Abstract
Biosecurity agencies need robust bioeconomic tools to help inform policy and allocate scarce management resources. They need to estimate the potential for each invasive alien species (IAS) to create negative impacts, so that relative and absolute comparisons can be made. Using pine processionary moth (Thaumetopoea pityocampa sensu lato) as an example, these needs were met by combining species niche modelling, dispersal modelling, host impact and economic modelling. Within its native range (the Mediterranean Basin and adjacent areas), T. pityocampa causes significant defoliation of pines and serious urticating injuries to humans. Such severe impacts overseas have fuelled concerns about its potential impacts, should it be introduced to New Zealand. A stochastic bioeconomic model was used to estimate the impact of PPM invasion in terms of pine production value lost due to a hypothetical invasion of New Zealand by T. pityocampa. The bioeconomic model combines a semi-mechanistic niche model to develop a climate-related damage function, a climate-related forest growth model, and a stochastic spread model to estimate the present value (PV) of an invasion. Simulated invasions indicate that Thaumetopoea pityocampa could reduce New Zealand’s merchantable and total pine stem volume production by 30%, reducing forest production by between NZ$1,550 M to NZ$2,560 M if left untreated. Where T. pityocampa is controlled using aerial application of an insecticide, projected losses in PV were reduced, but still significant (NZ$30 M to NZ$2,210 M). The PV estimates were more sensitive to the efficacy of the spray program than the potential rate of spread of the moth. Our novel bioeconomic method provides a refined means of estimating potential impacts of invasive alien species, taking into account climatic effects on asset values, the potential for pest impacts, and pest spread rates.
Introduction
Invasive alien species (IAS) are a major problem worldwide. Despite the efforts of biosecurity agencies and an international phytosanitary legal framework for the management of invasion pathways, the rate at which these species are invading new territories appears to be increasing rapidly [1], [2]. Globalisation is having a major impact on the spread of crop pests with increased trade and travel providing new dispersal pathways for pests [3]. In addition to increasing the dispersal options for pests, globalisation of agriculture, silviculture, and horticulture is homogenising the distribution patterns of plant hosts across continents. In combination, these processes are increasing pest risk profiles in terms of both threats and vulnerabilities [4].
The rate of biological invasions has fuelled demands from biosecurity agencies for information on the potential distribution and impacts of IAS in terms of economics, human health and biodiversity. Climate has long been recognised as an important environmental determinant of the distribution of pests [5], [6], [7]. Process-based niche models, such as CLIMEX, have utilised these relationships to project the potential distribution and relative abundance of a wide range of invasive insects, weeds and pathogens under both current and future climatic conditions [8]–[13].
It is one thing for a biosecurity agency to know that a pest could establish within its jurisdiction, but another to be able and willing to respond to it should an incursion eventuate. IAS incursion responses are costly, and agencies have a finite capacity to respond. When faced with an incursion, they must decide whether to 1) attempt an eradication, 2) develop a campaign to slow the spread of the organism, 3) attempt to contain it, 4) allow the organism to spread without a coordinated response, and let land managers and local authorities manage the organism to reduce impacts to acceptable levels, or 5) allow the organism to spread, but mobilise a coordinated response such as a biological control programme [14], [15]. To assist them in this social resource allocation problem it is becoming more commonplace to see benefit cost analyses being used to guide these biosecurity response decisions [16], [17], [18].
Despite their common use in pest risk assessment, the potential of process-based niche models to inform these responses has not yet been fully realised. Although potential distribution is a useful input to pest risk assessment, in that it delineates an area in which the pest could potentially be problematical [19], [20], this information alone provides little insight into expected spatial and temporal variation in pest abundance, and consequent damage to crop species or other valuable assets. Such projections would be of considerable use to both biosecurity agencies and primary producers for quantifying and mitigating pest impacts and making decisions on allocation of resources. The development of bioeconomic models that can estimate impacts of pests on hosts, within a framework that spatially constrains pest distribution would therefore represent a major methodological advance.
It has been proposed recently that pine processionary moths are a species complex that consists of two species: Thaumetopoea pityocampa and T. wilkinsoni (Lepidoptera: Thaumetopoeidae) [21]. For consistency with the existing literature, we use here the previous nomenclature (T. pityocampa) to refer to the species complex, T. pityocampa sensu lato. Thaumetopoea pityocampa cause major defoliation of pines across southern Europe and around the Mediterranean Sea. Previous research has described the climatic requirements of T. pityocampa [22], [23] and this information was used to project the likely effects of a changing climate on its distribution in France [24]. Increases in outbreak severity and northward range expansion of the species, over the last few decades, are thought to be due to reduced winter mortality caused by a warming climate [23], [24].
Artificial woodlands appear more susceptible to attack by T. pityocampa than natural woodlands [25], and Pinus radiata appears more susceptible to attack by T. pityocampa than native European Pinus spp. [26], [27], [28]. Through time, defoliation appears to cycle [29] and this may be due to an unknown factor that reduces larval survival on plants that have suffered repeated defoliation [30].
Although the relationship between climate and host damage has not yet been quantified, defoliation of pines by T. pityocampa has been shown to result in growth reductions of up to 83% [29]. Should T. pityocampa be accidentally introduced into the southern hemisphere where its preferred host P. radiata is the most widely planted of all plantation species [31], there is considerable scope for serious economic damage. New Zealand is possibly highly vulnerable to damage from T. pityocampa as it has a significant plantation resource (1.8 million ha), of which approximately 90% comprises P. radiata [31].
In this contribution we demonstrate a novel analytical framework combining ecological and economic modelling, to estimate the economic impacts of an invasive organism as a basis for informing policy-level decision-making. We use New Zealand as a case study, and simulate the impact of an invasion by T. pityocampa on volume and present value of the plantation estate under current climate, assuming control or no control of T. pityocampa using insecticide. Finally, we discuss the utility of this case study as a generic approach for quantifying economic impacts of pests on important crop species.
Materials and Methods
The analytical framework consists of five components, 1) a climate based host productivity model, 2) a niche model describing the climate suitability for the pest species, T. pityocampa, 3) a climate-based host damage function, 4) a spread model and 5) a partial budget economic model.
Climate-based Host Growth Model
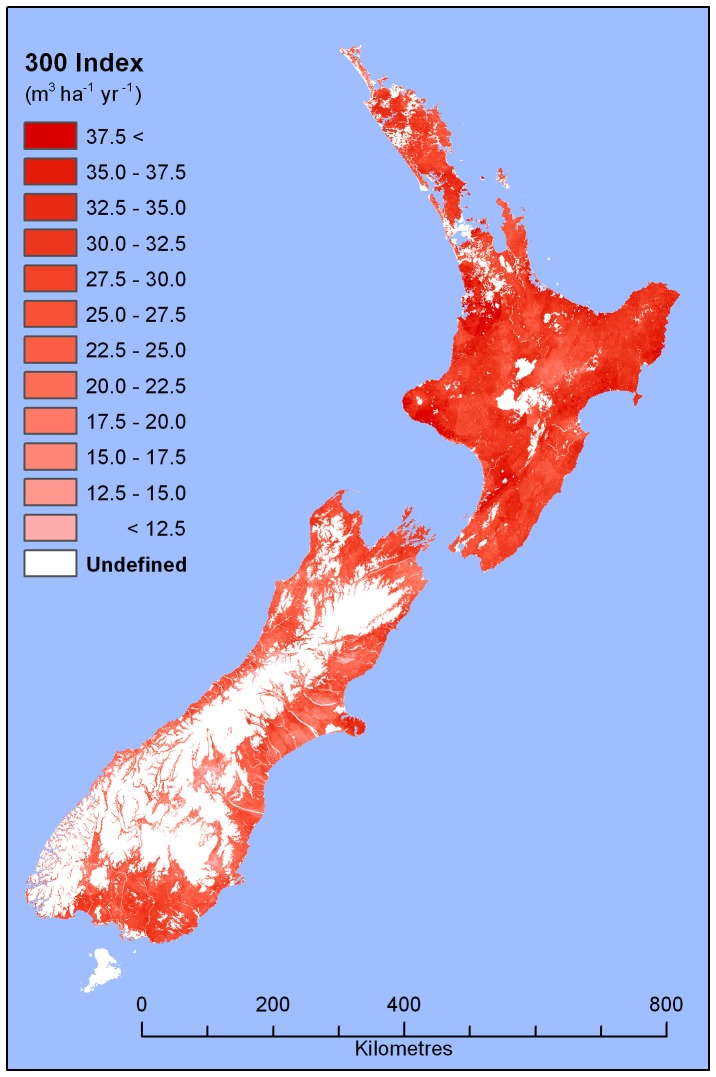
The 300 Index defines the volume mean annual increment (MAI) for P. radiata at age 30 years with a reference stem density of 300 stems ha−1 (Fig. 1) [32], [33]. We used the 300 Index to define site productivity, as this provides a standardised measure of volume that is independent of age and stand density [33]. For the 300 Index, growth is expressed on an annual basis (averaged over a 30 year period), which corresponds well to the frequency over which damage is typically recorded. Another advantage of using the 300 Index is that volume translates readily into fiscal value, allowing potential economic losses resulting from T. pityocampa damage to be estimated.
Figure 1. Spatial variation in 300 Index (mean annual volume increment at age 30 for a standard regime grown at 300 stems ha−1) for Pinus radiata in New Zealand [32]. Projection: New Zealand map grid.
The MAI described by the 300 Index is a total annual stem volume flux. There is typically 15% breakage in harvesting, and approximately 10% of stands are unstocked (MW, pers. obs.). Therefore estimates of MAI for stands both with and without T. pityocampa were reduced by 25% per hectare to determine the merchantable MAI. All economic analyses of damage described below relate to the merchantable MAI.
Niche Modelling for Thaumetopoea Pityocampa
The CLIMEX model
CLIMEX is a dynamic species niche model that integrates modelled weekly responses of a population to climate in order to create a series of climatic suitability indices that can be mapped or graphed [34], [35]. CLIMEX uses an annual Growth Index (GIA) to describe the potential for population growth as a function of soil moisture and temperature during favourable conditions. It uses up to eight stress indices (cold, wet, hot, dry, cold-wet, cold-dry, hot-wet and hot-dry) to simulate the ability of the population to survive unfavourable conditions. CLIMEX also includes a mechanism for defining the minimum amount of thermal accumulation (number of degree days) during the growing season that is necessary for population persistence (PDD).
The growth and stress indices are calculated weekly and then combined into an overall annual index of climatic suitability, the Ecoclimatic Index (EI), which gives an overall measure of the potential of a given location to support a permanent population of the species [35]. The EI ranges from 0 for locations at which the species is not able to persist, to a theoretical maximum of 100 for locations that are climatically perfect for the species [35]. Due to seasonality in climate, it is usually only near the equator where climatic conditions are stable enough that values of 100 are ever attained [12].
The stress parameters for CLIMEX models are generally fitted to known distribution data using an iterative manual process. This involves adjusting growth and stress parameters and then comparing model results to the known distribution of the species, and including consideration of any additional information about the species being modelled, such as minimum and maximum temperatures for growth. In setting these parameters, consideration is also given to the biological plausibility of the selected parameters. This process allows models to be developed that accord with the known biology of the species. The growth parameters of CLIMEX models generally have less influence on the modelled potential range of the species (EI ≥1) than they do on the climate suitability patterns within that range. Although growth parameters can be fitted from simple presence records, direct observation of the species growth response to climatic variables are most useful for estimating these values. Another option is to use observed phenological patterns to inform these growth responses [36].
Fitting CLIMEX parameters for Thaumetopoea pityocampa
Due to its pest status in Europe, there has been a significant amount of research effort applied to understanding the current and potential distribution of T. pityocampa in its native range, as well as the factors limiting it, including an ad hoc correlative model [22], [24] and laboratory studies into survival and feeding behaviour at low temperatures [37], [38]. Whilst the latter studies may inform niche modelling for international pest risk assessment, the ability of a correlative species distribution model to provide robust projections into a novel climate is highly questionable [39], [40].
In fitting the CLIMEX parameters for the T. pityocampa model, three sources of data were considered: distribution data that indicated locations where the climate was suitable for persistence, the published results of a set of laboratory experiments, and previous modelling of its Pinus spp. hosts. The model accounted for the effects of the nest building behaviour of T. pityocampa on its temperature relations, which allows the larvae to overwinter without entering diapause or quiescence [38]. The larvae build silk nests on sun-exposed branches in which they rest during the day. If night-time temperatures are suitable they forage at night. This behaviour and biogenic heating of the nests means that the larvae experience a microclimate that is significantly different from the Stevenson screen air-temperatures used to develop long-term climatic records [41]. One effect of this factor is that fitted relationships between presence and air temperature could result in an accurate model, but not be indicative of the true temperature-presence relationship.
The climate data used in the CLIMEX modelling was the CliMond 10′ global historical dataset centred on 1975 (CM10_1975H_V1_WO) [42]. This dataset includes monthly average data for minimum and maximum air temperature, precipitation, and relative humidity recorded at 09∶00 and 15∶00. Whilst the climate averages in the CM10_1975H_V1_WO dataset are centred on 1975, they include data from 1951–2000 for precipitation [43], and 1961–1990 for temperature and humidity [44].
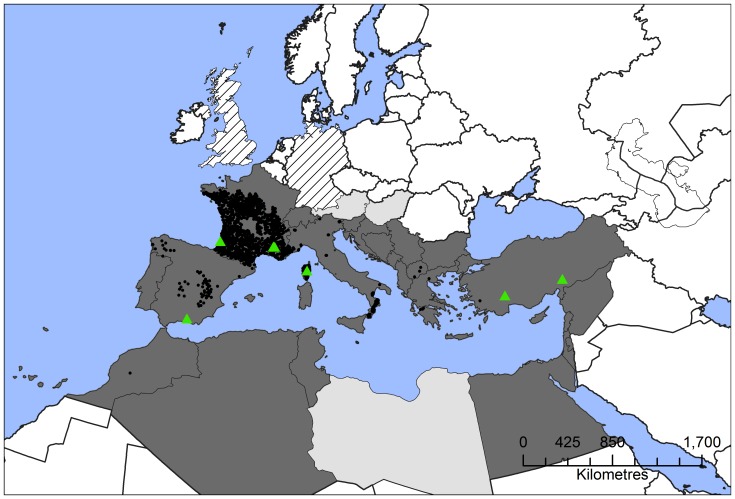
Distribution data for T. pityocampa (Fig. 2) were assembled from a literature review and various databases including the entry for processionaria del pino in the Atlas Linguistico Y Etnographico de Castilla-La Mancha [21], [25], [45], [46], [47]. Noting the recent range expansion by T. pityocampa, presumably in response to climate warming [23], [24], we took care to define a geographic dataset describing species presence that corresponded as closely as possible to the climate dataset used for fitting our niche model (Fig. 2). The CLIMEX stress parameters were fitted to this pre-1990 European data and all of the Middle-East and North African data. The parameters used to model the distribution of T. pityocampa are described in Table 1. The fitted parameters were applied to the same historical climatology dataset for New Zealand to define its potential distribution there. This method ignores both the recent range expansion of T. pityocampa and the recent climatic changes [23]. Therefore, the resulting model should be adequate and appropriate when it is applied to different climatic datasets. By projecting the potential range in New Zealand using the historical climate, we are likely to be biasing our results slightly conservatively.
Figure 2. Known distribution of Thaumetopoea pityocampa in Europe, Asia and North Africa, combining records from before and after 1990.
Black circles indicate known locations for T. pityocampa, green triangles are locations from which impact data has been reported. Countries are shaded to indicate areas where T. pityocampa has been reported as occurring regularly (dark grey), seldom found (light grey), presence unknown (cross-hatched) and absent (white). Projection: Albers equal-area.
Table 1. CLIMEX parameter values used for Thaumetopoea pityocampa. Parameter mnemonics are taken from Sutherst et al. (2007b).
| Index | Parameter | Valuea |
| Temperature | DV0 = lower threshold | 5.6°C |
| DV1 = lower optimum temperature | 18.0°C | |
| DV2 = upper optimum temperature | 25.0°C | |
| DV3 = upper threshold | 30.0°C | |
| Moisture | SM0 = lower soil moisture threshold | 0.25 |
| SM1 = lower optimum soil moisture | 0.40 | |
| SM2 = upper optimum soil moisture | 0.80 | |
| SM3 = upper soil moisture threshold | 2.00 | |
| Cold stress | TTCS = temperature threshold | −4°C |
| THCS = stress accumulation rate | −0.05 Week−1 | |
| DTCS = degree day threshold | 17.0°C Days | |
| DHCS = stress accumulation rate | −0.00025 Week−1 | |
| Heat stress | TTHS = temperature threshold | 32.0°C |
| THHS = stress accumulation rate | 0.005 Week−1 | |
| Dry stress | SMDS = threshold soil moisture | 0.2 |
| HDS = stress accumulation rate | −0.005 Week−1 | |
| Generations | PDD = degree-day thresholdb | 600°C Days |
Values without units are dimensionless indices of a 100 mm single bucket soil moisture profile.
Minimum annual total number of degree-days above DV0 needed for population persistence.
Growth parameters
The temperature growth parameters for T. pityocampa were mostly derived from published observations and development rate experiments. The lower temperature limit for development DV0 was set to 5.6°C in order to allow sufficient development of the moth at apparently suitable northern European locations (Fig 2). This is consistent with the rule developed by Battisti et al. [23], whereby for feeding to occur, daytime temperatures need to reach 9°C (the activation temperature) followed by a night time temperatures that do not fall below 0°C. The lower and upper temperature optima (DV1 and DV2) were set to 18 and 25°C respectively, representing a bracketing of the upper value for the maximum temperature under which processions occur [48]. The upper temperature limit for development (DV3) was set to 30°C, as at elevated temperatures around 30 to 32°C no larval activity is noted [41] and in some cases mortality is observed [48].
The lower soil moisture for growth (SM0) was set to a value of 0.25 that allowed appropriate persistence at the dry range limits in North Africa. The upper soil moisture level for growth (SM3) was taken from a previous model indicating the maximum soil moisture suitable for Pinus spp. [49]. The soil moisture optima were fitted to give a growth index that broadly agreed with observations of maximum tree growth impact throughout the suitable range.
Stress parameters
Geographical distribution data most strongly influences the fitting of the stress parameters, as these most directly influence the ability of a species to persist in an area. The primary limiting factor for T. pityocampa in Europe is cold stress. Two forms of cold stress appear to affect T. pityocampa in different parts of its range. Whilst the supercooling point of individuals of T. pityocampa has been shown to be −7°C [38], the ecological limit for colony survival are temperatures of approximately −5°C [37]. A cold stress temperature threshold of −4°C was found to accord well with the distribution records. Since larvae need to forage during the cool season, but may only do so when their nests become warm, a degree day cold stress was fitted to the northern range boundary in Europe. This mechanism required 15 degree days per week above 5.6°C to stave off cold stress. This set of degree day parameters is likely to be consistent with the observation by Hoch et al. [38] that feeding did not occur until daily temperatures reached 9°C day/0°C night. The difference in thresholds may be because Hoch et al. (2009) used a square wave day/night time temperature pattern, while a circadian daily temperature cycle occurs in the field and is used within CLIMEX. Another possible reason for this slight disparity is differences between instantaneous nest temperatures and long-term average screen temperatures as they relate to survival and feeding behaviours of T. pityocampa.
According to the model, the combination of lethal cold temperature and degree day cold stresses limited the ability of T. pityocampa to occupy sites in the Pyrenees and north of the Massif Central in southern France. It also prevented occupation of southern Britain. Seasonal dry stress appears to affect T. pityocampa in central Spain and the south of Italy and Greece. Dry stress becomes limiting in northern Africa where the dry stress parameters were fitted to the single known point location at Marrakech in Morocco [47]. Heat stress appears to affect T. pityocampa in parts of southern Spain and at Marrakech. The heat stress parameters were fitted to limit its distribution in these regions. The paucity of data in these hot dry regions means that model results in the hot and dry extremes should be considered indicative, rather than reliable.
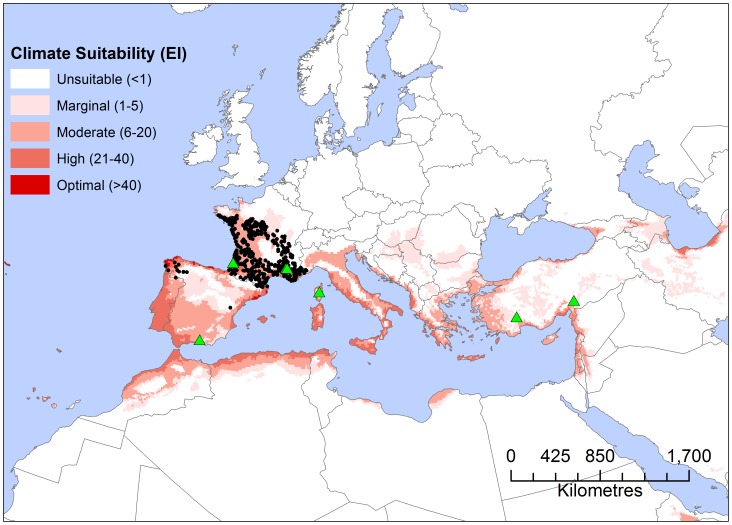
Using the parameter values described in Table 1, the projected potential distribution within Europe, Asia and north Africa (locations with an EI≥1) fitted known observations reasonably well (Fig. 3). The shape of the northern range limit is well characterised, and was clearly reproduced in the CLIMEX model. The few reported location points for T. pityocampa that did not accord with the model results were mainly found in areas of dissected terrain (e.g. the northern limits of the Massif Centrale in France). This same model misfit problem was encountered by Robinet et al., [24] using a generalised additive model, suggesting that there is a problem with the distribution data, the climate data or both around the massif Central and the pre-1990 northern French range boundary for T. pityocampa. Another option is that these points could fall within the modelled climatically suitable zone if the model were applied to a sufficiently fine resolution climatological surface. This scaling issue has been explored previously [50], and it is unlikely to have a serious impact on the resulting model as it affects marginally suitable locations covering a small area in dissected terrain. Whilst the hot and dry range limits of T. pityocampa are less well defined with occurrence records, the model nonetheless matches the known pre-1990 distribution throughout the Mediterranean Basin.
Figure 3. Europe showing known and potential range of occurrences of Thaumetopoea pityocampa.
Thaumetopoea pityocampa up to 1990 (black dots) and modelled climatic suitability for T. pityocampa under historical (1961–1990) climate averages as modelled using CLIMEX Ecoclimatic index (EI). Projection: Albers equal-area. Green triangles are locations from which impact data has been reported.
Climate-based Growth Impact Function
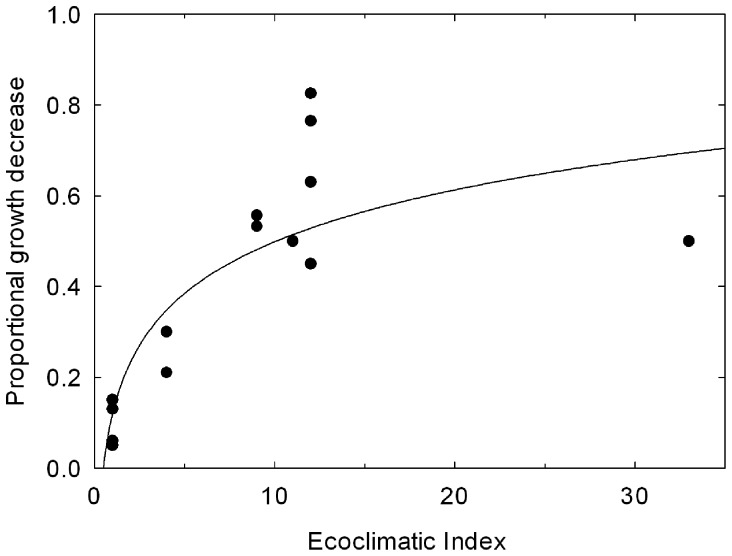
Values for variables describing the annual percentage of growth decrease (radial, diametric and circumference) over the five years following attacks by T. pityocampa compared to non-attacked trees were used in this study to define growth losses attributable to T. pityocampa. Reports from fourteen sites across Europe were used to build the relationship between modelled EI values and the level of growth impact on a range of pine species [29], [51]–[54]. These pine species did not include P. radiata, which is a preferred host and highly susceptible to attack and, because of this, rarely planted in Europe today, except in Spain.
Values of EI at these locations were extracted and regressed against the reported damage values. Where necessary the reported damage values were transformed into a proportional volume growth reduction. For the fourteen sites, EI accounted for 67.9% of the variance in the damage (Fig. 4). The following fitted logarithmic function was used to model annual proportional growth losses (Di) as a function of climate suitability for T. pityocampa (EI) in each 10′ cell (i):
| (1) |
Figure 4. Growth impacts from Thaumetopoea pityocampa as a function of climate suitability modelled using CLIMEX.
The location of sites with reported growth impacts used to develop the relationship are indicated in Figure 2.
The curvature of the fitted function is somewhat sensitive to the point with the highest climate suitability (50% growth impact with an EI of 33). However, including this point in the analysis ensures that the function does not reach 100% impact (a biologically reasonable feature).
As P. radiata is more susceptible to T. pityocampa than many other pine species [26], [27], [28], we assumed that the damage function described above would provide a conservative estimate of damage. The damage function (Equation 1) was applied to the Ecoclimatic Index results for each 10′ climate station cell in New Zealand to create a polygon fishnet dataset of P.radiata potential proportional growth reduction.
Spread Modelling
Model overview
Given the lack of knowledge about the likely point of entry of T. pityocampa in New Zealand, we make no assumptions about where the initial incursion occurs. Similarly, because of the large inherent uncertainty regarding the spread of invasive organisms over long time scales [55], [56] we make no attempt to explicitly simulate the spread of T. pityocampa. Instead, we use the spatially implicit spread model developed by Waage et al. [57]. In this model, the spread function is applied to the total area of host plantations, and subsequently, the total proportion of this area that is occupied at time t is used to apportion the cell-specific growth impact function to all cells containing pine plantations based upon their areal extent.
The spread model of Waage et al. [57] assumes that an invasive pest species spreads by a diffusive process such that area occupied by the population expands following the function:
| (2) |
Where 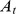 is the area occupied at time
is the area occupied at time  ;
; 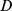 is the population diffusion coefficient (e.g. km2 yr−1);
is the population diffusion coefficient (e.g. km2 yr−1);  is the intrinsic rate of population growth (yr−1) specified as a PERT function [58], [59], [60]. This spread model assumes that the population is in a homogenous environment and expands at an equal rate in each direction.
is the intrinsic rate of population growth (yr−1) specified as a PERT function [58], [59], [60]. This spread model assumes that the population is in a homogenous environment and expands at an equal rate in each direction.
As the area involved in an initial site expansion and the population density within that area increases, so too does the likelihood of a random satellite outbreak some distance from the original site:
| (3) |
Here 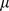 is the rate of satellite generation specified as a PERT function, and
is the rate of satellite generation specified as a PERT function, and 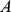 is the occupied area. Once generated, each satellite population grows and expands in the same manner as the original population. The total area of the original site occupied and the number of satellites grows until
is the occupied area. Once generated, each satellite population grows and expands in the same manner as the original population. The total area of the original site occupied and the number of satellites grows until 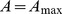 (maximum habitable area), at which point total area occupied remains constant.
(maximum habitable area), at which point total area occupied remains constant.
Simulations
In total, five scenarios were examined. The first two assumed that T. pityocampa was fully dispersed throughout suitable areas in New Zealand, and as such make no assumptions around spread. These two equilibrium scenarios are Equilibrium No Control (no control) and Equilibrium with Control (infestations are controlled using Bacillus thuringiensis var. Kurstaki, Btk). In addition three invasion spread scenarios were simulated: 1) No control: an unmanaged invasion by T. pityocampa, 2) Little control: invasion by T. pityocampa that is managed ineffectively [maximum rate of population growth (rm) reduced by 50%], and 3) Good control: invasion by T. pityocampa that is managed effectively (rm reduced by 95%). For the three scenarios in which Btk is applied it is assumed that the insecticide completely negates the negative impact of T. pityocampa on growth over the area occupied by the moth.
For each invasion scenario a set of simulations were run using the spread model of Waage et al. [57]. Parameters within the spread model were specified as distributions, and a Latin hypercube sampling algorithm used to sample from each distribution. In each of 2 000 model iterations one value was sampled from each distribution within the model and calculations performed using this set of parameters. To test their sensitivity, parameters rm (the maximum rate of population growth) and μ (the rate at which satellite populations are generated each year) were then sampled from uniform distributions described in Table 2 with a maximum (minimum) of +95 per cent (−95 per cent) of their mean values using Monte Carlo simulation.
Table 2. Parameter values used in spread simulations using the spread model of Waage et al. [57].
| Scenario | Parameter | ||
| rm (year−1) | μ (year−1) | D (ha year−1) | |
| No control | 0.2–0.5 | 0–0.00001 | 1 996 |
| Little control | 0.1–0.25 | 0–0.00001 | 1 996 |
| Good control | 0.01–0.025 | 0–0.00001 | 1 996 |
rm is the maximum rate of population growth μ is the rate at which satellite populations are generated each year, and D is the diffusion coefficient.
Specification of Parameter Values in Models
Spread rate
Whilst it is possible for male moths of T. pityocampa to fly 20 km [61], female moths are short-lived, and can only fly short distances before ovipositing a single batch of eggs [62], [63], [64]. An analysis of the historical spread of T. pityocampa over 32 years in the comparatively flat Paris Basin indicated an average annual radial rate of spread of 2.7 km yr−1, though more recently rates of 5.6 km year−1 have been observed [23]. When translocated outside of the current range, T. pityocampa larvae were able to survive conditions colder than presently occupied, which indicates that the observed spread could be limited by the dispersal rates of the female moths, and the present range is lagged behind the retreating climatic limits. The diffusion coefficient in Equation 2
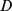 can be derived from the Mean Dispersal Distance (
can be derived from the Mean Dispersal Distance (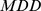 ) [65]:
) [65]:
| (4) |
where D is the diffusion coefficient (ha yr−1), and MDD is the average radial rate of spread (5.6 km year−1), which yields a value of 1 996 ha year−1.
In a survey of spread rates for 27 Lepidoptera, Waage et al. [57] noted that only one, Pieris rapae exhibited significant acceleration in its range expansion that indicated that long-distance jump dispersal was likely to be a significant factor in its invasion. In that survey, observed rates of radial range expansion were predominantly less than 10 km yr−1, which agrees with the estimate from Battisti et al [23].
Fecundity and population growth
Pimentel et al. [64] undertook a study of the population dynamics of T. pityocampa. They found that the fecundity of a winter population of T. pityocampa was 197.4±45.4, with 98.4±1% viability. Whilst Pimentel et al. [64] noted approximately 13% parasitism, this was in the native range, and is likely to overestimate the attack rates in a new environment unless the parasitoids are also translocated with T. pityocampa. Predation resulted in approximately 5% of nests having no survivors, with survival of larvae in other nests of 32.2±22.8%. Taken together, these results indicate an average value for the net reproductive rate, r of approximately 1.47 year−1, derived using Equation 5.
| (5) |
where F is average fecundity of each egg batch (197) [64], RF is the (assumed) female sex ratio for eggs (0.5), V is average egg viability (0.98) [64], SNestPredation is the assumed average fraction of nests with at least one survivor (0.95), SLarvalPredation is the average fraction of larvae surviving predation (0.32) [64], and M is the assumed mating success rate (0.05). The value of M for the No Control scenario was selected after consideration of the observed mating success rate for gypsy moth (Lymantria dispar L.) under low density conditions such as those experienced in the region of the invasion front [66]. The biotype of L. dispar studied by Robinet et al. shares with T, pityocampa the qualities of highly mobile males and sessile females [66]. In T. pityocampa, the females lay a single batch of eggs; colonies are formed from one or several batches of eggs [64]. Note that the reproductive rate variable rm included in the model of Waage et al. [57] is equal to r –1 as it is included within a difference equation framework, hence a value of 1.45 for r is equivalent to a value of 0.45 for rm. The carrying capacity of pine forests for T. pityocampa is estimated to be 0.95 nests per tree×1 000 trees ha−1×1 adult female per nest. It was assumed that only one female reaches the adult stage.
The annual rate of generation of satellite populations of T. pityocampa (μ) was very low (Table 2). This reflects the assumption that during an incursion, plantation hygiene would minimise, but not eliminate, long distance dispersal of eggs or pupae well ahead of the invasion front, creating new invasion foci.
Partial Budget Model of Economic Damage from Thaumetopoea pityocampa
The 300 Index spatial dataset describing P. radiata productivity throughout New Zealand [32] and a shapefile defining the spatial extent of the New Zealand pine plantation estate were spatially intersected with the 10′ cells from the CLIMEX analysis. These paired values were extracted into an Excel spreadsheet for subsequent analyses.
The total potential loss to pine plantations from T. pityocampa in m3 yr−1 was calculated using the following equation:
| (6) |
(where A is the area (m2) planted to forest in each cell and MAIi is the area-weighted average 300 Index value in m3 ha−1 yr−1 , and OF is the outbreak or attack frequency reported by Laurent-Hervouet [29] for T. pityocampa (0.5 yr−1), and n is the total number of 10′ cells in New Zealand that are climatically suitable for T. pityocampa under historical conditions.
The present gross value of this production loss GVPL, was calculated as,
| (7) |
where VS is the stumpage value (presently NZ$55.00).
We assumed that Btk was applied aerially to infested forests throughout the 30 year period, at a cost of NZ$120 ha−1 (ca. 60 € ha−1) [67]. It was also assumed that Btk completely negates any detrimental impact of T. pityocampa on crop volume growth.
Under each scenario, the impact of T. pityocampa on the tree growth rates across the New Zealand plantation estate was then estimated using discounted cashflow analysis. Analyses were undertaken to estimate discounted annual losses over a 30 year period from 2010 to 2040 (the invasion was assumed to start in 2010). In this analysis, it was assumed that forest rotations and harvests would occur on a rolling basis and so the forest age class would have a stable distribution. The discount rate used was 7% per annum.
Results
Potential Distribution of Thaumetopoea pityocampa within Current Plantations
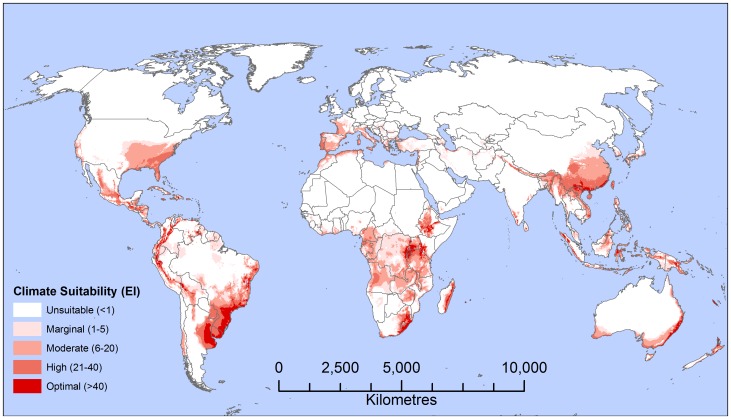
To verify the climate suitability model for T. pityocampa, the CLIMEX Ecoclimatic Index was compared with the reported distribution in Europe and North Africa (Fig. 3). The visual fit is satisfactory, with good model specificity (few false positives, Fig. 3). Elsewhere in the world, the CLIMEX model indicates that the potential range includes warm temperate, Mediterranean and moist sub-tropical climates. Notably, the most favourable climates for T. pityocampa appear to lie outside of its native range, in eastern South America, pockets of southern and central eastern Africa, southern China and eastern Australia (Fig. 5).
Figure 5. Global climate suitability for Thaumetopoea pityocampa modelled using CLIMEX Ecoclimatic index (EI).
Projection: Robinson.
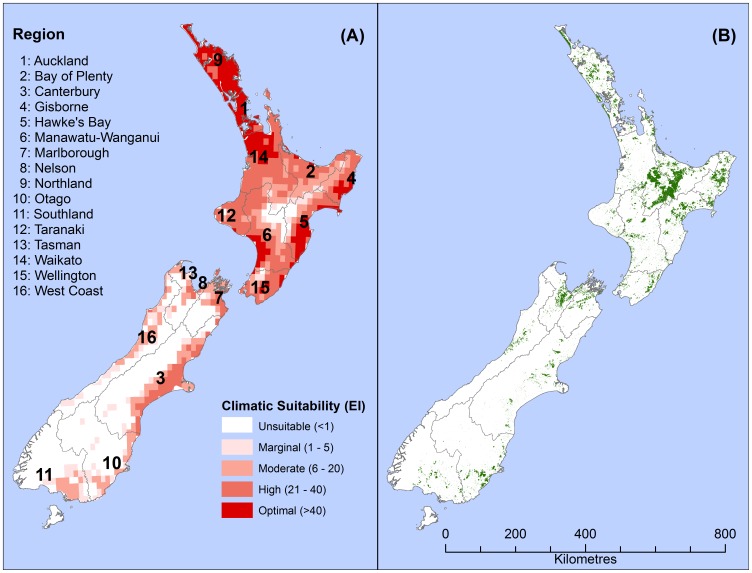
In New Zealand, under current climate, most of the coastal North Island and a strip along the east coast of the South Island appear suitable for T. pityocampa (Fig. 6A). With the exception of part of the central North Island forests, most plantations in the North Island were projected to have a suitable climate for the moth (Fig. 6B). In total 65% of the total plantation area within New Zealand was projected to be suitable for T. pityocampa. The proportion of plantation area suitable for T. pityocampa declined with latitude (Fig. 6A).
Figure 6. New Zealand map showing: (a) climatic suitability, by region, under current climate for Thaumetopoea pityocampa using CLIMEX Ecoclimatic Index (EI), and (b) existing (green) pine plantations [69].
Projection: New Zealand map grid.
Rate of spread of Thaumetopoea pityocampa
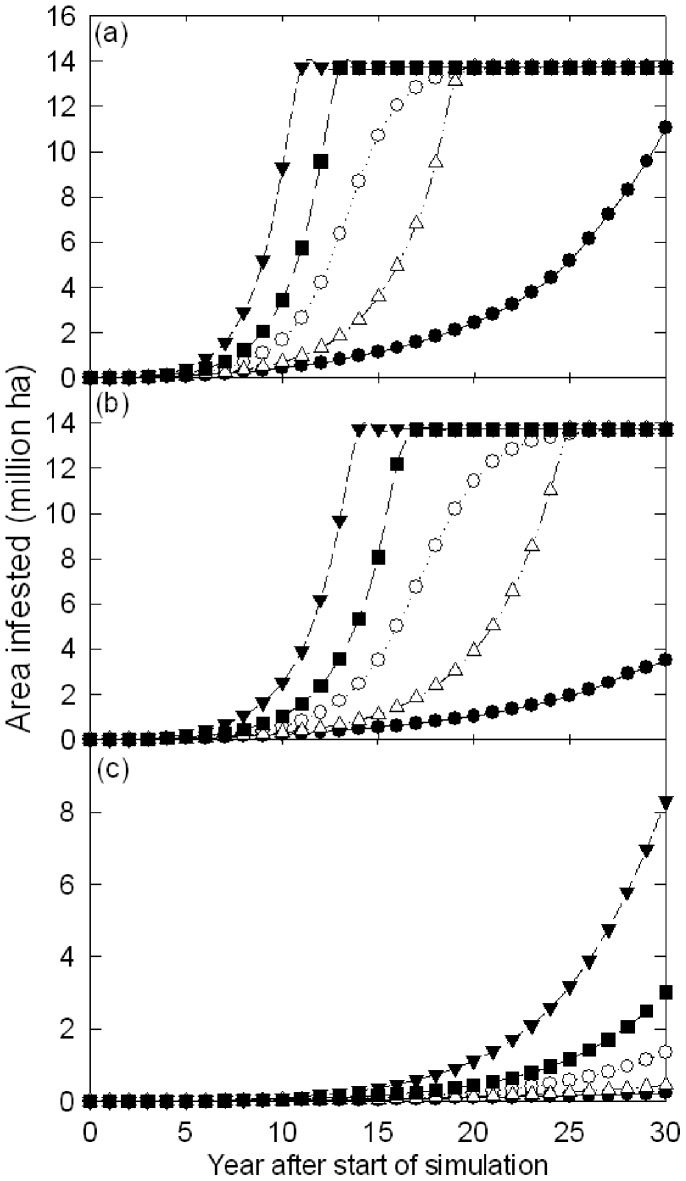
The modelled rate of spread of T. pityocampa varied widely between scenarios (Fig 7A). Examination of the median simulations showed that 20 years after the simulation starts the moth occupies, 99% (Fig. 7A), 83% (Fig. 7B) and <2% (Fig. 7C) of the climatically suitable area, respectively, for the no control, little control and good control scenarios respectively. Although there is variation around the average for these scenarios, this variation is much smaller than the differences that exist between scenarios (Fig. 7). Under the least conservative no control scenario, sampled from the 95 percentile, the moth completely occupies the climatically suitable area within 13 years of the start of the simulation (Fig. 7A).
Figure 7. Change in area infested by Thaumetopoea pityocampa from the simulation start under the (a) no control (b) little control and (c) good control spread scenarios.
On all figures the lines represent trajectories from spread simulations showing the minimum, (filled circles), maximum (filled triangles), 5th (open triangles), 50th (open circles), and 95th (closed squares) percentiles. Note the variation in scale between figure panels.
Variation in production losses with and without Thaumetopoea pityocampa.
Assuming Full Occupancy of the Climatically Suitable Area
The Equilibrium No Control simulation indicates that if the moth was presently fully dispersed throughout its potential range and left untreated, merchantable timber production would be reduced nationally by 29.9% or 8.8×106 m3 yr−1, which is equivalent to NZ$482 million yr−1. In the absence of control efforts, potential regional losses are likely to vary from 76% of harvest revenue in Northland forests to nil in some Southland forests. This figure represents the annual recurrent losses in 2010 terms that we could expect in the absence of any efforts to reduce the damage.
Under the Equilibrium with Control scenario, expected annual financial losses are NZ$72 million yr−1. Given that volume losses are assumed to be nil in stands treated with Btk this figure represents the annual cost of spraying the affected area with Btk. Comparing the two scenarios indicates an average net saving of 85% through the application of control measures.
Under the spread scenarios, without any controls, the total present value (PV) losses ranged from NZ$1,550 M to NZ$2,560 M (Table 3). Under the assumption that T. pityocampa is controlled using aerial application of Btk, the combined costs due to production losses and spray costs were reduced significantly, but nonetheless remain substantial, particularly if the control is only moderately effective (Table 3).
Table 3. Present value of damage to commercial forestry operations in New Zealand due to the spread of Thaumetopoea pityocampa over 30 years.
| Spread Scenario | Present value of impacts (NZ$M) | Present Value of treatment costs (NZ$M) | Present value of impacts and control costs (NZ$M) | ||||||
| 5% | Median | 95% | 5% | Median | 95% | 5% | Median | 95% | |
| No control | 1550 | 2098 | 2560 | – | – | – | 1550 | 2098 | 2560 |
| Little control | 884 | 1441 | 1920 | 132 | 215 | 287 | 1016 | 1657 | 2207 |
| Good control | 27 | 58 | 113 | 4 | 9 | 17 | 31 | 67 | 129 |
Values are discounted at 7% and accumulated over the 30 year period. 5%, median and 95% are results for different quantiles in the stochastic spread simulations.
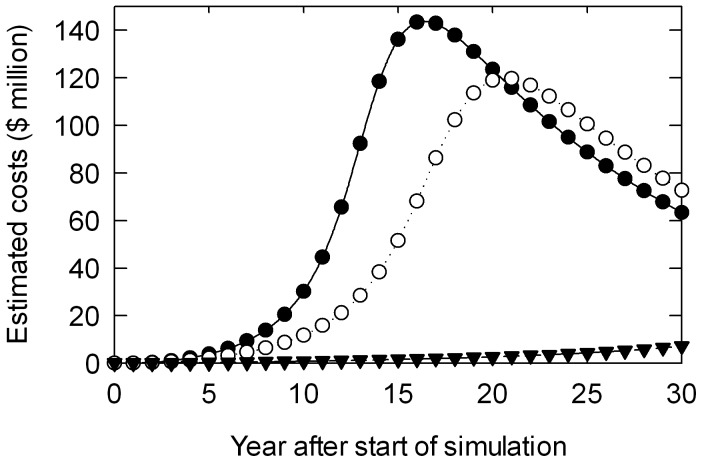
The effect of the discounting rate interacting with the spreading impacts is apparent in the declining costs in later years in the No Control and Little Control scenarios in Figure 8. As potential impacts accrue as the pest spreads, the marginal value of these additional costs declines through time. The extreme high value in suppressing effectively the rate of spread of the pest is clearly apparent in the cost savings from effective control of the spread of T. pityocampa compared with doing nothing (Fig. 8).
Figure 8. Variation in simulated annual costs due to Thaumetopoea pityocampa (discounted back to simulation start) under scenarios assuming no control (closed circles) little control (open circles) and good control (closed triangles).
All scenarios used simulations with a median spread rate as sampled from the 50th percentile.
Discussion
What are the Likely Costs of Invasion?
CLIMEX projections indicate that about 60% of New Zealand’s plantation forest area is climatically suitable for T. pityocampa under the current climate. Should this pine defoliator become established in New Zealand, outbreaks are likely to occur at a high frequency because P. radiata is a preferred host and it is highly susceptible to attack. This situation is likely to be exacerbated as T. pityocampa would experience release from the effects of at least some of its natural enemies, which contrasts with the situation in its native range [68]. If left untreated, the potential damage resulting from an invasion by T. pityocampa is expected to be significant (NZ$1,550 M –2,560 M, Table 3). It would appear cost-effective to spray Btk to reduce the impacts of T. pityocampa even with little control.
The cost estimates above only account for direct losses of stumpage value due to defoliation, and control costs to mitigate these losses. However, other damage would be incurred if this insect became established. For example, there would be considerably less carbon sequestration and loss of recreational value as well as human health costs because the urticating hairs of T. pityocampa cause serious dermatitis. An attempt to value these costs has been made previously [67].
Assuming that T. pityocampa invaded New Zealand and had become fully dispersed throughout its potential range, it would cost approximately NZ$72 M yr−1 to spray Btk over the 1.15 million ha of vulnerable forests. Using a 7% discount rate, these recurrent costs total NZ$966 M in PV across the thirty years of this case study. Even under the most optimistic (low cost) scenarios with a low rate of spread due to effective control (Table 3, Fig. 8), some effort at preventing the introduction and ensuring that cost-effective and socio-politically acceptable control techniques are available for deployment at short notice are clearly warranted. The amount of effort justified will also depend upon the probability of entry and establishment in New Zealand by T. pityocampa, [18], and consideration of the benefits of preventative measures in excluding other pests such as gypsy moth.
Accidental shipments of T. pityocampa egg masses with sea containers originating from infested regions are probably the highest risk introduction pathway. Larvae are vulnerable to starvation and environmental mortality factors, adults are too short-lived, and rigid biosecurity measures prevent the movement of soil that could harbour pupae. There are frequent outbreaks of T. pityocampa in southern Europe, which suggests that there is a high propagule pressure from its native region. These outbreaks often affect pines in urban and semi-urban areas in close proximity to industrial sites with shipping container activity [28]. It is conceivable that pine needles carrying egg masses could accidentally be transported on the surface or inside a shipping container. Incursions of several other Lepidoptera, previously considered low risk have recently occurred in New Zealand, including the white-spotted tussock moth, painted apple moth (Teia anartoides) and gum leaf skeletoniser (Uraba lugens). Even if T. pityocampa did not become established in New Zealand in the near future, the rate of defoliator introductions in New Zealand and overseas indicates that there is a high risk of such an invasion. Ultimately, this study should serve as a case study of a potentially invasive insect pest of pines.
The niche model indicates that the global potential distribution of T. pityocampa is extensive (Fig. 5), and includes most of the regions of the world where P. radiata is grown commercially. Interestingly, the apparently most climatically suitable areas of the world for T. pityocampa occur in regions outside of its native range (mostly in the southern hemisphere). These mostly sub-tropical regions experience climates that have less extreme seasonality than that of the Mediterranean basin. This curiosity may be explained by the fact that the native range of T. pityocampa is geographically isolated from areas with a sub-tropical climate, and should it invade such a region with a less extreme climate than its native range, it may indeed thrive. However, whilst suitable hosts are grown in these regions (e.g. P. radiata in eastern Australia), indicating some degree of likely climate suitability, it is prudent to treat climate suitability projections in these areas with some added caution as there may be some negative life-history factor associated with warm-wet conditions that have not been taken into account in the niche model.
How Effective was the Modelling Method, and what did it tell us that couldn’t otherwise be Answered?
Compared with traditional economic pest risk assessments, the method demonstrated here offers significant enhancements. Traditional cost-benefit approaches ignore variability in pest population dynamics due to differences in climate throughout the area in which the vulnerable crops are grown. Typically, these analyses do not even use a species niche model for the pest to estimate which cropping regions are vulnerable to pest impacts. The CLIMEX model allowed us to identify which forests were vulnerable to attack from T. pityocampa, and to construct the climate-based growth impact function. Combining the 300 Index values, the CLIMEX niche model and the growth impact function allowed us to quantify potential tree growth rate impacts in a spatially-explicit and defensible manner, and to identify regions of highest priority for protection. The growth impact function is a relatively simple technique that captures climatic variability in pest suitability without resorting to the complicated and expensive method of modelling the pest population dynamics directly. By including consideration of the rate of spread of the pest, the potential costs were discounted appropriately, and the different control strategies that affect the spread rate could be evaluated.
How Transferrable and Useful is the Method?
The data requirements for this project were significant, and it is unlikely that this type of analysis would be undertaken routinely for pest risk assessments. Of the different information components required to undertake this analysis, the CLIMEX analysis could be considered the most practiced and available. Whilst high quality fine resolution modelled productivity surfaces such as the 300 Index dataset are likely to remain rare, crop productivity maps based on crop production statistics are becoming more commonplace [e.g., www.mapspam.info (global), Eurostat (regional), and Cropscape (USA)]. The spatial resolution of the crop productivity maps are typically coarser than the 300 Index surface, though they appear adequate for combining with niche models for the type of analysis described here.
The climate-impact function is an attractive compromise between expensive detailed process-based population dynamics modelling on the one hand, and using a simplistic estimate of average pest impacts on the other. For relatively little effort, it provides a means of spatially matching pest impacts with productivity. As with the niche model, having generated the model once, it is able to be applied to pest risk assessments in any jurisdiction. However, for this type of analysis to become more commonplace, reporting of pest impact would be best studied and reported using standardised methods and measures.
Discounting the costs throughout the simulated invasion had a significant impact on the cost estimates, so it is clearly desirable to include plausible estimates of the rate of spread of invaders throughout pest risk areas. To do this routinely, the potential rates of spread of pests across landscapes may need to be better studied and documented. Whilst it is inconceivable that there would ever be a useful library of documented pest spread rates for every pest of economic concern, it is possible that a study of spread rates for different organisms could be used to develop a useful trait-based means of estimating useful rates of spread classes for invasive species.
The method of linking climate suitability, spread rates and host-impact demonstrated here appears useful and practical for estimating the potential costs of invasive pests. Whilst it has been applied to a case where impacts are considered for a single host crop, it can be easily extended to accommodate multiple hosts, so long as the impact functions can be calibrated for each crop, and the abundance of each crop is known throughout the pest risk area. The method described here can also be readily applied to pests threatening non-productive assets such as natural ecosystems using appropriate pricing mechanisms such as the hedonic pricing technique or the valuation of ecosystem services. Linking the niche modelling and economic analytical tools together as we have done in this paper provides an open and transparent means by which different pest threats can be gauged in terms of their likely economic impacts, and allows different pest threats to be compared.
Acknowledgments
We are grateful to Dr. James Turner for providing useful help on the economic analysis, Ms Samantha Alcaraz for collating distribution data for T. pityocampa, and Drs Libby Pinkard, Helen Murphy and Wopke van der Werf for useful comments on a draft of the manuscript.
Funding Statement
This work was partly funded by the New Zealand Foundation for Research, Science & Technology through contract C02X0501 [the Better Border Biosecurity (B3) programme (www.b3nz.org)], and contract C09X0905 (Beating Environmental Weeds II) and the New Zealand Ministry of Agriculture and Forestry. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kriticos DJ, Phillips CB, Suckling DM (2005) Improving border biosecurity: Potential economic benefits to New Zealand. N.Z. Plant Prot. 58: 1–6. [Google Scholar]
- 2. Ricciardi A (2007) Are modern biological invasions an unprecedented form of global change? Conserv. Biol. 21: 329–336. [DOI] [PubMed] [Google Scholar]
- 3. Pimentel D, McNair S, Janecka J, Wightman J, Simmonds C, et al. (2001) Economic and environmental threats of alien plant, animal, and microbe invasions. Agriculture, Ecosystems and Environment 84: 1–20. [Google Scholar]
- 4. Brasier CM (2008) The biosecurity threat to the UK and and global environment from international trade in plants. Plant Pathol. 57: 792–808. [Google Scholar]
- 5.Andrewartha HG, Birch LC (1954) The distribution and abundance of animals. Chicago: University of Chicago Press.
- 6. De Wolf ED, Isard SA (2007) Disease cycle approach to plant disease prediction. Annual Review of Phytopathology 45: 203–220. [DOI] [PubMed] [Google Scholar]
- 7.Wallin JR, Waggoner PE (1950) The influence of climate on the development and spread of Phytophthora infestans in artificially inoculated potato plots. Plant Dis. Reptr. Suppl. 190: 19–33.
- 8. Kriticos DJ, Yonow T, McFadyen RE (2005) The potential distribution of Chromolaena odorata (Siam weed) in relation to climate. Weed Res. 45: 246–254. [Google Scholar]
- 9. Yonow T, Kriticos DJ, Medd RW (2004) The potential geographic range of Pyrenophora semeniperda . Phytopathology 94: 805–812. [DOI] [PubMed] [Google Scholar]
- 10. Yonow T, Sutherst RW (1998) The geographical distribution of the Queensland fruit fly, Bactrocera (Dacus) tryoni, in relation to climate. Australian Journal of Agricultural Research 49: 935–953. [Google Scholar]
- 11. Watt MS, Kriticos DJ, Manning LK (2009) The current and future potential distribution of Melaleuca quinquenervia . Weed Research 49: 381–390. [Google Scholar]
- 12. Stephens AEA, Kriticos DJ, Leriche A (2007) The current and future potential geographic distribution of the Oriental fruit fly, Bactrocera dorsalis, (Diptera: Tephritidae). Bulletin of Entomological Research 97: 369–378. [DOI] [PubMed] [Google Scholar]
- 13. Sutherst RW, Bourne AS (2009) Modelling non-equilibrium distributions of invasive species: a tale of two modelling paradigms. Biol. Inv. 11: 1231–1237. [Google Scholar]
- 14. Sharov AA (2004) Bioeconomics of managing the spread of exotic pest species with barrier zones. Risk Anal. 24: 879–892. [DOI] [PubMed] [Google Scholar]
- 15. Fraser RW, Cook DC, Mumford JD, Wilby A, Waage JK (2006) Managing outbreaks of invasive species: Eradication versus suppression. International Journal of Pest Management 52: 261–268. [Google Scholar]
- 16.Plant Health Australia (2009) PLANTPLAN: Australian Emergency Plant Pest Response Plan. Canberra, ACT: Plant Health Australia. 197 p.
- 17. Cook DC, Thomas MB, Cunningham SA, Anderson DL, De Barro PJ (2007) Predicting the economic impact of an invasive species on an ecosystem service. Ecol. Appl. 17: 1832–1840. [DOI] [PubMed] [Google Scholar]
- 18. Cook DC, Matheson AC (2008) An estimate of the potential economic impact of pine pitch canker in Australia. Australian Forestry 71: 107–112. [Google Scholar]
- 19.FAO (2006) International standards for phytosanitary measures: 1 to 24. Rome: Secretariat of the International Plant Protection Convention.
- 20.Standards Australia (2006) National Post-Border Weed Risk Management Protocol. Sydney: CRC Australian Weed Management, Adelaide and Standards Australia International Ltd.
- 21. Salvato P, Battisti A, Concato S, Masutti L, Patarnello T, et al. (2002) Genetic differentiation in the winter pine processionary moth (Thaumetopoea pityocampa wilkinsoni complex), inferred by AFLP and mitochondrial DNA markers. Molecular Ecology 11: 2435–2444. [DOI] [PubMed] [Google Scholar]
- 22.Huchon H, Démolin G (1970) La bioécologie de la processionaire du pin. Dispersion potentielle. Revue Forestière Française, spécial ‘La lutte biologique en forêt’: 220–234.
- 23. Battisti A, Stastny M, Netherer S, Robinet C, Schopf A, et al. (2005) Expansion of geographic range in the pine processionary moth caused by increased winter temperature. Ecol. Appl. 15: 2084–2096. [Google Scholar]
- 24. Robinet C, Baier P, Pennerstorfer J, Schopf A, Roques A (2007) Modelling the effects of climate change on the potential feeding activity of Thaumetopoea pityocampa (Den. & Schiff.) (Lep., Notodontidae) in France. Global Ecol. Biogeogr. 16: 460–471. [Google Scholar]
- 25. Palmeri V, Pulvirenti A, Zappalà L (2005) La processionaria dei pini nei boschi della dorsale appenninica della Calabria. Forest@ 2: 345–357. [Google Scholar]
- 26. Buxton RD (1983) Forest management and the pine processionary moth. Outlook on Agriculture 12: 34–39. [Google Scholar]
- 27. Cobos-Suarez JM, Ruiz-Urrestarazu MM (1990) Problemas fitosanitarios de la especie Pinus radiata D. Don en Espana, con especial referencia al Pais Vasco. Bol. San. Veg. Plagas 16: 37–53. [Google Scholar]
- 28.Brockerhoff EG, Jactel H, Goldarazena A, Berndt L, Bain J (2007) Risk assessment of European pests of Pinus radiata. Report No. 12216 for New Zealand Forest Health Research Collaborative. Christchurch: Ensis/Scion.
- 29. Laurent-Hervouet N (1986) Radial growth losses in some species of Pinus due to 2 forest defoliators. 1. Case of the pine processionary moth in the Mediterranean area. Annales Des Sciences Forestieres 43: 239–262. [Google Scholar]
- 30. Hódar J, Zamora R, Castro J, Baraza E (2004) Feast and famine: previous defoliation limiting survival of pine processionary caterpillar Thaumetopoea pityocampa in Scots pine Pinus sylvestris . Acta Oecologia 26: 203–210. [Google Scholar]
- 31.Ministry of Agriculture and Forestry (2010) National Exotic Forest Description. Wellington: Ministry of Agriculture and Forestry.
- 32. Palmer DJ, Hock BK, Kimberley MO, Watt MS, Lowe DJ, et al. (2009) Comparison of spatial prediction techniques for developing Pinus radiata productivity surfaces across New Zealand. Forest Ecology and Management 258: 2046–2055. [Google Scholar]
- 33. Kimberley MO, West G, Dean M, Knowles L (2005) Site Productivity: The 300 Index - a volume productivity index for radiata pine. New Zealand Journal of Forestry 50: 13–18. [Google Scholar]
- 34. Sutherst RW, Maywald GF (1985) A computerised system for matching climates in ecology. Agriculture, Ecosystems and Environment 13: 281–299. [Google Scholar]
- 35.Sutherst RW, Maywald GF, Kriticos DJ (2007) CLIMEX Version 3: User’s Guide. Hearne Scientific Software Pty Ltd. Available: http://www.Hearne.com.au.
- 36.de Villiers M, Hattingh V, Kriticos DJ (2012) Combining field phenological observations with distribution data to model the potential range distribution of the fruit fly Ceratitis rosa Karsch (Diptera: Tephritidae). Bulletin of Entomological Research. [DOI] [PubMed]
- 37. Buffo E, Battisti A, Stastny M, Larsson S (2007) Temperature as a predictor of survival of the pine processionary moth in the Italian Alps. Agric. For. Entomol. 9: 65–72. [Google Scholar]
- 38. Hoch G, Toffolo EP, Netherer S, Battisti A, Schopf A (2009) Survival at low temperature of larvae of the pine processionary moth Thaumetopoea pityocampa from an area of range expansion. Agric. For. Entomol. 11: 313–320. [Google Scholar]
- 39. Duncan RP, Cassey P, Blackburn TM (2009) Do climate envelope models transfer? A manipulative test using dung beetle introductions. Proceedings of the Royal Society B-Biological Sciences 276: 1449–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Webber BL, Yates CJ, Le Maitre DC, Scott JK, Kriticos DJ, et al. (2011) Modelling horses for novel climate courses: insights from projecting potential distributions of native and alien Australian acacias with correlative and mechanistic models. Div. and Distrib. 17: 978–1000. [Google Scholar]
- 41. Breuer M, Devkota B (1990) Studies on the importance of nest temperature of Thaumetopoea pityocampa (Den. & Schiff.) (Lep., Thaumetopoeidae). J. Appl. Entomol. 109: 331–335. [Google Scholar]
- 42. Kriticos DJ, Webber BL, Leriche A, Ota N, Bathols J, et al. (2012) CliMond: global high resolution historical and future scenario climate surfaces for bioclimatic modelling. Methods in Ecology and Evolution 3: 53–64. [Google Scholar]
- 43. Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25: 1965–1978. [Google Scholar]
- 44. New M, Lister D, Hulme M, Makin I (2002) A high-resolution data set of surface climate over global land areas. Clim. Res. 21: 1–25. [Google Scholar]
- 45.CTGREF-INRA (1980) La chenille processionaire du pin. Organisation de la surveillance en forêt à partir. Saint Martin d’Hères, INRA, Station de Zoologie Forestière, Avignon: Ministère de l’Agriculture, CTGREF, Division de la Protection de la Nature.
- 46.EPPO/CABI (1996) Thaumetopoea pityocampa. Quarantine Pests for Europe: CAB international, Wallingford (GB). 532–537.
- 47. Schmidt GH, Mirchev P, Tsankov G (1997) The egg parasitoids of Thaumetopoea pityocampa in the Atlas mountains near Marrakech (Morocco). Phytoparasitica 25: 275–281. [Google Scholar]
- 48. Astiaso Gallart F (1992) Plagas de insectos en las masas forestales españolas. Boletin del Servicio de Plagas Forestales 4: 122–130. [Google Scholar]
- 49. Watt MS, Kriticos DJ, Alcaraz S, Brown A, Leriche A (2009) The hosts and potential geographic range of Dothistroma needle blight. Forest Ecology and Management 257: 1505–1519. [Google Scholar]
- 50. Kriticos DJ, Leriche A (2009) The effects of spatial data precision on fitting and projecting species niche models. Ecography 33: 115–127. [Google Scholar]
- 51. Lemoine B (1977) Contribution à la mesure des pertes de production causées par la chenille processionnaire (Thaumetopoea pityocampa Schiff.) au Pin maritime dans les Landes de Gascogne. Annales Des Sciences Forestieres 34: 205–214. [Google Scholar]
- 52.Markalas S (1998) Biomass production of Pinus pinaster after defoliation by the pine processionary moth (Thaumetopoea pityocampa Schiff.). Population Dynamics, Impacts, and Integrated Management of Forest Defoliating Insects - Proceedings. 292–302.
- 53. Carus S (2004) Impact of defoliation by the pine processionary moth (Thaumetopoea pityocampa) on radial, height and volume growth of Calabrian pine (Pinus brutia) trees in Turkey. Phytoparasitica 32: 459–469. [Google Scholar]
- 54. Kanat M, Alma MH, Sivrikaya F (2005) Effect of defoliation by Thaumetopoea pityocampa (Den. & Schiff.) (Lepidoptera: Thaumetopoeidae) on annual diameter increment of Pinus brutia Ten. in Turkey. Annals of Forest Science 62: 91–94. [Google Scholar]
- 55.Melbourne BA, Hastings A (2009) Highly variable spread rates in replicated biological invasions: Fundamental limits to predictability. Science 325. [DOI] [PubMed]
- 56. Pitt JPW, Kriticos DJ, Dodd MB (2011) Limits to simulating the future spread of invasive species: Buddleja davidii in New Zealand. Ecol. Model. 222: 1880–1887. [Google Scholar]
- 57.Waage JK, Fraser RW, Mumford JD, Cook DC, Wilby A (2005) A New Agenda for Biosecurity London: Department for Food, Environment and Rural Affairs. 198 p.
- 58.Shigesada N, Kawasaki K (1997) Biological invasions: Theory and practice. May RM, Harvey PH, editors. Oxford: Oxford University Press. 205 p.
- 59.Hengeveld R (1989) The Dynamics of Biological Invasions. London: Chapman and Hall.
- 60.Lewis MA (1997) Variability, Patchiness, and Jump Dispersal in the Spread of an Invading Population”. In: Tilman D, Kareiva P, editors. Spatial Ecology: The Role of Space in Population Dynamics and Interspecific Interactions. New Jersey: Princeton University Press. 46–74.
- 61. Pimentel C, Ferreira C, Nilsson JÅ (2010) Latitudinal gradients and the shaping of life-history traits in a gregarious caterpillar. Biol. J. Linn. Soc. 100: 224–236. [Google Scholar]
- 62. Zhang QH, Paiva MR (1998) Female calling behaviour and male response to the sex pheromone in Thaumetopoea pityocampa (Den. & Schiff.) (Lep., Thaumetopoeidae). J. Appl. Entomol.-Z. Angew. Entomol. 122: 353–360. [Google Scholar]
- 63. Kerdelhue C, Magnoux E, Lieutier F, Roques A, Rousselet J (2006) Comparative population genetic study of two oligophagous insects associated with the same hosts. Heredity 97: 38–45. [DOI] [PubMed] [Google Scholar]
- 64. Pimentel C, Calvão T, Santos M, Ferreira C, Neves M, et al. (2006) Establishment and expansion of a Thaumetopoea pityocampa (Den. & Schiff.) (Lep. Notodontidae) population with a shifted life cycle in a production pine forest, Central-Coastal Portugal. Forest Ecology and Management 233: 108–115. [Google Scholar]
- 65. Andow DA, Kareiva P, Levin SA, Okubo A (1990) Spread of invading organisms. Landscape Ecology 4: 177–188. [Google Scholar]
- 66. Robinet C, Lance DR, Thorpe KW, Onufrieva KS, Tobin PC, et al. (2008) Dispersion in time and space affect mating success and Allee effects in invading gypsy moth populations. J. Anim. Ecol. 77: 966–973. [DOI] [PubMed] [Google Scholar]
- 67. Gatto P, Zocca A, Battisti A, Barrento MJ, Branco M, et al. (2009) Economic assessment of managing processionary moth in pine forests: A case-study in Portugal. J. Environ. Manage. 90: 683–691. [DOI] [PubMed] [Google Scholar]
- 68. Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 17: 164–170. [Google Scholar]
- 69.Ministry for Environment. Land Cover Database version 2 (LCDB2) Available: www.mfe.govt.nz. Accessed 2009 Jun 25.