Abstract
Gymnotus (Gymnotiformes, Gymnotidae) is the most diverse known Neotropical electric knife fish genus. Cytogenetic studies in Gymnotus demonstrate a huge karyotypic diversity for this genus, with diploid numbers ranging from 34 to 54. The NOR are also variable in this genus, with both single and multiple NORs described. A common interpretation is that the single NOR pair is a primitive trait while multiple NORs are derivative. However this hypothesis has never been fully tested. In this report we checked if the NOR-bearing chromosome and the rDNA site are homeologous in different species of the genus Gymnotus: G. carapo (2n = 40, 42, 54), G. mamiraua (2n = 54), G. arapaima (2n = 44), G. sylvius (2n = 40), G. inaequilabiatus (2n = 54) and G. capanema (2n = 34), from the monophyletic group G. carapo (Gymnotidae-Gymnotiformes), as well as G. jonasi (2n = 52), belonging to the G1 group. They were analyzed with Fluorescence in situ hybridization (FISH) using 18S rDNA and whole chromosome probes of the NOR-bearing chromosome 20 (GCA20) of G. carapo (cytotype 2n = 42), obtained by Fluorescence Activated Cell Sorting. All species of the monophyletic G. carapo group show the NOR in the same single pair, confirmed by hybridization with CGA20 whole chromosome probe. In G. jonasi the NORs are multiple, and located on pairs 9, 10 and 11. In G. jonasi the GCA20 chromosome probe paints the distal half of the long arm of pair 7, which is not a NOR-bearing chromosome. Thus these rDNA sequences are not always in the homeologous chromosomes in different species thus giving no support to the hypothesis that single NOR pairs are primitive traits while multiple NORs are derived. The separation of groups of species in the genus Gymnotus proposed by phylogenies with morphologic and molecular data is supported by our cytogenetic data.
Introduction
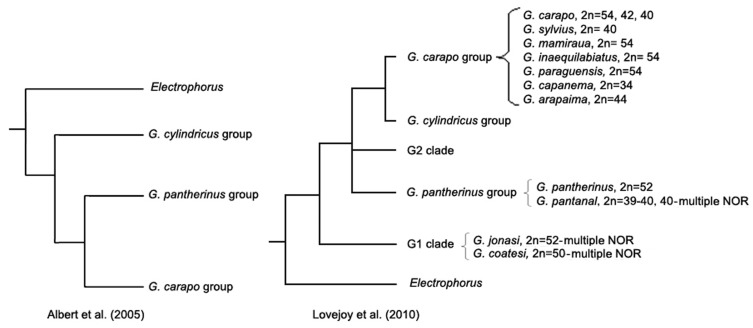
Gymnotus (Gymnotiformes, Gymnotidae) is the most diverse known Neotropical electric knife fish genus. It currently holds 37 valid described species [1] and is distributed within a large geographic region, from the south of Mexico to the north of Argentina. There are 19 species described in the Amazon region in Brazil [1]. This genus contains three groups: G. carapo, G. pantherinus and G. cylindricus [2]. This subdivision can be found in the phylogeny of Albert et al. [3], where G. cylindricus is the basal group. However, Lovejoy et al. [4] proposed a new phylogeny with five groups, where the G. pantherinus group is divided into groups G1, G2 and G. pantherinus. In the consensus phylogeny, G1 is the basal group while G. cylindricus and G. carapo group are the more derived condition, with the G. pantherinus and G2 groups as sister groups. All studies support the monophyly of the G. carapo group (Figure 1).
Figure 1. Phylogeny of the genus Gymnotus adapted from [3] and [4] with chromosomal information cited in this paper.
Cytogenetic studies in Gymnotus demonstrate a huge karyotypic diversity for this genus. Diploid numbers range from 34 chromosomes in G. capanema to 54 in G. cf. carapo, G. mamiraua, G. paraguensis and G. inaequilabiatus. Variations in the karyotypic formula among species and populations result from differences in chromosome morphology (see [1], [5]). On the other hand, sex-related polymorphisms are found only in G. pantanal, which has a X1X1X2X2/X1X2Y multiple sex chromosome system [6].
NORs, composed of rRNA genes 5,8S, 18S and 28S, are important markers for evolutionary chromosome studies [7]. Interspecific and intraspecific polymorphism in NORs have been documented in several groups, with variation in the number of NORs per genome, in the chromosomal location of NOR sites, in the relative sizes of individual NORs, and in the number of active NOR sites per cell [8]. These events are commonly seen in fish, where the rDNA loci have been shown to be highly dynamic. For example, three NOR chromosomes, including one W sex chromosome, occur in some species of Triportheus [9]; several chromosomes with rDNA are known in Salmo truta [10]; similar variation is found in different populations of Characidium gomesi [11], and in Astyanax scabripinnis [12]. Variations in the number, size and position of rDNA loci on the sex chromosome (varying from two to eight) have also been observed in Salvelinus alpinus [13].
In Gymnotiformes polymorphisms are described in the location of the NORs, mainly involving paracentric inversions in the karyotype of the G. carapo group [14]. However, the presence of a single NOR (one pair) is the most common trait, as found in Apteronotus albifrons, Sternopygus macrurus, Eigenmannia sp, E. virescens, Steatogenys elegans, S. duidae, Gymnotus carapo, G. paraguensis, G. sylvius, G. cf. carapo, G. inaequilabiatus, G. pantherinus, G. mamiraua and G. arapaima [1], [5], [15]–[25]. In Electrophorus electricus a single NOR was found on a sample from River Araguaia and three NOR-bearing chromosomes were identified in a fish from River Amazonas [26]. On the other hand, in G. pantanal [27], G. jonasi [22] and G. coatesi [28] the NORs are multiple (more than one pair).
A common evolutionary interpretation of this variation is attributed to Hsu et al. [29] who assume that a single NOR pair is a primitive (plesiomorphic) condition while multiple NORs are a derived (apomorphic) trait [16], [30]–[33]. For this hypothesis to be correct two conditions must be achieved: 1) for species with a single NOR the NOR-bearing chromosomes must be homeologous in the different species within the same taxonomic group. Since it is the primitive condition, it must have appeared once and spread over the species. 2) A phylogenetic study should confirm that primitive species have a single NOR pair while derivate species have multiple NORs. The achievement of only one of these conditions is not enough to confirm the hypothesis. With time this old hypothesis became very popular but (or maybe because) until recently there were no tools to test it. Without the precise identification of chromosome homeologies in fish karyotypes, the comparative analysis in different species has been made on the assumption that there is homeology among chromosomes of similar size, morphology and presence of NORs. The absence of compartmentalization in GC or AT rich regions (for revision, see [34]) explains the absence of G-banding in these organisms and the difficulty in obtaining whole chromosome probes by FACS compared to the situation in mammals [35]–[38]. Nagamachi et al. [39] demonstrated this by using whole chromosome probes of Gymnotus carapo (2n = 42). The chromosomes were separated in groups of pairs with similar size. Only the NOR-bearing chromosome (pair GCA20) was individually separated because of its large amount of repetitive sequences.
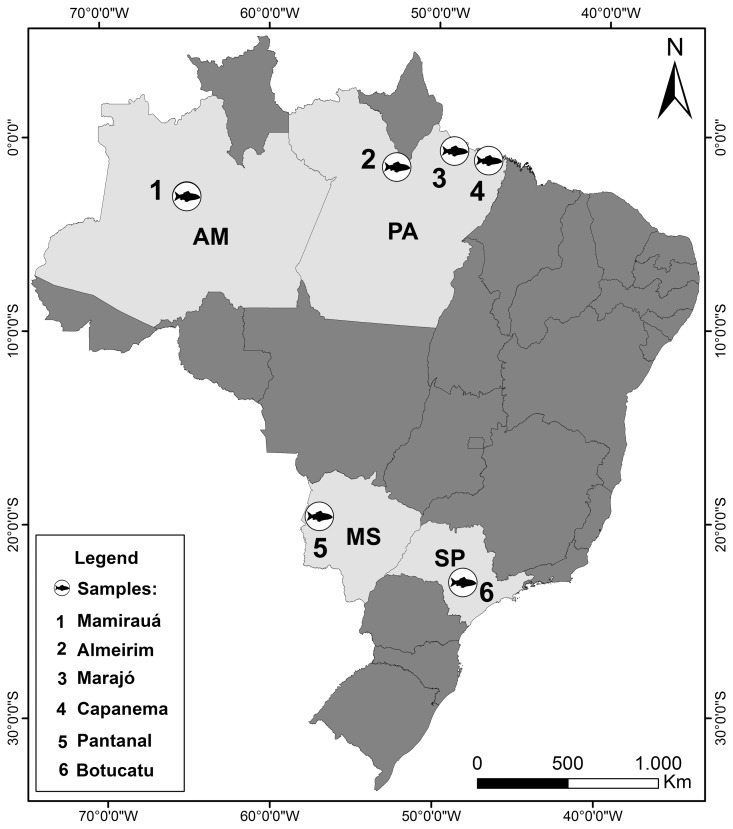
In the present study we check the homeology of the association, sites of rDNA with the NOR-bearer chromosome, by doing dual-color FISH with 18S rDNA and whole NOR-bearer chromosome probes (GCA 20, [39]) on different species of Gymnotus from different places in Brazil (Figure 2). Also, we analyzed the results on a phylogenetic perspective, to test the hypothesis that the species with single NOR pairs are primitive. We give results for G. carapo (GCA, 2n = 40, 42 and 54), G. mamiraua (GMA, 2n = 54), G. arapaima (GAR, 2n = 44), G. sylvius (GSY, 2n = 40), G. inaequilabiatus (GIN, 2n = 54), G. capanema (GCP, 2n = 34) and G. jonasi (GJO, 2n = 52). The results of FISH using only 18S rDNA probes were already reported for most of the species [1], [5], [25], apart from the samples from Almeirim and Marajo Island which are new results (Table 1, Figure 2).
Figure 2. A map showing the places where the samples were collected.
Table 1. Some information on the species analyzed in this work.
| Species | Coordinates | Locality | 2n | KF |
| G. mamiraua (GMA) | 03°01′41.8″S/064°51′16.6″W | Mamirauá-AM | 54 | 46m/sm+8st/a |
| G. carapo (GCA) | 00°42′03.2S/049°10′42.1W | Marajó-PA | 42 | 30m/sm+12st/a |
| G. carapo (GCA) | 01° 31′ 34.2″S/052°33′37.9″W | Almeirim-PA | 40 | 34m/sm+6st/a |
| G. cf. carapo (GCA) | 19°34′34″S/57°02′17″W | Pantanal-MS | 54 | 38m/+12sm+/4st |
| G. arapaima (GAR) | 03°02′49.1″S/064°51′02.2″W | Mamirauá-AM | 44 | 26m/sm+18st/a |
| G. capanema (GCP) | 01°11′45″S/47°10′51″W | Capanema-PA | 34 | 20m/sm+14st/a |
| G. jonasi (GJO) | 03° 02′49.1″S/064° 51′ 02.2″W | Mamirauá-AM | 52 | 52 (12 m-sm+40st-a) |
| G. sylvius (GSY) | 22° 59′25″S/48°25′40″W | Botucatu-SP | 40 | 22m/+12sm/+6st |
| G. inaequilabiatus (GIN) | 22° 59′25″S/48°25′40″W | Botucatu-SP | 54 | 42m/+10sm/+2a |
2n = diploid number and KF = karyotypic formula.
Results
FISH with 18S rDNA probe demonstrate the location of the NOR sites confirming previous publications that these occur in the same positions revealed by Ag-NO3 staining in the species GCA, GMA, GAR, GSY, GIN and GCP. New information is given for GCA samples from Almeirim and Marajo Island (Table 1, Figure 2). In GJO hybridization is found in three acrocentric pairs (two pairs with interstitial signals in the long arm and one pair in the short arm).
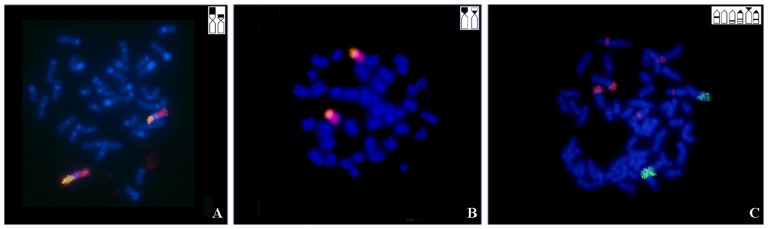
In all the species studied, chromosome painting with the whole NOR-bearing chromosome probe (GCA20) produced a signal in just one chromosome pair. The results with dual color FISH demonstrate the association sites of the rDNA with NOR-bearing chromosome in the species of Gymnotus carapo group, that has single NOR. Figure 3 shows examples of this for the species G. capanema (Fig. 3A) and G. sylvius (Fig. 3B). This result was found on the other species with exception of GJO, where the hybridization is in the distal half of the long arm of a large acrocentric chromosome, pair 7, which is not one of the NOR-bearing chromosomes (Fig. 3C).
Figure 3. 18S rDNA probe FISH (green) and whole NOR-bearing chromosome probe FISH (red) in A) G. capanema; B) G. sylvius and C) G. jonasi. The ideogram on each image shows the position of NOR.
Discussion
In the present work we have used FISH to map NOR numbers and locations on a (partly resolved) Gymnotus species tree and characterized the autosome that bears it.
Using the rDNA probe we confirmed that, with the exception of GJO, which has rDNA sequences in three chromosome pairs, all the species have a NOR in just one chromosome pair. The multiple NOR trait has been found only in two other species previously: G. pantanal (GPN) that has rDNA in three chromosomes [27] and G. coatesi (GCO) that has at least 7 pairs of NOR-bearing chromosomes [28]. There is the possibility that these species can be polymorphic for the number of NORs. However, the genus Gymnotus has been studied by others and us in many papers in recent years. In reference [17] we studied 10 G. carapo with 2n = 42 and 7 with 2n = 40. In reference [22] we analyzed 12 G. arapaima, 23 G. mamiraua, 2 G. jonasi and 9 G. capanema; in reference [5] it were analyzed 23 G. sylvius, 18 G. inaquelabiatus, 3 G. pantherinus and 3 G. c/carapo. There are no NOR polymorphism on any of theses samples. So we believe that we can rule out this possibility.
The results of dual color FISH with the 18S rDNA and the whole NOR-bearing chromosome probe, GCA20, demonstrate painting in only one chromosome pair in all the species analyzed, with conserved synteny among different karyotypes. In the G. carapo group, the hybridization of this probe extended all over the NOR-bearing chromosome (Figure 3A, 3B), showing that the chromosome/rDNA locus association is conserved in this group of species. In GJO this association was not found (Figure 3C), since the probe of GCA20 painted half a large acrocentric pair (pair 7), which is not one of the NOR-bearing chromosomes (pairs 9, 10 and 11). These results show that the karyotype of GJO has a different organization of rDNA sites and that the GCA20 is associated with another chromosome in a translocation or in a tandem fusion rearrangement.
If we accept the phylogeny proposed by Lovejoy et al. [4], where the group G. carapo is the most derived and group G1 is the most basal, we may conclude that the single NOR with the conserved chromosome/rDNA locus association shared by the G. carapo group is a synapomorphy for the group. Gymnotus pantherinus also has a single NOR chromosome, similar to the one in the G. carapo group. In GPN there are three NOR-bearing chromosomes (one pair and an additional chromosome from another pair), where one is similar to that found in the G. carapo group. Although GPN has not being analyzed by Lovejoy et al. [4], it probably belongs to the group G2 as it is a sister species of G. anguillaris [27]. If GPA and GPN share the chromosome/rDNA locus association, this association is likely to have happened before the appearance of the G. carapo group.
In the classification of Albert et al. [3] all species belong to the group G. pantherinus, except G. cylindricus and the G. carapo group. In the alternative classification [4] the G. pantherinus group is split into three monophyletic groups. Our data show that, while the G. carapo group has homogeneity of NORs, the G. pantherinus group is heterogeneous. Species GPA has a NOR similar to the G. carapo group, while multiple NORs are found in GJO ([22], present study) and GCO [28], apart from GPN [27], already mentioned. Besides this, our studies demonstrate that GJO and GCO have multiple NORs with different patterns of distribution of rDNA loci, involving different chromosomes. The same can be said about the “single NOR” trait, for Sternopygus also has a single NOR, but the chromosome involved is different from that in the G. carapo group (data not shown), as well as in Electrophorus [26]. So it cannot be claimed a priori that the single or multiple NOR conditions can be primitive or derived per se, since each of these conditions in one species can have an origin and composition very divergent from the one found in other species.
The NOR heterogeneity observed in the G. pantherinus group of Albert et al. [3], in contradiction with the homogeneity of the G. carapo group, suggests that the G. pantherinus group is composed of more than one monophyletic group, supporting the phylogeny of Lovejoy et al. [4]. Actually, Albert et al. [4] included many species in the G. pantherinus group because it was not possible to define precisely their phylogenetic relationships using only morphology data. Anyway, these results show clearly the huge mobility of the rDNA cistrons.
Additional studies, with detailed analysis using whole chromosome and rDNA probes in species from different families of Gymnotiformes, will permit more precise conclusions on the differentiation and distribution of NORs in this order. The study of GJO using FISH with other whole chromosome probes as described in Nagamachi et al. [39] will allow an improved understanding of the amount of chromosomal divergence between these species.
Materials and Methods
Samples were collected in rivers from the Amazon basin (Pará and Amazonas states), Paranapanema basin (São Paulo state) and Paraguay basin (Mato Grosso do Sul state) (Table 1, Figure 2). Some of these specimens were previously studied [1], [5], [17], [20], [21]. Samples were deposited at the Museu de Ciências e Tecnologia da Pontifícia Universidade Católica do Rio Grande do Sul (GCA from Marajó), at Museu Paraense Emílio Goeldi (GCA from Almeirim and GCP from Capanema), at Museu da Reserva de Desenvolvimento Sustentável Mamirauá (GMA, GAR and GJO from Mamirauá) and at the fish collection from the Laboratório de Biologia e Genética de Peixes (LBP) from UNESP from Botucatu, vouchers 11160 (GSY), 9836 (G. cf. carapo) and 11154 (GIN).
The chromosome preparations were obtained following [40]. The Ag-NO3 staining followed [41]. The FISH with the 18S rDNA probe from Prochilodus argenteus was made according to [42]. The whole chromosome probe of the NOR-bearing chromosome (GCA20) of G. carapo (cytotype 2n = 42) was obtained by Fluorescence Activated Cell Sorting [39]. Dual color FISH was made using the (GCA20) probe, labeled with dUTP-Cy3, and the 18S rDNA probe, labeled with Digoxigenin and detected with Anti-Digoxigenin-FITC. The detection followed [43], with modifications [39]. The images were captured using a CCD Axiocam Mrm camera connected to a Zeiss Axiophot microscope and Axiovision 3.0 software, where the images were pseudocolored. Brightness and contrast levels were adjusted using Adobe Photoshop 7.1.
Ethics Statement
This study was analyzed by the Ethics Committee on Animal Research from the UNESP University and got the authorization number 395-CEUA. JCP has a Permanent License for Collecting Zoological Samples in Brazil, Permit Number 13248, ICMBio (Chico Mendes Institute for the Conservation of Biodiversity, Brazilian Ministry for Environment).
Acknowledgments
We thank the IDSM (Instituto de Desenvolvimento Sustentável Mamirauá) for logistic support to collect samples; Dr. William G. R. Crampton for the taxonomic identification of the Amazon sample; Dr. Pedro Manoel Galetti Junior for providing the DNAr18S Prochilodus argenteus; Ms. Ricardo Utsunomia for suggestions. Collecting was authorized by IBAMA (Instituto Brasileiro do Meio Ambiente) permit 020/2005 (IBAMA Registration: 207419).
Funding Statement
Most of this research was supported by FAPESPA (Pará Foundation for Supporting Science) through the National Excellence on Research Program (PRONEX, TO 011/2008) on a project coordinated by J. C. Pieczarka. Funding to the Brazilian authors was provided by CNPq, CAPES, UFPA, FAPESPA and FAPESP. Wellcome Trust provided a grant to M. A. Ferguson-Smith, the CNPq-Brazil provided Post-Doctoral scholarships to C. Y. Nagamachi and J. C. Pieczarka and a Doctoral scholarship to S. S. R. Milhomem. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Milhomem SSR, Crampton WGR, Pieczarka JC, Shetka GH, Silva DS, et al. (2012) Gymnotus capanema, a new species of electric knife fish (Gymnotiformes -Gymnotidae) from eastern Amazonia, with comments on an unusual karyotype. Journal of Fish Biology 80: 802–815. [DOI] [PubMed] [Google Scholar]
- 2. Albert JS, Miller RR (1995) Gymnotus maculosus: a new species of electric fish from Middle America (Teleostei: Gymnotoidei), with a key to the species of Gymnotus . Proceedings Biological Society Washington 108: 662–678. [Google Scholar]
- 3. Albert JS, Crampton WGR, Thorsen DH, Lovejoy NR (2005) Phylogenetic systematics and historical biogeography of the Neotropical electric fish Gymnotus (Teleostei: Gymnotidae). Systematics and Biodiversity 4: 375–417. [Google Scholar]
- 4. Lovejoy NR, Lester K, Crampton WGR, Marques FPL, Albert JS (2010) Phylogeny, biogeography and electric signal evolution of Neotropical knifefishes of the genus Gymnotus (Osteichthyes: Gymnotidae). Molecular Phylogenetics and Evolution 54: 278–290. [DOI] [PubMed] [Google Scholar]
- 5. Scacchetti PC, Pansonato-Alves JC, Utsunomia R, Oliveira C, Foresti F (2011) Karyotypic diversity in four species of the genus Gymnotus Linnaeus, 1758 (Teleostei, Gymnotiformes, Gymnotidae): physical mapping of ribosomal genes and telomeric sequences. Comparative Cytogenetics 3: 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Margarido VP, Bellafronte E, Moreira-Filho O (2007) Cytogenetic analysis of three sympatric Gymnotus (Gymnotiformes, Gymnotidae) species verifies invasive species in the Upper Paraná River basin, Brazil. Journal of Fish Biology 70: 155–164. [Google Scholar]
- 7.Geoffrey MC (2002) A célula: uma abordagem molecular, 2rd edn. Artmed: Porto Alegre.
- 8.Gold JR (1984) Silver-staining and heteromorphism of chromosomal nucleolus organizer regions in north American cyprinid fishes. Copeia 133–139.
- 9. Artoni RF, Betollo LAC (2002) Evolutionary aspects of the ZZ/ZW sex chromosome system in the Characidae fish, genus Triportheus. A monophyletic state and NOR location on the W chromosome. Heredity 89: 15–19. [DOI] [PubMed] [Google Scholar]
- 10. Pendás AM, Móran P, García-Vázquez E (1993) Ribosomal RNA genes are interspersed throughout a heterochromatic chromosome arm in Atlantic salmon. Cytogenetics and Cell Genetics 63: 128–130. [DOI] [PubMed] [Google Scholar]
- 11. Machado TC, Pansonato-Alves JC, Pucci MB, Nogaroto V, Almeida MC, et al. (2011) Chromosomal painting and ZW sex chromosomes differentiation in Characidium (Characiformes, Crenuchidae). BMC Genetics 12: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferro DAM, Néo DM, Moreira-Filho O, Bertollo LAC (2001) Nucleolar organizing regions, 18S and 5S rDNA in Astyanax scabripinnis (Pisces, Characidae): populations distribution and functional diversity. Genetica 110: 55–62. [DOI] [PubMed] [Google Scholar]
- 13. Reed KM, Phillips RB (1997) Polymorphism of the nucleolus organizer region (NOR) on the putative sex chromosomes of Arctic char (Salvelinus alpinus) is not sex related. Chromosome research 5: 221–227. [DOI] [PubMed] [Google Scholar]
- 14. Porto JIR, Feldberg E, Nakayama C, Falcão JN (1992) A checklist of chromosome numbers and karyotypes of Amazonian freshwater fishes. Revue d'Hydrobiologie Tropicale 25: 287–299. [Google Scholar]
- 15. Almeida-Toledo LF, Foresti F (1985) As regiões organizadoras de nucléolo em peixes. Ciência e Cultura 3: 448–453. [Google Scholar]
- 16. Fernandes-Matioli FMC, Marchetto MCN, Almeida-Toledo LF, Toledo-Filho SA (1998) High intraspecific karyological conservation in four species of Gymnotus (Pisces: Gymnotiformes) from Southeastern Brazilian basins. Caryologia 3: 221–234. [Google Scholar]
- 17. Milhomem SSR, Pieczarka JC, Crampton WGR, Silva DS, Souza ACP, et al. (2008) Chromosomal evidence for a cryptic species in the Gymnotus carapo species-complex (Gymnotiformes, Gymnotidae). BMC Genetic 9: 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fernandes-Matioli FMC, Almeida-Toledo LF, Toledo-Filho SA (1997) Extensive nucleolus organizer region polymorphism in Gymnotus carapo (Gymnotoidei, Gymnotidae). Cytogenetics and Cell Genetics 78: 236–239. [DOI] [PubMed] [Google Scholar]
- 19. Foresti F, Almeida-Toledo LF, Toledo-Filho SA (1981) A Polymorphic nature of nucleolus organizer regions on fishes. Cytogenetic and Cell Genetic 31: 137–144. [DOI] [PubMed] [Google Scholar]
- 20. Sánchez S, Laudicina A, Jorge LC (2004) A new report of multiple sex chromosome system in the order Gymnotiformes (Pisces). Cytologia 69: 155–160. [Google Scholar]
- 21. Milhomem SSR, Pieczarka JC, Crampton WGR, Souza ACP, Carvalho JR Jr, et al. (2007) Differences in karyotype between two sympatric species of Gymnotus (Gymnotiformes: Gymnotidae) from the eastern amazon of Brazil. Zootaxa 1397: 55–62. [Google Scholar]
- 22. Milhomem SSR, Crampton WGR, Pieczarka JC, Silva DS, Cardoso AL, et al. (2012) Chromosomal and electric signal diversity in three sympatric electric knifefish species (Gymnotus, Gymnotidae) from the Central Amazon Floodplain. Reviews in Fish Biology and Fisheries 22(2): 485–497. [Google Scholar]
- 23. Silva DS, Milhomem SSR, Souza ACP, Pieczarka JC, Nagamachi CY (2008) A conserved karyotype of Sternopygus macrurus (Sternopygidae, Gymnotiformes) in the Amazon region: differences from other hydrographic basins suggest cryptic speciation. Micron 39: 1251–1254. [DOI] [PubMed] [Google Scholar]
- 24. Silva DS, Milhomem SSR, Pieczarka JC, Nagamachi CY (2009) Cytogenetic studies in Eigenmannia virescens (Sternopygidae, Gymnotiformes) and new inference on the origin of sex chromosome in the Eigenmannia genus. BMC Genetics 10: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cardoso AL, Pieczarka JC, Feldberg E, Milhomem SSR, Moreira-Almeida T, et al. (2011) Chromosomal characterization of two species of genus Steatogenys (Gymnotiformes: Rhamphichthyoidea: Steatogenini) from the Amazon basin: sex chromosomes and correlations with Gymnotiformes phylogeny. Reviews in Fish Biology and Fisheries 21: 613–621. [Google Scholar]
- 26. Fonteles SBA, Lopes CE, Akama A, Fernandes FMC, Porto-Foresti F, et al. (2008) Cytogenetic characterization of the strongly electric Amazonian eel, Electrophorus electricus (Teleostei, Gymnotiformes), from the Brazilian rivers Amazon and Araguaia. Genetics and Molecular Biology 31: 227–230. [Google Scholar]
- 27. Fernandes FMC, Albert JS, Daniel-Silva MFZ, Lopes CE, Crampton WGR, et al. (2005) A new Gymnotus (Teleostei: Gymnotiformes: Gymnotidae) from the Pantanal Matogrossense of Brazil and adjacent drainages: continued documentation of a cryptic fauna. Zootaxa 933: 1–14. [Google Scholar]
- 28.Machado MA, Milhomem SSR, Cardoso AL, Pieczarka JC, Nagamachi CY (2011) Descrição cariotípica de Gymnotus coatesi (Gymnotidae, Gymnotiformes) da Amazônia Oriental. Resumos do XIV Simpósio de Citogenética e Genética de Peixes. CT 122.
- 29. Hsu TC, Spirito SE, Pardue ML (1975) Distribution of 18S+28S Ribosomal Genes in Mammalian Genomes. Chromosoma 53: 25–36. [DOI] [PubMed] [Google Scholar]
- 30. Dergam JA, Bertollo LAC (1990) Karyotypic diversification in Hoplias malabaricus (Osteichthyes, Erythrinidae) of the São Francisco and Alto Paraná basins, Brazil. Brazilian Journal of Genetics 13(4): 755–766. [Google Scholar]
- 31. Martins-Santos IC, Tavares MG (1996) Chromosomal analysis of Roeboides paranensis (Pisces, Characidae) from the Paraná river. Brazilian Journal of Genetics 19(2): 271–274. [Google Scholar]
- 32. Delana AM, CoLuccia E, Cannas R, Pesci P, Fonnesu A, et al. (2006) Colocalization of the ribosomal gene families in Conger conger (Anguilliformes, Congridae). Italian Journal of Zoology 73(1): 1–5. [Google Scholar]
- 33. Marques DK, Venere PC, Galetti Junior PM (2006) Chromosomal characterization of the bonytongue Arapaima gigas (Osteoglossiformes: Arapaimidae). Neotropical Ichthyology 4(2): 215–218. [Google Scholar]
- 34.Sharma OP, Tripathi NK, Sharma KK (2002) A review of chromosome banding in fishes. In Some aspects of chromosome structure and function Edited by: Sobti RC, Obe G, Athwal RS. Narosa Publishing House, New Delhi, India; 109–122.
- 35. Yang F, O’Brien PCM, Wienberg J, Neitzel H, Lin CC, et al. (1997) Chromosomal evolution of the Chinese muntjac (Muntiacus reevesi). Chromosoma 106: 37–43. [DOI] [PubMed] [Google Scholar]
- 36. Ferguson-Smith MA, Yang F, O’Brien PC (1998) Comparative mapping using chromosome sorting and painting. Ilar Journal 39: 68–76. [DOI] [PubMed] [Google Scholar]
- 37. Dumas F, Bigoni F, Stone G, Sineo L, Stanyon R (2005) Mapping genomic rearrangements in titi monkeys by chromosome flow sorting and multidirectional in-situ hybridization. Chromosome Research 13: 85–96. [DOI] [PubMed] [Google Scholar]
- 38. Pieczarka JC, Nagamachi CY, O'Brien PCM, Yang F, Rens W, et al. (2005) Reciprocal chromosome painting between two South American bats: Carollia brevicauda and Phyllostomus hastatus (Phyllostomidae, Chiroptera). Chromosome Research 13: 339–347. [DOI] [PubMed] [Google Scholar]
- 39. Nagamachi CY, Pieczarka JC, Milhomem SSR, O’Brien PCM, Souza ACP, et al. (2010) Multiple rearrangements in cryptic species of electric knifefish, Gymnotus carapo (Gymnotidae, Gymnotiformes) revealed by chromosome painting. BMC Genetics 11: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bertollo LAC, Takashi CS, Moreira-Filho O (1978) Citotaxonomic considerations on Hoplias lacerdae (Pisces, Erythrinidae). Brazilian Journal of Genetics 2: 103–120. [Google Scholar]
- 41. Howell WM, Black DA (1980) Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Experientia 36: 1014–1015. [DOI] [PubMed] [Google Scholar]
- 42. Hatanaka T, Galetti Jr PM (2004) Mapping of the 18S and 5S ribosomal RNA genes in the fish Prochilodus argenteus Agassiz, 1829 (Characiformes, Prochilodontidae). Genetica 122: 239–244. [DOI] [PubMed] [Google Scholar]
- 43. Yang F, Carter NP, Shi L, Ferguson-Smith MA (1995) A comparative study of karyotypes of muntjacs by chromosome painting. Chromosoma 103: 642–652. [DOI] [PubMed] [Google Scholar]