo, TN, Nakagomi, R, Adachi, H, Fujiwara, K, Okabe, M, Suzuki, T, et al. (2001) LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers. Gastroenterology 120:1689–1699 [DOI] [PubMed] [Google Scholar]
- Adachi H, Suzuki T, Abe M, N Asano, H Mizutamari, M Tanemoto, T Nishio, T Onogawa, T Toyohara, S Kasai, et al. (2003) Molecular characterization of human and rat organic anion transporter OATP-D. Am J Physiol Renal Physiol 285:F1188–F1197 [DOI] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF. (2008) Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies. CNS Neurol Disord Drug Targets 7:243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF, Dempsey RJ. (2006a) Lipids and lipidomics in brain injury and diseases. AAPS J 8:E314–E321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF, Larsen EC, Chen X, Sun D, Tsao FH. (2006b) CDP-choline significantly restores phosphatidylcholine levels by differentially affecting phospholipase A2 and CTP: phosphocholine cytidylyltransferase after stroke. J Biol Chem 281:6718–6725 [DOI] [PubMed] [Google Scholar]
- Aloisi F. (2001) Immune function of microglia. Glia 36:165–179 [DOI] [PubMed] [Google Scholar]
- Angeletti RH, Bergwerk AJ, Novikoff PM, Wolkoff AW. (1998) Dichotomous development of the organic anion transport protein in liver and choroid plexus. Am J Physiol 275:C882–C887 [DOI] [PubMed] [Google Scholar]
- Arai K, Lok J, Guo S, Hayakawa K, Xing C, Lo EH. (2011) Cellular mechanisms of neurovascular damage and repair after stroke. J Child Neurol 26:1193–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrup J, Siesjö BK, Symon L. (1981) Thresholds in cerebral ischemia—the ischemic penumbra. Stroke 12:723–725 [DOI] [PubMed] [Google Scholar]
- Azzi G, Bernaudin JF, Bouchaud C, Bellon B, Fleury-Feith J. (1990) Permeability of the normal rat brain, spinal cord and dorsal root ganglia microcirculations to immunoglobulins G. Biol Cell 68:31–36 [DOI] [PubMed] [Google Scholar]
- Bahn A, Ljubojevic M, Lorenz H, Schultz C, Ghebremedhin E, Ugele B, Sabolic I, Burckhardt G, Hagos Y. (2005) Murine renal organic anion transporters mOAT1 and mOAT3 facilitate the transport of neuroactive tryptophan metabolites. Am J Physiol Cell Physiol 289:C1075–C1084 [DOI] [PubMed] [Google Scholar]
- Baldwin K, Orr S, Briand M, Piazza C, Veydt A, McCoy S. (2010) Acute ischemic stroke update. Pharmacotherapy 30:493–514 [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. (2004) The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 16:1–13 [DOI] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Waagepetersen HS. (2006) The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem 98:641–653 [DOI] [PubMed] [Google Scholar]
- Banerjee N, Allen C, Bendayan R. (2012) Differential role of organic anion-transporting polypeptides in estrone-3-sulphate uptake by breast epithelial cells and breast cancer cells. J Pharmacol Exp Ther 342:510–519 [DOI] [PubMed] [Google Scholar]
- Banks WA. (2008) Delivery of peptides to the brain: emphasis on therapeutic development. Biopolymers 90:589–594 [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Ehrensing CA. (1993a) Endogenous peptide Tyr-Pro-Trp-Gly-NH2 (Tyr-W-MIF-1) is transported from the brain to the blood by peptide transport system-1. J Neurosci Res 35:690–695 [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Harrison LM, Zadina JE. (1996a) Perinatal treatment of rats with opiates affects the development of the blood-brain barrier transport system PTS-1. Neurotoxicol Teratol 18:711–715 [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Komaki G, Arimura A. (1993b) Passage of pituitary adenylate cyclase activating polypeptide1-27 and pituitary adenylate cyclase activating polypeptide1-38 across the blood-brain barrier. J Pharmacol Exp Ther 267:690–696 [PubMed] [Google Scholar]
- Banks WA, Uchida D, Arimura A, Somogyvári-Vigh A, Shioda S. (1996b) Transport of pituitary adenylate cyclase-activating polypeptide across the blood-brain barrier and the prevention of ischemia-induced death of hippocampal neurons. Ann N Y Acad Sci 805:270–277, discussion 277–279 [DOI] [PubMed] [Google Scholar]
- Barone E, Cenini G, Di Domenico F, Martin S, Sultana R, Mancuso C, Murphy MP, Head E, Butterfield DA. (2011) Long-term high-dose atorvastatin decreases brain oxidative and nitrosative stress in a preclinical model of Alzheimer disease: a novel mechanism of action. Pharmacol Res 63:172–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone E, Mancuso C, Di Domenico F, Sultana R, Murphy MP, Head E, Butterfield DA. (2012) Biliverdin reductase-A: a novel drug target for atorvastatin in a dog pre-clinical model of Alzheimer disease. J Neurochem 120:135–146 [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz AM, Fricker G, Miller DS. (2004) Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol 66:413–419 [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz AM, Lucking JR, Yang X, Pollack GM, Miller DS. (2008) Coordinated nuclear receptor regulation of the efflux transporter, Mrp2, and the phase-II metabolizing enzyme, GSTpi, at the blood-brain barrier. J Cereb Blood Flow Metab 28:1222–1234 [DOI] [PubMed] [Google Scholar]
- Bauer B, Yang X, Hartz AM, Olson ER, Zhao R, Kalvass JC, Pollack GM, Miller DS. (2006) In vivo activation of human pregnane X receptor tightens the blood-brain barrier to methadone through P-glycoprotein up-regulation. Mol Pharmacol 70:1212–1219 [DOI] [PubMed] [Google Scholar]
- Bendayan R, Ronaldson PT, Gingras D, Bendayan M. (2006) In situ localization of P-glycoprotein (ABCB1) in human and rat brain. J Histochem Cytochem 54:1159–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma GJ, Muizelaar JP. (1992) Cerebral blood flow, cerebral blood volume, and cerebrovascular reactivity after severe head injury. J Neurotrauma 9 (Suppl 1):S333–S348 [PubMed] [Google Scholar]
- Bronger H, König J, Kopplow K, Steiner HH, Ahmadi R, Herold-Mende C, Keppler D, Nies AT. (2005) ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood-tumor barrier. Cancer Res 65:11419–11428 [DOI] [PubMed] [Google Scholar]
- Bsibsi M, Ravid R, Gveric D, van Noort JM. (2002) Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol 61:1013–1021 [DOI] [PubMed] [Google Scholar]
- Buckwalter MS, Wyss-Coray T. (2004) Modelling neuroinflammatory phenotypes in vivo. J Neuroinflammation 1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AM, Jones HC, Abbott NJ. (1990) Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol 429:47–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Barone E, Di Domenico F, Cenini G, Sultana R, Murphy MP, Mancuso C, Head E. (2012) Atorvastatin treatment in a dog preclinical model of Alzheimer's disease leads to up-regulation of haem oxygenase-1 and is associated with reduced oxidative stress in brain. Int J Neuropsychopharmacol 15:981–987 [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E. (2009) Injury and repair mechanisms in ischemic stroke: considerations for the development of novel neurotherapeutics. Curr Opin Investig Drugs 10:644–654 [PubMed] [Google Scholar]
- Chan GN, Hoque MT, Cummins CL, Bendayan R. (2011) Regulation of P-glycoprotein by orphan nuclear receptors in human brain microvessel endothelial cells. J Neurochem 118:163–175 [DOI] [PubMed] [Google Scholar]
- Cheng X, Maher J, Chen C, Klaassen CD. (2005) Tissue distribution and ontogeny of mouse organic anion transporting polypeptides (Oatps). Drug Metab Dispos 33:1062–1073 [DOI] [PubMed] [Google Scholar]
- Chi Y, Schuster VL. (2010) The prostaglandin transporter PGT transports PGH(2). Biochem Biophys Res Commun 395:168–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou R. (2009) 2009 Clinical Guidelines from the American Pain Society and the American Academy of Pain Medicine on the use of chronic opioid therapy in chronic noncancer pain: what are the key messages for clinical practice? Pol Arch Med Wewn 119:469–477 [PubMed] [Google Scholar]
- Choudhuri S, Cherrington NJ, Li N, Klaassen CD. (2003) Constitutive expression of various xenobiotic and endobiotic transporter mRNAs in the choroid plexus of rats. Drug Metab Dispos 31:1337–1345 [DOI] [PubMed] [Google Scholar]
- Chu XY, Bleasby K, Yabut J, Cai X, Chan GH, Hafey MJ, Xu S, Bergman AJ, Braun MP, Dean DC, et al. (2007) Transport of the dipeptidyl peptidase-4 inhibitor sitagliptin by human organic anion transporter 3, organic anion transporting polypeptide 4C1, and multidrug resistance P-glycoprotein. J Pharmacol Exp Ther 321:673–683 [DOI] [PubMed] [Google Scholar]
- Dagenais C, Ducharme J, Pollack GM. (2001) Uptake and efflux of the peptidic delta-opioid receptor agonist. Neurosci Lett 301:155–158 [DOI] [PubMed] [Google Scholar]
- Dagenais C, Graff CL, Pollack GM. (2004) Variable modulation of opioid brain uptake by P-glycoprotein in mice. Biochem Pharmacol 67:269–276 [DOI] [PubMed] [Google Scholar]
- Dallas S, Miller DS, Bendayan R. (2006) Multidrug resistance-associated proteins: expression and function in the central nervous system. Pharmacol Rev 58:140–161 [DOI] [PubMed] [Google Scholar]
- Dallas S, Ronaldson PT, Bendayan M, Bendayan R. (2004) Multidrug resistance protein 1-mediated transport of saquinavir by microglia. Neuroreport 15:1183–1186 [DOI] [PubMed] [Google Scholar]
- Dallas S, Zhu X, Baruchel S, Schlichter L, Bendayan R. (2003) Functional expression of the multidrug resistance protein 1 in microglia. J Pharmacol Exp Ther 307:282–290 [DOI] [PubMed] [Google Scholar]
- Dauchy S, Miller F, Couraud PO, Weaver RJ, Weksler B, Romero IA, Scherrmann JM, De Waziers I, Declèves X. (2009) Expression and transcriptional regulation of ABC transporters and cytochromes P450 in hCMEC/D3 human cerebral microvascular endothelial cells. Biochem Pharmacol 77:897–909 [DOI] [PubMed] [Google Scholar]
- Davson H, Hollingsworth G, Segal MB. (1970) The mechanism of drainage of the cerebrospinal fluid. Brain 93:665–678 [DOI] [PubMed] [Google Scholar]
- Deierborg T, Roybon L, Inacio AR, Pesic J, Brundin P. (2010) Brain injury activates microglia that induce neural stem cell proliferation ex vivo and promote differentiation of neurosphere-derived cells into neurons and oligodendrocytes. Neuroscience 171:1386–1396 [DOI] [PubMed] [Google Scholar]
- del Rio-Hortega P (1932). Microglia, in Cytology and Cellular Pathology of the Nervous System (Penfield W ed) pp 481–584, Paul P. Hocher, New York. [Google Scholar]
- Derynck R, Zhang YE. (2003) Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425:577–584 [DOI] [PubMed] [Google Scholar]
- Dickinson T, Mitchell R, Robberecht P, Fleetwood-Walker SM. (1999) The role of VIP/PACAP receptor subtypes in spinal somatosensory processing in rats with an experimental peripheral mononeuropathy. Neuropharmacology 38:167–180 [DOI] [PubMed] [Google Scholar]
- Dickson DW, Mattiace LA, Kure K, Hutchins K, Lyman WD, Brosnan CF. (1991) Microglia in human disease, with an emphasis on acquired immune deficiency syndrome. Lab Invest 64:135–156 [PubMed] [Google Scholar]
- Dogrukol-Ak D, Kumar VB, Ryerse JS, Farr SA, Verma S, Nonaka N, Nakamachi T, Ohtaki H, Niehoff ML, Edwards JC, et al. (2009) Isolation of peptide transport system-6 from brain endothelial cells: therapeutic effects with antisense inhibition in Alzheimer and stroke models. J Cereb Blood Flow Metab 29:411–422 [DOI] [PubMed] [Google Scholar]
- Dohgu S, Yamauchi A, Takata F, Naito M, Tsuruo T, Higuchi S, Sawada Y, Kataoka Y. (2004) Transforming growth factor-beta1 upregulates the tight junction and P-glycoprotein of brain microvascular endothelial cells. Cell Mol Neurobiol 24:491–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KP, Cekanaviciute E, Mamer LE, Buckwalter MS. (2010) TGFβ signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke. J Neuroinflammation 7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egleton RD, Davis TP. (1999) Transport of the delta-opioid receptor agonist [D-penicillamine2,5] enkephalin across the blood-brain barrier involves transcytosis1. J Pharm Sci 88:392–397 [DOI] [PubMed] [Google Scholar]
- Egleton RD, Davis TP. (2005) Development of neuropeptide drugs that cross the blood-brain barrier. NeuroRx 2:44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ad B, Lavie P. (2005) Effect of sleep apnea on cognition and mood. Int Rev Psychiatry 17:277–282 [DOI] [PubMed] [Google Scholar]
- Faustino JV, Wang X, Johnson CE, Klibanov A, Derugin N, Wendland MF, Vexler ZS. (2011) Microglial cells contribute to endogenous brain defenses after acute neonatal focal stroke. J Neurosci 31:12992–13001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgenhauer K. (1974) Protein size and cerebrospinal fluid composition. Klin Wochenschr 52:1158–1164 [DOI] [PubMed] [Google Scholar]
- Feng Y, Rhodes PG, Bhatt AJ. (2011) Dexamethasone pre-treatment protects brain against hypoxic-ischemic injury partially through up-regulation of vascular endothelial growth factor A in neonatal rats. Neuroscience 179:223–232 [DOI] [PubMed] [Google Scholar]
- Feurstein D, Kleinteich J, Heussner AH, Stemmer K, Dietrich DR. (2010) Investigation of microcystin congener-dependent uptake into primary murine neurons. Environ Health Perspect 118:1370–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R, Cidlowski JA. (2006) SLCO/OATP-like transport of glutathione in FasL-induced apoptosis: glutathione efflux is coupled to an organic anion exchange and is necessary for the progression of the execution phase of apoptosis. J Biol Chem 281:29542–29557 [DOI] [PubMed] [Google Scholar]
- Fujiwara K, Adachi H, Nishio T, Unno M, Tokui T, Okabe M, Onogawa T, Suzuki T, Asano N, Tanemoto M, et al. (2001) Identification of thyroid hormone transporters in humans: different molecules are involved in a tissue-specific manner. Endocrinology 142:2005–2012 [DOI] [PubMed] [Google Scholar]
- Gao B, Hagenbuch B, Kullak-Ublick GA, Benke D, Aguzzi A, Meier PJ. (2000) Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J Pharmacol Exp Ther 294:73–79 [PubMed] [Google Scholar]
- Gao B, Huber RD, Wenzel A, Vavricka SR, Ismair MG, Remé C, Meier PJ. (2005) Localization of organic anion transporting polypeptides in the rat and human ciliary body epithelium. Exp Eye Res 80:61–72 [DOI] [PubMed] [Google Scholar]
- Gao B, Stieger B, Noé B, Fritschy JM, Meier PJ. (1999) Localization of the organic anion transporting polypeptide 2 (Oatp2) in capillary endothelium and choroid plexus epithelium of rat brain. J Histochem Cytochem 47:1255–1264 [DOI] [PubMed] [Google Scholar]
- Gazzin S, Strazielle N, Schmitt C, Fevre-Montange M, Ostrow JD, Tiribelli C, Ghersi-Egea JF. (2008) Differential expression of the multidrug resistance-related proteins ABCb1 and ABCc1 between blood-brain interfaces. J Comp Neurol 510:497–507 [DOI] [PubMed] [Google Scholar]
- Genter MB, Krishan M, Augustine LM, Cherrington NJ. (2010) Drug transporter expression and localization in rat nasal respiratory and olfactory mucosa and olfactory bulb. Drug Metab Dispos 38:1644–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghersi-Egea JF, Strazielle N. (2001) Brain drug delivery, drug metabolism, and multidrug resistance at the choroid plexus. Microsc Res Tech 52:83–88 [DOI] [PubMed] [Google Scholar]
- Glaeser H, Mandery K, Sticht H, Fromm MF, König J. (2010) Relevance of conserved lysine and arginine residues in transmembrane helices for the transport activity of organic anion transporting polypeptide 1B3. Br J Pharmacol 159:698–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloth FM., 3rd (2011) Pharmacological management of persistent pain in older persons: focus on opioids and nonopioids. J Pain 12 (3 Suppl 1):S14–S20 [DOI] [PubMed] [Google Scholar]
- Goldberg M, De Pittà M, Volman V, Berry H, Ben-Jacob E. (2010) Nonlinear gap junctions enable long-distance propagation of pulsating calcium waves in astrocyte networks. PLOS Comput Biol 6:e1000909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. (2002) Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J 21:1743–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube M, Köck K, Karner S, Reuther S, Ritter CA, Jedlitschky G, Kroemer HK. (2006a) Modification of OATP2B1-mediated transport by steroid hormones. Mol Pharmacol 70:1735–1741 [DOI] [PubMed] [Google Scholar]
- Grube M, Köck K, Oswald S, Draber, K, Meissner, K, Eckel, L, Böhm, M, Felix, SB, Vogelgesang, S, Jedlitschky, G, et al. (2006b) Organic anion transporting polypeptide 2B1 is a high-affinity transporter for atorvastatin and is expressed in the human heart. Clin Pharmacol Ther 80:607–620 [DOI] [PubMed] [Google Scholar]
- Gui C, Hagenbuch B. (2008) Amino acid residues in transmembrane domain 10 of organic anion transporting polypeptide 1B3 are critical for cholecystokinin octapeptide transport. Biochemistry 47:9090–9097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui C, Hagenbuch B. (2009) Role of transmembrane domain 10 for the function of organic anion transporting polypeptide 1B1. Protein Sci 18:2298–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas DA. (2002) An update on analgesics for the management of acute postoperative dental pain. J Can Dent Assoc 68:476–482 [PubMed] [Google Scholar]
- Hackett PH. (1999) High altitude cerebral edema and acute mountain sickness. A pathophysiology update. Adv Exp Med Biol 474:23–45 [DOI] [PubMed] [Google Scholar]
- Hagenbuch B. (2007) Cellular entry of thyroid hormones by organic anion transporting polypeptides. Best Pract Res Clin Endocrinol Metab 21:209–221 [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. (2003) The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta 1609:1–18 [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. (2004) Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch 447:653–665 [DOI] [PubMed] [Google Scholar]
- Hamabe W, Maeda T, Kiguchi N, Yamamoto C, Tokuyama S, Kishioka S. (2007) Negative relationship between morphine analgesia and P-glycoprotein expression levels in the brain. J Pharmacol Sci 105:353–360 [DOI] [PubMed] [Google Scholar]
- Hänggi E, Grundschober AF, Leuthold S, Meier PJ, St-Pierre MV. (2006) Functional analysis of the extracellular cysteine residues in the human organic anion transporting polypeptide, OATP2B1. Mol Pharmacol 70:806–817 [DOI] [PubMed] [Google Scholar]
- Hartz AM, Bauer B. (2010) Regulation of ABC transporters at the blood-brain barrier: new targets for CNS therapy. Mol Interv 10:293–304 [DOI] [PubMed] [Google Scholar]
- Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. (1998) ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol 141:199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hau VS, Huber JD, Campos CR, Davis RT, Davis TP. (2004) Effect of lambda-carrageenan-induced inflammatory pain on brain uptake of codeine and antinociception. Brain Res 1018:257–264 [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. (2005) The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57:173–185 [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Ocheltree SM, Norwood KM, Egleton RD. (2007) Decreased blood-brain barrier permeability to fluorescein in streptozotocin-treated rats. Neurosci Lett 411:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BT, Sykes DB, Miller DS. (2010) Rapid, reversible modulation of blood-brain barrier P-glycoprotein transport activity by vascular endothelial growth factor. J Neurosci 30:1417–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Pu H, Andras IE, Eum SY, Yamauchi A, Hennig B, Toborek M. (2006) HIV-TAT protein upregulates expression of multidrug resistance protein 1 in the blood-brain barrier. J Cereb Blood Flow Metab 26:1052–1065 [DOI] [PubMed] [Google Scholar]
- Hayashi K, Pu H, Tian J, Andras IE, Lee YW, Hennig B, Toborek M. (2005) HIV-Tat protein induces P-glycoprotein expression in brain microvascular endothelial cells. J Neurochem 93:1231–1241 [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Nomura M, Yamagishi S, Harada S, Yamashita J, Yamamoto H. (1997) Induction of various blood-brain barrier properties in non-neural endothelial cells by close apposition to co-cultured astrocytes. Glia 19:13–26 [PubMed] [Google Scholar]
- Heuer H, Visser TJ. (2009) Minireview: Pathophysiological importance of thyroid hormone transporters. Endocrinology 150:1078–1083 [DOI] [PubMed] [Google Scholar]
- Hirano M, Maeda K, Shitara Y, Sugiyama Y. (2006) Drug-drug interaction between pitavastatin and various drugs via OATP1B1. Drug Metab Dispos 34:1229–1236 [DOI] [PubMed] [Google Scholar]
- Hirrlinger J, Dringen R. (2005) Multidrug resistance protein 1-mediated export of glutathione and glutathione disulfide from brain astrocytes. Methods Enzymol 400:395–409 [DOI] [PubMed] [Google Scholar]
- Ho RH, Tirona RG, Leake BF, Glaeser H, Lee W, Lemke CJ, Wang Y, Kim RB. (2006) Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology 130:1793–1806 [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Gale SD, Weaver LK. (2006) Brain atrophy and cognitive impairment in survivors of Acute Respiratory Distress Syndrome. Brain Inj 20:263–271 [DOI] [PubMed] [Google Scholar]
- Hsiang B, Zhu Y, Wang Z, Wu Y, Sasseville V, Yang WP, Kirchgessner TG. (1999) A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of a liver-specific human organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl-CoA reductase inhibitor transporters. J Biol Chem 274:37161–37168 [DOI] [PubMed] [Google Scholar]
- Huang L, Wang Y, Grimm S. (2006) ATP-dependent transport of rosuvastatin in membrane vesicles expressing breast cancer resistance protein. Drug Metab Dispos 34:738–742 [DOI] [PubMed] [Google Scholar]
- Huber RD, Gao B, Sidler Pfändler MA, Zhang-Fu W, Leuthold S, Hagenbuch B, Folkers G, Meier PJ, Stieger B. (2007) Characterization of two splice variants of human organic anion transporting polypeptide 3A1 isolated from human brain. Am J Physiol Cell Physiol 292:C795–C806 [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Brown K, Coats, BD, Shridhar, M, Sholar, PW, Patel, SJ, Crysdale, NY, Harrison, JA, Maier, SF, et al. (2008) Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4). Eur J Neurosci 28:20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara H, Kubota H, Lindberg RL, Leppert D, Gloor SM, Errede M, Virgintino D, Fontana A, Yonekawa Y, Frei K. (2008) Endothelial cell barrier impairment induced by glioblastomas and transforming growth factor beta2 involves matrix metalloproteinases and tight junction proteins. J Neuropathol Exp Neurol 67:435–448 [DOI] [PubMed] [Google Scholar]
- Jahan R, Vinuela F. (2009) Treatment of acute ischemic stroke: intravenous and endovascular therapies. Expert Rev Cardiovasc Ther 7:375–387 [DOI] [PubMed] [Google Scholar]
- Jakas A, Horvat S. (2004) The effect of glycation on the chemical and enzymatic stability of the endogenous opioid peptide, leucine-enkephalin, and related fragments. Bioorg Chem 32:516–526 [DOI] [PubMed] [Google Scholar]
- Janzer RC, Raff MC. (1987) Astrocytes induce blood-brain barrier properties in endothelial cells. Nature 325:253–257 [DOI] [PubMed] [Google Scholar]
- Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. (2010) The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain 11:1230–1239 [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Ballard C. (2001) Stroke and cognition. Curr Atheroscler Rep 3:334–339 [DOI] [PubMed] [Google Scholar]
- Kanai N, Lu R, Satriano JA, Bao Y, Wolkoff AW, Schuster VL. (1995) Identification and characterization of a prostaglandin transporter. Science 268:866–869 [DOI] [PubMed] [Google Scholar]
- Kardash KJ, Sarrazin F, Tessler MJ, Velly AM. (2008) Single-dose dexamethasone reduces dynamic pain after total hip arthroplasty. Anesth Analg 106:1253–1257 [DOI] [PubMed] [Google Scholar]
- Kassan M, Montero MJ, Sevilla MA. (2010) In vitro antioxidant activity of pravastatin provides vascular protection. Eur J Pharmacol 630:107–111 [DOI] [PubMed] [Google Scholar]
- Kato K, Shirasaka Y, Kuraoka E, Kikuchi A, Iguchi M, Suzuki H, Shibasaki S, Kurosawa T, Tamai I. (2010) Intestinal absorption mechanism of tebipenem pivoxil, a novel oral carbapenem: involvement of human OATP family in apical membrane transport. Mol Pharm DOI: 10.1021/mp100130b [published ahead of print]. [DOI] [PubMed] [Google Scholar]
- Keep RF, Smith DE. (2011) Choroid plexus transport: gene deletion studies. Fluids Barriers CNS 8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattab MM. (2006) TEMPOL, a membrane-permeable radical scavenger, attenuates peroxynitrite- and superoxide anion-enhanced carrageenan-induced paw edema and hyperalgesia: a key role for superoxide anion. Eur J Pharmacol 548:167–173 [DOI] [PubMed] [Google Scholar]
- Kielian T. (2006) Toll-like receptors in central nervous system glial inflammation and homeostasis. J Neurosci Res 83:711–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA. (1990) Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci 10:1583–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindla J, Rau TT, Jung R, Fasching PA, Strick R, Stoehr R, Hartmann A, Fromm MF, König J. (2011) Expression and localization of the uptake transporters OATP2B1, OATP3A1 and OATP5A1 in non-malignant and malignant breast tissue. Cancer Biol Ther 11:584–591 [DOI] [PubMed] [Google Scholar]
- Kis B, Isse T, Snipes JA, Chen L, Yamashita H, Ueta Y, Busija DW. (2006) Effects of LPS stimulation on the expression of prostaglandin carriers in the cells of the blood-brain and blood-cerebrospinal fluid barriers. J Appl Physiol 100:1392–1399 [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. (2003) Involvement of human organic anion transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport across intestinal apical membrane. J Pharmacol Exp Ther 306:703–708 [DOI] [PubMed] [Google Scholar]
- König J, Cui Y, Nies AT, Keppler D. (2000a) Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J Biol Chem 275:23161–23168 [DOI] [PubMed] [Google Scholar]
- König J, Cui Y, Nies AT, Keppler D. (2000b) A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am J Physiol Gastrointest Liver Physiol 278:G156–G164 [DOI] [PubMed] [Google Scholar]
- König J, Glaeser H, Keiser M, Mandery K, Klotz U, Fromm MF. (2011) Role of organic anion-transporting polypeptides for cellular mesalazine (5-aminosalicylic acid) uptake. Drug Metab Dispos 39:1097–1102 [DOI] [PubMed] [Google Scholar]
- Kopplow K, Letschert K, König J, Walter B, Keppler D. (2005) Human hepatobiliary transport of organic anions analyzed by quadruple-transfected cells. Mol Pharmacol 68:1031–1038 [DOI] [PubMed] [Google Scholar]
- Kraft ME, Glaeser H, Mandery K, König J, Auge D, Fromm MF, Schlötzer-Schrehardt U, Welge-Lüssen U, Kruse FE, Zolk O. (2010) The prostaglandin transporter OATP2A1 is expressed in human ocular tissues and transports the antiglaucoma prostanoid latanoprost. Invest Ophthalmol Vis Sci 51:2504–2511 [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Becker MB. (2003) Regulation of drug and bile salt transporters in liver and intestine. Drug Metab Rev 35:305–317 [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Hagenbuch B, Stieger B, Schteingart CD, Hofmann AF, Wolkoff AW, Meier PJ. (1995) Molecular and functional characterization of an organic anion transporting polypeptide cloned from human liver. Gastroenterology 109:1274–1282 [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F, Fattinger K, Meier PJ, Hagenbuch B. (2001) Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology 120:525–533 [DOI] [PubMed] [Google Scholar]
- Kusuhara H, Sugiyama Y. (2005) Active efflux across the blood-brain barrier: role of the solute carrier family. NeuroRx 2:73–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuz D, Mousa SA, Schäfer M, Stein C, Machelska H. (2007) Relative contribution of peripheral versus central opioid receptors to antinociception. Brain Res 1160:30–38 [DOI] [PubMed] [Google Scholar]
- Lebrin F, Deckers M, Bertolino P, Ten Dijke P. (2005) TGF-beta receptor function in the endothelium. Cardiovasc Res 65:599–608 [DOI] [PubMed] [Google Scholar]
- Lee G, Schlichter L, Bendayan M, Bendayan R. (2001) Functional expression of P-glycoprotein in rat brain microglia. J Pharmacol Exp Ther 299:204–212 [PubMed] [Google Scholar]
- Leggas M, Adachi M, Scheffer GL, Sun D, Wielinga P, Du G, Mercer KE, Zhuang Y, Panetta JC, Johnston B, et al. (2004) Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol 24:7612–7621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuthold S, Hagenbuch B, Mohebbi N, Wagner CA, Meier PJ, Stieger B. (2009) Mechanisms of pH-gradient driven transport mediated by organic anion polypeptide transporters. Am J Physiol Cell Physiol 296:C570–C582 [DOI] [PubMed] [Google Scholar]
- Li L, Lee TK, Meier PJ, Ballatori N. (1998) Identification of glutathione as a driving force and leukotriene C4 as a substrate for oatp1, the hepatic sinusoidal organic solute transporter. J Biol Chem 273:16184–16191 [DOI] [PubMed] [Google Scholar]
- Li L, Meier PJ, Ballatori N. (2000) Oatp2 mediates bidirectional organic solute transport: a role for intracellular glutathione. Mol Pharmacol 58:335–340 [DOI] [PubMed] [Google Scholar]
- Li L, Nouraldeen A, Wilson AG. (2012) Evaluation of transporter-mediated hepatic uptake in a non-radioactive high-throughput assay: a study of kinetics, species difference and plasma protein effect. Xenobiotica DOI: 10.3109/00498254.2012.713146 [published ahead of print]. [DOI] [PubMed] [Google Scholar]
- Lim C, Alexander MP, LaFleche G, Schnyer DM, Verfaellie M. (2004) The neurological and cognitive sequelae of cardiac arrest. Neurology 63:1774–1778 [DOI] [PubMed] [Google Scholar]
- Liu S, Levine SR, Winn HR. (2010) Targeting ischemic penumbra: part I—from pathophysiology to therapeutic strategy. J Exp Stroke Transl Med 3:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobysheva NV, Tonshin AA, Selin AA, Yaguzhinsky LS, Nartsissov YR. (2009) Diversity of neurodegenerative processes in the model of brain cortex tissue ischemia. Neurochem Int 54:322–329 [DOI] [PubMed] [Google Scholar]
- Lochhead JJ, McCaffrey G, Quigley CE, Finch J, DeMarco KM, Nametz N, Davis TP. (2010) Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab 30:1625–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead JJ, McCaffrey G, Sanchez-Covarrubias L, Finch JD, Demarco KM, Quigley CE, Davis TP, Ronaldson PT. (2012) Tempol modulates changes in xenobiotic permeability and occludin oligomeric assemblies at the blood-brain barrier during inflammatory pain. Am J Physiol Heart Circ Physiol 302:H582–H593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram LC, Taylor FR, Strand KA, Frank MG, Sholar P, Harrison JA, Maier SF, Watkins LR. (2011) Prior exposure to glucocorticoids potentiates lipopolysaccharide induced mechanical allodynia and spinal neuroinflammation. Brain Behav Immun 25:1408–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Pittman QJ. (2003) Talking back: dendritic neurotransmitter release. Trends Neurosci 26:255–261 [DOI] [PubMed] [Google Scholar]
- Mahagita C, Grassl SM, Piyachaturawat P, Ballatori N. (2007) Human organic anion transporter 1B1 and 1B3 function as bidirectional carriers and do not mediate GSH-bile acid cotransport. Am J Physiol Gastrointest Liver Physiol 293:G271–G278 [DOI] [PubMed] [Google Scholar]
- Maloney-Wilensky E, Gracias V, Itkin A, Hoffman K, Bloom S, Yang W, Christian S, LeRoux PD. (2009) Brain tissue oxygen and outcome after severe traumatic brain injury: a systematic review. Crit Care Med 37:2057–2063 [DOI] [PubMed] [Google Scholar]
- Manchikanti L, Fellows B, Ailinani H, Pampati V. (2010) Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician 13:401–435 [PubMed] [Google Scholar]
- Mandery K, Sticht H, Bujok K, Schmidt I, Fahrmayr C, Balk B, Fromm MF, Glaeser H. (2011) Functional and structural relevance of conserved positively charged lysine residues in organic anion transporting polypeptide 1B3. Mol Pharmacol 80:400–406 [DOI] [PubMed] [Google Scholar]
- Marin JJ, Mangas D, Martinez-Diez MC, El-Mir MY, Briz O, Serrano MA. (2003) Sensitivity of bile acid transport by organic anion-transporting polypeptides to intracellular pH. Biochim Biophys Acta 1611:249–257 [DOI] [PubMed] [Google Scholar]
- Martinez-Becerra P, Briz O, Romero MR, Macias RI, Perez MJ, Sancho-Mateo C, Lostao MP, Fernandez-Abalos JM, Marin JJ. (2011) Further characterization of the electrogenicity and pH sensitivity of the human organic anion-transporting polypeptides OATP1B1 and OATP1B3. Mol Pharmacol 79:596–607 [DOI] [PubMed] [Google Scholar]
- Mason BL, Pariante CM, Jamel S, Thomas SA. (2010) Central nervous system (CNS) delivery of glucocorticoids is fine-tuned by saturable transporters at the blood-CNS barriers and nonbarrier regions. Endocrinology 151:5294–5305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheny CJ, Ali RY, Yang X, Pollack GM. (2004) Effect of prototypical inducing agents on P-glycoprotein and CYP3A expression in mouse tissues. Drug Metab Dispos 32:1008–1014 [PubMed] [Google Scholar]
- Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. (2010) The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 58:1094–1103 [DOI] [PubMed] [Google Scholar]
- Mayerl S, Visser TJ, Darras VM, Horn S, Heuer H. (2012) Impact of Oatp1c1 deficiency on thyroid hormone metabolism and action in the mouse brain. Endocrinology 153:1528–1537 [DOI] [PubMed] [Google Scholar]
- McCaffrey G, Staatz WD, Sanchez-Covarrubias L, Finch JD, Demarco K, Laracuente ML, Ronaldson PT, Davis TP. (2012) P-glycoprotein trafficking at the blood-brain barrier altered by peripheral inflammatory hyperalgesia. J Neurochem 122:962–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Abt F, Mokrab Y, Mizuguchi K. (2005) Organic anion transporting polypeptides of the OATP/SLCO superfamily: identification of new members in nonmammalian species, comparative modeling and a potential transport mode. J Membr Biol 208:213–227 [DOI] [PubMed] [Google Scholar]
- Ménochet K, Kenworthy KE, Houston JB, Galetin A. (2012) Use of mechanistic modeling to assess interindividual variability and interspecies differences in active uptake in human and rat hepatocytes. Drug Metab Dispos 40:1744–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer zu Schwabedissen HE, Tirona RG, Yip CS, Ho RH, Kim RB. (2008) Interplay between the nuclear receptor pregnane X receptor and the uptake transporter organic anion transporter polypeptide 1A2 selectively enhances estrogen effects in breast cancer. Cancer Res 68:9338–9347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkaichi T, Suzuki T, Onogawa T, Tanemoto M, Mizutamari H, Okada M, Chaki T, Masuda S, Tokui T, Eto N, et al. (2004) Isolation and characterization of a digoxin transporter and its rat homologue expressed in the kidney. Proc Natl Acad Sci USA 101:3569–3574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP. (2002) Neuroendocrine aspects of the response to stress. Metabolism 51 (6 Suppl 1):5–10 [DOI] [PubMed] [Google Scholar]
- Minich T, Riemer J, Schulz JB, Wielinga P, Wijnholds J, Dringen R. (2006) The multidrug resistance protein 1 (Mrp1), but not Mrp5, mediates export of glutathione and glutathione disulfide from brain astrocytes. J Neurochem 97:373–384 [DOI] [PubMed] [Google Scholar]
- Miyagawa M, Maeda K, Aoyama A, Sugiyama Y. (2009) The eighth and ninth transmembrane domains in organic anion transporting polypeptide 1B1 affect the transport kinetics of estrone-3-sulfate and estradiol-17beta-D-glucuronide. J Pharmacol Exp Ther 329:551–557 [DOI] [PubMed] [Google Scholar]
- Miyajima M, Kusuhara H, Fujishima M, Adachi Y, Sugiyama Y. (2011) Organic anion transporter 3 mediates the efflux transport of an amphipathic organic anion, dehydroepiandrosterone sulfate, across the blood-brain barrier in mice. Drug Metab Dispos 39:814–819 [DOI] [PubMed] [Google Scholar]
- Mougey EB, Feng H, Castro M, Irvin CG, Lima JJ. (2009) Absorption of montelukast is transporter mediated: a common variant of OATP2B1 is associated with reduced plasma concentrations and poor response. Pharmacogenet Genomics 19:129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus J, Risau W, Wolburg H. (1991) Induction of blood-brain barrier characteristics in bovine brain endothelial cells by rat astroglial cells in transfilter coculture. Ann N Y Acad Sci 633:578–580 [DOI] [PubMed] [Google Scholar]
- Nishio T, Adachi H, Nakagomi R, Tokui T, Sato E, Tanemoto M, Fujiwara K, Okabe M, Onogawa T, Suzuki T, et al. (2000) Molecular identification of a rat novel organic anion transporter moat1, which transports prostaglandin D(2), leukotriene C(4), and taurocholate. Biochem Biophys Res Commun 275:831–838 [DOI] [PubMed] [Google Scholar]
- Noé B, Hagenbuch B, Stieger B, Meier PJ. (1997) Isolation of a multispecific organic anion and cardiac glycoside transporter from rat brain. Proc Natl Acad Sci USA 94:10346–10350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noé J, Portmann R, Brun ME, Funk C. (2007) Substrate-dependent drug-drug interactions between gemfibrozil, fluvastatin and other organic anion-transporting peptide (OATP) substrates on OATP1B1, OATP2B1, and OATP1B3. Drug Metab Dispos 35:1308–1314 [DOI] [PubMed] [Google Scholar]
- Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. (2004) Functional characterization of pH-sensitive organic anion transporting polypeptide OATP-B in human. J Pharmacol Exp Ther 308:438–445 [DOI] [PubMed] [Google Scholar]
- Obaidat A, Roth M, Hagenbuch B. (2012) The expression and function of organic anion transporting polypeptides in normal tissues and in cancer. Annu Rev Pharmacol Toxicol 52:135–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki S, Takizawa T, Takanaga H, Terasaki N, Kitazawa T, Sasaki M, Abe T, Hosoya K, Terasaki T. (2003) In vitro study of the functional expression of organic anion transporting polypeptide 3 at rat choroid plexus epithelial cells and its involvement in the cerebrospinal fluid-to-blood transport of estrone-3-sulfate. Mol Pharmacol 63:532–537 [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Tomi M, Hata T, Nagai Y, Hori S, Mori S, Hosoya K, Terasaki T. (2005) Dominant expression of androgen receptors and their functional regulation of organic anion transporter 3 in rat brain capillary endothelial cells; comparison of gene expression between the blood-brain and -retinal barriers. J Cell Physiol 204:896–900 [DOI] [PubMed] [Google Scholar]
- Ose A, Kusuhara H, Endo C, Tohyama K, Miyajima M, Kitamura S, Sugiyama Y. (2010) Functional characterization of mouse organic anion transporting peptide 1a4 in the uptake and efflux of drugs across the blood-brain barrier. Drug Metab Dispos 38:168–176 [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Dussor GO, Porreca F. (2010) Central modulation of pain. J Clin Invest 120:3779–3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald S, König J, Lütjohann D, Giessmann T, Kroemer HK, Rimmbach C, Rosskopf D, Fromm MF, Siegmund W. (2008) Disposition of ezetimibe is influenced by polymorphisms of the hepatic uptake carrier OATP1B1. Pharmacogenet Genomics 18:559–568 [DOI] [PubMed] [Google Scholar]
- Ott M, Fricker G, Bauer B. (2009) Pregnane X receptor (PXR) regulates P-glycoprotein at the blood-brain barrier: functional similarities between pig and human PXR. J Pharmacol Exp Ther 329:141–149 [DOI] [PubMed] [Google Scholar]
- Pelligrino DA, Vetri F, Xu HL. (2011) Purinergic mechanisms in gliovascular coupling. Semin Cell Dev Biol 22:229–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper MS. (1997) Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev 8:21–43 [DOI] [PubMed] [Google Scholar]
- Pizzagalli F, Hagenbuch B, Stieger B, Klenk U, Folkers G, Meier PJ. (2002) Identification of a novel human organic anion transporting polypeptide as a high affinity thyroxine transporter. Mol Endocrinol 16:2283–2296 [DOI] [PubMed] [Google Scholar]
- Pizzagalli F, Varga Z, Huber RD, Folkers G, Meier PJ, St-Pierre MV. (2003) Identification of steroid sulfate transport processes in the human mammary gland. J Clin Endocrinol Metab 88:3902–3912 [DOI] [PubMed] [Google Scholar]
- Price TO, Ercal N, Nakaoke R, Banks WA. (2005) HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Res 1045:57–63 [DOI] [PubMed] [Google Scholar]
- Raivich G, Bohatschek M, Kloss CU, Werner A, Jones LL, Kreutzberg GW. (1999) Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Res Brain Res Rev 30:77–105 [DOI] [PubMed] [Google Scholar]
- Rao VV, Dahlheimer JL, Bardgett ME, Snyder AZ, Finch RA, Sartorelli AC, Piwnica-Worms D. (1999) Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drug-permeability barrier. Proc Natl Acad Sci USA 96:3900–3905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S. (2003) Molecular insights on the cerebral innate immune system. Brain Behav Immun 17:13–19 [DOI] [PubMed] [Google Scholar]
- Roberts J, Ossipov MH, Porreca F. (2009) Glial activation in the rostroventromedial medulla promotes descending facilitation to mediate inflammatory hypersensitivity. Eur J Neurosci 30:229–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LM, Black DS, Raman C, Woodford K, Zhou M, Haggerty JE, Yan AT, Cwirla SE, Grindstaff KK. (2008) Subcellular localization of transporters along the rat blood-brain barrier and blood-cerebral-spinal fluid barrier by in vivo biotinylation. Neuroscience 155:423–438 [DOI] [PubMed] [Google Scholar]
- Roemer D, Pless J. (1979) Structure activity relationship of orally active enkephalin analogues as analgesics. Life Sci 24:621–624 [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee (2011) Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 123:e18–e209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson PT, Ashraf T, Bendayan R. (2010) Regulation of multidrug resistance protein 1 by tumor necrosis factor alpha in cultured glial cells: involvement of nuclear factor-kappaB and c-Jun N-terminal kinase signaling pathways. Mol Pharmacol 77:644–659 [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Bendayan R. (2008) HIV-1 viral envelope glycoprotein gp120 produces oxidative stress and regulates the functional expression of multidrug resistance protein-1 (Mrp1) in glial cells. J Neurochem 106:1298–1313 [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Davis TP. (2011) Targeting blood-brain barrier changes during inflammatory pain: an opportunity for optimizing CNS drug delivery. Ther Deliv 2:1015–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson PT, Davis TP. (2012) Blood-brain barrier integrity and glial support: mechanisms that can be targeted for novel therapeutic approaches in stroke. Curr Pharm Des 18:3624–3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson PT, Demarco KM, Sanchez-Covarrubias L, Solinsky CM, Davis TP. (2009) Transforming growth factor-beta signaling alters substrate permeability and tight junction protein expression at the blood-brain barrier during inflammatory pain. J Cereb Blood Flow Metab 29:1084–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson PT, Finch JD, Demarco KM, Quigley CE, Davis TP. (2011) Inflammatory pain signals an increase in functional expression of organic anion transporting polypeptide 1a4 at the blood-brain barrier. J Pharmacol Exp Ther 336:827–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson PT, Lee G, Dallas S, Bendayan R. (2004) Involvement of P-glycoprotein in the transport of saquinavir and indinavir in rat brain microvessel endothelial and microglia cell lines. Pharm Res 21:811–818 [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Persidsky Y, Bendayan R. (2008) Regulation of ABC membrane transporters in glial cells: relevance to the pharmacotherapy of brain HIV-1 infection. Glia 56:1711–1735 [DOI] [PubMed] [Google Scholar]
- Roth M, Obaidat A, Hagenbuch B. (2012) OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol 165:1260–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sai Y, Kaneko Y, Ito S, Mitsuoka K, Kato Y, Tamai I, Artursson P, Tsuji A. (2006) Predominant contribution of organic anion transporting polypeptide OATP-B (OATP2B1) to apical uptake of estrone-3-sulfate by human intestinal Caco-2 cells. Drug Metab Dispos 34:1423–1431 [DOI] [PubMed] [Google Scholar]
- Salvemini D, Doyle TM, Cuzzocrea S. (2006) Superoxide, peroxynitrite and oxidative/nitrative stress in inflammation. Biochem Soc Trans 34:965–970 [DOI] [PubMed] [Google Scholar]
- Satlin LM, Amin V, Wolkoff AW. (1997) Organic anion transporting polypeptide mediates organic anion/HCO3- exchange. J Biol Chem 272:26340–26345 [DOI] [PubMed] [Google Scholar]
- Sato K, Sugawara J, Sato T, Mizutamari H, Suzuki T, Ito A, Mikkaichi T, Onogawa T, Tanemoto M, Unno M, et al. (2003) Expression of organic anion transporting polypeptide E (OATP-E) in human placenta. Placenta 24:144–148 [DOI] [PubMed] [Google Scholar]
- Saunders NR, Habgood MD, Dziegielewska KM. (1999) Barrier mechanisms in the brain, I. Adult brain. Clin Exp Pharmacol Physiol 26:11–19 [DOI] [PubMed] [Google Scholar]
- Scafidi S, Douglas RM, Farahani R, Banasiak KJ, Haddad GG. (2007) Prostaglandin transporter expression in mouse brain during development and in response to hypoxia. Neuroscience 146:1150–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild L, Reiser G. (2005) Oxidative stress is involved in the permeabilization of the inner membrane of brain mitochondria exposed to hypoxia/reoxygenation and low micromolar Ca2+. FEBS J 272:3593–3601 [DOI] [PubMed] [Google Scholar]
- Schuster VL. (2002) Prostaglandin transport. Prostaglandins Other Lipid Mediat 68-69:633–647 [DOI] [PubMed] [Google Scholar]
- Seelbach MJ, Brooks TA, Egleton RD, Davis TP. (2007) Peripheral inflammatory hyperalgesia modulates morphine delivery to the brain: a role for P-glycoprotein. J Neurochem 102:1677–1690 [DOI] [PubMed] [Google Scholar]
- Seki T, Yun J, Oh SP. (2003) Arterial endothelium-specific activin receptor-like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ Res 93:682–689 [DOI] [PubMed] [Google Scholar]
- Sharom FJ, Yu X, Lu P, Liu R, Chu JW, Szabo K, Müller M, Hose CD, Monks A, Váradi A, et al. (1999) Interaction of the P-glycoprotein multidrug transporter (MDR1) with high affinity peptide chemosensitizers in isolated membranes, reconstituted systems, and intact cells. Biochem Pharmacol 58:571–586 [DOI] [PubMed] [Google Scholar]
- Shirasaka Y, Suzuki K, Nakanishi T, Tamai I. (2010) Intestinal absorption of HMG-CoA reductase inhibitor pravastatin mediated by organic anion transporting polypeptide. Pharm Res 27:2141–2149 [DOI] [PubMed] [Google Scholar]
- Shirasaka Y, Suzuki K, Shichiri M, Nakanishi T, Tamai I. (2011) Intestinal absorption of HMG-CoA reductase inhibitor pitavastatin mediated by organic anion transporting polypeptide and P-glycoprotein/multidrug resistance 1. Drug Metab Pharmacokinet 26:171–179 [DOI] [PubMed] [Google Scholar]
- Simpson IA, Carruthers A, Vannucci SJ. (2007) Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab 27:1766–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector R, Johanson CE. (1989) The mammalian choroid plexus. Sci Am 261:68–74 [DOI] [PubMed] [Google Scholar]
- Speth C, Dierich MP, Sopper S. (2005) HIV-infection of the central nervous system: the tightrope walk of innate immunity. Mol Immunol 42:213–228 [DOI] [PubMed] [Google Scholar]
- St-Pierre MV, Hagenbuch B, Ugele B, Meier PJ, Stallmach T. (2002) Characterization of an organic anion-transporting polypeptide (OATP-B) in human placenta. J Clin Endocrinol Metab 87:1856–1863 [DOI] [PubMed] [Google Scholar]
- Steckelbroeck S, Nassen A, Ugele B, Ludwig M, Watzka M, Reissinger A, Clusmann H, Lütjohann D, Siekmann L, Klingmüller D, et al. (2004) Steroid sulfatase (STS) expression in the human temporal lobe: enzyme activity, mRNA expression and immunohistochemistry study. J Neurochem 89:403–417 [DOI] [PubMed] [Google Scholar]
- Stedman CA, Liddle C, Coulter SA, Sonoda J, Alvarez JG, Moore DD, Evans RM, Downes M. (2005) Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc Natl Acad Sci USA 102:2063–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A, Yassouridis A, Szopko C, Helmke K, Stein C. (1999) Intraarticular morphine versus dexamethasone in chronic arthritis. Pain 83:525–532 [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kato Y, Tsuji A. (2006) Role of SLC xenobiotic transporters and their regulatory mechanisms PDZ proteins in drug delivery and disposition. J Control Release 116:238–246 [DOI] [PubMed] [Google Scholar]
- Sugiyama D, Kusuhara H, Taniguchi H, Ishikawa S, Nozaki Y, Aburatani H, Sugiyama Y. (2003) Functional characterization of rat brain-specific organic anion transporter (Oatp14) at the blood-brain barrier: high affinity transporter for thyroxine. J Biol Chem 278:43489–43495 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Onogawa T, Asano N, Mizutamari H, Mikkaichi T, Tanemoto M, Abe M, Satoh F, Unno M, Nunoki K, et al. (2003) Identification and characterization of novel rat and human gonad-specific organic anion transporters. Mol Endocrinol 17:1203–1215 [DOI] [PubMed] [Google Scholar]
- Svoboda M, Riha J, Wlcek K, Jaeger W, Thalhammer T. (2011) Organic anion transporting polypeptides (OATPs): regulation of expression and function. Curr Drug Metab 12:139–153 [DOI] [PubMed] [Google Scholar]
- Sykes D, Sweet DH, Lowes S, Nigam SK, Pritchard JB, Miller DS. (2004) Organic anion transport in choroid plexus from wild-type and organic anion transporter 3 (Slc22a8)-null mice. Am J Physiol Renal Physiol 286:F972–F978 [DOI] [PubMed] [Google Scholar]
- Tamai I, Nezu J, Uchino H, Sai Y, Oku A, Shimane M, Tsuji A. (2000) Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem Biophys Res Commun 273:251–260 [DOI] [PubMed] [Google Scholar]
- Tamai I, Nozawa T, Koshida M, Nezu J, Sai Y, Tsuji A. (2001) Functional characterization of human organic anion transporting polypeptide B (OATP-B) in comparison with liver-specific OATP-C. Pharm Res 18:1262–1269 [DOI] [PubMed] [Google Scholar]
- Tao-Cheng JH, Nagy Z, Brightman MW. (1987) Tight junctions of brain endothelium in vitro are enhanced by astroglia. J Neurosci 7:3293–3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taogoshi T, Nomura A, Murakami T, Nagai J, Takano M. (2005) Transport of prostaglandin E1 across the blood-brain barrier in rats. J Pharm Pharmacol 57:61–66 [DOI] [PubMed] [Google Scholar]
- Tirona RG, Leake BF, Wolkoff AW, Kim RB. (2003) Human organic anion transporting polypeptide-C (SLC21A6) is a major determinant of rifampin-mediated pregnane X receptor activation. J Pharmacol Exp Ther 304:223–228 [DOI] [PubMed] [Google Scholar]
- Tokui T, Nakai D, Nakagomi R, Yawo H, Abe T, Sugiyama Y. (1999) Pravastatin, an HMG-CoA reductase inhibitor, is transported by rat organic anion transporting polypeptide, oatp2. Pharm Res 16:904–908 [DOI] [PubMed] [Google Scholar]
- Tournier N, Declèves X, Saubaméa B, Scherrmann JM, Cisternino S. (2011) Opioid transport by ATP-binding cassette transporters at the blood-brain barrier: implications for neuropsychopharmacology. Curr Pharm Des 17:2829–2842 [DOI] [PubMed] [Google Scholar]
- Treiber A, Schneiter R, Häusler S, Stieger B. (2007) Bosentan is a substrate of human OATP1B1 and OATP1B3: inhibition of hepatic uptake as the common mechanism of its interactions with cyclosporin A, rifampicin, and sildenafil. Drug Metab Dispos 35:1400–1407 [DOI] [PubMed] [Google Scholar]
- Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, Terasaki T. (2011) Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J Neurochem 117:333–345 [DOI] [PubMed] [Google Scholar]
- Urquhart BL, Tirona RG, Kim RB. (2007) Nuclear receptors and the regulation of drug-metabolizing enzymes and drug transporters: implications for interindividual variability in response to drugs. J Clin Pharmacol 47:566–578 [DOI] [PubMed] [Google Scholar]
- Vadivelu N, Mitra S, Hines RL. (2011) Peripheral opioid receptor agonists for analgesia: a comprehensive review. J Opioid Manag 7:55–68 [DOI] [PubMed] [Google Scholar]
- Vaidyanathan S, Camenisch G, Schuetz H, Reynolds C, Yeh CM, Bizot MN, Dieterich HA, Howard D, Dole WP. (2008) Pharmacokinetics of the oral direct renin inhibitor aliskiren in combination with digoxin, atorvastatin, and ketoconazole in healthy subjects: the role of P-glycoprotein in the disposition of aliskiren. J Clin Pharmacol 48:1323–1338 [DOI] [PubMed] [Google Scholar]
- Vallejo R, Tilley DM, Vogel L, Benyamin R. (2010) The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract 10:167–184 [DOI] [PubMed] [Google Scholar]
- van Vliet EA, Redeker S, Aronica E, Edelbroek PM, Gorter JA. (2005) Expression of multidrug transporters MRP1, MRP2, and BCRP shortly after status epilepticus, during the latent period, and in chronic epileptic rats. Epilepsia 46:1569–1580 [DOI] [PubMed] [Google Scholar]
- Vangilder RL, Rosen CL, Barr TL, Huber JD. (2011) Targeting the neurovascular unit for treatment of neurological disorders. Pharmacol Ther 130:239–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma MV, Rotter CJ, Chupka J, Whalen KM, Duignan DB, Feng B, Litchfield J, Goosen TC, El-Kattan AF. (2011) pH-sensitive interaction of HMG-CoA reductase inhibitors (statins) with organic anion transporting polypeptide 2B1. Mol Pharm 8:1303–1313 [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. (2000) Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev 52:269–324 [PubMed] [Google Scholar]
- Wagner M, Halilbasic E, Marschall HU, Zollner G, Fickert P, Langner C, Zatloukal K, Denk H, Trauner M. (2005) CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology 42:420–430 [DOI] [PubMed] [Google Scholar]
- Wang P, Hata S, Xiao Y, Murray JW, Wolkoff AW. (2008) Topological assessment of oatp1a1: a 12-transmembrane domain integral membrane protein with three N-linked carbohydrate chains. Am J Physiol Gastrointest Liver Physiol 294:G1052–G1059 [DOI] [PubMed] [Google Scholar]
- Wang X, Sykes DB, Miller DS. (2010) Constitutive androstane receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier. Mol Pharmacol 78:376–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, et al. (2004) A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther 309:869–878 [DOI] [PubMed] [Google Scholar]
- Watabe T, Nishihara A, Mishima K, Yamashita J, Shimizu K, Miyazawa K, Nishikawa S, Miyazono K. (2003) TGF-beta receptor kinase inhibitor enhances growth and integrity of embryonic stem cell-derived endothelial cells. J Cell Biol 163:1303–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Rice KC, Maier SF. (2009) The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci 30:581–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver YM, Hagenbuch B. (2010) Several conserved positively charged amino acids in OATP1B1 are involved in binding or translocation of different substrates. J Membr Biol 236:279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Chigurupati S, Arumugam TV, Jo DG, Li H, Chan SL. (2011) Notch activation enhances the microglia-mediated inflammatory response associated with focal cerebral ischemia. Stroke 42:2589–2594 [DOI] [PubMed] [Google Scholar]
- Westholm DE, Salo DR, Viken KJ, Rumbley JN, Anderson GW. (2009a) The blood-brain barrier thyroxine transporter organic anion-transporting polypeptide 1c1 displays atypical transport kinetics. Endocrinology 150:5153–5162 [DOI] [PubMed] [Google Scholar]
- Westholm DE, Stenehjem DD, Rumbley JN, Drewes LR, Anderson GW. (2009b) Competitive inhibition of organic anion transporting polypeptide 1c1-mediated thyroxine transport by the fenamate class of nonsteroidal antiinflammatory drugs. Endocrinology 150:1025–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis CL, Leach L, Clarke GJ, Nolan CC, Ray DE. (2004a) Reversible disruption of tight junction complexes in the rat blood-brain barrier, following transitory focal astrocyte loss. Glia 48:1–13 [DOI] [PubMed] [Google Scholar]
- Willis CL, Nolan CC, Reith SN, Lister T, Prior MJ, Guerin CJ, Mavroudis G, Ray DE. (2004b) Focal astrocyte loss is followed by microvascular damage, with subsequent repair of the blood-brain barrier in the apparent absence of direct astrocytic contact. Glia 45:325–337 [DOI] [PubMed] [Google Scholar]
- Windass AS, Lowes S, Wang Y, Brown CD. (2007) The contribution of organic anion transporters OAT1 and OAT3 to the renal uptake of rosuvastatin. J Pharmacol Exp Ther 322:1221–1227 [DOI] [PubMed] [Google Scholar]
- Witt KA, Davis TP. (2006) CNS drug delivery: opioid peptides and the blood-brain barrier. AAPS J 8:E76–E88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt KA, Gillespie TJ, Huber JD, Egleton RD, Davis TP. (2001) Peptide drug modifications to enhance bioavailability and blood-brain barrier permeability. Peptides 22:2329–2343 [DOI] [PubMed] [Google Scholar]
- Witt KA, Mark KS, Huber J, Davis TP. (2005) Hypoxia-inducible factor and nuclear factor kappa-B activation in blood-brain barrier endothelium under hypoxic/reoxygenation stress. J Neurochem 92:203–214 [DOI] [PubMed] [Google Scholar]
- Witt KA, Slate CA, Egleton RD, Huber JD, Yamamura HI, Hruby VJ, Davis TP. (2000) Assessment of stereoselectivity of trimethylphenylalanine analogues of delta-opioid [D-Pen(2),D-Pen(5)]-enkephalin. J Neurochem 75:424–435 [DOI] [PubMed] [Google Scholar]
- Wolka AM, Huber JD, Davis TP. (2003) Pain and the blood-brain barrier: obstacles to drug delivery. Adv Drug Deliv Rev 55:987–1006 [DOI] [PubMed] [Google Scholar]
- Wood WG, Eckert GP, Igbavboa U, Müller WE. (2010) Statins and neuroprotection: a prescription to move the field forward. Ann N Y Acad Sci 1199:69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Ma J, Han JD, Wang N, Chen YG. (2006) Distinct regulation of gene expression in human endothelial cells by TGF-beta and its receptors. Microvasc Res 71:12–19 [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Sugie M, Okada M, Mikkaichi T, Toyohara T, Abe T, Goto J, Hishinuma T, Shimada M, Mano N. (2010) Transport of estrone 3-sulfate mediated by organic anion transporter OATP4C1: estrone 3-sulfate binds to the different recognition site for digoxin in OATP4C1. Drug Metab Pharmacokinet 25:314–317 [DOI] [PubMed] [Google Scholar]
- Yang L, Shah K, Wang H, Karamyan VT, Abbruscato TJ. (2011a) Characterization of neuroprotective effects of biphalin, an opioid receptor agonist, in a model of focal brain ischemia. J Pharmacol Exp Ther 339:499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wang H, Shah K, Karamyan VT, Abbruscato TJ. (2011b) Opioid receptor agonists reduce brain edema in stroke. Brain Res 1383:307–316 [DOI] [PubMed] [Google Scholar]
- Zhang J, Huang W, Chua SS, Wei P, Moore DD. (2002) Modulation of acetaminophen-induced hepatotoxicity by the xenobiotic receptor CAR. Science 298:422–424 [DOI] [PubMed] [Google Scholar]