Abstract
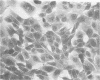
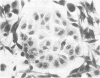
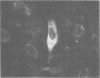
The in vitro transformation of hamster embryo fibroblasts by herpes simplex virus type 1 (HSV-1) after exposure of the virus to UV irradiation is described. Cell transformation was induced by 2 out of 12 strains of HSV-1 that were tested for transforming potential. Cells transformed by the KOS strain of HSV-1 were not oncogenic when injected into newborn Syrian hamsters. However, cells transformed by HSV-1 strain 14-012 induced tumors in 47% of the newborn hamsters injected. HSV-specific antigens were found in the cytoplasm of cells transformed by both virus strains. Sera from tumor-bearing hamsters contained HSV-1- and HSV-2-neutralizing antibodies as well as antibodies which reacted specifically with HSV antigens by the indirect immunofluorescence technique. Hamster oncornavirus antigens were not detected by immunofluorescence methods. These observations represent the first evidence of the oncogenic potential of HSV-1.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bronson D. L., Graham B. J., Ludwig H., Benyesh-Melnick M., Biswal N. Studies on the relatedness of herpes viruses through DNA-RNA hybridization. Biochim Biophys Acta. 1972 Jan 18;259(1):24–34. doi: 10.1016/0005-2787(72)90470-4. [DOI] [PubMed] [Google Scholar]
- Churchill A. E., Biggs P. M. Agent of Marek's disease in tissue culture. Nature. 1967 Jul 29;215(5100):528–530. doi: 10.1038/215528a0. [DOI] [PubMed] [Google Scholar]
- Darai G., Munk K. Human embryonic lung cells abortively infected with Herpes virus hominis type 2 show some properties of cell transformation. Nat New Biol. 1973 Feb 28;241(113):268–269. doi: 10.1038/newbio241268a0. [DOI] [PubMed] [Google Scholar]
- Duff R., Rapp F. Oncogenic transformation of hamster cells after exposure to herpes simplex virus type 2. Nat New Biol. 1971 Sep 8;233(36):48–50. doi: 10.1038/newbio233048a0. [DOI] [PubMed] [Google Scholar]
- Duff R., Rapp F. Properties of hamster embryo fibroblasts transformed in vitro after exposure to ultraviolet-irradiated herpes simplex virus type 2. J Virol. 1971 Oct;8(4):469–477. doi: 10.1128/jvi.8.4.469-477.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPSTEIN M. A., ACHONG B. G., BARR Y. M. VIRUS PARTICLES IN CULTURED LYMPHOBLASTS FROM BURKITT'S LYMPHOMA. Lancet. 1964 Mar 28;1(7335):702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- Geder L., Skinner G. R. Differentiation between type 1 and type 2 strains of herpes simplex virus by an indirect immunofluorescent technique. J Gen Virol. 1971 Aug;12(2):179–182. doi: 10.1099/0022-1317-12-2-179. [DOI] [PubMed] [Google Scholar]
- Glaser R., Duff R. G., Rapp F. Ultrastructural characterization of hamster cells transformed following exposure to ultraviolet-irradiated herpes simplex virus type 2. Cancer Res. 1972 Dec;32(12):2803–2806. [PubMed] [Google Scholar]
- Henle G., Henle W., Diehl V. Relation of Burkitt's tumor-associated herpes-ytpe virus to infectious mononucleosis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):94–101. doi: 10.1073/pnas.59.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle G., Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. 1966 Mar;91(3):1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Diehl V., Kohn G., Zur Hausen H., Henle G. Herpes-type virus and chromosome marker in normal leukocytes after growth with irradiated Burkitt cells. Science. 1967 Sep 1;157(3792):1064–1065. doi: 10.1126/science.157.3792.1064. [DOI] [PubMed] [Google Scholar]
- Huebner R. J., Todaro G. J. Oncogenes of RNA tumor viruses as determinants of cancer. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1087–1094. doi: 10.1073/pnas.64.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt R. D., Meléndez L. V., King N. W., Gilmore C. E., Daniel M. D., Williamson M. E., Jones T. C. Morphology of a disease with features of malignant lymphoma in marmosets and owl monkeys inoculated with Herpesvirus saimiri. J Natl Cancer Inst. 1970 Feb;44(2):447–465. [PubMed] [Google Scholar]
- Kieff E., Hoyer B., Bachenheimer S., Roizman B. Genetic relatedness of type 1 and type 2 herpes simplex viruses. J Virol. 1972 May;9(5):738–745. doi: 10.1128/jvi.9.5.738-745.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyon W., Kraiselburd E., Davis D., Mann J. Transfer of thymidine kinase to thymidine kinaseless L cells by infection with ultraviolet-irradiated herpes simplex virus. J Virol. 1971 Jun;7(6):813–820. doi: 10.1128/jvi.7.6.813-820.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naib Z. M., Nahmias A. J., Josey W. E., Kramer J. H. Genital herpetic infection. Association with cervical dysplasia and carcinoma. Cancer. 1969 Apr;23(4):940–945. doi: 10.1002/1097-0142(196904)23:4<940::aid-cncr2820230432>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Nazerian K., Solomon J. J., Witter R. L., Burmester B. R. Studies on the etiology of Marek's disease. II. Finding of a herpesvirus in cell culture. Proc Soc Exp Biol Med. 1968 Jan;127(1):177–182. doi: 10.3181/00379727-127-32650. [DOI] [PubMed] [Google Scholar]
- RAPP F. VARIANTS OF HERPES SIMPLEX VIRUS: ISOLATION, CHARACTERIZATION, AND FACTORS INFLUENCING PLAQUE FORMATION. J Bacteriol. 1963 Nov;86:985–991. doi: 10.1128/jb.86.5.985-991.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp F., Conner R., Glaser R., Duff R. Absence of leukosis virus markers in hamster cells transformed by herpes simplex virus type 2. J Virol. 1972 Jun;9(6):1059–1063. doi: 10.1128/jvi.9.6.1059-1063.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls W. E., Tompkins W. A., Melnick J. L. The association of herpesvirus type 2 and carcinoma of the uterine cervix. Am J Epidemiol. 1969 May;89(5):547–554. doi: 10.1093/oxfordjournals.aje.a120967. [DOI] [PubMed] [Google Scholar]
- Schneweis K. E., Nahmias A. J. Antigens of Herpes simplex virus type 1 and 2-immunodiffusion and inhibition passive hemagglutination studies. Z Immunitatsforsch Exp Klin Immunol. 1971 Jun;141(5):471–487. [PubMed] [Google Scholar]
- Wolfe L. G., Falk L. A., Deinhardt F. Oncogenicity of herpesvirus saimiri in marmoset monkeys. J Natl Cancer Inst. 1971 Nov;47(5):1145–1162. [PubMed] [Google Scholar]