Abstract
Purpose.
To test the in vivo activity of a peptide derived from the protein transducing domain of the human immunodeficiency virus (HIV) Tat protein, TAT-Cd0, in a murine herpes simplex type 1 (HSV-1) keratitis model.
Methods.
The efficacy of TAT-Cd0 was assessed in a postinfection treatment model with different concentrations (1 mg/mL, 0.1 mg/mL, 0.01 mg/mL) of the peptide in one of four delivery vehicles: artificial tears, PBS, methylcellulose, and aquaphor cream. Treatment began within 4 or 24 hours postinfection. Viral titers in the tear film were determined by plaque assay.
Results.
TAT-Cd0 reduced the severity of keratitis in all of the delivery vehicles tested when treatment started, 4 hours postinfection. Peptide in the tears or PBS delivery vehicle had the most significant reduction in disease severity and delayed the onset of vascularization and stromal keratitis. The percentage of mice presenting with disease was also significantly reduced and viral titers were reduced by 1 log at 24 hours postinfection in mice treated with 1 mg/mL TAT-Cd0, suggesting that inhibiting replication early is sufficient to achieve clinical effects. Lower concentrations were not effective and delaying treatment by 24 hours was also not effective.
Conclusions.
This study shows that TAT-Cd0 is an effective antiviral against HSV-1 strain KOS when applied shortly postinfection and that aqueous-based formulations are more suitable.
A cationic peptide derived from protein transducing domain of tat possesses in vivo antiviral activity in a mouse ocular model of HSV-1 keratitis.
Introduction
Herpes simplex virus type 1 (HSV-1) is an enveloped, double-stranded DNA virus responsible for significant primary and secondary infection of mucous membranes and epithelia. In addition, HSV-1 establishes latent infections in sensory ganglia and reactivation of the virus can result in the recurrence of disease throughout the life of the host.1 HSV-1 can also infect the cornea and is the leading cause of infectious blindness in developed countries, with an estimated 8.4 to 13.2 new cases per 100,000 people per year.2 Although HSV-1 and HSV-2 can both cause ocular disease, 95% of ocular herpes is caused by HSV-1, with the remaining percentage consisting of HSV-2 ocular infections of neonates.1,3 Pathologic manifestations of HSV-1 ocular infections include blepharitis, neovascularization in the cornea, and stromal keratitis, which is an immunopathologic response resulting in the clouding of the eye.3–7 Blindness is most commonly the result of recurrent manifestations and ultimately may require corneal transplantation to restore vision.2,8
Treating ocular HSV infection often requires a combination treatment of topical antivirals to inactivate viral replication and topical corticosteroids to treat the immunopathologic responses leading to stromal disease.3 Currently, trifluridine (TFT), a nucleoside analog, is approved for use in ocular HSV-1 infections but has displayed some toxicity at higher doses and when used for prolonged periods of time.9,10 Although significant clinical resistance to TFT has yet to be observed, resistant strains can be isolated in culture.11 Corticosteroids, which suppress the immune system, should be used only in conjunction with an antiviral and can create problems such as steroid-induced glaucoma.12 These limitations reveal a need for the development of new therapies with reduced toxicity and alternative mechanisms of action.
We previously described a novel antiviral peptide, TAT-Cd0, which was derived from the protein transduction domain of the HIV Tat protein and is among a class of molecules called cell-penetrating peptides (CPPs).13 A study of the antiviral properties of TAT found that the addition of a cysteine residue and an amide to the C-terminal end of the peptide, as well as synthesizing the peptide using d-amino acids improved its ability to block entry (half-maximal effective concentration [EC50] = 0.6 μM) and improved the virucidal activity of the peptide.14 The resulting peptide, called TAT-Cd0 (NH2GRKKRRQRRRCCONH2), contains multiple positive charges and is therefore highly hydrophilic. The antiviral activity of TAT-Cd0 is not cell type dependent, and the peptide has been shown to have low cytotoxicity in culture and in a murine eye model.15,16 TAT-Cd0 also inactivates virus in solution (EC50 = 34 μM) and treatment of cells prior to infection can make them resistant to infection (EC50 = 0.4 μM).14 Based on these properties, TAT-Cd0 has the potential to be an effective antiviral with alternative mechanisms of action compared with currently available drugs. One goal of this study was to assess the efficacy of TAT-Cd0 in a murine model of HSV-1–induced ocular disease.
Previously we tested the antiviral activity of a modified theta defensin, RC-2, which was also highly cationic, in a mouse model of HSV-1 keratitis using PBS with 2% methylcellulose as the vehicle, and found it had modest activity.17 Changes in the formulation might alter the efficacy of cationic peptides. Since TAT-Cd0 is highly cationic, we also wanted to test several formulations to determine if formulating TAT-Cd0 in different delivery vehicles would affect the antiviral properties of the peptide. The TAT-Cd0 peptide was formulated (1 mg/mL) in one of four delivery vehicles: (1) cream, consisting of a hydrophobic emulsion of water, mineral oil, lanolin, ceresin wax, bisabolol, petrolatum, panthenol, and alcohol; (2) lubricant eye drops (Tears Naturale II; Alcon Laboratories, Fort Worth, TX), an artificial tear solution consisting of dextran and hypromellose; (3) phosphate buffered saline (PBS); or (4) PBS with 2% methylcellulose.
In this report, we show that TAT-Cd0 significantly reduced ocular disease in all delivery vehicles tested when treatment was initiated within 4 hours of infection. PBS and artificial tears appeared to be the optimal delivery vehicles for topical application of peptide. Viral titers were significantly reduced on day 1 postinfection, suggesting early inhibition of replication can have a significant effect on disease. Treatment with doses of 0.01 and 0.1 mg/mL did not significantly affect disease, nor did delaying the onset of treatment by 24 hours. The results suggest that TAT-Cd0 has significant antiviral activity in vivo and that further study is warranted.
Materials and Methods
Peptides
TAT-Cd0 was synthesized at the University of Wisconsin-Madison as described previously.18 The purity of the peptides exceeded 90% as determined by analytical high performance liquid chromatography. Peptide concentrations were determined from absorbance readings at both 215 and 225 nm and were corrected for the purity of each batch.13,14,18
Cells and Viruses
African green monkey kidney cells (Vero cells) were grown in Dulbecco's modified Eagle's medium (DMEM), supplemented with 5.0% serum (1:1 v/v mixture of fetal bovine serum, S11150 [Atlanta Biologicals, Atlanta, GA] and bovine calf serum, SH30072.03 [Hyclone, Ogden, UT]), 10 mL/L of penicillin/streptomycin (100 U/mL of penicillin, 100 μg/mL of streptomycin sulfate, G1146; Sigma-Aldrich, St. Louis, MO) and 10 mL/L of 250 μg/mL Amphotericin B (30-003CF; Cellgro/Mediatech, Manassas, VA). Cells used for viral titering were cultured in DMEM supplemented with 5% serum as described earlier and switched to DMEM supplemented with 2% serum for titering. All cell cultures were incubated at 37°C in a 5% CO2 atmosphere. The KOS strain of HSV-1 was utilized throughout this study. High-titer virus stocks were prepared by infecting Vero cell monolayers in 10-cm plates at a multiplicity of infection of 0.1 and harvesting the cells when cytopathic effect reached 90% to 100%.19 The cells were then pelleted and freeze-thawed three times in a dry ice–ethanol bath and centrifuged at 2000g for 10 minutes to remove cell debris. The supernatants were then applied to a cushion of 36% sucrose (Reaction Stop Buffer [RSB]; 10 mM Tris HCl, pH 7.4; 10 mM NaCl, 3 mM MgCl2; Promega Corp., Madison, WI) and centrifuged at 13,500 rpm for 80 minutes to pellet the virions. The pellet was resuspended in RSB and the cushion step was repeated. The purified virion pellet was resuspended in RSB, aliquoted, and stored at −80°C. Titers were determined by plaque assay on Vero cell monolayers.
Animal Inoculation
Four to 6-week-old female BALB/C mice (Harlan Sprague-Dawley, Indianapolis, IN) were used for this study. Groups consisted of 10 mice each. For all inoculations, examinations, treatments, and sample collections, mice were anesthetized with isoflurane (#57319-47406; Phoenix Pharmaceutical, St. Joseph, MO). The right eyes of the mice were examined microscopically prior to infection for corneal defects and those with defects were removed from the study. The remaining mice were then randomly assigned to each group. Under anesthesia, six to ten scratches forming a cross-hatch pattern were made on the cornea using a 30-gauge needle, taking care not to puncture the eye. For the studies involving different formulations, a 5-μL drop of DMEM containing 1.0 × 105 plaque-forming unit (PFU) of HSV-1 KOS was applied to the scarified cornea, and the eyelids were closed twice over the cornea to ensure complete application. For the dose–response and 24-hour postinfection studies, the mice were infected with 1 × 106 PFU of HSV-1 strain KOS. The left eyes remained uninfected and untreated throughout the course of the study. To maintain comfort and provide analgesia, the mice were injected intraperitoneally with 0.01 mg/kg of buprenorphine (Butrans, #0409-2012-32 [0.3 mg/mL]; Hospira, Lake Forest, IL) twice daily on days 0 through 7. Control groups included mice infected with KOS and treated with vehicle in the absence of peptide, as well as mice that were infected and not treated. These studies adhere to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and National Institutes of Health guidelines for the use of animals in research.
Formulation of Peptide for Therapy
Treatment groups included mice infected with KOS and then treated with 1 mg/mL of TAT-Cd0 peptide in each of the four delivery vehicles: (1) PBS vehicle (sterile #21-040-CV; Cellgro/Mediatech); (2) cream vehicle composed of 70% w/w healing ointment (Aquaphor; Smith and Nephew, Lachine, QC, Canada), 15% w/w mineral oil, and 15% w/w water mixed completely to form an emulsion20; (3) artificial tear solution vehicle (Tears Naturale II; Alcon); and (4) gel vehicle composed of 2% methylcellulose in PBS.
Peptide Treatment
Treatment with peptide began within 4 hours of infection, except for the group in which treatment was delayed until 24 hours postinfection. The mice were dosed five times daily at 2- to 4-hour intervals for seven consecutive days. Mice were anesthetized with isoflurane prior to each dosing. For treatment with tears and PBS, 3 μL of either solution was applied directly to the infected eye in the manner described previously.17 Due to its viscosity, 5 μL of the methylcellulose gel was used to ensure full coverage over the eye. For cream treatments, a small bead of the cream was applied to the eye using the end of a pipette tip as described previously.20 After each dosing, the eyelids were closed twice over the cornea and the animals returned to the cage.
Disease Scoring
On days 1, 3, 5, 7, 9, 11, 13, and 15 postinfection, ocular disease severity was scored on a number scale as previously described, based on three disease parameters.17,20,21 Briefly, blepharitis, or swelling of the eyelid, was scored: 1+, puffy eyelids; 2+, puffy eyelids with some crusting; 3+, eye swollen shut with severe crusting; and 4+, eye completely swollen shut and crusted over. Neovascularization, the growth of blood vessels into the cornea, was scored: 1+, <25% of the cornea involved; 2+, 25% to 50% corneal involvement; and 3+, >50% corneal involvement. Stromal keratitis was scored: 1+, cloudiness, some iris detail visible; 2+, iris detail obscured; 3+, cornea totally opaque; and 4+, corneal perforation.
Measuring Virus Titers
At days 1, 3, 5, 7, and 9 postinfection, tear film samples were collected and titered for infectious virus. Mice were anesthetized with isoflurane and the infected corneas were flushed with 10 μL DMEM containing 2% serum. This was then added to 190 μL DMEM containing 2% serum and stored at −80°C. Each sample was thawed then serially diluted 10-fold and quantified using a standard plaque assay.17
Analysis of Disease Scores and Titers
Statistical analyses were conducted using commercial analytical software (SigmaPlot 11.0; Systat Software, Chicago, IL). At the designated time points, raw scores for each disease parameter were recorded for each mouse in a group. The mean disease scores were calculated for each group from the raw scores and analyzed for statistical significance. Mean peak disease scores (MPDS) were calculated as previously described.19,21 Tear film titers were averaged from raw titer data for each mouse in a group and SE was calculated. Kruskal–Wallis one-way ANOVA on ranks was used to compare effects between the peptide in each of the four delivery vehicles and between doses of peptide. The Mann–Whitney rank sum test was used for pairwise comparisons of the average disease scores and MPDS of groups treated with peptide in each of delivery vehicles against vehicle only controls or untreated infected mice. Values of P < 0.05 were deemed significant unless otherwise stated.
Results
Effect of TAT-Cd0 on Ocular Disease
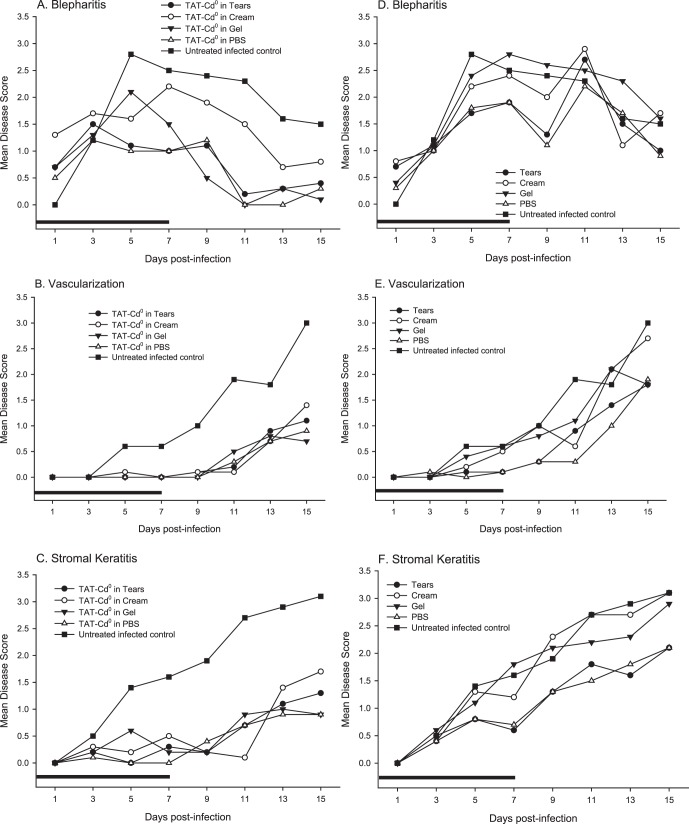
To determine the effects of TAT-Cd0 in a murine model of HSV-1, we infected mice with the KOS strain of HSV-1 and beginning 4 hours postinfection, treated them for 7 days with TAT-Cd0 in one of four delivery vehicles. Figures 1A–C show the mean disease scores for peptide, and the vehicle only controls are shown in Figures 1D–F. Blepharitis developed on day 1 for all treatment groups (Fig. 1A). Blepharitis scores peaked on days 5 and 7 for mice treated with TAT-Cd0 in the more viscous gel and cream formulations, respectively. For mice treated with peptide in PBS and artificial tear solution, blepharitis peaked on day 3 for both vehicles and the disease scores were significantly lower (P < 0.05) compared with untreated infected controls from day 5 to the end of the study. By day 15, blepharitis was not observed in mice treated with TAT-Cd0 in the tear, PBS, and gel formulations. A significant reduction in blepharitis was seen in mice treated with TAT-Cd0 in gel at day 7 to the end of the study. For the groups treated with TAT-Cd0 in cream, there was no significant reduction in blepharitis at any time during the study (Fig. 1A). As shown in Figure 1D, the vehicles alone did not significantly reduce the severity of blepharitis on any day, although the disease scores were lower.
Figure 1. .
Effect of TAT-Cd0 and vehicles only on the severity of ocular disease caused by HSV-1 (A–C) or vehicles only (D–F). Groups of mice (n = 10) were treated with TAT-Cd0 in one of four delivery vehicles and ocular disease was measured as described previously. Results presented are the average of individual scores. (A, D) Blepharitis, (B, E) neovascularization, and (C, F) stromal keratitis. Black bar: duration of treatment.
Stromal keratitis is an immunopathologic response aided by neovascularization in the cornea. These two disease characteristics often develop and progress simultaneously and are both first seen at approximately day 5 in a normal infection with HSV-1 KOS. Treatment with TAT-Cd0 in any of the four delivery vehicles delayed the onset and reduced the severity of neovascularization and stromal keratitis when compared with untreated infected controls (Figs. 1B, 1C). Neovascularization in all treatment groups was not detectable until day 11 and reached an average score of approximately 1.0 on day 15 (Fig. 1B). Neovascularization scores for mice treated with TAT-Cd0 in all formulations were significantly (P < 0.05) reduced compared with untreated infected controls beginning at day 5 through the end of the study (Fig. 1B). Mice treated with vehicle only controls had no significant reduction in neovascularization when compared with untreated infected mice (Fig. 1E). We also saw a trend where, after the treatment period ended at day 7, both stromal keratitis and neovascularization scores increased slowly, beginning at day 9, but remained significantly lower than scores of untreated infected controls.
Stromal keratitis in untreated infected mice was first detected at day 3, and continued to increase steadily through the end of the study. There was a significant reduction in the severity of stromal keratitis beginning at day 5 for mice treated with peptide in any of the four delivery vehicles (Fig. 1C). Mice treated with vehicle only controls had no significant reduction (P > 0.05) in stromal keratitis when compared with untreated infected mice, although disease scores in the PBS and tear groups were lower (Fig. 1F).
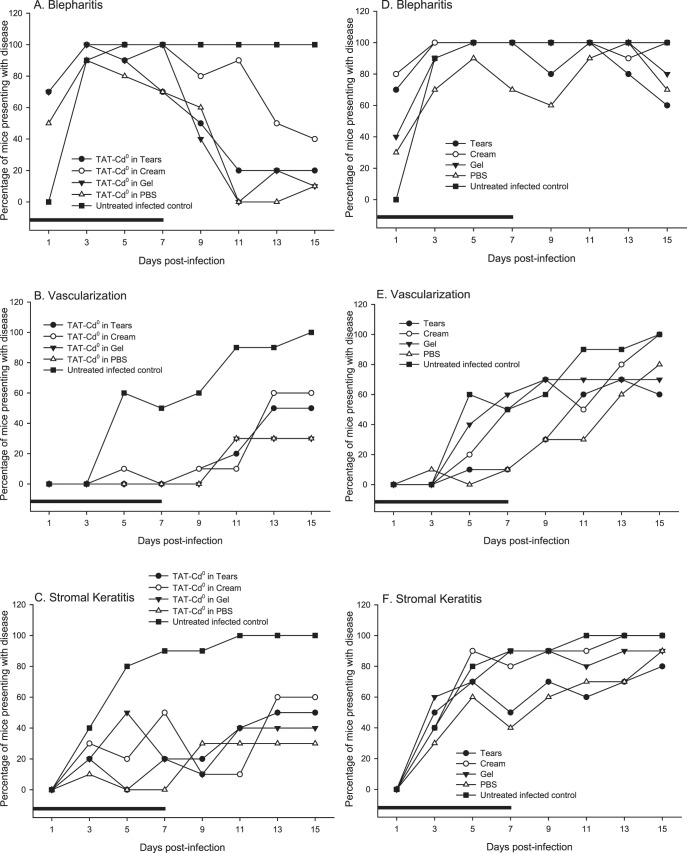
Percentage of Mice Presenting with Disease
At each time point, the percentage of mice with a measurable disease score was recorded (Figs. 2A–C). Ninety percent of mice in the untreated infected group presented with blepharitis at day 3 (Fig. 2A). This value rose to 100% on day 5 and remained constant through the end of the study. On day 3, 40% of mice in the untreated infected group presented with stromal keratitis, whereas 60% of the untreated infected mice presented with neovascularization, rising to 100% for both disease measures by day 11 (Figs. 2B, 2C). In mice treated with the vehicles only, the percentage of mice presenting with disease was not reduced when compared with untreated infected control mice for the three disease characteristics measured (Figs. 2D–F).
Figure 2. .
The percentage of mice developing disease when treated with TAT-Cd0 (A–C) or vehicles only (D–F). Groups of infected mice (n = 10) were treated either with TAT-Cd0 (A–C) in each of the four delivery vehicles, the delivery vehicles only (D–F), or were left untreated. (A, D) Blepharitis, (B, E) neovascularization, and (C, F) stromal keratitis. Black bar: duration of treatment.
TAT-Cd0 reduced the percentage of mice presenting with blepharitis in all treatment groups tested. Figure 2A shows the percentage of mice presenting with blepharitis. In mice treated with TAT-Cd0 in tear solution, 100% of mice had blepharitis at day 3 but this dropped to 20% by day 15. In the group treated with TAT-Cd0 in aquaphor cream, blepharitis had dropped to 40% of the mice by day 15. For mice treated with TAT-Cd0 in gel, 100% of the mice had blepharitis on day 3 and by day 15 this had dropped to 10%. In the group treated with TAT-Cd0 in PBS, 90% of the mice had blepharitis on day 3, dropping to 10% by day 15.
Figures 2B and 2C show the percentage of mice that presented with neovascularization and stromal keratitis, respectively. TAT-Cd0, regardless of vehicle, delayed the onset of neovascularization and stromal keratitis in all treatment groups tested when compared with untreated infected controls. In mice treated with TAT-Cd0 in tear solution, neovascularization became measurable at day 11, whereas stromal keratitis became measurable at day 7. These values rose and, by the end of the study, 50% of mice from the group treated with TAT-Cd0 in tear solution presented with neovascularization and stromal keratitis. In mice treated with TAT-Cd0 in PBS, the percentage of mice presenting with neovascularization and stromal keratitis by the end of the study was 30% and 40%, respectively (Figs. 2E, 2F). Treatment with TAT-Cd0 in gel showed 30% of mice presenting with neovascularization and 40% of mice presenting with stromal keratitis by day 15 (Figs. 2E, 2F). For the group treated with TAT-Cd0 in cream, 60% of mice in the group had neovascularization and stromal keratitis by day 15 (Figs. 2E, 2F).
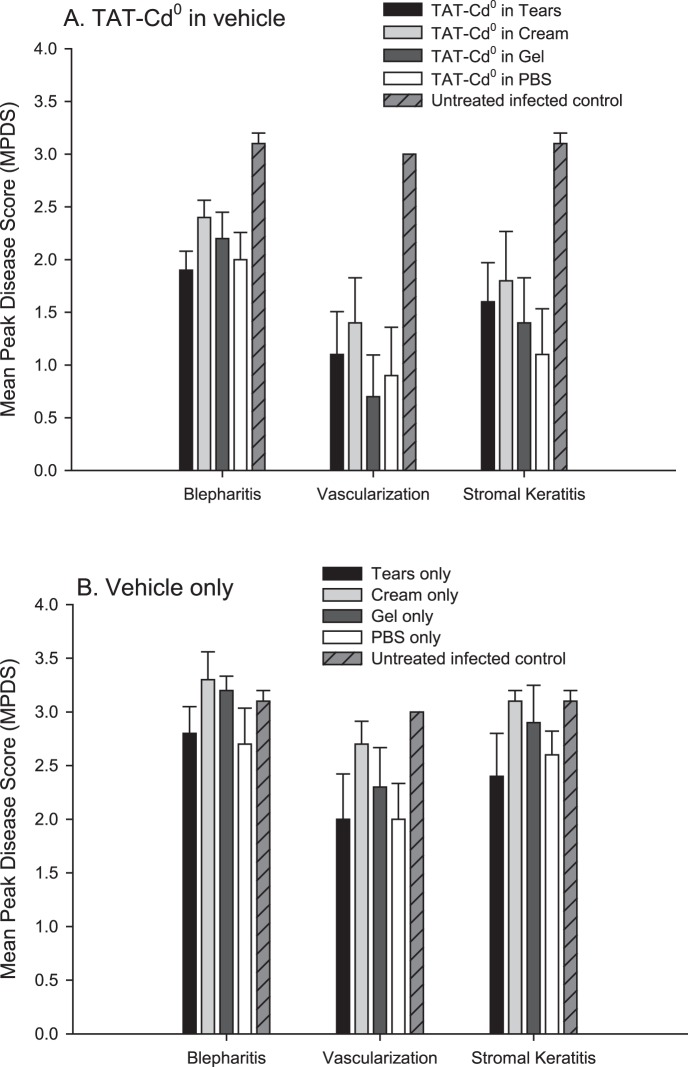
Mean Peak Disease Score
Treatment with TAT-Cd0, regardless of the delivery vehicle, significantly reduced (P < 0.05) MPDS scores for each disease parameter when compared against untreated infected controls (Fig. 3A). MPDS scores for mice treated with the delivery vehicles only were similar to untreated infected controls for the three disease parameters measured and were not statistically significant (P > 0.05) (Fig. 3B).
Figure 3. .
MPDS for all groups in the vehicle study. The highest disease score for each mouse through the entire study was determined and then averaged and the mean and SE were plotted. Statistical analysis of the MPDS data was carried out using the Mann–Whitney rank sum test to determine if the presence of TAT-Cd0 reduced MPDS scores when compared against vehicle only, controls, and untreated infected controls. (A) Treatment of mice with peptide in tears, PBS, cream, or gel. (B) Treatment of mice with vehicles only.
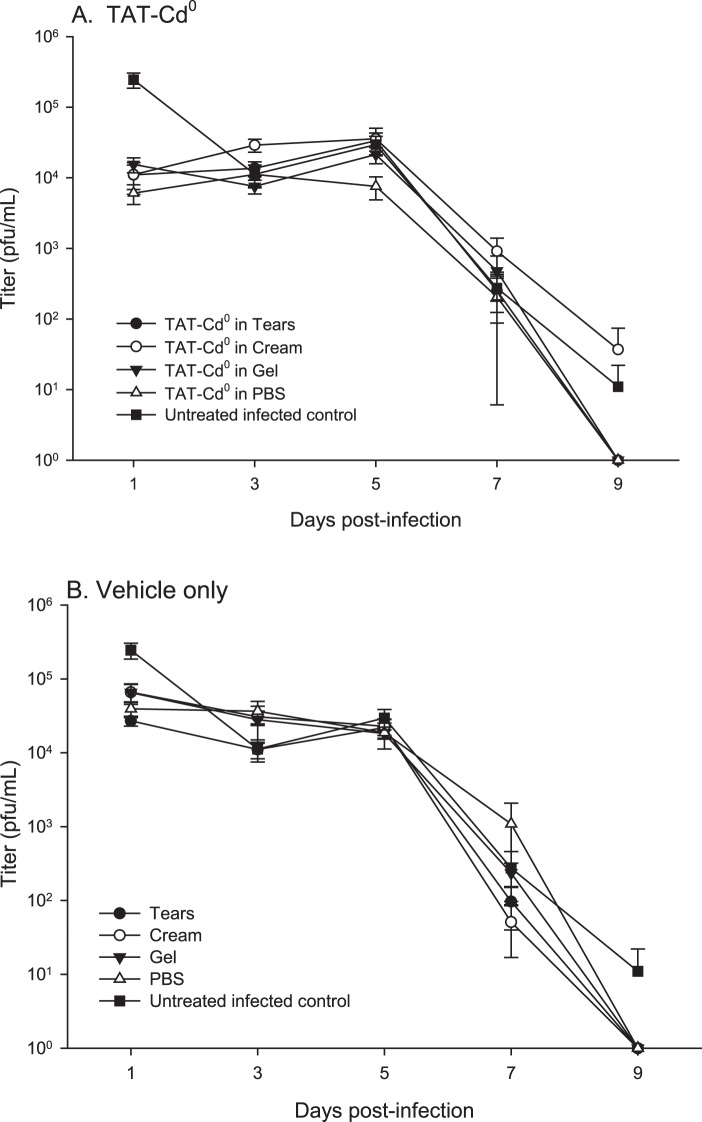
Viral Titers
Titers on day 1 for mice treated with TAT-Cd0 in any of the delivery vehicles were approximately 1 log lower than that of untreated infected controls (Fig. 4A, P < 0.05), whereas titers on day 1 for mice treated with vehicles only were one-half log lower than untreated infected controls (Fig. 4B, P < 0.05). For the remaining days of the study, titers were essentially equivalent and treatment with TAT-Cd0 did not significantly reduce viral titers when compared with mice treated with vehicle only controls and untreated infected mice. By day 9, virus had cleared in groups treated with TAT-Cd0 in gel, PBS, and artificial tears; however, infectious virus was still detectable in the infected untreated mice and mice treated with TAT-Cd0 in cream (Figs. 4A, 4B), although the difference was not significant (P > 0.05).
Figure 4. .
Effect of TAT-Cd0 on infectious virus titers. Tear film samples were collected as described and samples assessed for infectious virus using a standard plaque assay on Vero cells. (A) Treatment of mice with peptide in tears, PBS, cream, or gel. (B) Treatment of mice with vehicles only.
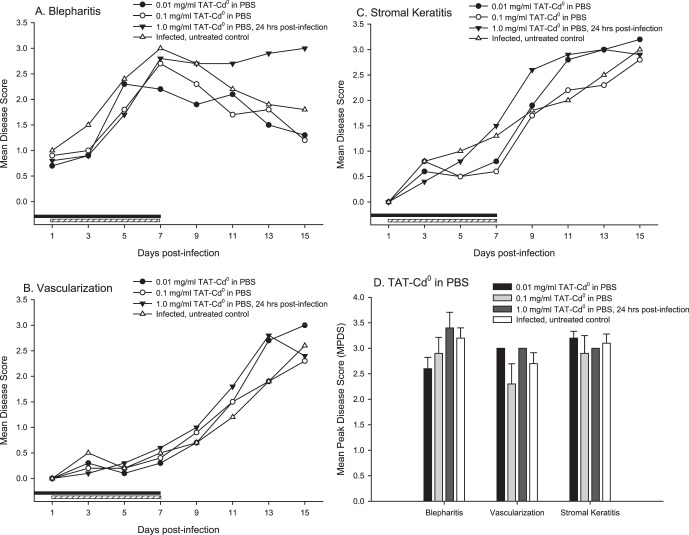
Treatment with Lower Doses of TAT-Cd0 and Delay of Treatment by 24 Hours
To determine if lower concentrations of TAT-Cd0 formulated in PBS were effective, infected mice were treated 5 times per day for 7 days with 0.01 and 0.1 mg/mL TAT-Cd0. We also tested whether delaying the onset of therapy by 24 hours had a significant effect. The results are shown in Figure 5. Overall, treatment with lower concentrations of TAT did not significantly reduce disease, although eye disease in the mice treated with 0.1 mg/mL was lower on day 13 than that in the mice given 0.01 mg/mL. All three disease parameters were more severe in the mice treated with 1 mg/mL TAT-Cd0 starting 24 hours postinfection. In addition, the percentage of animals with disease and viral titers were not significantly reduced in the mice treated with lower concentrations of TAT-Cd0, or where treatment was delayed (data not shown). Statistical analysis of the MPDS failed to show a significant effect in the treatment groups (Fig. 5D). These results suggest that concentrations below 1 mg/mL should not be used and that treatment should be initiated as soon as possible after infection.
Figure 5. .
The effect of treatment with lower concentrations of TAT-Cd0 and delaying the initiation of treatment by 24 hours. Mice (10/group) were infected with 1 × 106 PFU of HSV-1 strain KOS and treated with peptide at concentrations of 0.01 or 0.1 mg/mL in PBS beginning within 4 hours of infection. One group was treated with 1 mg/mL of TAT-Cd0 in PBS but treatment was initiated 24 hours postinfection. (A) Blepharitis; (B) corneal vascularization; (C) stromal keratitis; (D) MPDS scores for the three disease parameters. The MPDS scores were not significantly different (P > 0.05). Blepharitis scores for the 24-hour postinfection treatment group were significantly more severe on days 13 and 15 (P < 0.05). Black bar: duration of treatment, groups 1 and 2; hashed bar: duration of treatment, group 3.
Discussion
We previously showed that a modified CPP, TAT-Cd0, had antiviral activity in cell culture and was nontoxic to cells at concentrations several times the in vitro EC50 value.15 TAT-Cd0 is hydrophilic and contains nine positive charges that are important for the antiviral activity.14 Here we have shown that TAT-Cd0 significantly reduced disease in a mouse ocular model of herpes keratitis, when treatment was initiated within 4 hours of infection. We tested TAT-Cd0 in four different delivery vehicles, to determine if the delivery vehicles affected the antiviral activity of TAT-Cd0. The results showed that TAT-Cd0 reduced ocular disease in all of the vehicles tested. However, the data suggest that PBS or an artificial tear solution is a more effective delivery vehicle for TAT-Cd0 than gel or cream formulations.
Of the four vehicles used, aquaphor cream had the poorest performance against blepharitis, with higher disease scores and a higher percentage of mice presenting with blepharitis. This is not surprising given the highly cationic nature of TAT-Cd0. The highly viscous nature of the cream may prevent TAT-Cd0 from reaching the eyelids in effective concentrations where swelling is most prominent and we have seen this effect before when testing other antivirals in a cream vehicle.20 Although the cream vehicle affected blepharitis scores, it did not appear to impair the effect of TAT-Cd0 against neovascularization and stromal keratitis.
We found that after the treatment regimen ended on day 7, neovascularization and stromal keratitis began to increase beginning on day 11, indicating that TAT-Cd0 delayed the onset of stromal keratitis and neovascularization regardless of the vehicle used. Based on these findings, treating with TAT-Cd0 for more than 7 days may provide greater therapeutic effect.
There was a statistically significant 1-log drop in tear film titers at 24 hours postinfection for TAT-Cd0–treated groups regardless of vehicle and virus cleared by day 9 in all of the TAT-treatment groups, except the cream vehicle. Previous work with other antivirals has shown that as little as a 1-log reduction in viral titer could reduce the severity of ocular disease17,19–21 and this study suggests that even reducing the viral titers in the first 24 hours can have a significant effect on the disease. The data from this study reinforce the conclusion that achieving a significant therapeutic effect does not necessarily require complete inhibition of viral replication. In addition, the titer data indicate that treatment with less viscous vehicles resulted in a more rapid clearance of virus compared with the untreated infected controls.
Treatment with the tears and PBS vehicles reduced the severity of vascularization and corneal clouding, although the differences were not significant compared with the untreated control. Shortly after infection with HSV-1, IL-1α and IL-6 are quickly produced and both cytokines have been shown to induce vascular endothelial growth factor A (VEGF-A) production in nearby uninfected cells. The rise in VEGF-A leads to blood vessel formation in the cornea.6,22 IL-6 has also been shown to stimulate MIP-2 and MIP-1α production in cells, which aids in the recruitment of neutrophils to the site of infection.23 Along with neutrophils, CD4+ T cells are also recruited to the site of infection due to the upregulation of IL-6 and IL-12 early in infection. The lesion formation and clouding of the eye indicative of stromal keratitis are thought to be primarily due to the actions of CD4+ T cells, particularly the Th1 cell subset; however, recent evidence has shown that the Th17 cell subset plays a role in both the beginning and later phases of immunopathology and that IL-6 and TGF-β are responsible for Th17 cell differentiation through the production of IL-17.7,22–24 The invasion of neutrophils and T cells to the infected area is made easier through VEGF-A–induced neovascularization. In turn, the invading immune cells stimulate further vascularization through VEGF-A production and stimulate continued immune cell recruitment through the release of proinflammatory cytokines, resulting in tissue damage and lesion formation. Treatment with vehicle would flush cytokines from the eye and this might account for the slight effect on disease severity seen in the PBS and tears groups.
Treatment with 10- and 100-fold lower concentrations of TAT-Cd0 did not reduce the severity of ocular disease. The 10-fold lower concentration (0.1 mg/mL) appeared to reduce disease severity somewhat, suggesting a dose–response, but the differences were not significant. Pharmacokinetic studies to determine the amount of TAT-Cd0 in corneal tissue are needed to establish if, at lower doses, the concentrations are sufficient to achieve an antiviral effect.
When we delayed the initiation of TAT-Cd0 treatment by 24 hours, we found that there was no therapeutic effect. This is consistent with the titer data showing that significant reductions in viral titers were achieved only on day 1 postinfection. This suggests that inhibition of viral replication very early in infection interrupts critical events in the pathology. Whether this is due to reductions in the synthesis and release of critical proinflammatory mediators will require further testing.
The blepharitis scores were more severe on days 13 and 15 (P < 0.05) in the mice where treatment was delayed 24 hours. Disease scores were also slightly higher for stromal keratitis (but not significantly different). It is not clear how TAT-Cd0 treatment could lead to enhancement of disease, but this observation emphasizes that treatment should be initiated as soon as possible. At the present time, the possibility that delayed onset of treatment would exacerbate disease, reduces the potential clinical utility of TAT-Cd0.
The eye poses a unique challenge to effective drug delivery. Topical delivery of drugs or other compounds are preferred due to the ease of administration and patient compliance; however, the corneal and conjunctival epithelia serve as a static barrier to topical instillation of compounds, and have lower rates of diffusion compared with other tissues. Dynamic mechanisms in the precorneal tear film can clear the eye of compounds at a rate of 0.5 to 2.2 μL/min, with many topical drop solutions persisting on the cornea for only 5 minutes before being flushed away into the nasolacrimal duct.25–27 Many groups have found that therapeutic concentrations of drugs in the anterior segment of the eye (cornea, anterior chamber, iris, crystalline lens, and ciliary body) are still achievable and that higher concentrations of topically instilled drugs can help to increase the bioavailability of drugs to the anterior segments of the eye.9,25–28 In addition, the viscosity of the drug vehicle has been shown to play a role in increasing residence time on the cornea.29,30 Small proteins and organic solutes can passively diffuse into the cornea and conjunctiva, whereas active transport mechanisms for amino acids, peptides, larger proteins, and other solutes exist in the cornea for a number of different organisms. A number of groups have identified peptides and other small molecules with therapeutic activity when topically applied to the cornea, showing that with some modification to delivery vehicle or formulation, many types of compounds can be effective in treating corneal disease.17,27,28,31–35
A synthetic retrocyclin, RC-2, is a cationic, synthetic θ-defensin (molecular weight: 2041 daltons [Da]) that functions as an inhibitor of HSV infection.36 θ-Defensins are circular octadecapeptides consisting of two antiparallel β-sheets with six cysteines connecting the β-sheets to each other, forming a ladder-like array, and certain θ-defensins have shown activity against multiple viruses in cell culture.36–40 In our murine model, we previously reported that RC-2 had only a modest effect on keratitis when administered postinfection. RC-2 protected mice from infection when incubated with the virus prior to infection. Previous work has shown that θ-defensins act primarily as both attachment and entry inhibitors, with little effect on the virus after entry.36,38,40,41 TAT-Cd0 differs from RC-2 in that TAT-Cd0 predominantly exists as a dimer in solution and through this study has been shown to reduce disease when applied postinfection. In the dimer form, TAT-Cd0 has a molecular weight that is 956 Da greater than that of RC-2 and has 18 positive charges, whereas RC-2 has 5 positive charges and 4 hydrophobic isoleucine residues. Consequently, TAT-Cd0 has a higher charge-to-mass ratio than that of RC-2. Previous work with other derivatives of the TAT peptide showed that antiviral activity decreased if positively charged amino acids were substituted with negatively charged residues14,17 and it is possible that the higher charge-to-mass ratio of TAT-Cd0 contributes to increased efficacy when compared with RC-2. In addition, there may be sequence-specific attributes that distinguish TAT-Cd0 as a more effective antiviral than RC-2 when applied postinfection, and warrants further investigation.
In summary, we have shown that TAT-Cd0 is an effective antiviral when treatment is initiated shortly after infection and identified potential delivery vehicles for TAT-Cd0. Additional studies are needed to define the mechanisms by which TAT-Cd0 inhibits infection and pharmacokinetic studies are needed to determine tissue concentrations and half-life of TAT-Cd0 in the eye. Additional studies are also needed to determine why treating with TAT-Cd0 at 24 hours postinfection was not effective and may, in fact, exacerbate the disease.
Acknowledgments
The authors thank Hermann Bultmann for technical advice and preparation of the peptide formulations.
Footnotes
Supported by National Eye Institute/National Institutes of Health Grant R01EY018597, Core Grant for Vision Research P30EY016665, and unrestricted funds to the Department of Ophthalmology and Visual Sciences by Research to Prevent Blindness, Inc.
Disclosure: G.G. Jose, None; I.V. Larsen, None; J. Gauger, None; E. Carballo, None; R. Stern, None; R. Brummel, None; C.R. Brandt, None
References
- 1. Roizman B, Knipe D. Herpes simplex viruses and their replication. In: Knipe DM, Howley PM. eds Fields Virology. 4th ed Vol. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 2001: 2399–2460 [Google Scholar]
- 2. Liesegang TJ, Melton LJ, Daly PJ, Ilstrup DM. Epidemiology of ocular herpes simplex. Incidence in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989; 107: 1155–1159 [DOI] [PubMed] [Google Scholar]
- 3. Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001; 20: 1–13 [DOI] [PubMed] [Google Scholar]
- 4. Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012; 57: 448–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stuart PM, Summers B, Morris JE, Morrison LA, Leib DA. CD8(+) T cells control corneal disease following ocular infection with herpes simplex virus type 1. J Gen Virol. 2004; 85: 2055–2063 [DOI] [PubMed] [Google Scholar]
- 6. Suryawanshi A, Mulik S, Sharma S, Reddy PB, Sehrawat S, Rouse BT. Ocular neovascularization caused by herpes simplex virus type 1 infection results from breakdown of binding between vascular endothelial growth factor A and its soluble receptor. J Immunol. 2011; 186: 3653–3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suryawanshi A, Veiga-Parga T, Rajasagi NK, et al. Role of IL-17 and Th17 cells in herpes simplex virus-induced corneal immunopathology. J Immunol. 2011; 187: 1919–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Knickelbein JE, Hendricks RL, Charukamnoetkanok P. Management of herpes simplex virus stromal keratitis: an evidence-based review. Surv Ophthalmol. 2009; 54: 226–234 [DOI] [PubMed] [Google Scholar]
- 9. Imperia PS, Lazarus HM, Dunkel EC, et al. An in vitro study of ophthalmic antiviral agent toxicity on rabbit corneal epithelium. Antiviral Res. 1988; 9: 263–272 [DOI] [PubMed] [Google Scholar]
- 10. Lass J, Langston R, Foster C, Pavan-Langston D. Antiviral medications and corneal wound healing. Antiviral Res. 1984; 4: 143–157 [DOI] [PubMed] [Google Scholar]
- 11. Fardeau C, Langlois M, Mathys B, et al. Emergence of cross-resistant herpes simplex virus following topical drug therapy in rabbit keratitis. Curr Eye Res. 1991; 10: 151–158 [DOI] [PubMed] [Google Scholar]
- 12. Wilhelmus KR, Gee L, Hauck WW, et al. Herpetic Eye Disease Study. A controlled trial of topical corticosteroids for herpes simplex stromal keratitis. Ophthalmology. 1994; 101: 1883–1895 ; discussion 1895–1896 [DOI] [PubMed] [Google Scholar]
- 13. Bultmann H, Brandt CR. Peptides containing membrane transiting motifs inhibit virus entry. J Biol Chem. 2002; 277: 36018–36023 [DOI] [PubMed] [Google Scholar]
- 14. Bultmann H, Teuton J, Brandt CR. Addition of a C-terminal cysteine improves the anti-herpes simplex virus activity of a peptide containing the human immunodeficiency virus type 1 TAT protein transduction domain. Antimicrob Agents Chemother. 2007; 51: 1596–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Akkarawongsa R, Cullinan AE, Zinkel A, Clarin J, Brandt CR. Corneal toxicity of cell-penetrating peptides that inhibit herpes simplex virus entry. J Ocul Pharmacol Ther. 2006; 22: 279–289 [DOI] [PubMed] [Google Scholar]
- 16. Larsen IV, Brandt CR. A cationic TAT peptide inhibits herpes simplex virus type 1 infection of human corneal epithelial cells. J Ocul Pharmacol Ther. 2010; 26: 541–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brandt CR, Akkarawongsa R, Altmann S, et al. Evaluation of a theta-defensin in a murine model of herpes simplex virus type 1 keratitis. Invest Ophthalmol Vis Sci. 2007; 48: 5118–5124 [DOI] [PubMed] [Google Scholar]
- 18. Bultmann H, Busse JS, Brandt CR. Modified FGF4 signal peptide inhibits entry of herpes simplex virus type 1. J Virol. 2001; 75: 2634–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grau DR, Visalli RJ, Brandt CR. Herpes simplex virus stromal keratitis is not titer-dependent and does not correlate with neurovirulence. Invest Ophthalmol Vis Sci. 1989; 30: 2474–2480 [PubMed] [Google Scholar]
- 20. Brandt CR, Spencer B, Imesch P, Garneau M, Deziel R. Evaluation of a peptidomimetic ribonucleotide reductase inhibitor with a murine model of herpes simplex virus type 1 ocular disease. Antimicrob Agents Chemother. 1996; 40: 1078–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brandt CR, Coakley LM, Grau DR. A murine model of herpes simplex virus-induced ocular disease for antiviral drug testing. J Virol Methods. 1992; 36: 209–222 [DOI] [PubMed] [Google Scholar]
- 22. Biswas PS, Banerjee K, Kinchington PR, Rouse BT. Involvement of IL-6 in the paracrine production of VEGF in ocular HSV-1 infection. Exp Eye Res. 2006; 82: 46–54 [DOI] [PubMed] [Google Scholar]
- 23. Fenton RR, Molesworth-Kenyon S, Oakes JE, Lausch RN. Linkage of IL-6 with neutrophil chemoattractant expression in virus-induced ocular inflammation. Invest Ophthalmol Vis Sci. 2002; 43: 737–743 [PubMed] [Google Scholar]
- 24. Kim B, Sarangi PP, Azkur AK, Kaistha SD, Rouse BT. Enhanced viral immunoinflammatory lesions in mice lacking IL-23 respones. Microbes Infect. 2008; 10: 302–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ghate D, Edelhauser HF. Barriers to glaucoma drug delivery. J Glaucoma. 2008; 17: 147–156 [DOI] [PubMed] [Google Scholar]
- 26. Hughes PM, Olejnik O, Chang-Lin J-E, Wilson CG. Topical and systemic drug delivery to the posterior segments. Adv Drug Deliv Rev. 2005; 57: 2010–2032 [DOI] [PubMed] [Google Scholar]
- 27. Martin J, Malreddy P, Iwamoto T, et al. NC-1059: a channel-forming peptide that modulates drug delivery across in vitro corneal epithelium. Invest Ophthalmol Vis Sci. 2009; 50: 3337–3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maudgal PC, Clercq ED, Bernaerrs R, et al. Ocular penetration and efficacy of chloroethyldeoxyuridine against herpetic keratouveitis. Invest Ophthalmol Vis Sci. 1986; 27: 1453–1458 [PubMed] [Google Scholar]
- 29. Waltman SR, Patrowicz TC. Effects of hydroxypropyl methylcellulose and polyvinyl alcohol on intraocular penetration of topical fluorescein in man. Invest Ophthalmol Vis Sci. 1970; 9: 966–970 [PubMed] [Google Scholar]
- 30. Wilson CG. Topical drug delivery in the eye. Exp Eye Res. 2004; 78: 737–743 [DOI] [PubMed] [Google Scholar]
- 31. Anand BS, Hill JM, Dey S, et al. In vivo antiviral efficacy of a dipeptide acyclovir prodrug, Val-Val-Acyclovir, against HSV-1 epithelial and stromal keratitis in the rabbit eye model. Invest Ophthalmol Vis Sci. 2003; 44: 2529–2534 [DOI] [PubMed] [Google Scholar]
- 32. Heitz F, Morris MC, Divita G. Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. Br J Pharmacol. 2009; 157: 195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liuzzi M, Dezlel R, Moss N, et al. A potent peptidomimetic inhibitor of HSV ribonucleotide reductase with antiviral activity in vivo. Nature. 1994; 372: 695–698 [DOI] [PubMed] [Google Scholar]
- 34. Meyers-Elliot RH, Chitjian PA, Billups CB. Effects of cyclosporine A on clinical and immunological parameters in herpes simplex keratitis. Invest Ophthalmol Vis Sci. 1987; 28: 1170–1180 [PubMed] [Google Scholar]
- 35. Shuler RK, Dioguardi PK, Henjy C, et al. Scleral permeability of a small, single-stranded oligonucleotide. J Ocul Pharmacol Ther. 2004; 20: 159–168 [DOI] [PubMed] [Google Scholar]
- 36. Yasin B, Wang W, Pang M, et al. Theta defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J Virol. 2004; 78: 5147–5156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trabi M, Schirra H, Craik D. Three-dimensional structure of RTD-1, a cyclic antimicrobial defensin from Rhesus macaque leukocytes. Biochemistry. 2001; 40: 4211–4221 [DOI] [PubMed] [Google Scholar]
- 38. Cole A, Hong T, Boo L, et al. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc Natl Acad Sci U S A. 2002; 99: 1813–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Daher K, Selsted M, Lehrer R. Direct inactivation of viruses by human granulocyte defensins. J Virol. 1986; 60: 1068–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leikina E, Delanoe-Ayari H, Melikov K, et al. Carbohydrate-binding molecules inhibit viral fusion and entry by crosslinking membrane glycoproteins. Nat Immunol. 2005; 6: 995–1001 [DOI] [PubMed] [Google Scholar]
- 41. Gallo S, Wang W, Rawat S, et al. Theta-defensins prevent HIV-1 Env-mediated fusion by binding gp41 and blocking 6-helix bundle formation. J Biol Chem. 2006; 281: 18787–18792 [DOI] [PubMed] [Google Scholar]