Abstract
Purpose of review
To discuss recent findings on the role and regulation of macrophage polarization in obesity and atherosclerosis.
Recent findings
Macrophages infiltrate the vascular wall during atherosclerosis and adipose tissue during obesity. At least two distinct sub-populations with different functions, the classically (M1) and the alternatively (M2) activated macrophages, have been found in these tissues. Reciprocal skewing of macrophage polarization between the M1 and M2 states is a process modulated by diet, humoral and transcription factors, such as the nuclear receptor Peroxisome Proliferator-Activated Receptor gamma (PPARγ).
Summary
Recent literature highlights the importance not only of the number of infiltrated macrophages, but also their activation in the maintenance of the inflammation state. Identifying mechanisms and molecules able to modify the balance between M1 and M2 represents a promising field of research.
Keywords: Adipose Tissue, immunology, Animals, Humans, Inflammation, immunology, Macrophages, cytology, metabolism, Metabolic Diseases, immunology
Keywords: macrophages, obesity, atherosclerosis, nuclear receptors
INTRODUCTION
Monocyte/macrophages are heterogeneous, versatile cells which functionally adapt to specific microenvironmental signals. Differential expression of selected surface markers is used to identify monocyte and macrophage sub-populations. Many of the used markers differ, however, between mice and humans.
In humans, the presence of CD16 distinguishes two monocyte subsets. CD14+/CD16− monocytes (representing approximately 90% of circulating monocytes), which express high levels of the chemokine receptor CCR2 (MCP-1 receptor) and low levels of CX3CR1 (fractalkine receptor). CD14+/CD16+ monocytes display high CX3CR1 expression and a pro-inflammatory profile [1]. Mouse monocytes are distinguished on the basis of Ly6C antigen expression [2]. The short-lived CXCR1lowCCR2(+)Ly6Chigh inflammatory monocytes are actively recruited to inflamed tissues, while the CX3CR1highCCR2(−)Ly6Clow subset are characterized by a CX3CR1-dependent recruitment to non-inflamed tissues.
Diverse macrophage populations have also been described, but the relationship between the monocyte subsets and macrophage phenotype is unclear. Classically activated M1 macrophages, driven by Th1 cytokines (IFNγ, TNF) or by LPS recognition, produce high levels of IL-12 and IL-23 (in humans) [3] and low levels of IL-10, secrete pro-inflammatory cytokines (TNF, IL-6, IL1β) and exhibit potent microbicidal properties, thus conferring a Th1-like response. During the inflammatory response, inflammation is usually spatially and temporally counteracted by protective mechanisms operated by the alternatively activated M2 macrophages. The M2 alternative phenotype is typically driven by Th2 cytokines. 3 different sub-classes have been identified: IL-4 and IL-13 lead to M2a macrophages, immune complexes in combination with IL-1β or LPS drive the M2b subtype, while IL-10, TGFβ or glucocorticoids induce M2c macrophages. M2 macrophages are characterized by high IL-10 and TGFβ (especially prominent in M2c) and low IL-12 production, high endocytic clearance capacities, protecting surrounding tissues from a detrimental immune response [4]. An exception are the M2b macrophages, which are functionally anti-inflammatory, but still produce high amounts of inflammatory cytokines [5].
Moreover, human monocytes differentiated in the presence of granulocyte–macrophage colony stimulating factor (GM-CSF) or M-CSF, respectively, display M1 and M2 properties, and have been referred to as Mø1 and Mø2 [3]. More recently, the platelet factor-4 (CXCL4) was shown to induce a unique transcriptional profile generating M4 macrophages, characterized by reduced CD163 and other scavenger receptor expression as well as phagocytic capacity, while some M1 and M2 markers are over-represented [6•, 7]. This classification system represents the extremes of macrophage activation states and probably simplifies the in vivo situation where the full phenotypic spectrum between M1 and M2 can exist [8]. Moreover, an alternative classification of macrophage populations has been proposed based on three different macrophage homeostatic activities: host defence, wound healing and immune regulation [8]. This classification distinguishes classically activated macrophages, wound-healing macrophages (corresponding to the alternatively activated macrophages) and regulatory macrophages [8]. This latter polarization, induced by TLR agonists in combination with other stimuli (amongst which IgG immune complexes, prostaglandins, apoptotic cells), produces high levels of IL-10, thus resembling the M2b phenotype.
Although general properties of macrophages are conserved between species, there are some significant differences between mice and humans. For example markers such as Ym1 (also called chitinase 3-like 3), the transcription factor Found in Inflammatory Zone 1 (FIZZ1) and arginase 1 (Arg1), and more generally arginine metabolism, are characteristic of the M2 phenotype in mouse but not in human macrophages [9] (see table 1).
Table 1. Mouse and human macrophage sub-population markers.
Some markers are common amongst different macrophage subtypes, suggesting that overlapping phenotypes can exist. Caution is required when a novel macrophage sub-type is identified solely on the basis of the expression of one or two markers.
| Markers | Mouse | Human |
|---|---|---|
| M2 macrophages | ||
| Arginase 1 (Arg1) | + | |
| FIZZ1 | + | |
| Ym1 | + | |
| MGL1 | + | |
| AMAC1 | + | |
| MR (CD206) | + | + |
| CD163 | + | + |
| Stabilin 1 (stab1) | + | + |
| Selenoprotein 1 (sepp1) | + | + |
| CD163L1 | + | + |
| Pro-platelet basic protein (ppbp) | + | + |
| IL1R antagonist (IL1Ra) | + | + |
| IL-10 | + | + |
| CD209a | + | |
| CD150 | + | |
| HA macrophages | ||
| CD163 | + | |
| IL-10 | + | |
| Mox macrophages | ||
| Heme oxygenase -1 (HO-1) | + | |
| Sulforedoxin-1 (Srxn-1) | + | |
| Thioredoxin-1 reductase (Txnrd-1) | + |
Macrophage sub-populations in pathophysiology
The role of alternative macrophages in tumorigenesis is well established. Tumour-associated macrophages (TAM), display an alternative-like activation phenotype and play a detrimental pro-tumoural role [10]. In this review we will focus on the involvement of alternative macrophages in cardio-vascular and metabolic disorders such as atherosclerosis and obesity (figure 1).
Figure 1.
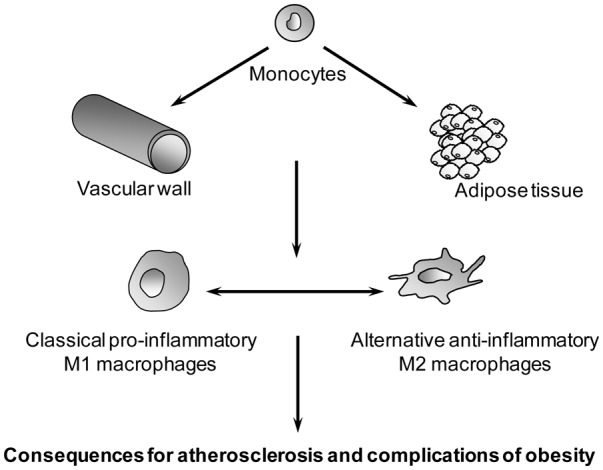
Circulating monocytes can infiltrate the vascular wall during atherosclerosis and adipose tissue during obesity and can differentiate in different macrophage sub-populations, exemplified by the extreme pro-inflammatory M1 and anti-inflammatory M2 macrophage subtypes
Macrophage sub-populations and atherosclerosis
Only few data report the presence/characterization of alternative macrophages within atherosclerotic plaques in mice. In apoE-deficient mice on chow diet, early lesions (20 weeks) contain Arg1-positive M2 macrophages, whereas Arg2-positive M1 macrophages appear and prevail in lesions of aged mice (55 weeks). Lesion progression is thus associated with the predominance of the M1 over the M2 phenotype [11]. M2 to M1 phenotype switching of pre-existing cells rather a direct recruitment of M1 macrophages likely occurs in this model. High-fat diet feeding of 10 week-old apoE-deficient mice induced the expression of the M2-associated genes, selenoprotein-1 (SEPP1), stabilin-1 (STAB1) and CD163 molecule-like-1 (CD163L1) in parallel with atherosclerotic plaque development. In contrast, expression of the M1-related marker, pro-platelet basic protein (PPBP), increased only in very early lesions (6 weeks of diet) returning to basal levels in late stages (12 weeks of diet) [12]. A distinct macrophage phenotype, strikingly different from the conventional M1 and M2 macrophages, has been reported in atherosclerotic lesions of LDL receptor-deficient mice [13••]. This phenotype, called Mox, is induced by oxydized phospholipids and is characterized by high expression of heme oxygenase 1 (HO-1), sulforedoxin-1 (Srnx-1) and thioredoxin-1 reductase (txnrd-1), all redox-regulatory genes under the control of the Nrf2 transcription factor [13••]. Mox macrophages represent approximately 30% of all macrophages in murine advanced atherosclerotic lesions.
Using the expression levels of different macrophage markers, as well as morphology and phenotypic analysis, several macrophage sub-populations have been identified in human atherosclerosis. In human coronary arteries, macrophages expressing both CD68 and CD14 appear, while a CD68+CD14− sub-population appear predominant in areas devoid of disease [14]. While the CD68+CD14+ cells express many pro-inflammatory genes, the CD68+CD14− cells express high levels of genes involved in reverse cholesterol transport (PPARγ, LXRα, ABCG1) and macrophage emigration (CCR7) from the vessel wall [14]. These two populations are similar to monocytes in vitro differentiated with M-CSF or GM-CSF, respectively [14]. Similarly, using the M-CSF (M2) vs GM-CSF (M1) macrophage model, the M2 phenotype-associated genes, SEPP1, STAB-1 and CD163L1, were found to be enriched in human atherosclerotic plaques compared to fatty streaks or normal arteries [12].
We identified CD68+MR− (M1) macrophages in the lipid core of human carotid atherosclerotic lesions, while CD68+MR+ (M2) macrophages prevail in the shoulder region as well as in the periphery of the plaque [15, 16]. CD68+MR+ macrophages appear smaller and contain several small lipid droplets in their cytoplasm, while CD68+MR− macrophages contain fewer, but bigger lipid droplets. In vitro IL4-polarized M2 macrophages are less competent to captate native and oxidized lipoproteins. Whereas CD68+MR+ macrophages display lower cholesterol-handling capacity, they are competent for phagocytosis, since the expression of opsonins and receptors involved in phagocytosis is high in these cells [16••]. Moreover, hemorrhaged atherosclerotic plaques contain hemorrhage-associated macrophages (HA-mac) [17], which contain more iron, express high levels of CD163 and thus highly scavenge the haemoglobin/haptoglobin complex which induces IL-10 secretion and monocyte differentiation to M2 macrophages. Differentiation to HA-mac was prevented by neutralizing IL-10 antibodies, indicating that IL-10 mediates an autocrine feedback mechanism. Whether these different populations represent completely different polarization states or whether they display overlapping phenotypes, is a challenging question.
Macrophage sub-populations and obesity
Substantial evidences at the cellular and molecular level indicate that obesity is a chronic low-grade inflammatory disease [18]. Monocytes infiltrate adipose tissue during obesity and differentiate in adipose tissue macrophages (ATM) [19]. ATM from lean mice express many genes characteristic of M2 macrophages, which may protect adipocytes from inflammation, while diet-induced obesity led to a shift in the activation state to an M1 pro-inflammatory state that contributes to insulin resistance [20–22]. Comparative studies revealed that the majority of adipose tissue-produced cytokines (TNF, IL-6), with the exception of leptin and adiponectin, are secreted by non-adipocyte cells and in particular by M1 polarized macrophages. These pro-inflammatory cytokines may contribute to the low-grade inflammatory state. Furthermore, while M2 ATM, which express N-acetyl-galactosamine specific lectin 1 (MGL1), are localized in the interstitial space, MGL1−/CD11c+ M1 ATM rather surround death adipocytes thus forming the crown-like structures (CLS) [23]. The CD11c surface molecule is considered as an M1 marker and its expression in ATM is considerably increased upon high-fat diet feeding [20, 24]. The obesity-induced switch from the M2 to M1 phenotype was attributed to a CCR2-dependent monocyte recruitment rather than to the conversion of pre-existing M2 macrophages [23].
However, this strict spatiotemporal polarization concept has been challenged more recently. Indeed, mouse epidydimal ATM recruited in response to a high fat-diet display a mixed M1/M2 phenotype and their transcription profile became more M2-like upon diet duration extension [25]. Using MR and CD11c as markers, three distinct ATM populations have been described [26]. Obesity promotes a shift from a predominant MR+CD11c− population (expressing marginally M1 and M2 markers, but high levels of MCP-1 and CCL7) to two MR− populations: MR−CD11c+ cells exhibiting a M1 inflammatory phenotype and MR−CD11c− cells expressing low levels of inflammatory markers and high levels of M2 markers such as Arg1 and Ym1 [26]. Analysis of chemokine receptors identified CCR2, CCR5, CCR3 and CX3CR1 to be expressed on both MR−CD11c+ and MR−CD11c−, whereas CCR7 and CCR9 were selectively expressed in MR−CD11c+and MR−CD11c−, respectively [26]. These data reveal previously unappreciated similarities between the murine and human ATM phenotypes.
In humans, the amount of ATM correlates with BMI, adipocyte size and total body fat mass [27]. Fat mass expansion is associated with the accumulation of anti-inflammatory or mixed M1/M2 polarized ATM [28], characterized by increased MMP activities, indicating a role in tissue remodelling [29]. Human ATM produce the anti-inflammatory cytokines IL-10 and IL-1Ra, but can also secrete pro-inflammatory cytokines such as TNF, IL-6 and IL-1β supporting a role of ATMs in obesity-induced adipocyte dysfunction and metabolic disorders [28]. Together with increased macrophage numbers, adipose tissue from obese subjects contain increased areas of fibrosis, when compared to lean subjects [30]. The majority of macrophages appear rather associated with fibrosis, than in CLS. CLS macrophages are predominantly M1, whereas other macrophages, particularly those in fibrotic areas, are CD150+, a M2c marker [30].
Regulation of macrophage polarization in metabolic disorders: lessons from humans and mice
Regulation by lifestyle and diet
In obese mice, progressive lipid accumulation in macrophages with age provokes a M2 to M1 polarization [31]. Nearly 50% of the ATM of obese mice accumulate lipids and resemble atherosclerotic foam cells. Lipid accumulation was associated with increased CD11c+ ATM (M1) and decreased CD209a+ ATM (M2). However, a unique subclass of lipid loaded CD209a+/CD11c+ has been identified. Diphtheria toxin–induced death of CD11c+ cells reduces CLS formation and improves insulin sensitivity [32], emphasizing the pathogenic role of CD11c+ M1 macrophages. Stimulation of obese db/db mice with resolvin D1, a docosahexaenoic acid-derived anti-inflammatory mediator, reduced the number of M1 macrophages resident in CLS and increased the percentage of MGL1+ macrophages [33].
Gastric surgery of obese subjects modulated the M1/M2 balance by increasing the number of M2 and decreasing the number of M1 macrophages in sub-cutaneous adipose tissue [34]. Weight stabilization by 6-month of very low calorie diet decreased the amount of ATM, without changing the relative proportion of M1/M2 macrophages [35]. The discrepancies between these two studies can be due to differences in the type of patient population and/or cell surface markers used to identify ATM sub-populations.
Exercise training promoted M1 to M2 phenotype switching of adipose tissue macrophages in high fat diet-induced obese mice [36] and enhanced the expression of M2 markers of circulating leukocytes in humans, an effect which may be related to an increased expression of PPARγ and its cofactors [37].
Regulation by humoral factors
Macrophage polarization can be modulated by different factors. Among these, C-reactive protein [38•], angiotensin type 1 receptor [39] and activin A [40] block the M2 phenotype, while adiponectin [41, 42], apolipoprotein E [43•], interleukin 33 (IL-33) [44] and angiotensin converting enzyme (ACE) [45] favour an M2 differentiation. IL-4 is one of the most potent M2-drivers. In adipose tissue, eosinophils are the major IL-4 producing cells and in their absence the macrophage M2 phenotype is greatly attenuated [46••].
Deficiency in CD40/tumor necrosis factor receptor-associated factor (TRAF)6 signalling skewed the immune response toward an anti-inflammatory M2 profile, thus preventing atherosclerosis [47]. In addition to immune-inflammatory factors, high density lipoproteins (HDL) also decrease the expression of pro-inflammatory factors and increase M2 markers (Arg1, MR, CD163) in mice, in an apoAI-dependent manner [48••].
Regulation by transcription factors
Transcription factor networks are important regulators of macrophage polarization. Among these, nuclear receptor family members, including the Peroxisome Proliferator-Activated Receptors (PPARα, PPARγ and PPARβ/δ) and the Liver X Receptors (LXRα and LXRβ) are highly expressed in macrophages where they control the inflammatory response [49]. Glass et al. reported that IL-4 induces PPARγ expression and activity by generating natural PPARγ ligands via the 12/15-lipoxygenase pathway [50]. Furthermore, IL-4 signalling enhances PPARγ activity through an interaction between PPARγ and the Signal Transducer and Activators of Transcription (STAT)6 on promoters of PPARγ-target genes [51]. In response to IL-4, STAT6 and the PPARγ-coactivator-1beta (PGC-1β) induce macrophage programs for fatty acid oxidation and mitochondrial biogenesis [52] (figure 2). IL-4 and IL-13 also induce PPARβ/δ expression through a STAT6 site in its promoter to activate alternative differentiation [53].
Figure 2.
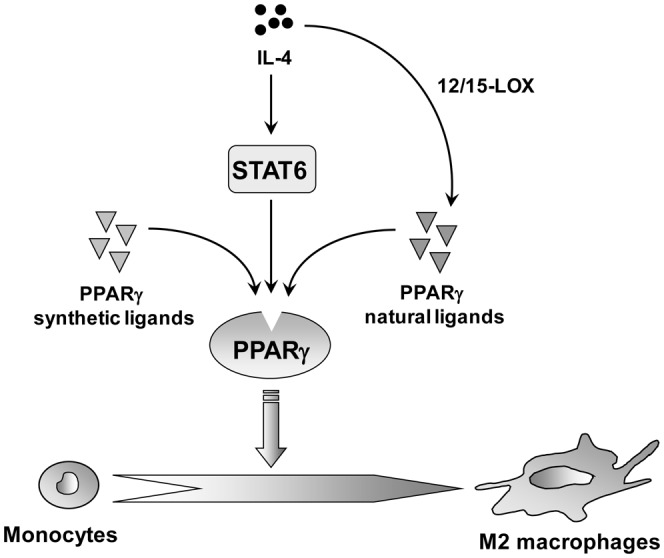
PPARγ activation promotes alternative macrophage differentiation.
IL-4 induces PPARγ expression and activity by generating natural PPARγ ligands via the 12/15-lipoxygenase (12/15 LOX) pathway PPARγ activation by its ligands promotes monocyte differentiation toward M2 macrophages in mice and humans.
The first evidence of modulation of macrophage polarization by nuclear receptors came from observations related to obesity. Macrophage-specific PPARγ deletion in Th2-oriented Balbc mice identified a role for PPARγ in the polarization of macrophages toward an alternative phenotype. Disruption of PPARγ in myeloid cells impairs the alternative differentiation, an effect associated with the development of diet-induced obesity, insulin resistance and glucose intolerance [54]. Myeloid PPARβ/δ-deficiency rendered macrophages unable to shift toward an alternative phenotype, causing inflammation and metabolic perturbations in adipocytes and hepatocytes leading to insulin resistance, increased adipocyte lipolysis and hepatosteatosis [53, 55].
However, there is some controversy, since Marathe et al. reported that Th1-oriented C57BL/6 mouse macrophages lacking PPARγ, PPARβ/δ or LXRα/β display unaltered expression profiles of alternative macrophage markers and presented normal glucose tolerance upon bone marrow transplantation [56]. This suggests that macrophage alternative polarization is not completely dependent on nuclear receptor signalling per se, and the effects can be modulated by the genetic background.
In obese mice, treatment with the PPARγ ligand rosiglitazone promotes lipid redistribution from macrophages towards adipocytes thus restoring a M2 phenotype [31]. In line, treatment with PPARγ ligands, rosiglitazone and pioglitazone, as well as telmisartan, an angiotensin II type 1 receptor blocker with PPARγ agonist activity [57], decreased the number of M1 macrophages in visceral adipose tissue [58] and enhanced the expression of M2 markers thus improving insulin sensitivity [25, 59].
Information on the role of nuclear receptors in macrophage polarization in the context of atherosclerosis is scarce. In the Reversa mouse model (Ldlr−/−Apob100/100Mttpfl/flMx1-Cre+/+), treatment with the PPARγ ligand pioglitazone during the phase of atherosclerosis regression significantly enhanced the expression of M2 markers in parallel with a reduction of plaque macrophage and lipid content [60].
PPARγ activation also promotes IL-4 induced M2 differentiation of primary human monocytes resulting in a more pronounced M2 anti-inflammatory activity [15]. In vivo administration of pioglitazone to humans increased MR expression in peripheral blood mononuclear cells [15]. In contrast to PPARγ, the expression of PPARα and PPARβ/δ does not correlate with the expression of M2 markers in atherosclerotic plaques [61]. Moreover, PPARα or PPARβ/δ activation does not enhance IL-4-promoted alternative macrophage differentiation of human monocytes [61].
Despite their important role in the inflammatory response, whether LXRs influence macrophage polarization has not yet been reported. However, LXRα expression and signalling is altered in human IL-4 polarized M2 macrophages [16••]. These cells display a reduced capacity to handle and efflux cellular cholesterol due to low expression levels of LXRα and its target genes, ABCA1 and ApoE [16••]. The decreased LXRα expression and activity in M2 macrophages is likely related to an enhanced 15-lipoxygenase activity. As a consequence, PPARγ activation has no effect on the cholesterol efflux pathways. By contrast, PPARγ activation induces the expression of thrombospondin-1 in M2 macrophages, thus enhancing their phagocytic activity [16••].
Glucocorticoid receptor (GR) activation induces a unique M2 phenotype, characterized by the high expression levels of CD36, CD163, IL-10 and IL-1 receptor type II and enhanced antioxidative homeostasis and iron recycling [62, 63]. Treatment of high-fat diet-fed mice with dexamethasone prevented ATM accumulation, an effect most pronounced for the F4/80+/CD11b+/CD11c+ M1 subset [64].
Other nuclear receptors can also control macrophage polarization. As an example, the mineralocorticoid receptor (MR) is necessary for efficient macrophage polarization by inflammatory cytokines [65]. Macrophages differentiated from MR-deficient myeloid cells display a transcription profile of alternative activation and protect against cardiac hypertrophy and fibrosis.
CONCLUSIONS
Although significant progress has been made in characterizing the phenotype and functions of macrophage sub-populations during obesity and atherosclerosis development, much remains to be discovered. It appears of crucial importance to define a panel of markers in mice and humans allowing identification of the macrophage sub-types in different microenvironments. Investigations on the role of these different macrophages in adipose tissue and vascular wall patho-physiology and identification of endogenous as well as novel synthetic molecules controlling the M1/M2 macrophage balance, among which potential nuclear receptor ligands, represent exciting areas of future research which may lead to possible therapeutic applications.
KEY POINTS.
Macrophages are a heterogeneous cell population exhibiting a wide spectrum of phenotypes;
Classically M1 and alternatively M2 activated macrophages are found in adipose tissue and atherosclerotic plaques of the vascular wall;
M1 and M2 macrophages are phenotypically and functionally different;
Macrophage M1 to M2 skewing is transcriptionally regulated by nuclear receptors including PPARγ
Acknowledgments
Grants from the Région Nord-Pas de Calais/FEDER (CPER N. 1449), COST actions BM0602 and BM0904, the Agence Nationale de la Recherche (AlMHA project), the transatlantic Leducq HDL Network, the Fondation Coeur et Artères and the European Community’s 7th Framework Programme (FP7/2007-2013, grant agreement n° 201608) are acknowledged.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Ziegler-Heitbrock L, Ancuta P, Crowe S, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 2.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 3.Verreck FA, de Boer T, Langenberg DM, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci U S A. 2004;101:4560–5. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantovani A, Sica A, Sozzani S, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Anderson CF, Gerber JS, Mosser DM. Modulating macrophage function with IgG immune complexes. J Endotoxin Res. 2002;8:477–81. doi: 10.1179/096805102125001118. [DOI] [PubMed] [Google Scholar]
- 6•.Gleissner CA, Shaked I, Little KM, Ley K. CXC chemokine ligand 4 induces a unique transcriptome in monocyte-derived macrophages. J Immunol. 2010;184:4810–8. doi: 10.4049/jimmunol.0901368. This paper reports a unique macrophage transcriptome induced by CXCL4, distinct from known macrophage sub-types. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gleissner CA, Shaked I, Erbel C, et al. CXCL4 downregulates the atheroprotective hemoglobin receptor CD163 in human macrophages. Circ Res. 2010;106:203–11. doi: 10.1161/CIRCRESAHA.109.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–11. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–7. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Khallou-Laschet J, Varthaman A, Fornasa G, et al. Macrophage plasticity in experimental atherosclerosis. PLoS One. 2010;5:e8852. doi: 10.1371/journal.pone.0008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brocheriou I, Maouche S, Durand H, et al. Antagonistic regulation of macrophage phenotype by M-CSF and GM-CSF: implication in atherosclerosis. Atherosclerosis. 2011;214:316–24. doi: 10.1016/j.atherosclerosis.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 13••.Kadl A, Meher AK, Sharma PR, et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107:737–46. doi: 10.1161/CIRCRESAHA.109.215715. This study characterizes Mox macrophages in murine atherosclerotic lesions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waldo SW, Li Y, Buono C, et al. Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am J Pathol. 2008;172:1112–26. doi: 10.2353/ajpath.2008.070513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouhlel MA, Derudas B, Rigamonti E, et al. PPARg activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metabolism. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 16••.Chinetti-Gbaguidi G, Baron M, Bouhlel MA, et al. Human Atherosclerotic Plaque Alternative Macrophages Display Low Cholesterol Handling but High Phagocytosis Because of Distinct Activities of the PPAR{gamma} and LXRα Pathways. Circ Res. 2011;108:985–95. doi: 10.1161/CIRCRESAHA.110.233775. This study characterizes the LXR/PPARγ signalling pathway in human alternative macrophages from atherosclerotic lesions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyle JJ, Harrington HA, Piper E, et al. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am J Pathol. 2009;174:1097–108. doi: 10.2353/ajpath.2009.080431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–88. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lumeng C, Bodzin J, Saltiel A. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 22.Fujisaka S, Usui I, Bukhari A, et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58:2574–82. doi: 10.2337/db08-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–46. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen MT, Favelyukis S, Nguyen AK, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–92. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 25.Shaul ME, Bennett G, Strissel KJ, et al. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes. 2010;59:1171–81. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeyda M, Gollinger K, Kriehuber E, et al. Newly identified adipose tissue macrophage populations in obesity with distinct chemokine and chemokine receptor expression. Int J Obes (Lond) 2010;34:1684–94. doi: 10.1038/ijo.2010.103. [DOI] [PubMed] [Google Scholar]
- 27.Curat CA, Wegner V, Sengenes C, et al. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49:744–7. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 28.Zeyda M, Farmer D, Todoric J, et al. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 2007;31:1420–1428. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- 29.Bourlier V, Zakaroff-Girard A, Miranville A, et al. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation. 2008;117:806–15. doi: 10.1161/CIRCULATIONAHA.107.724096. [DOI] [PubMed] [Google Scholar]
- 30.Spencer M, Yao-Borengasser A, Unal R, et al. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab. 2010;299:E1016–27. doi: 10.1152/ajpendo.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prieur X, Mok CY, Velagapudi VR, et al. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes. 2011;60:797–809. doi: 10.2337/db10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patsouris D, Li PP, Thapar D, et al. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–9. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hellmann J, Tang Y, Kosuri M, et al. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. Faseb J. 2011 doi: 10.1096/fj.10-178657. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aron-Wisnewsky J, Tordjman J, Poitou C, et al. Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab. 2009;94:4619–23. doi: 10.1210/jc.2009-0925. [DOI] [PubMed] [Google Scholar]
- 35.Kovacikova M, Sengenes C, Kovacova Z, et al. Dietary intervention-induced weight loss decreases macrophage content in adipose tissue of obese women. Int J Obes (Lond) 2011;35:91–98. doi: 10.1038/ijo.2010.112. [DOI] [PubMed] [Google Scholar]
- 36.Kawanishi N, Yano H, Yokogawa Y, Suzuki K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc Immunol Rev. 2010;16:105–18. [PubMed] [Google Scholar]
- 37.Yakeu G, Butcher L, Isa S, et al. Low-intensity exercise enhances expression of markers of alternative activation in circulating leukocytes: roles of PPARgamma and Th2 cytokines. Atherosclerosis. 2010;212:668–73. doi: 10.1016/j.atherosclerosis.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 38•.Devaraj S, Jialal I. C-Reactive Protein Polarizes Human Macrophages to an M1 Phenotype and Inhibits Transformation to the M2 Phenotype. Arterioscler Thromb Vasc Biol. 2011;1(6):397–402. doi: 10.1161/ATVBAHA.111.225508. This study describes a role for CRP in promoting macrophage differentiation toward an M1 phenotype. These data identify a novel pro-inflammatory property of CRP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma LJ, Corsa BA, Zhou J, et al. Angiotensin type 1 receptor modulates macrophage polarization and renal injury in obesity. Am J Physiol Renal Physiol. 2011 doi: 10.1152/ajprenal.00468.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sierra-Filardi E, Puig-Kroger A, Blanco FJ, et al. Activin A skews macrophage polarization by promoting a pro-inflammatory phenotype and inhibiting the acquisition of anti-inflammatory macrophage markers. Blood. 2011 doi: 10.1182/blood-2010-09-306993. [DOI] [PubMed] [Google Scholar]
- 41.Lovren F, Pan Y, Quan A, et al. Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. Am J Physiol Heart Circ Physiol. 2010;299:H656–63. doi: 10.1152/ajpheart.00115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mandal P, Pratt BT, Barnes M, et al. Molecular Mechanism for Adiponectin-dependent M2 Macrophage Polarization: LINK BETWEEN THE METABOLIC AND INNATE IMMUNE ACTIVITY OF FULL-LENGTH ADIPONECTIN. J Biol Chem. 2011;286:13460–9. doi: 10.1074/jbc.M110.204644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Baitsch D, Bock HH, Engel T, et al. Apolipoprotein E Induces Antiinflammatory Phenotype in Macrophages. Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.111.222745. In Press. This paper reports the ability of apoE to induce the M2 phenotype in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller AM, Asquith DL, Hueber AJ, et al. Interleukin-33 induces protective effects in adipose tissue inflammation during obesity in mice. Circ Res. 2010;107:650–8. doi: 10.1161/CIRCRESAHA.110.218867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kohlstedt K, Trouvain C, Namgaladze D, Fleming I. Adipocyte-derived lipids increase angiotensin-converting enzyme (ACE) expression and modulate macrophage phenotype. Basic Res Cardiol. 2011;106:205–15. doi: 10.1007/s00395-010-0137-9. [DOI] [PubMed] [Google Scholar]
- 46••.Wu D, Molofsky AB, Liang HE, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–7. doi: 10.1126/science.1201475. This study reports a crucial role for eosinophils, as major IL-4-producing cells in white adipose tissue, in metabolic homeostasis through the maintenance of macrophage alternative activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lutgens E, Lievens D, Beckers L, et al. Deficient CD40-TRAF6 signaling in leukocytes prevents atherosclerosis by skewing the immune response toward an antiinflammatory profile. J Exp Med. 2010;207:391–404. doi: 10.1084/jem.20091293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48••.Feig JE, Rong JX, Shamir R, et al. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci U S A. 2011;108(17):7166–71. doi: 10.1073/pnas.1016086108. This is the first study to establish HDL as in vivo regulator of macrophage plasticity in a mouse model of atherosclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rigamonti E, Chinetti-Gbaguidi G, Staels B. Regulation of macrophage functions by PPAR-alpha, PPAR-gamma, and LXRs in mice and men. Arterioscler Thromb Vasc Biol. 2008;28:1050–9. doi: 10.1161/ATVBAHA.107.158998. [DOI] [PubMed] [Google Scholar]
- 50.Huang JT, Welch JS, Ricote M, et al. Interleukin-4-dependent production of PPAR-γ ligands in macrophages by 12/15 lipoxygenase. Nature. 1999;400:378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 51.Szanto A, Balint BL, Nagy ZS, et al. STAT6 transcription factor is a facilitator of the nuclear receptor PPARgamma-regulated gene expression in macrophages and dendritic cells. Immunity. 2010;33:699–712. doi: 10.1016/j.immuni.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vats D, Mukundan L, Odegaard JI, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang K, Reilly SM, Karabacak V, et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–95. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–20. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, et al. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marathe C, Bradley MN, Hong C, et al. Preserved glucose tolerance in high fat diet-fed C57BL/6 mice transplanted with PPARgamma −/−, PPARdelta −/−, PPARgamma delta −/− or LXRalpha beta −/− bone marrow. J Lipid Res. 2009;50:214–224. doi: 10.1194/jlr.M800189-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsumura T, Kinoshita H, Ishii N, et al. Telmisartan Exerts Antiatherosclerotic Effects by Activating Peroxisome Proliferator-Activated Receptor-{gamma} in Macrophages. Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.110.222067. In Press. [DOI] [PubMed] [Google Scholar]
- 58.Fujisaka S, Usui I, Kanatani Y, et al. Telmisartan Improves Insulin Resistance and Modulates Adipose Tissue Macrophage Polarization in High-Fat-Fed Mice. Endocrinology. 2011;152(5):1789–99. doi: 10.1210/en.2010-1312. [DOI] [PubMed] [Google Scholar]
- 59.Stienstra R, Duval C, Keshtkar S, et al. Peroxisome proliferator-activated receptor gamma activation promotes infiltration of alternatively activated macrophages into adipose tissue. J Biol Chem. 2008;283:22620–7. doi: 10.1074/jbc.M710314200. [DOI] [PubMed] [Google Scholar]
- 60.Feig JE, Parathath S, Rong JX, et al. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation. 2011;123:989–98. doi: 10.1161/CIRCULATIONAHA.110.984146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bouhlel MA, Brozek J, Derudas B, et al. Unlike PPARgamma, PPARalpha or PPARbeta/delta activation does not promote human monocyte differentiation toward alternative macrophages. Biochem Biophys Res Commun. 2009;386:459–62. doi: 10.1016/j.bbrc.2009.06.047. [DOI] [PubMed] [Google Scholar]
- 62.Ehrchen J, Steinmuller L, Barczyk K, et al. Glucocorticoids induce differentiation of a specifically activated, anti-inflammatory subtype of human monocytes. Blood. 2007;109:1265–74. doi: 10.1182/blood-2006-02-001115. [DOI] [PubMed] [Google Scholar]
- 63.Vallelian F, Schaer CA, Kaempfer T, et al. Glucocorticoid treatment skews human monocyte differentiation into a hemoglobin-clearance phenotype with enhanced heme-iron recycling and antioxidant capacity. Blood. 2010;116:5347–56. doi: 10.1182/blood-2010-04-277319. [DOI] [PubMed] [Google Scholar]
- 64.Patsouris D, Neels JG, Fan W, et al. Glucocorticoids and thiazolidinediones interfere with adipocyte-mediated macrophage chemotaxis and recruitment. J Biol Chem. 2009;284:31223–35. doi: 10.1074/jbc.M109.041665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Usher MG, Duan SZ, Ivaschenko CY, et al. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest. 2010;120:3350–64. doi: 10.1172/JCI41080. [DOI] [PMC free article] [PubMed] [Google Scholar]


