Abstract
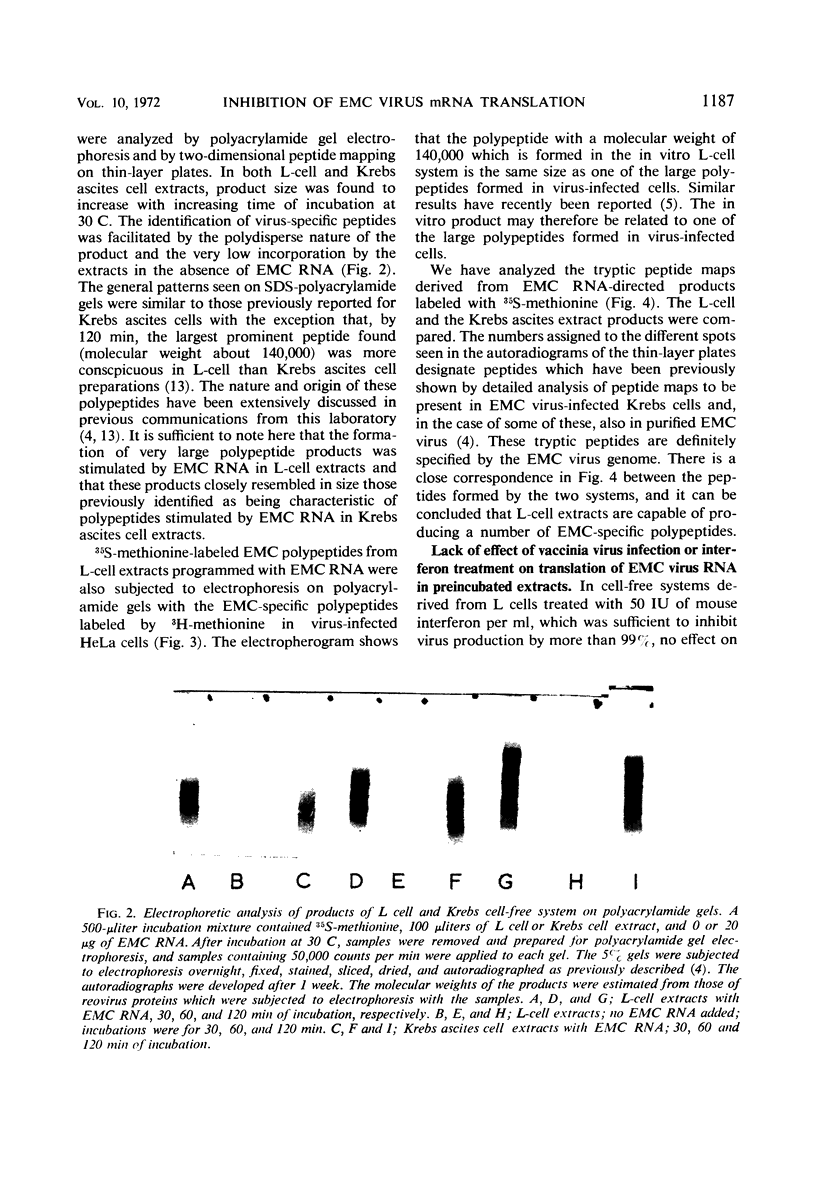
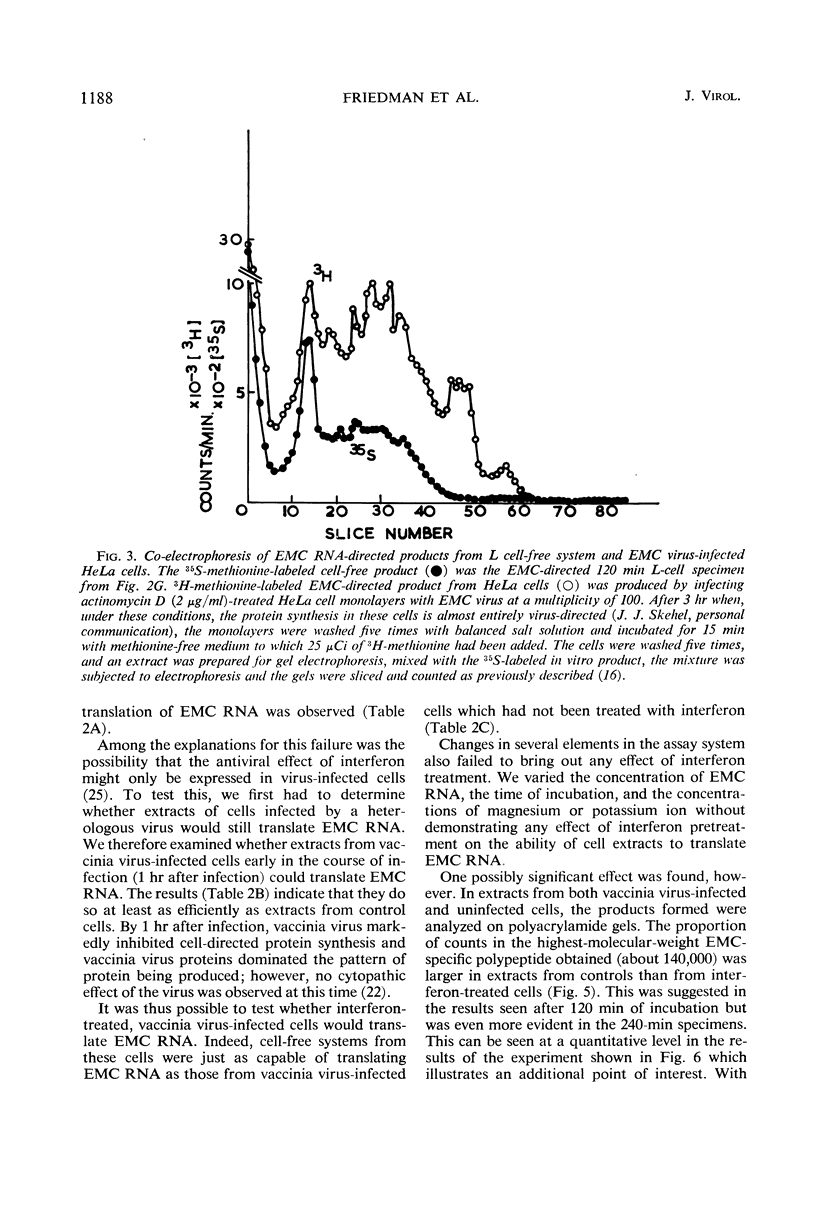
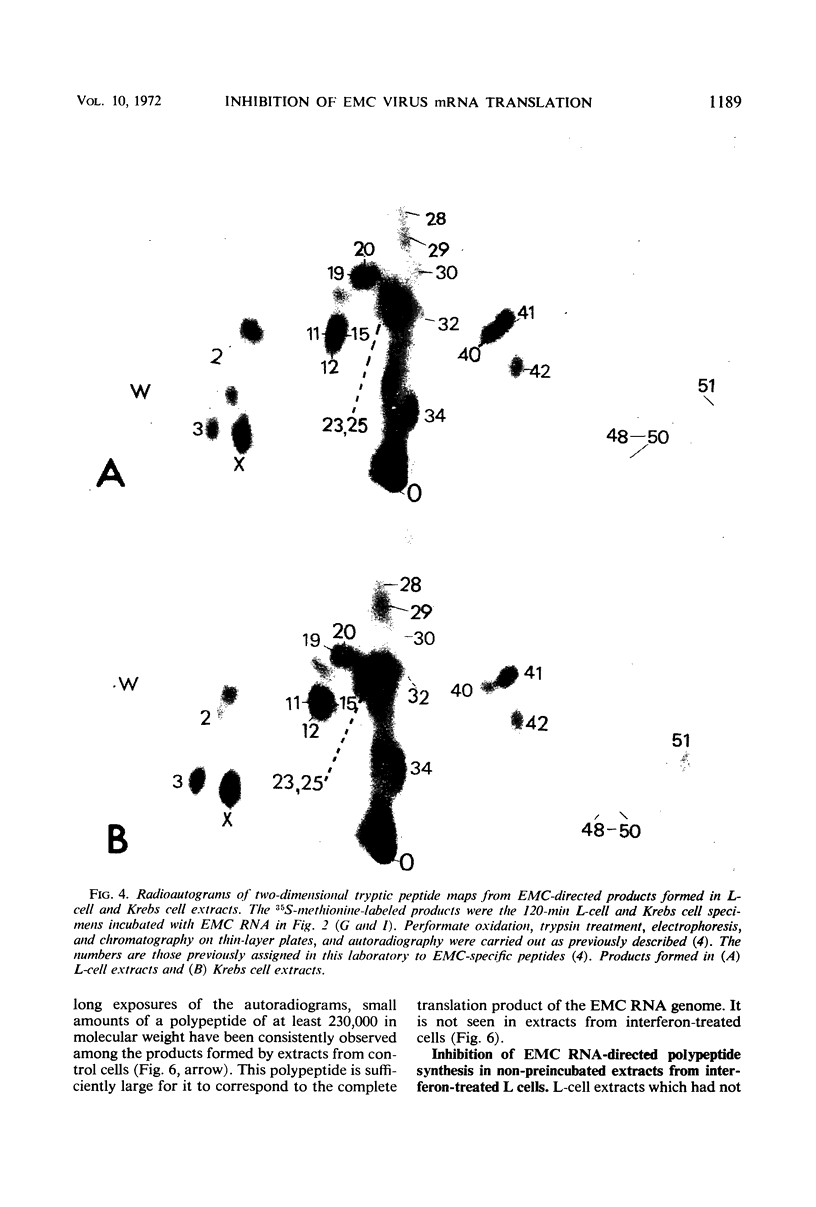
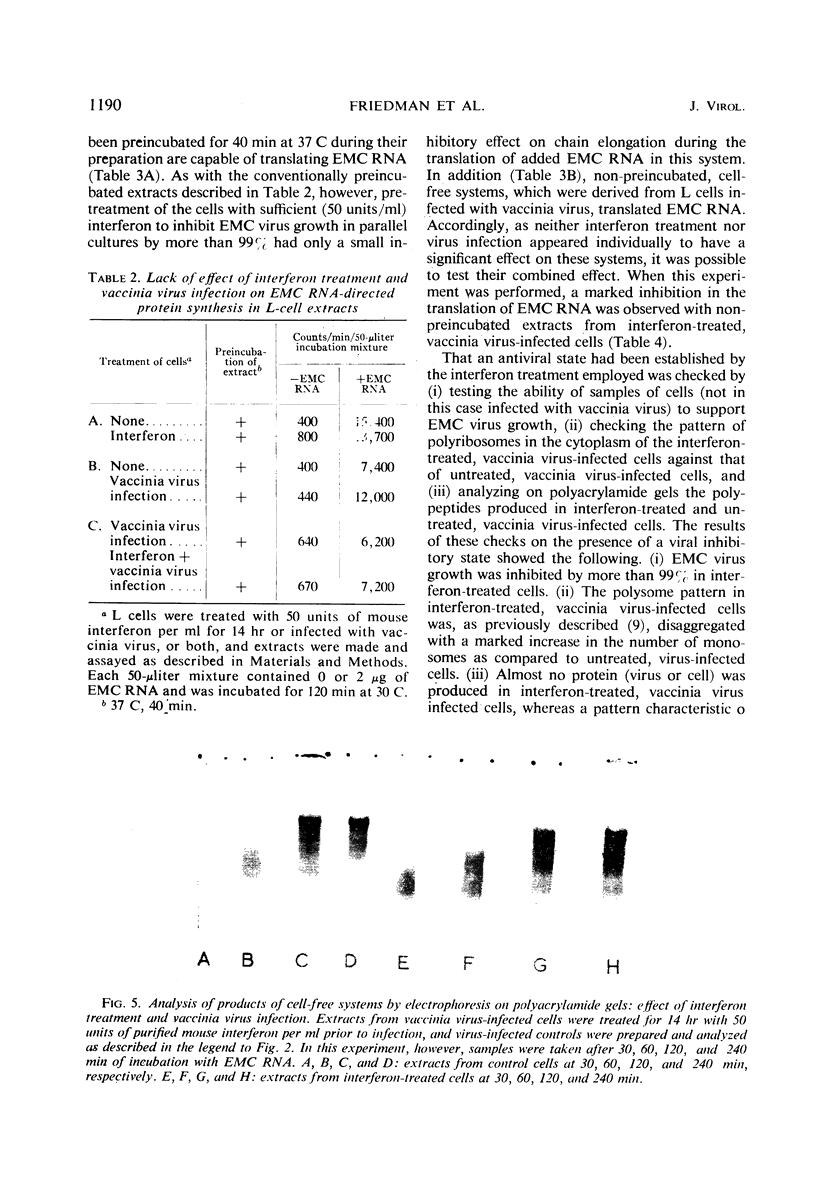
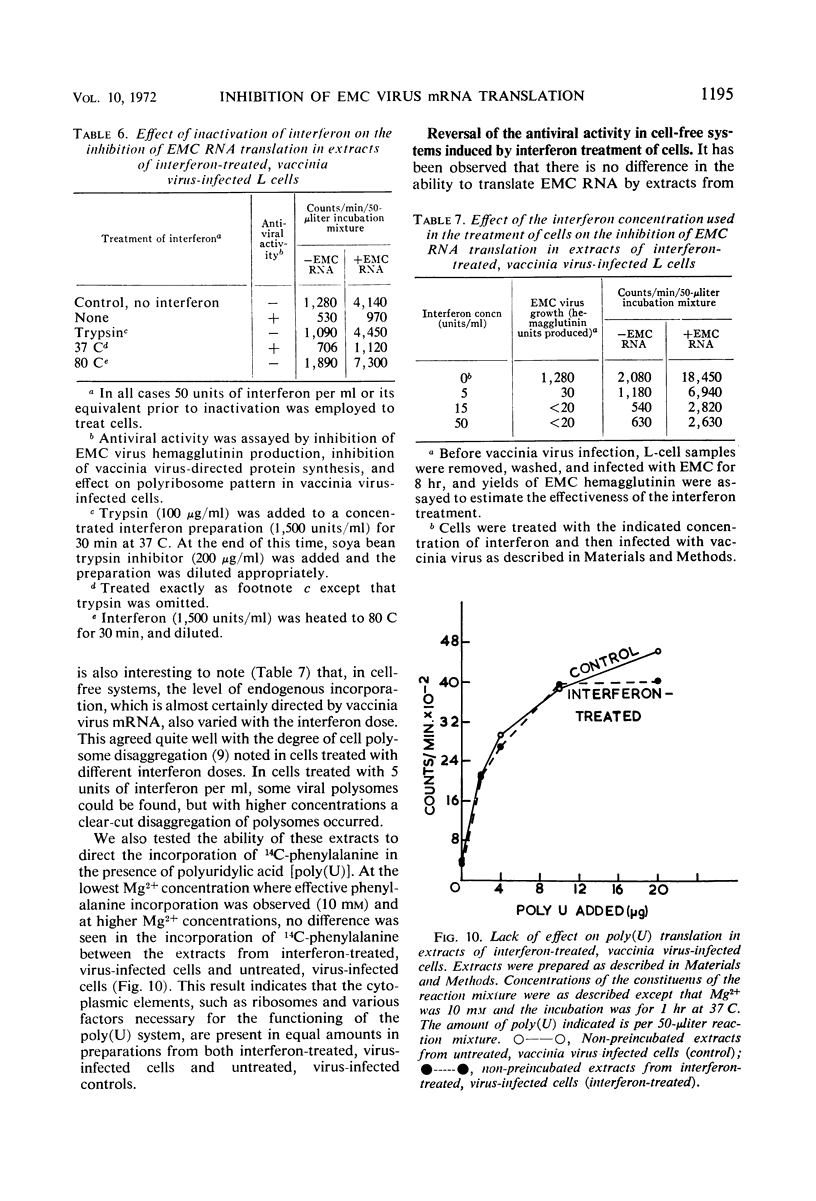
Encephalomyocarditis (EMC) virus ribonucleic acid (RNA) stimulated the incorporation of 14C-amino acids into polypeptides in cell-free systems using preincubated S10 extracts from L cells. Incorporation was linear for over 2 hr. Analysis of the tryptic peptides derived from the polypeptide products formed in response to EMC RNA showed them to be virus specific. The major product, a polypeptide of 140,000 in molecular weight, migrated on sodium dodecyl sulfate-polyacrylamide gels with one of the virus-specific polypeptides present in EMC-infected cells. A minor component of molecular weight about 230,000 may correspond to the product of complete translation of the EMC virus genome. Little or no effect of interferon or vaccinia virus infection was observed in the preincubated, cell-free system. The EMC RNA-stimulated incorporation of 14C-amino acids into polypeptides was not inhibited in extracts derived from L cells early in virus infection, from interferon-treated cells, or from cells subjected to both treatments. Interferon treatment did appear to have a slight inhibitory effect on chain elongation in this system. However, treatment of cells with highly purified interferon before virus infection caused a decrease of about 80% in the capacity of non-preincubated cell extracts to translate added EMC RNA. This effect did not extend to the translation of polyuridylic acid and could be reversed by preincubation of the extracts at 37 C for 20 min. The inhibition of translation was manifest at interferon concentrations as low as 5IU/ml, and in this respect closely paralleled the inhibition of virus growth. Inactivation of the antiviral activity of the interferon by heating or digestion with trypsin also abolished the effect on cell-free protein synthesis. The EMC-specific polypeptides formed in reduced amounts in extracts of interferon-treated vaccinia-infected cells were smaller than those formed in extracts of untreated, vaccinia-infected cells. Thus, inhibition of initiation or elongation of polypeptides, or both, can be demonstrated in cell-free systems employing non-preincubated extracts from interferon-treated, virus-infected cells. These results indicate that antiviral activity of interferon is directed against the translation of viral messenger RNA.
Full text
PDF

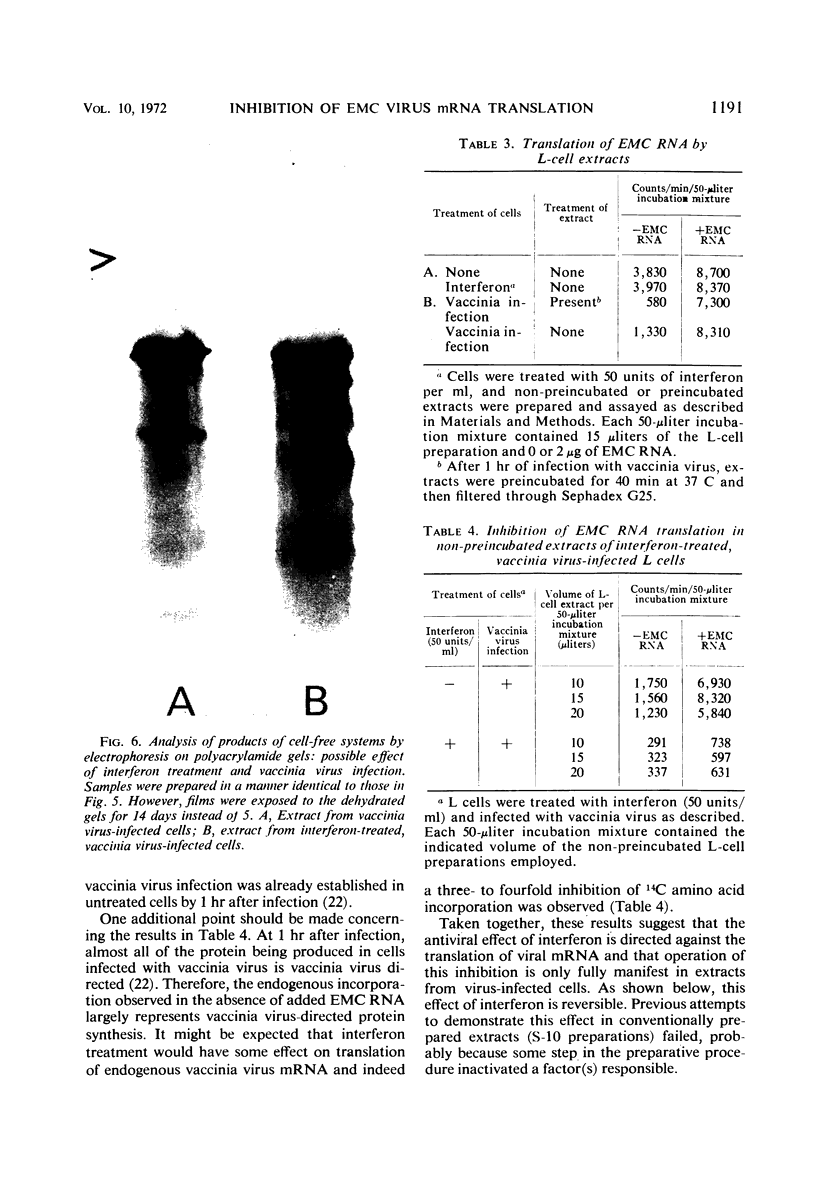
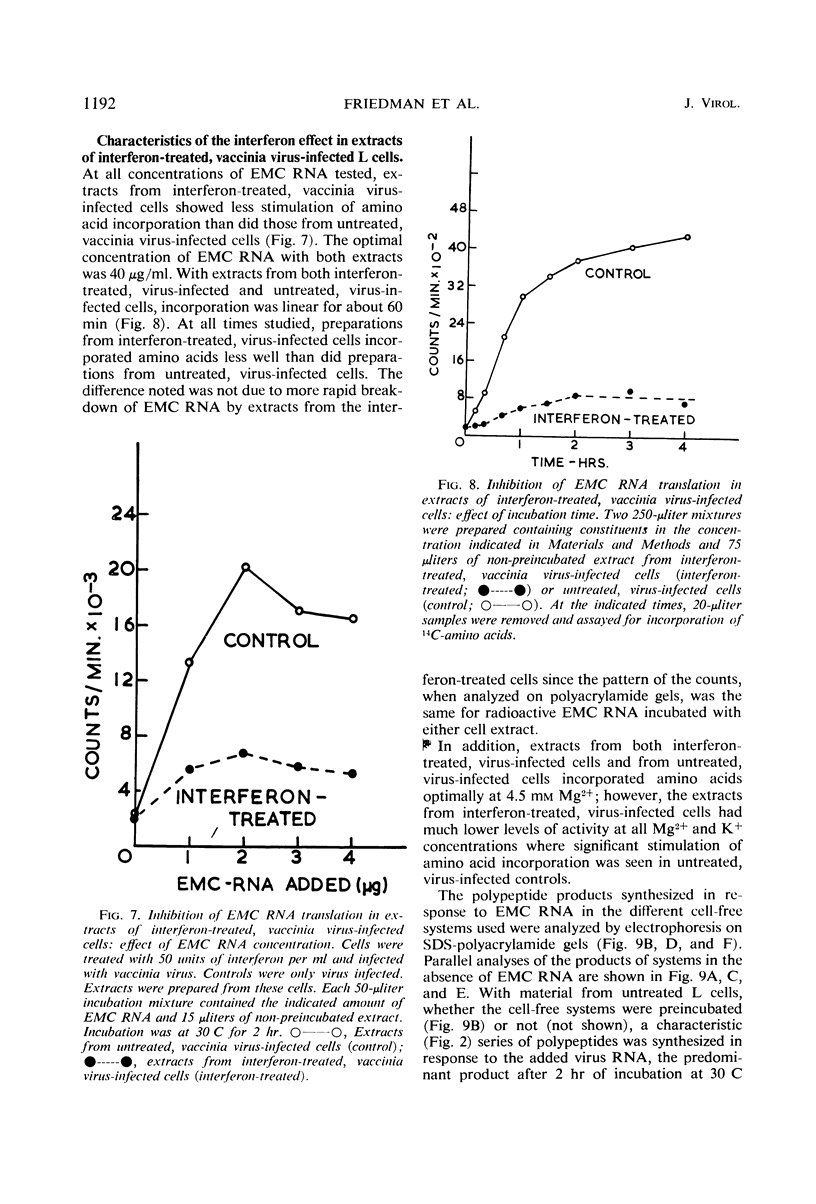
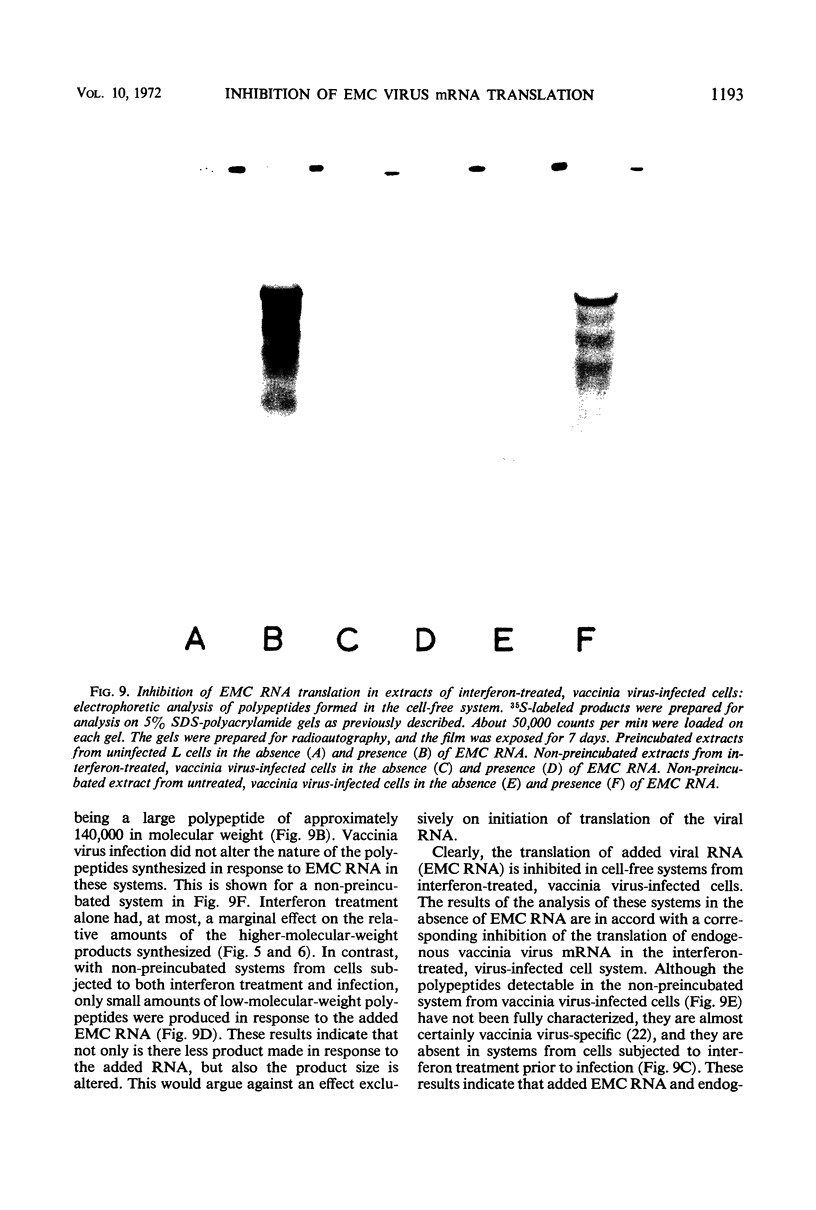
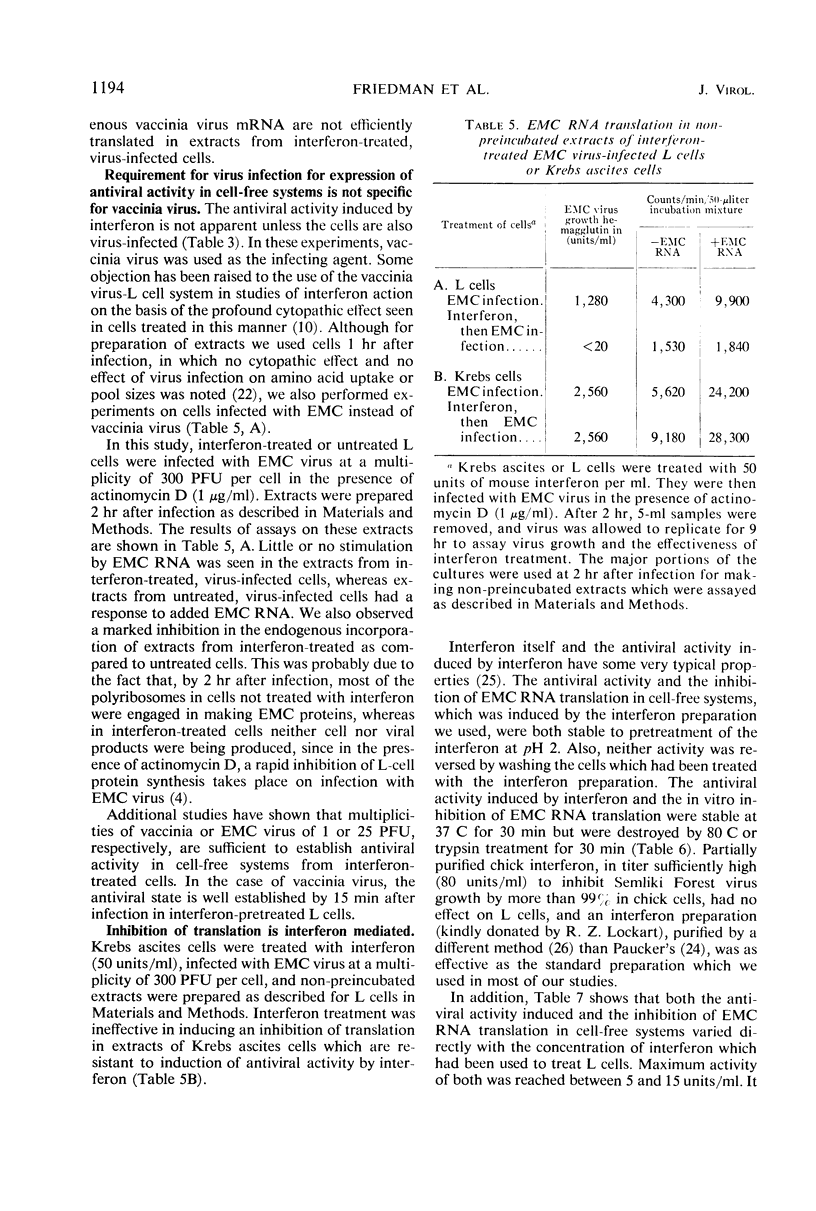



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKER Y., JOKLIK W. K. MESSENGER RNA IN CELLS INFECTED WITH VACCINIA VIRUS. Proc Natl Acad Sci U S A. 1964 Apr;51:577–585. doi: 10.1073/pnas.51.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialy H. S., Colby C. Inhibition of early vaccinia virus ribonucleic acid synthesis in interferon-treated chicken embryo fibroblasts. J Virol. 1972 Feb;9(2):286–289. doi: 10.1128/jvi.9.2.286-289.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W. A., Levy H. B. The recognition of viral RNA by mammalian ribosomes. An effect of interferon. Biochim Biophys Acta. 1968 Feb 26;155(2):437–443. doi: 10.1016/0005-2787(68)90189-5. [DOI] [PubMed] [Google Scholar]
- Dobos P., Kerr I. M., Martin E. M. Synthesis of capsid and noncapsid viral proteins in response to encephalomyocarditis virus ribonucleic acid in animal cell-free systems. J Virol. 1971 Oct;8(4):491–499. doi: 10.1128/jvi.8.4.491-499.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggen K. L., Shatkin A. J. In vitro translation of cardiovirus ribonucleic acid by mammalian cell-free extracts. J Virol. 1972 Apr;9(4):636–645. doi: 10.1128/jvi.9.4.636-645.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantes K. H. Purification of interferon from chick embryonic allantoic fluids and fibroblast tissue infected with influenza virus. J Gen Virol. 1967 Jul;1(3):257–267. doi: 10.1099/0022-1317-1-3-257. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Esteban R. M., Metz D. H., Tovell D. R., Kerr I. M., Williamson R. Translation of RNA by L cell extracts: Effect of interferon. FEBS Lett. 1972 Aug 15;24(3):273–277. doi: 10.1016/0014-5793(72)80371-5. [DOI] [PubMed] [Google Scholar]
- Friedman R. M. Inhibition of arbovirus protein synthesis by interferon. J Virol. 1968 Oct;2(10):1081–1085. doi: 10.1128/jvi.2.10.1081-1085.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joklik W. K., Merigan T. C. Concerning the mechanism of action of interferon. Proc Natl Acad Sci U S A. 1966 Aug;56(2):558–565. doi: 10.1073/pnas.56.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungwirth C., Horak I., Bodo G., Lindner J., Schultze B. The synthesis of poxvirus-specific RNA in interferon-treated cells. Virology. 1972 Apr;48(1):59–70. doi: 10.1016/0042-6822(72)90114-6. [DOI] [PubMed] [Google Scholar]
- Kates J., Beeson J. Ribonucleic acid synthesis in vaccinia virus. I. The mechanism of synthesis and release of RNA in vaccinia cores. J Mol Biol. 1970 May 28;50(1):1–18. doi: 10.1016/0022-2836(70)90100-2. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E., Tovell D. R. Characterization of the polypeptides formed in response to encephalomyocarditis virus ribonucleic acid in a cell-free system from mouse ascites tumor cells. J Virol. 1972 Jul;10(1):73–81. doi: 10.1128/jvi.10.1.73-81.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Martin E. M. Simple method for the isolation of encephalomyocarditis virus ribonucleic acid. J Virol. 1972 Mar;9(3):559–561. doi: 10.1128/jvi.9.3.559-561.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M. Protein synthesis in cell-free systems: an effect of interferon. J Virol. 1971 Apr;7(4):448–459. doi: 10.1128/jvi.7.4.448-459.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Sonnabendja, Martin E. M. Proein-synthetic activity of ribosomes from interferon-treated cells. J Virol. 1970 Feb;5(2):132–144. doi: 10.1128/jvi.5.2.132-144.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin J. G., Friedman R. M. Analysis of arbovirus ribonucleic acid forms by polyacrylamide gel electrophoresis. J Virol. 1971 Apr;7(4):504–514. doi: 10.1128/jvi.7.4.504-514.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN E. M., MALEC J., SVED S., WORK T. S. Studies on protein and nucleic acid metabolism in virus-infected mammalian cells. 1. Encephalomyocarditis virus in Krebs II mouse-ascites-tumour cells. Biochem J. 1961 Sep;80:585–597. doi: 10.1042/bj0800585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus P. I., Engelhardt D. L., Hunt J. M., Sekellick M. J. Interferon action: inhibition of vesicular stomatitis virus RNA synthesis induced by virion-bound polymerase. Science. 1971 Nov 5;174(4009):593–598. doi: 10.1126/science.174.4009.593. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Salb J. M. Molecular basis of interferon action: inhibition of viral RNA translation. Virology. 1966 Nov;30(3):502–516. doi: 10.1016/0042-6822(66)90126-7. [DOI] [PubMed] [Google Scholar]
- Mathews M., Korner A. Mammalian cell-free protein synthesis directed by viral ribonucleic acid. Eur J Biochem. 1970 Dec;17(2):328–338. doi: 10.1111/j.1432-1033.1970.tb01170.x. [DOI] [PubMed] [Google Scholar]
- Metz D. H., Esteban M. Interferon inhibits viral protein synthesis in L cells infected with vaccinia virus. Nature. 1972 Aug 18;238(5364):385–388. doi: 10.1038/238385a0. [DOI] [PubMed] [Google Scholar]
- Oxman M. N., Levin M. J. Interferon and transcription of early virus-specific RNA in cells infected with simian virus 40. Proc Natl Acad Sci U S A. 1971 Feb;68(2):299–302. doi: 10.1073/pnas.68.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paucker K., Berman B. J., Golgher R. R., Stancek D. Purification, characterization, and attempts at isotopic labeling of mouse interferon. J Virol. 1970 Feb;5(2):145–152. doi: 10.1128/jvi.5.2.145-152.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Gosser L. B., Lockart R. Z., Jr Priming: a nonantiviral function of interferon. J Virol. 1971 Jun;7(6):792–801. doi: 10.1128/jvi.7.6.792-801.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R., Morrison M., Lanyon G., Eason R., Paul J. Properties of mouse globin messenger ribonucleic acid and its preparation in milligram quantities. Biochemistry. 1971 Aug 3;10(16):3014–3021. doi: 10.1021/bi00792a005. [DOI] [PubMed] [Google Scholar]