Abstract
We assessed the clinical usefulness of human epidermal growth factor receptor-2 extracellular domain (HER2ECD) as a biomarker for detecting cancer and monitoring disease status and for predicting the efficacy of anticancer treatment in breast cancer. Five-hundred and eighty serum samples from 252 patients with breast cancer were examined for the concentration of HER2ECD to compare with conventional tumor markers (CEA, CA15-3, NCC-ST439 and BCA225). Also, in 19 patients with HER2-overexpressed advanced or recurrent breast cancer who were treated with trastuzumab, clinical outcomes were evaluated retrospectively to determine whether their serum HER2ECD levels predict clinical responses. The proportion of patients with elevated HER2ECD levels was 15.1%, which was compatible with those with elevated conventional marker levels. In patients with HER2-overexpressed breast cancer, the positive rate of HER2ECD was significantly higher (24.1%) than those of conventional markers (7.4–12.9%), suggesting the usefulness of HER2ECD for detecting cancer in this population. HER2-overexpressed patients responding to trastuzumab (12 of 19 patients) showed significantly higher serum HER2ECD level (p = 0.033) and longer time to progression (TTP) (p = 0.039) and overall survival (OS) (p = 0.031) than did patients not responding (seven patients). Furthermore, higher response rates were observed in patients with elevated HER2ECD levels than in patients without elevated HER2ECD levels (91.3% vs. 14.3%, p = 0.032), whereas there was no difference in survival between the two groups. The results suggest that HER2ECD is a useful biomarker not only for detecting breast cancer recurrence but also for predicting tumor responses to trastuzumab.
Keywords: breast cancer, HER2ECD, trastuzumab, biomarker, predictive factor
Introduction
Biomarkers are useful for early detection of cancer by screening and for monitoring cancer status in patients during and after anticancer treatment as tumor markers. In breast cancer, CEA, CA15–3, NCC-ST439 and BCA225 are now clinically available tumor markers in daily clinical practice. However, these tumor marker levels are not always elevated when cancer has developed or relapsed.1,2 Novel tumor markers that are more sensitive to cancer status are needed. Furthermore, some biomarkers such as hormone receptor, HER2 gene and Ki67 proliferation parameters have a crucial role in predicting prognosis of cancer patients and the efficacy of anticancer therapeutics.3-6 HER2 gene product is a transmembrane receptor tyrosine kinase glycoprotein of approximately 185 kDa that belongs to the HER family including the human epidermal growth factor receptor (EGFR).7,8 HER2 protein is activated by phosphorylation of tyrosine residues, resulting in the regulation of cell growth and differentiation through signaling cascades.9,10 Amplified HER2 gene or overexpressed HER2 protein is observed in approximately 15–30% of primary breast cancers and these patients showed short survival or poor prognosis.11-13
Trastuzumab is a humanized mouse monoclonal antibody that binds to the extracellular domain of the HER2 molecule.14 It has been clinically approved as the world’s first humanized monoclonal antibody breast cancer therapeutic agent in 1998 by the USA Food and Drug Administration (FDA), and it was subsequently approved in Japan for use in a metastatic setting in 2001 and in an adjuvant setting in 2008.15 Administration of trastuzumab in combination with anticancer cytotoxic agents has shown good therapeutic efficacy in HER2-overexpressed metastatic breast cancer.16
Immunohistochemistry (IHC) and fluorescent in situ hybridization (FISH) are commonly used to determine the HER2 overexpression in breast cancer tissue.17,18 However, a tumor sample cannot always be obtained to examine HER2 status, especially in patients with recurrent or metastatic breast cancer. HER2 extracellular domain (HER2ECD), which is shed from the whole HER2 molecule on breast cancer cells, has been detected in sera of patients with breast cancer.19,20 The Siemens Serum HER2 test measures the serum concentration of this protein using a CLIA method.21,22 Since the measurement of serum HER2ECD levels is a simple, noninvasive and reproducible method, we assessed its availability as a biomarker for indicating the efficacy of anticancer treatment or for monitoring cancer status including progression or regression of cancer. According to a systemic review by Carney et al.,23,24 the positive rates of serum HER2ECD in primary and metastatic breast cancer were 18.5% (0–38) and 43% (23–80), respectively. These results are compatible with the positivity of other conventional tumor markers. A systemic review by the National Academy of Clinical Biochemistry (NACB),25 however, showed that serum HER2ECD level has lower sensitivity in monitoring cancer status as a tumor marker than does CA15–3 or CEA. As a prognostic factor, elevated serum HER2ECD level indicates a poor prognosis, i.e., short overall and disease-free survival.26 It has also been reported that elevated serum HER2ECD level predicted resistance to hormonal therapy and chemotherapy.27-29 However, whether serum HER2ECD level predicts the efficacy of trastuzumab has been controversial,30,31 more precise evaluation is needed to determine whether HER2ECD is a clinically useful biomarker for monitoring cancer status or predicting tumor responses in breast cancer.
In this study, serum HER2ECD levels were measured in patients with primary and metastatic breast cancers by using the Siemens Serum HER2 test to compare the ability of HER2ECD level to reflect cancer status with the abilities of serum levels of conventional tumor markers including CA15–3, NCC-ST439, BCA225 and CEA. Also, the ability of HER2ECD to predict the therapeutic efficacy of trastuzumab was assessed by comparing its levels in patients with HER2-overexpressed advanced or relapsed breast cancer responding to trastuzumab regimens and patients not responding to the regimens.
Materials and Methods
Serum samples
Five-hundred and eighty serum samples were obtained from 252 patients with primary breast cancer (stages 0–3) and recurrent or metastatic breast cancer who were treated at the Department of Respiratory, Breast and Endocrine Surgery of Kagawa University Hospital from July 2007–December 2011. All of the patients were diagnosed with breast cancer by histological examination of specimens from the primary or metastatic lesions. Clinical staging of the patients was determined on the basis of findings by imaging tests including mammography, sonography, CT (CT), magnetic resonance imaging (MRI) and bone scan or physical examination. Blood samples to examine serum levels of tumor markers were obtained at the time of initial diagnosis of primary or recurrent breast cancer. Serial measurements were done in patients with advanced or recurrent breast cancer while they received anticancer therapeutics including cytotoxic or molecular-targeting agents. Patient characteristics are shown in Table 1.
Table 1. Clinicopathologic features of patients with breast cancer studied.
| All patients | HER2(-) | HER2(+) | HER2(?) | ||
|---|---|---|---|---|---|
|
No. patients |
|
252 |
117 |
85 |
50 |
|
Median age |
|
59.1 |
58.1 |
59.0 |
69.5 |
|
Stage |
0 |
13 |
5 |
7 |
1 |
| 1 |
63 |
29 |
17 |
17 |
|
| 2 |
84 |
39 |
31 |
14 |
|
| 3 |
27 |
12 |
14 |
1 |
|
| 4 or rec. |
66 |
32 |
17 |
17 |
|
|
HR-positive (%) |
|
180 (71.4) |
94 (81.3) |
48 (56.5)* |
38 (78.0) |
| Ductal carcinoma (%) | 194 (91.9) | 86 (86.9) | 68 (97.1) | 40 (95.2) |
p = 0.0015, when compared with HER2-negative patients
Measurements of serum tumor marker levels
Serum HER2ECD levels were measured as described previously22 by the ADVIA Centaur Serum HER2 test (Siemens Healthcare Diagnostics) using the ADVIA Centaur XP system, which is a fully automated, chemiluminescence immunoassay. Results are available in approximately 18 min.
Serum levels of CA15–3, CEA, NCC-ST439 and BCA225 were measured using the CA15–3 RIA kit TFB (TFB, Inc.) on the ARC950 instrument (Hitachi-Aloka Medical, Ltd.), the Abbott CEA assay on the Abbott ARCHITECT I 2000SR (Abbott Japan Co. Ltd.), the NCC-ST439-EIA kit (Kainos Co.) on AP-X (Kyowa Medex Co. Ltd.) and the BCA225-EIA kit (Medical and Biological Laboratories Co.) on SJ eia Auto Reader III (Eidia Co., Ltd.), respectively. The cut-off values for serum HER2ECD, CA15–3, CEA, NCC-ST439 and BCA225 were 15.2 ng/mL, 30.0 U/ml, 5.0 ng/ml, 7.0 U/ml and 160.0 U/ml, respectively. Each cut-off value was determined according to the manufacturer’s instructions.
HER2, estrogen receptor (ER) and progesterone receptor (PgR) status
HER2 expression in the tumor samples was tested by IHC using a Hercep Test kit (DAKO). The HER2 IHC score is based on the intensity of membrane staining, ranging from 0 (negative) to 3+ (strong positive). A score of 2+ on IHC was further evaluated by FISH in which the tissue slide was hybridized using the PathVysion HER2 DNA probe kit (Abbott Japan) according to the manufacturer’s instructions. Patients with either HER2 IHC sore of 3+ or FISH score greater than 2.0 were indicated for trastuzumab-based therapy. The patients were divided into three groups by status of HER2 expression in the tumor, HER2-positive, -negative or -unknown. Patients with HER2IHC score of 0 and 1+ were referred to as HER2-negative and patients with HER2 score of 2+ and 3+ were referred to as HER2-positive.
Testing for tissue ER and PgR status was performed with Ventana CONFIRM anti-ER (SP1) and anti-PgR (1E2) monoclonal antibody kits, respectively, according to the manufacturer’s instructions. Patients with positively stained cancer cells of ≥ 1% in the primary tumor were determined to be ER- or PgR-positive.
Evaluation of therapeutic efficacy
Tumor responses were assessed by physical examination, CT or MRI according to the Response Evaluation Criteria in Solid Tumors (RECIST) every 2–3 mo during the treatment. A complete response (CR) was defined as the absence of evidence of disease, a partial response (PR) was defined as a reduction in the sum of diameters of target lesions by 30% or more, and a progressive disease (PD) was defined as an increase in the sum of target lesions by 20% or more or the presence of a new lesion. Tumor marker levels were examined once a month while the patients received treatment.
Statistical analysis
We used the U Test by Mann-Whitney or standard chi-square procedures for comparison of two groups. The effect of baseline characteristics on the risk of progression or death was calculated using Kaplan-Meier survival analysis and the log-rank test of significance. A 95% confidence interval (CI) for the median of each variable was computed using the method of Brookmeyer and Crowley. We defined p < 0.05 as significant; all p values were two-sided. SPSS statistical software system (SPSS Inc.) was used for all calculations.
Ethical consideration
This research was in compliance with the guidelines of the Ethics Committee at Kagawa University Hospital and conformed to the provision of the Declaration of Helsinki in 1995. We received informed consent from all of the study patients.
Results
Positivity of patients with elevated tumor marker levels
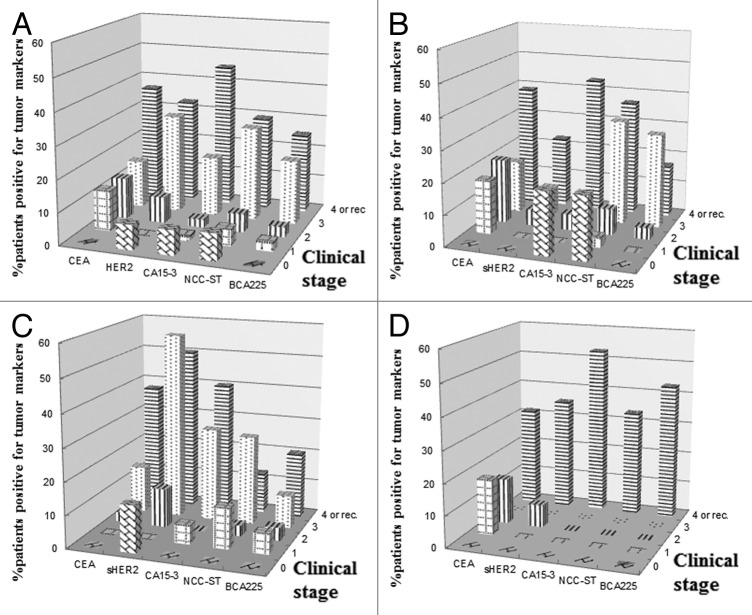
Five hundred and eighty blood samples from 252 patients with primary, recurrent or metastatic breast cancer were examined for serum levels of HER2ECD and conventional tumor markers, i.e., CEA, CA15–3, NCC-ST439 and BCA225. Clinicopathologic features of the patients are shown in Table 1. The patients were divided into three groups according to HER2 status in the primary or metastatic tumor. There was no information on HER2 status in 50 patients because most of the patients had been referred to our hospital from local hospitals in which they had received an operation for their primary breast cancer and their HER2 status had not been tested, and because some of the patients received an operation in our hospital when HER2 testing was not approved in Japan and resected samples from those patients were not available for the test. Furthermore, most of the patients had small metastatic lesions (< 1 cm in size) or lesions located at sites, such as the liver, lung, mediastinal node and bone, in which minimally invasive biopsies such as biopsy by a transbronchial or transcutaneous approach were not indicated. Median age of the patients was 59.1 y (34–88). Since the HER2-unknown group has the smallest number of patients, it included only one patient each in clinical stages 1 and 3. Hormone receptor (HR)-positive patients accounted for 71.4% of all of the patients examined. The proportion of patients with HR-positive tumors in the HER2-positive group, however, was much lower than that in the HER2-negative group (56.6 for HER2-positive vs. 81.3% for HER2-negative patients; p = 0.0015, Table 1). Table 2 shows percentages of patients with elevated serum levels of each marker. Patients with elevated HER2ECD levels accounted for 15.1% of all patients, being compatible with data from other conventional tumor markers (10.9–18.3%). The positivity of each tumor marker was calculated in all patients divided into groups according to HER2 status in the primary or metastatic tumor (Table 2). The percentage of patients with elevated CEA levels in the HER2-positive group was significantly lower than that in the HER2-negative group (p = 0.047). In contrast, the percentage of patients with elevated HER2ECD levels in the HER2-positive group was much higher than that in the HER2-negative group (p = 0.027). Figure 1A shows the percentages of patients with elevated levels of each tumor marker by clinical stages. Positive rates of all of the tumor markers increased with advance in disease stage. Serum levels of HER2ECD, CA15–3 and NCCST439 were elevated in less than 10% of the patients with stage 0. This finding was thought to be due to either non-specific detection or unrelated lesions producing these markers. However, the reason could not be determined from the clinical data in this study. In the HER2-negative patients, the percentage of patients with elevated HER2ECD levels was much lower than the percentages of patients with elevated levels of other markers (Fig. 1B). However, it should be noted that patients with HER2ECD-elevated recurrent or metastatic cancer in this group accounted for 22.6% of the patients in this group. In the HER2-positive group, in contrast, the percentage of patients with elevated HER2ECD levels was significantly higher than the percentages of patients with elevated levels of other markers (Fig. 1C). In the HER2-unknown group, although the population of this group was too small to evaluate the positivity of tumor markers, the percentage of patients with HER2ECD-elevated recurrent or metastatic breast cancer was compatible with that of patients with elevated levels of other markers (Fig. 1D). Figure 2 shows percentages of patients with elevated levels of at least one tumor marker including conventional markers and HER2ECD compared with those of patients with elevated levels of at least one conventional marker by clinical stages. Although adding HER2ECD to the conventional markers did not affect the detection rates of patients with elevated levels of at least one tumor marker in all patients or in the HER2-negative patients, it raised the positive rates from 50 to 71.4% in stage 3 and from 43.8 to 81.2% in recurrent or metastatic stage in the HER2-positive patients (Fig. 2). Similar results were obtained in the HER2-unknown group (data not shown).
Table 2. Percentages of patients with elevated levels of each tumor marker.
| all | HER2(-) | HER2(+) | p value§ | HER2(?) | p value§ | |
|---|---|---|---|---|---|---|
|
CEA |
18 |
23.2 |
9.4 |
0.047 |
22.4 |
0.533 |
|
CA15–3 |
15.3 |
14.8 |
13.3 |
0.427 |
20.4 |
0.29 |
|
NCCST439 |
14.6 |
17.9 |
10.5 |
0.197 |
12.9 |
0.663 |
|
BCA225 |
10.9 |
8.7 |
7.6 |
0.44 |
15.8 |
0.272 |
| HER2ECD | 15.1 | 8.7 | 23.5 | 0.027 | 14 | 0.294 |
§ The positive rate was compared with that in the HER2-negative patients
Figure 1. Percentages of patients with elevated levels of each tumor marker. Percentages of patients with elevated levels of each tumor marker were calculated by clinical stages in all patients (A) and in HER2-negative (B), HER2-overexpressed (C) and HER2-unknown patients (D).
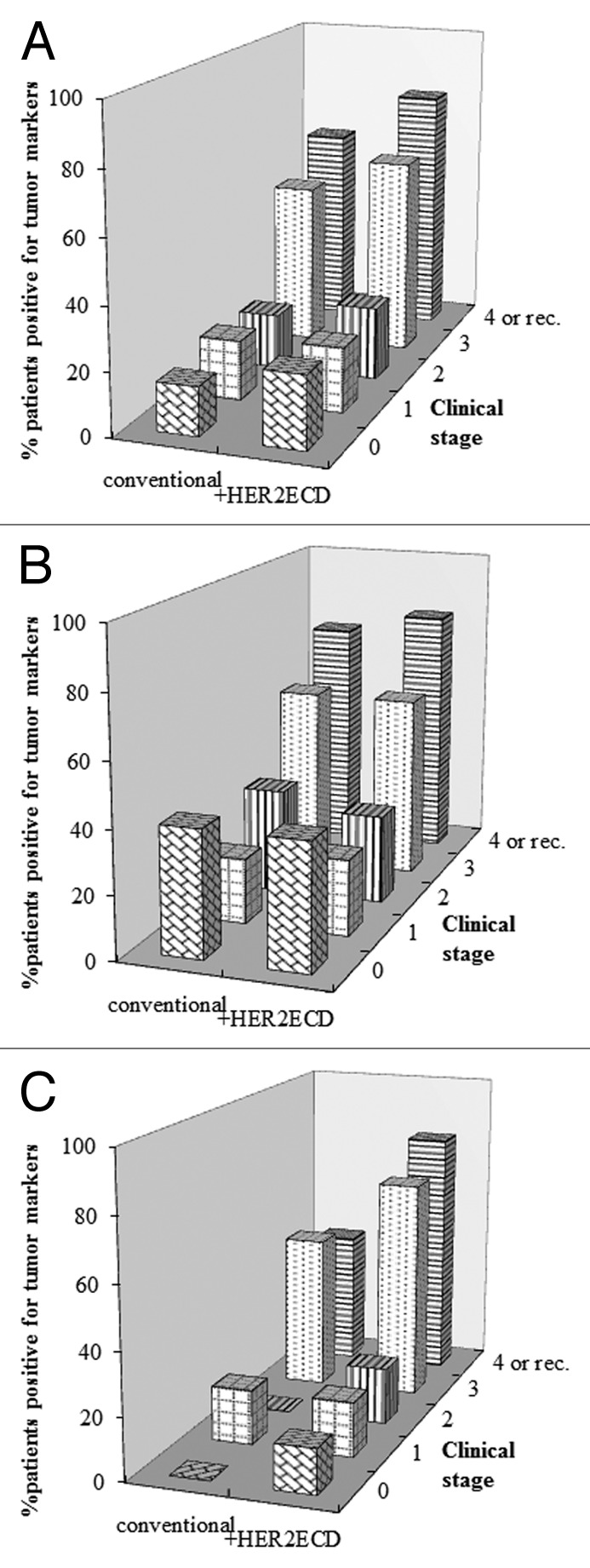
Figure 2. Effects of using HER2ECD in addition to conventional markers on detection rates of breast cancer. Percentage of patients with elevated levels of at least one tumor marker including conventional markers and HER2ECD was compared with percentages of patients with elevated levels of at least one conventional tumor marker by clinical stages. Detection rates were compared in all patients (A) and in HER2-negative (B) and HER2-overexpressed patients (C).
Serum levels of HER2ECD were compared in the three HER2 status groups. HER2ECD level in HER2-positive patients was significantly higher than that in HER2-negative patients (16.6 ± 26.8 vs. 11.4 ± 3.3 ng/ml, p = 0.037, data not shown). HER2ECD level in HER2-unknown patients was also higher, but the difference was not statistically significant (14.2 ± 13.9 ng/ml, p = 0.088, data not shown).
Predictive factors for responses to trastuzumab treatment in HER2-overexpressed breast cancer
Nineteen patients with advanced or recurrent HER2-overexpressed breast cancer were treated with trastuzumab regimens. Twelve of these patients showed clinical responses including complete response (CR) and partial response (PR) to the treatment, and the remaining seven patients did not respond. All of the patients who responded to the treatment showed markedly reduced levels of HER2ECD within a month after the initiation of treatment (data not shown). Clinicopathologic features of the patients were shown in Table 3. In both responders and non-responders, more than a half of the patients received a combination of trastuzumab and taxanes (either paclitaxcel or docetaxel). To determine which factors were responsible for tumor responses to trastuzumab, we compared the clinicopathologic factors in responders and non-responders (Table 4). Although age, number of metastatic sites and hormone receptor status in the tumor were not different between the two groups, there were statistically significant differences in positive rates of elevated HER2ECD levels, serum HER2ECD levels and percentages of cancer cells positive for HER2 protein in the tumor between responders and non-responders (91.7% vs. 14.3% in positive rates of elevated HER2ECD levels, p = 0.006; 50.1 ± 65.6 vs. 10.8 ± 5.3 ng/ml in serum HER2ECD levels, p = 0.032; 81.1 ± 14.6% vs. 53.4 ± 21.0% in percentages of HER2-positive cancer cells, p = 0.019). When clinical outcomes were compared in the two groups, median TTP and OS were significantly prolonged in responders compared with those in non-responders (TTP: 17.3 vs. 5.0 mo, p = 0.039; OS: p = 0.061, Fig. 3A and B). To determine whether HER2ECD status predicts clinical outcomes of patients with HER2-overexpressed breast cancer who were treated with trastuzumab regimens, we compared ORR, TTP and OS between patients with and those without elevated HER2ECD levels. As a result, ORR was significantly higher in patients with elevated HER2ECD levels than in patients without elevated HER2ECD levels (91.7% vs. 14.3%, p = 0.032). However, there was no significant difference in median TTP or OS between the two groups (Table 5).
Table 3. Clinicopathologic features of patients with HER2-overexpressed advanced or recurrent breast cancer who received trastuzumab regimens.
| Pts. no | Age | No. metastatic site | HR status+ | %HER2+ cells | HER2ECD (ng/ml) | Regimen§ | Response |
|---|---|---|---|---|---|---|---|
| 1 |
39 |
1 |
N |
100 |
34.2 |
PAC/H |
CR |
| 2 |
48 |
2 |
P |
68 |
46.7 |
X/H |
CR |
| 3 |
61 |
2 |
P |
68 |
23.6 |
PAC/H |
CR |
| 4 |
82 |
2 |
N |
66 |
29.6 |
S1/H |
PR |
| 5 |
63 |
4 |
P |
82 |
47.9 |
VINO/H |
PR |
| 6 |
40 |
2 |
P |
NT* |
24.4 |
X/H |
PR |
| 7 |
38 |
3 |
N |
NT |
19 |
PAC/H |
PR |
| 8 |
59 |
1 |
N |
98 |
51.7 |
PAC/H |
PR |
| 9 |
51 |
1 |
P |
72 |
11.8 |
PAC/H |
PR |
| 10 |
59 |
1 |
N |
95 |
40.9 |
PAC/H |
PR |
| 11 |
57 |
1 |
N |
NT |
17 |
PAC/H |
PR |
| 12 |
46 |
4 |
P |
NT |
254.1 |
DOC/H |
PR |
| 13 |
69 |
1 |
P |
42 |
6.9 |
PAC/H |
SD |
| 14 |
54 |
3 |
N |
38 |
22 |
X/H |
PD |
| 15 |
41 |
3 |
N |
47 |
5.6 |
DOC/H |
PD |
| 16 |
52 |
4 |
N |
NT |
9.9 |
PAC/H |
PD |
| 17 |
69 |
2 |
N |
50 |
9.5 |
H alone |
SD |
| 18 |
54 |
1 |
P |
90 |
11.1 |
X/H |
SD |
| 19 | 76 | 2 | N | NT | 10.4 | PAC/H | PD |
+ P, positive; N, negative; *not tested; §PAC, paclitaxcel; DOC, docetaxel; X, capecitabine; H, trasutumab
Table 4. Comparison of clinicopathologic factors in patients responding to and those not responding to trastuzumab regimens.
| Responders | Non-responders | p value | |
|---|---|---|---|
|
N |
12 |
7 |
|
|
age |
53.6 ± 12.6 |
59.3 ± 12.3 |
0.353 |
|
no. metastatic site |
2.0 ± 1.1 |
2.28 ± 1.1 |
0.3 |
|
HR-positivity (%) |
6 (50%) |
2 (28.6%) |
0.447 |
|
HER2ECD positivity (%) |
11 (91.7%) |
1 (14.3%) |
0.006 |
|
%HER2-positive cells |
81.1 ± 14.6 |
53.4 ± 21.0 |
0.019 |
| HER2ECD level (ng/ml) | 50.1 ± 65.6 | 10.8 ± 5.3 | 0.032 |
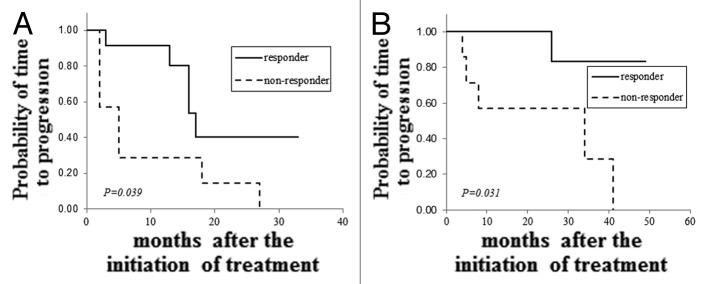
Figure 3. Comparison of clinical outcomes in patients responding to and patients not responding to trastuzumab regimens. TTP (A) and OS (B) were compared between patients with HER2-overexpressed advanced or recurrent breast cancer responding to and patients not responding to trastuzumab regimens.
Table 5. Comparison of clinical outcomes in patients with and those without elevated HER2ECD levels.
| HER2ECD(+) | HER2ECD(-) | p value | |
|---|---|---|---|
|
N |
12 |
7 |
|
|
response rate |
91.7 |
14.3 |
0.032 |
|
Median TTP (M) |
16 (2–33) |
5 (2–27) |
0.221 |
| Median OS (M) | NC+ (3–49) | 34 (5–41) | 0.226 |
+ not calculated
Discussion
In this study, we assessed the ability of HER2ECD to detect breast cancer and to monitor cancer status as a tumor marker by comparing its serum level with those of conventional tumor markers (CEA, CA15–3, NCC-ST439 and BCA225). Positive rate of HER2ECD in all patients with breast cancer studied was 15.1%, which was compatible with those of other markers ranging from 10.9–18.3% (Table 2). In HER2-negative patients, positive rate of HER2ECD was much lower in each clinical stage than those of other markers (Fig. 1B). Thus, this marker did not contribute to increase in detection rates of breast cancer as a tumor marker in this subset. In contrast, positive rate of HER2ECD in HER2-positive patients, especially in patients with clinical stages of 3 or 4 and in patients with recurrent cancer, were significantly higher than those of other markers (Fig. 1C). It should be noted that seven (22.6%) of the patients with HER2-negative metastatic or recurrent breast cancer showed elevated HER2ECD levels (Fig. 1B), suggesting that the disseminated or relapsed tumors were derived from HER2-overexpressed cancer cells in the primary tumor in which HER2-negative cells were dominant or that primary cancer cells without HER2 overexpression might transform into HER2-overexpressed cells when the patients relapse. However, we could not rule out the possibility of aberrant production of HER2 protein in the liver resulting from reactions to nonmalignant hepatic disorders or metastatic tumors. Molina et al. reported that serum levels of HER2ECD were elevated in 40–60% of patients with nonmalignant hepatic disorders such as hepatitis or cirrhosis and in some patients with metastatic tumors in the liver.32 In fact, three of the seven patients had multiple metastatic lesions in the liver and none of them had been examined for HER2 expression in the liver by biopsy. By adding HER2ECD to the conventional tumor markers for clinical use, detection rates of breast cancer by any marker in clinical stage of 3 or 4 and in recurrent cancer were increased from 50 to 71.4% and from 43.8 to 81.3%, respectively (Fig. 2C). Therefore, HER2ECD is thought to be useful for detecting recurrence or monitoring disease status in HER2-overexpressed breast cancer. In terms of early detection of breast cancer by this marker, whereas the marker level was elevated in 7.7% of patients with noninvasive breast cancer and the levels had decreased to the normal level after operation in all of the patients, it seemed unlikely that HER2ECD released from noninvasive breast cancer cells is detectable in sera. Also, we could not exclude the possibility of non-specific detection by the marker or of external production of HER2 protein such as that in non-breast malignancies or inflammatory diseases. Kashiwaba et al. examined HER2ECD levels in sera from 123 healthy donors33 and determined the cutoff level to be 15.2 ng/ml. They reported that only one of the donors examined showed a serum level of HER2ECD above the cut-off level, suggesting that this marker level is not commonly elevated in the general population.
Trastuzumab is a humanized monoclonal antibody directed against the HER2 gene product and has shown favorable clinical efficacy in HER2-overexpressed breast cancer.34-37 The combination of trastuzumab and anticancer cytotoxic agents is a gold standard for treatment of HER2-overexpressed breast cancer in adjuvant as well as metastatic settings. However, although the majority of HER2-overexpressed patients have responded to this trastuzumab regimen, patients do not always show good responses despite high levels of HER2 expression in the tumor. As one of the mechanisms of the acquisition of resistance to trastuzumab by cancer cells, increase in truncated HER2 molecules (HER2p95) by shedding HER2ECD in which epitopes of trastuzumab are situated has been reported.38 Thus, due to the neutralization of trastuzumab by free HERECD in sera and the loss of epitopes for trastuzumab on the HER2 protein, cancer cells are thought to acquire resistance to trastuzumab. In this study, more than 90% of HER2-overexpressed patients who showed tumor responses to trastuzumab regimens (Table 4). In contrast, only one of the seven patients who did not respond to the regimen had elevated HER2ECD levels. This finding seems to be contradictory with the mechanism described above. We do not have any data from this study that can explain the discrepancy, but we speculate that the amount of administered trastuzumab antibody is sufficient to bind to all of the free HER2ECD in sera and that the excess amount of the antibody binds to HER2 protein before this HER2ECD is shed from the whole HER2 molecule, resulting in decrease in truncated HER2p95, which accelerates phospholyration of tyrosine residues in the molecule. This mechanism has been proved by Molina et al.39 Data obtained by comparing clinicopathologic factors of HER2-overexpressed patients responding to and those not responding to trastuzumab regimens suggest that highly expressed HER2 protein in the tumor and elevated serum HER2ECD levels may be essential for obtaining favorable responses to the regimen. A comparison of clinical outcomes in responders and non-responders showed that both TTP and OS were significantly prolonged in responders compared with those in non-responders (Fig. 3A and B). Also, to determine whether HER2ECD is useful as a biomarker for predicting responses to trastuzumab, we compared clinical outcomes in HER2-overexpressed patients with and without elevated HERECD levels who were treated with trastuzumab regimens (Table 5). Patients with elevated HER2ECD levels showed a significantly higher response rate than that of patients without elevated HER2ECD levels (91.7% vs. 14.3%, p = 0.032). However, there was no difference in either TTP or OS between the two groups. The results suggest that elevated serum HER2ECD levels may predict clinical responses to trastuzumab, but do not predict survival of patients who were treated with trastuzumab regimens. This might be partly because the number of patients studied was too small and the duration of follow-up of patients was too short for precise evaluation. There has been a review article concerning the usefulness of HER2ECD by Leary et al.40 They suggested that HER2ECD is useful for detecting breast cancer regardless of HER2 status. However, the proportion of breast cancers detected by serum HER2ECD levels varied across the studies from 3–34% and from 23–62% in primary and metastatic breast cancer, respectively. The diversity in the positivity of HER2ECD elevation was due to the variety of cut-off levels and unselected population examined. In this study, we determined the proportion of patients with HER2-overexpressed breast cancer who showed elevated serum HER2ECD levels in each clinical stage and compared the proportion with that of patients with HER2-normal breast cancer. More than half of patients with HER2-overexpressed stage 3 or recurrent or metastatic breast cancer showed elevated levels of HER2ECD, suggesting the usefulness of HER2ECD for detecting relapse of breast cancer after an operation or for monitoring cancer status during or after treatment in neoadjuvant or metastatic settings. In terms of the usefulness of this biomarker as a predictor of benefit from trastuzumab regimens in metastatic settings, many studies addressing HER2ECD levels in patients with metastatic breast cancer who were treated with trastuzumab regimens demonstrated no correlation between baseline HER2ECD levels and benefit from trastuzumab-based treatment. For example, some investigators have reported that low levels of HER2ECD were predictable in tumor responses to trastuzumab. In contrast, some have reported that high levels of HER2ECD predicted tumor responses. Thus, the usefulness of HER2ECD as a predictor of benefit from trastuzumab remains to be determined. The results of this study are compatible with data obtained by Kostler et al.,30 suggesting that high HER2ECD levels predict high response rates. Prospective studies with a larger number of patients and for a longer duration of follow-up are needed to obtain a final conclusion about the usefulness of HER2ECD as a predictive biomarker for survival effects of trastuzumab-based therapeutics.
In conclusion, HER2ECD is useful for detecting breast cancer recurrence and monitoring disease status as a tumor marker, and addition of this marker to conventional tumor markers increases detection rates of HER2-overexpressed metastatic or recurrent breast cancer from 50% to more than 80%. Furthermore, HER2ECD is expected to be useful for predicting at least tumor responses to trastuzumab regimens as a predictive biomarker in patients with HER2-overexpressed breast cancer.
Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan (Nos. 10671249, 13671380, 14571262 and 15591340).
Glossary
Abbreviations:
- CR
complete response
- CT
computed tomography
- ECFR
epidermal growth factor receptor-1
- ER
estrogen receptor
- HER2
human epidermal growth factor receptor-2
- HER2ECD
human epidermal growth factor receptor-2 extracellular domain
- HR
hormone receptor
- IHC
immunohistochemistry
- OS
overall survival
- ORR
objective response rate
- PD
progressive disease
- PgR
progesterone receptor
- PR
partial response
- RECIST
Response Evaluation Criteria in Solid Tumors
- SD
stable disease
- TTF
time to treatment failure
- TTP
time to progression
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/22626
References
- 1.Cheung KL, Graves CR, Robertson JF. Tumour marker measurements in the diagnosis and monitoring of breast cancer. Cancer Treat Rev. 2000;26:91–102. doi: 10.1053/ctrv.1999.0151. [DOI] [PubMed] [Google Scholar]
- 2.Bast RC, Jr., Ravdin P, Hayes DF, Bates S, Fritsche H, Jr., Jessup JM, et al. American Society of Clinical Oncology Tumor Makers Expert Panel: 2000 update of recommendations for the use of tumor makers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1865–78. doi: 10.1200/JCO.2001.19.6.1865. [DOI] [PubMed] [Google Scholar]
- 3.Molina R, Jo J, Filella X, Zanon G, Pahisa J, Mu noz M, et al. c-erbB-2 oncoprotein, CEA, and CA 15.3 in patients with breast cancer: prognostic value. Breast Cancer Res Treat. 1998;51:109–19. doi: 10.1023/A:1005734429304. [DOI] [PubMed] [Google Scholar]
- 4.Berry DA, Cirrincione C, Henderson IC, Citron ML, Budman DR, Goldstein LJ, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295:1658–67. doi: 10.1001/jama.295.14.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pritchard KI, Shepherd LE, O’Malley FP, Andrulis IL, Tu D, Bramwell VH, et al. National Cancer Institute of Canada Clinical Trials Group HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med. 2006;354:2103–11. doi: 10.1056/NEJMoa054504. [DOI] [PubMed] [Google Scholar]
- 6.Penault-Llorca F, André F, Sagan C, Lacroix-Triki M, Denoux Y, Verriele V, et al. Ki67 expression and docetaxel efficacy in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27:2809–15. doi: 10.1200/JCO.2008.18.2808. [DOI] [PubMed] [Google Scholar]
- 7.Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 1986;232:1644–6. doi: 10.1126/science.3012781. [DOI] [PubMed] [Google Scholar]
- 8.Hynes NE, Stern DF. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta. 1994;1198:165–84. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 9.Riese DJ, 2nd, Stern DF. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays. 1998;20:41–8. doi: 10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 10.Yarden Y. The EGFR family and its ligands in human cancer: signaling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37(Suppl 4):3–8. doi: 10.1016/S0959-8049(01)00230-1. [DOI] [PubMed] [Google Scholar]
- 11.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 12.Sjögren S, Inganäs M, Lindgren A, Holmberg L, Bergh J. Prognostic and predictive value of c-erbB-2 overexpression in primary breast cancer, alone and in combination with other prognostic markers. J Clin Oncol. 1998;16:462–9. doi: 10.1200/JCO.1998.16.2.462. [DOI] [PubMed] [Google Scholar]
- 13.Gabos Z, Sinha R, Hanson J, Chauhan N, Hugh J, Mackey JR, et al. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol. 2006;24:5658–63. doi: 10.1200/JCO.2006.07.0250. [DOI] [PubMed] [Google Scholar]
- 14.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–26. doi: 10.1200/JCO.20.3.719. [DOI] [PubMed] [Google Scholar]
- 15.Roche PC, Ingle JN. Increased HER2 with U.S. Food and Drug Administration-approved antibody. J Clin Oncol. 1999;17:434. doi: 10.1200/JCO.1999.17.1.434. [DOI] [PubMed] [Google Scholar]
- 16.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 17.Pauletti G, Dandekar S, Rong H, Ramos L, Peng H, Seshadri R, et al. Assessment of methods for tissue-based detection of the HER-2/neu alteration in human breast cancer: a direct comparison of fluorescence in situ hybridization and immunohistochemistry. J Clin Oncol. 2000;18:3651–64. doi: 10.1200/JCO.2000.18.21.3651. [DOI] [PubMed] [Google Scholar]
- 18.Press MF, Hung G, Godolphin W, Slamon DJ. Sensitivity of HER-2/neu antibodies in archival tissue samples: potential source of error in immunohistochemical studies of oncogene expression. Cancer Res. 1994;54:2771–7. [PubMed] [Google Scholar]
- 19.Zabrecky JR, Lam T, McKenzie SJ, Carney W. The extracellular domain of p185/neu is released from the surface of human breast carcinoma cells, SK-BR-3. J Biol Chem. 1991;266:1716–20. [PubMed] [Google Scholar]
- 20.Leitzel K, Teramoto Y, Sampson E, Mauceri J, Langton BC, Demers L, et al. Elevated soluble c-erbB-2 antigen levels in the serum and effusions of a proportion of breast cancer patients. J Clin Oncol. 1992;10:1436–43. doi: 10.1200/JCO.1992.10.9.1436. [DOI] [PubMed] [Google Scholar]
- 21.Lüftner D, Cheli C, Mickelson K, Sampson E, Possinger K. ADVIA Centaur HER-2/neu shows value in monitoring patients with metastatic breast cancer. Int J Biol Markers. 2004;19:175–82. doi: 10.1177/172460080401900301. [DOI] [PubMed] [Google Scholar]
- 22.Kuroda N, Kontani K, Kajikawa T, Taminato T. Study of the measurement of serum extracellular domain of HER-2/neu protein with CLIA method. Rinsho Byori. 2010;58:541–52. [PubMed] [Google Scholar]
- 23.Carney WP, Neumann R, Lipton A, Leitzel K, Ali S, Price CP. Potential clinical utility of serum HER-2/neu oncoprotein concentrations in patients with breast cancer. Clin Chem. 2003;49:1579–98. doi: 10.1373/49.10.1579. [DOI] [PubMed] [Google Scholar]
- 24.Carney WP. The Emerging Role of monitoring serum HER-2/neu oncoprotein levels in women with metastatic breast cancer. Lab Med. 2003;34:58–64. doi: 10.1309/TY6M-DWB0-G2BU-6D5F. [DOI] [Google Scholar]
- 25.Sturgeon CM, Duffy MJ, Stenman UH, Lilja H, Brünner N, Chan DW, et al. National Academy of Clinical Biochemistry National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin Chem. 2008;54:e11–79. doi: 10.1373/clinchem.2008.105601. [DOI] [PubMed] [Google Scholar]
- 26.Hayes DF, Yamauchi H, Broadwater G, Cirrincione CT, Rodrigue SP, Berry DA, et al. Cancer and Leukemia Group B Circulating HER-2/erbB-2/c-neu (HER-2) extracellular domain as a prognostic factor in patients with metastatic breast cancer: Cancer and Leukemia Group B Study 8662. Clin Cancer Res. 2001;7:2703–11. [PubMed] [Google Scholar]
- 27.Lipton A, Ali SM, Leitzel K, Demers L, Chinchilli V, Engle L, et al. Elevated serum Her-2/neu level predicts decreased response to hormone therapy in metastatic breast cancer. J Clin Oncol. 2002;20:1467–72. doi: 10.1200/JCO.20.6.1467. [DOI] [PubMed] [Google Scholar]
- 28.Colomer R, Llombart-Cussac A, Lloveras B, Ramos M, Mayordomo JI, Fernández R, et al. High circulating HER2 extracellular domain levels correlate with reduced efficacy of an aromatase inhibitor in hormone receptor-positive metastatic breast cancer: a confirmatory prospective study. Cancer. 2007;110:2178–85. doi: 10.1002/cncr.23043. [DOI] [PubMed] [Google Scholar]
- 29.Colomer R, Montero S, Lluch A, Ojeda B, Barnadas A, Casado A, et al. Circulating HER2 extracellular domain and resistance to chemotherapy in advanced breast cancer. Clin Cancer Res. 2000;6:2356–62. [PubMed] [Google Scholar]
- 30.Köstler WJ, Schwab B, Singer CF, Neumann R, Rücklinger E, Brodowicz T, et al. Monitoring of serum Her-2/neu predicts response and progression-free survival to trastuzumab-based treatment in patients with metastatic breast cancer. Clin Cancer Res. 2004;10:1618–24. doi: 10.1158/1078-0432.CCR-0385-3. [DOI] [PubMed] [Google Scholar]
- 31.Ali SM, Carney WP, Esteva FJ, Fornier M, Harris L, Köstler WJ, et al. Serum HER-2/neu Study Group Serum HER-2/neu and relative resistance to trastuzumab-based therapy in patients with metastatic breast cancer. Cancer. 2008;113:1294–301. doi: 10.1002/cncr.23689. [DOI] [PubMed] [Google Scholar]
- 32.Molina R, Jo J, Filella X, Bruix J, Castells A, Hague M, et al. Serum levels of C-erbB-2 (HER-2/neu) in patients with malignant and non-malignant diseases. Tumour Biol. 1997;18:188–96. doi: 10.1159/000218029. [DOI] [PubMed] [Google Scholar]
- 33.Kashiwaba M, Hayakawa Y, Takiyama I, Inaba R, Itoh N, Saito K. Nihon Gekagakkaizasshi. 2005;106:587. [Google Scholar]
- 34.Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, et al. HERA study team 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 35.Buzdar AU, Valero V, Ibrahim NK, Francis D, Broglio KR, Theriault RL, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007;13:228–33. doi: 10.1158/1078-0432.CCR-06-1345. [DOI] [PubMed] [Google Scholar]
- 36.Valero V, Forbes J, Pegram MD, Pienkowski T, Eiermann W, von Minckwitz G, et al. Multicenter phase III randomized trial comparing docetaxel and trastuzumab with docetaxel, carboplatin, and trastuzumab as first-line chemotherapy for patients with HER2-gene-amplified metastatic breast cancer (BCIRG 007 study): two highly active therapeutic regimens. J Clin Oncol. 2011;29:149–56. doi: 10.1200/JCO.2010.28.6450. [DOI] [PubMed] [Google Scholar]
- 37.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 38.Scaltriti M, Rojo F, Ocaña A, Anido J, Guzman M, Cortes J, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99:628–38. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 39.Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744–9. [PubMed] [Google Scholar]
- 40.Leary AF, Hanna WM, van de Vijver MJ, Penault-Llorca F, Rüschoff J, Osamura RY, et al. Value and limitations of measuring HER-2 extracellular domain in the serum of breast cancer patients. J Clin Oncol. 2009;27:1694–705. doi: 10.1200/JCO.2008.17.3989. [DOI] [PubMed] [Google Scholar]