Abstract
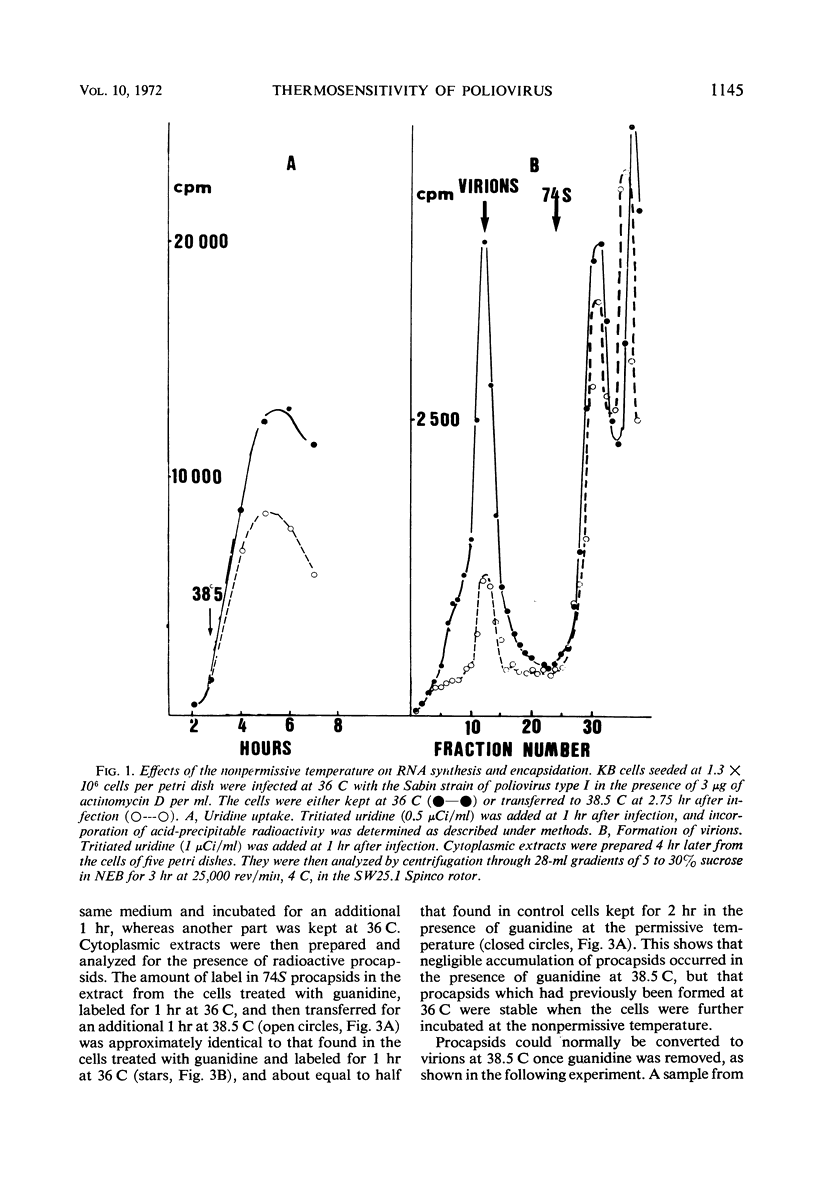
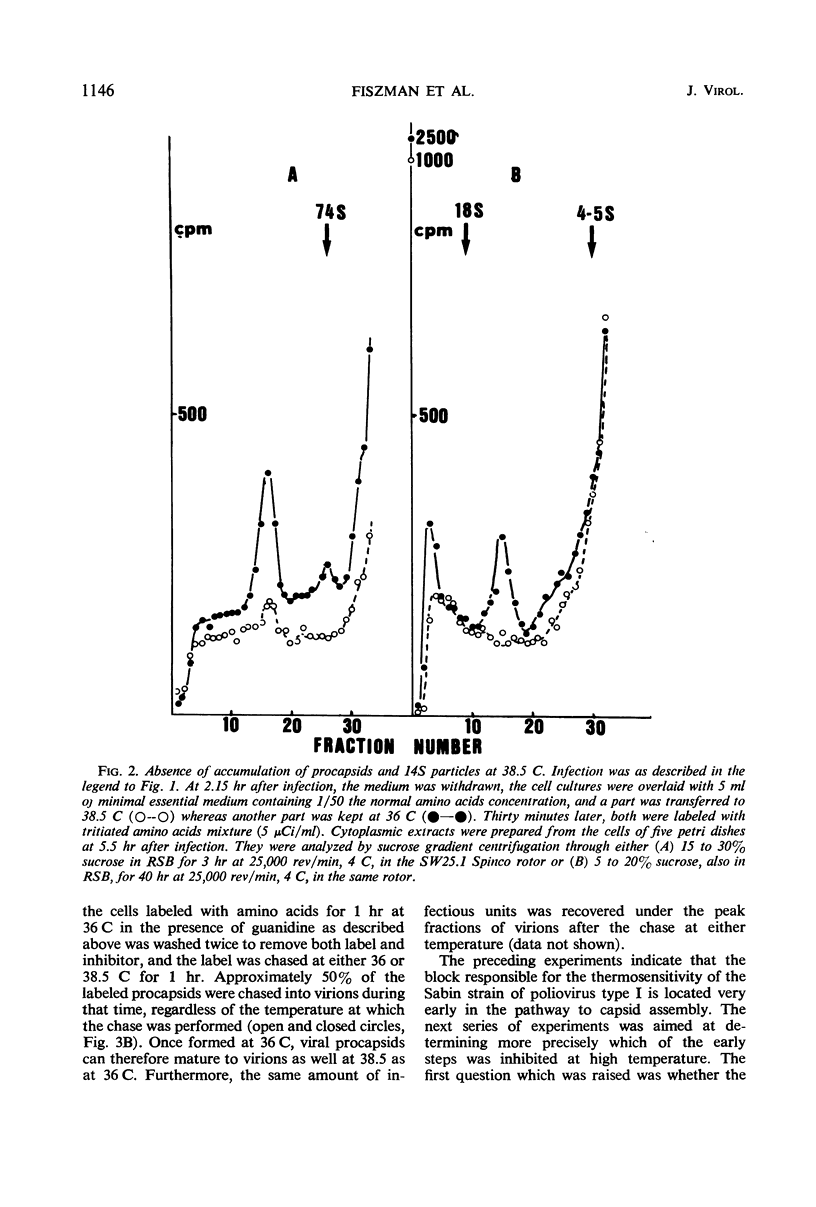
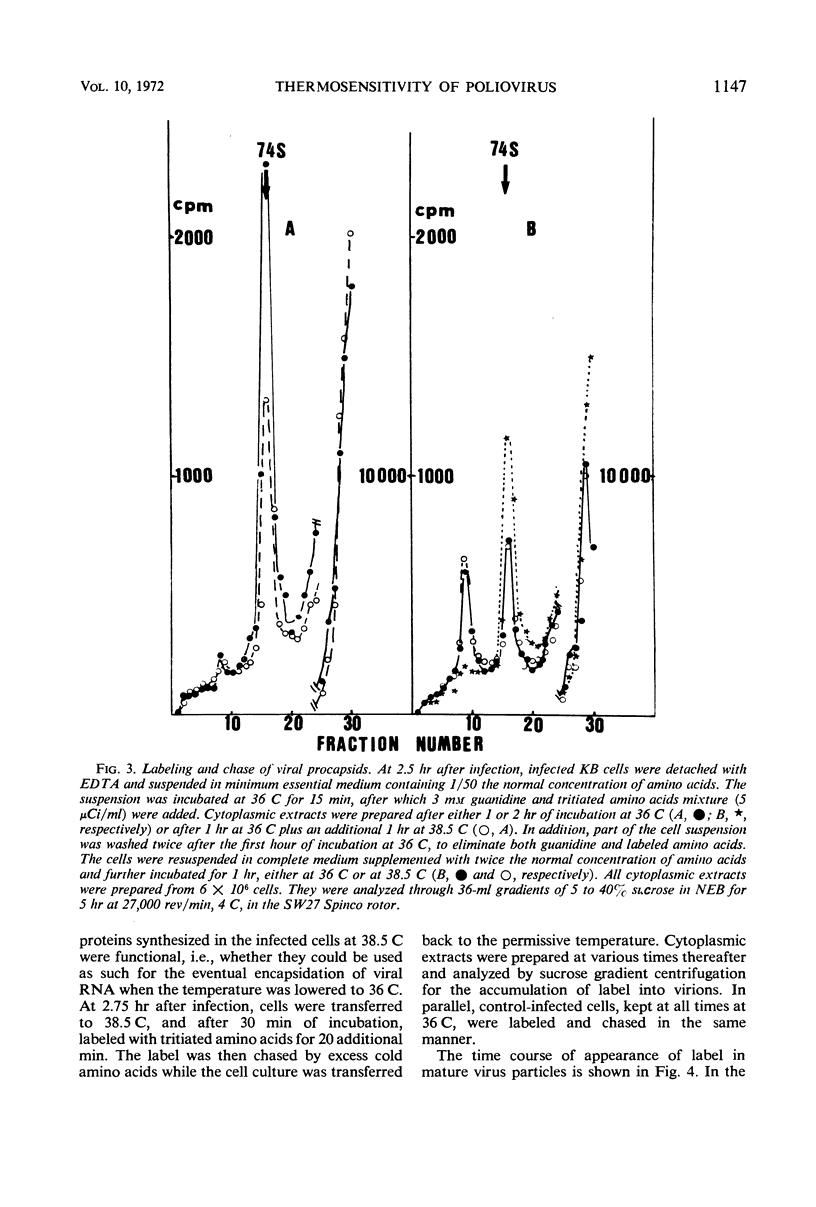
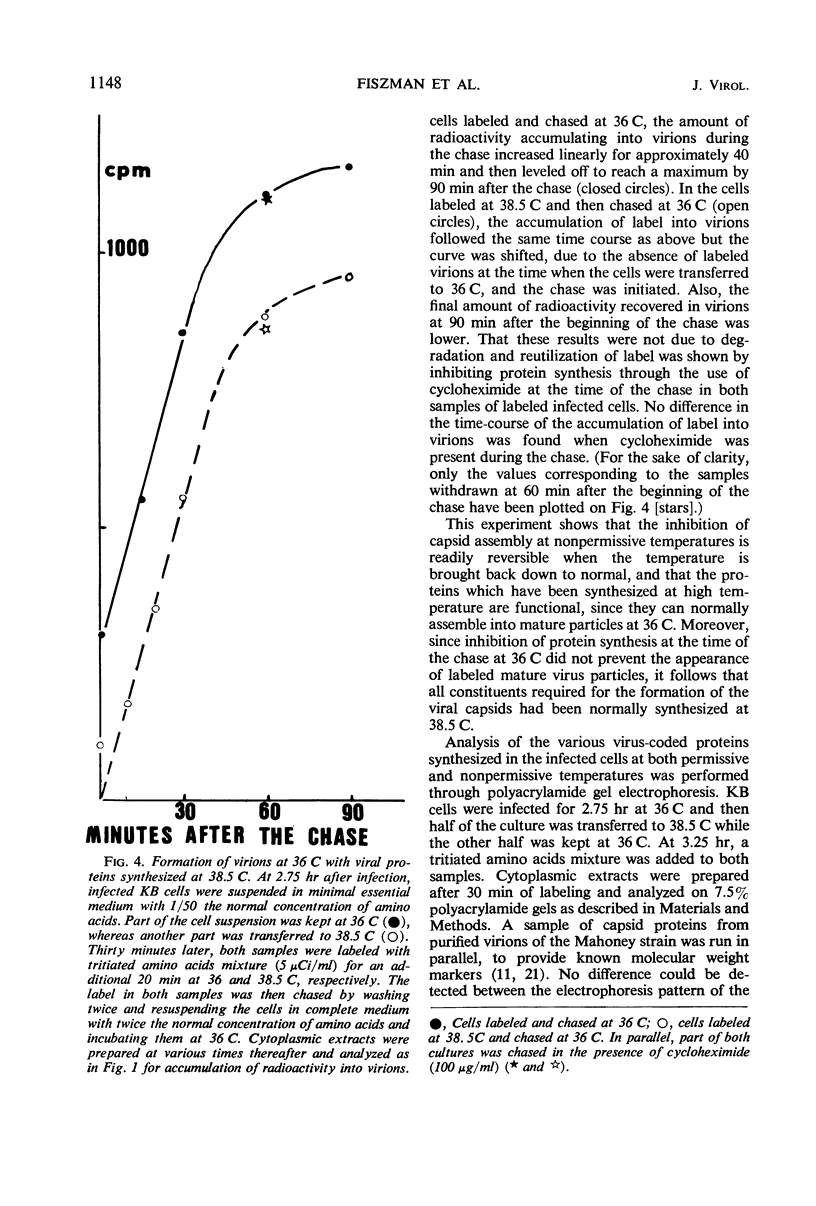
The thermosensitive defect of the Sabin LSc2ab strain of poliovirus type I was studied. Transfer of infected KB cells from 36 to 38.5 C resulted in 30% inhibition of viral RNA replication but in 90% inhibition of formation of virions. Neither 74S procapsids nor 14S particles were detected in the cells transferred to the non-permissive temperature. However, procapsids, once accumulated at 36 C, were normally stable at 38.5 C and could transform into virions at that temperature. Viral proteins synthesized at the nonpermissive temperature were not different from those synthesized at permissive temperature, as judged from their pattern in polyacrylamide gel electrophoresis and from the fact that they normally matured into virions when the infected cells were brought back to permissive temperature, even under conditions of inhibition of protein synthesis. This leads to the conclusion that the defect in the Sabin strain studied lies in the assembly of its viral capsid proteins into capsomeres.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Girard M., Darnell J. E. Aspects of the synthesis of poliovirus RNA and the formation of virus particles. Virology. 1966 Jun;29(2):179–189. doi: 10.1016/0042-6822(66)90024-9. [DOI] [PubMed] [Google Scholar]
- Borun T. W., Scharff M. D., Robbins E. Preparation of mammalian polyribosomes with the detergent Nonidet P-40. Biochim Biophys Acta. 1967 Nov 21;149(1):302–304. doi: 10.1016/0005-2787(67)90715-0. [DOI] [PubMed] [Google Scholar]
- Fiszman M. Y., Bucchini D., Girard M., Lwoff A. Inhibition of poliovirus RNA synthesis by supraoptimal temperatures. J Gen Virol. 1970 Feb;6(2):293–304. doi: 10.1099/0022-1317-6-2-293. [DOI] [PubMed] [Google Scholar]
- Fiszman M., Bucchini D., Girard M. Purification of the Sabin strain of poliovirus type I through treatment with sarkozyl. J Virol. 1971 May;7(5):687–689. doi: 10.1128/jvi.7.5.687-689.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel-Conrat H. Reconstitution of viruses. Annu Rev Microbiol. 1970;24:463–478. doi: 10.1146/annurev.mi.24.100170.002335. [DOI] [PubMed] [Google Scholar]
- Garfinkle B. D., Tershak D. R. Effect of temperature on the cleavage of polypeptides during growth of LSc poliovirus. J Mol Biol. 1971 Aug 14;59(3):537–541. doi: 10.1016/0022-2836(71)90318-4. [DOI] [PubMed] [Google Scholar]
- Garfinkle D. B., Tershak D. R. Degradation of poliovirus polypeptides in vivo. Nat New Biol. 1972 Aug 16;238(85):206–208. doi: 10.1038/newbio238206a0. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Kiehn E. D. Specific cleavage of viral proteins as steps in the synthesis and maturation of enteroviruses. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1015–1022. doi: 10.1073/pnas.60.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Morphogenesis of poliovirus. I. Association of the viral RNA with coat protein. J Mol Biol. 1968 Apr 28;33(2):369–378. doi: 10.1016/0022-2836(68)90195-2. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LWOFF A. The thermosensitive critical event of the viral cycle. Cold Spring Harb Symp Quant Biol. 1962;27:159–174. doi: 10.1101/sqb.1962.027.001.018. [DOI] [PubMed] [Google Scholar]
- Lab M., Kirn A. Variations de la thermosensibilité du développement du virus Sindbis en fonction du système cellularie. C R Acad Sci Hebd Seances Acad Sci D. 1969 May 28;268(21):2624–2627. [PubMed] [Google Scholar]
- Lwoff A. Death and transfiguration of a problem. Bacteriol Rev. 1969 Sep;33(3):390–403. doi: 10.1128/br.33.3.390-403.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips B. A. In vitro assembly of polioviruses. I. Kinetics of the assembly of empty capsids and the role of extracts from infected cells. Virology. 1969 Dec;39(4):811–821. doi: 10.1016/0042-6822(69)90018-x. [DOI] [PubMed] [Google Scholar]
- Phillips B. A., Summers D. F., Maizel J. V., Jr In vitro assembly of poliovirus-related particles. Virology. 1968 Jun;35(2):216–226. doi: 10.1016/0042-6822(68)90262-6. [DOI] [PubMed] [Google Scholar]
- Priess H., Eggers H. J. Synthesis and activity of RNA polymerase of a temperature sensitive poliovirus mutant at an elevated temperature. Nature. 1968 Dec 7;220(5171):1047–1048. doi: 10.1038/2201047a0. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Hebert R. R., Hartman K. A. Ribonucleoprotein complexes formed between bacteriophage MS2 RNA and MS2 protein in vitro. J Mol Biol. 1967 May 14;25(3):455–463. doi: 10.1016/0022-2836(67)90198-2. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Aug;54(2):505–513. doi: 10.1073/pnas.54.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Evidence for large precursor proteins in poliovirus synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):966–971. doi: 10.1073/pnas.59.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tershak D. R. Synthesis of ribonucleic acid in cells infected with LSc poliovirus at elevated temperatures. J Virol. 1969 Mar;3(3):297–303. doi: 10.1128/jvi.3.3.297-303.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



