Abstract
Denatured adenovirus DNA was retained by hydroxyapatite columns under conditions generally used for selective retention of double-stranded DNA, probably due to several partially complementary sequences within single-stranded DNA. It was found that addition of formamide reduced the fraction of sonically treated, denatured adenovirus DNA bound to hydroxyapatite from about 30% to less than 1%. This led to a study of the effect of formamide on the melting temperature (Tm) of double-stranded DNA in solution or bound to hydroxyapatite. The Tm of DNA decreases 0.56 C/1% formamide, a value determined in buffered solutions with purified formamide.
Full text
PDF
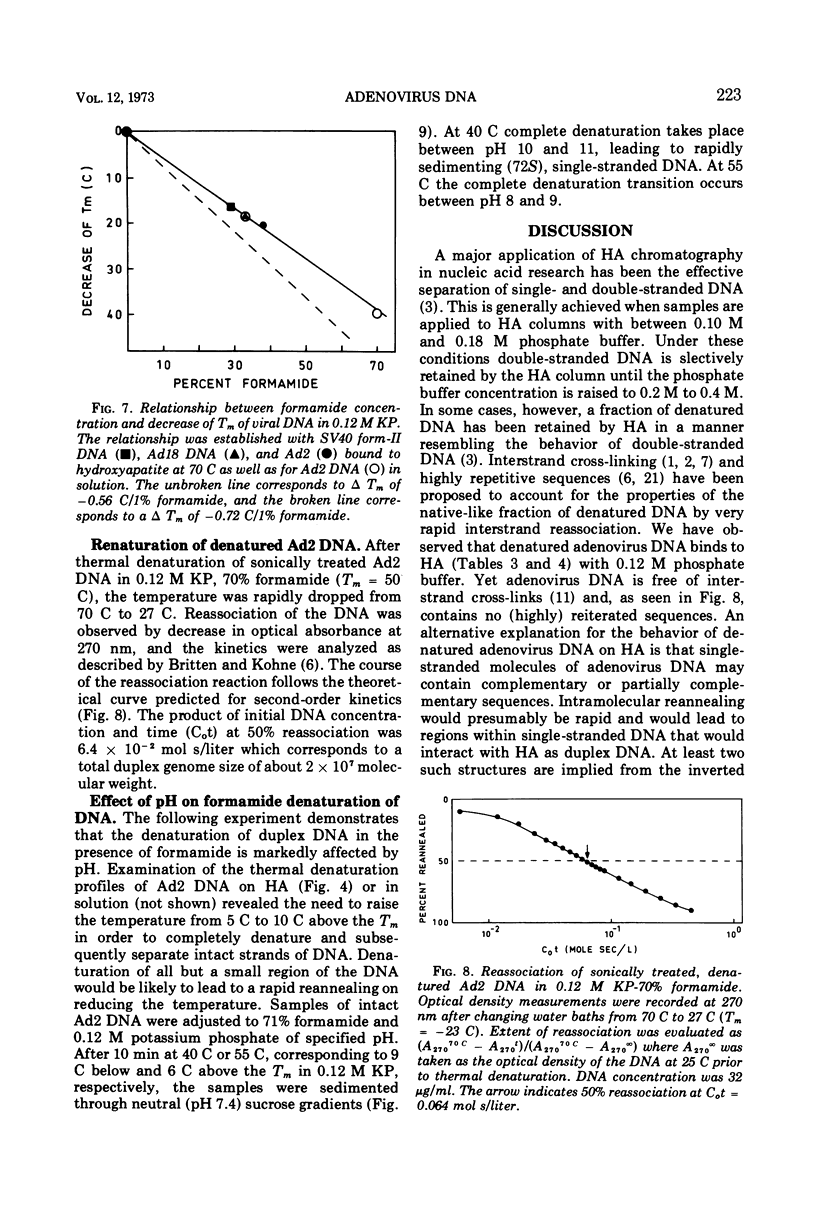

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M. Characterization of a naturally occurring, cross-linked fraction of DNA. II. Origin of the cross-linkage. J Mol Biol. 1968 Mar 14;32(2):405–421. doi: 10.1016/0022-2836(68)90018-1. [DOI] [PubMed] [Google Scholar]
- Alberts B. M., Doty P. Characterization of a naturally occurring, cross-linked fraction of DNA. 1. Nature of the cross-linkage. J Mol Biol. 1968 Mar 14;32(2):379–403. doi: 10.1016/0022-2836(68)90017-x. [DOI] [PubMed] [Google Scholar]
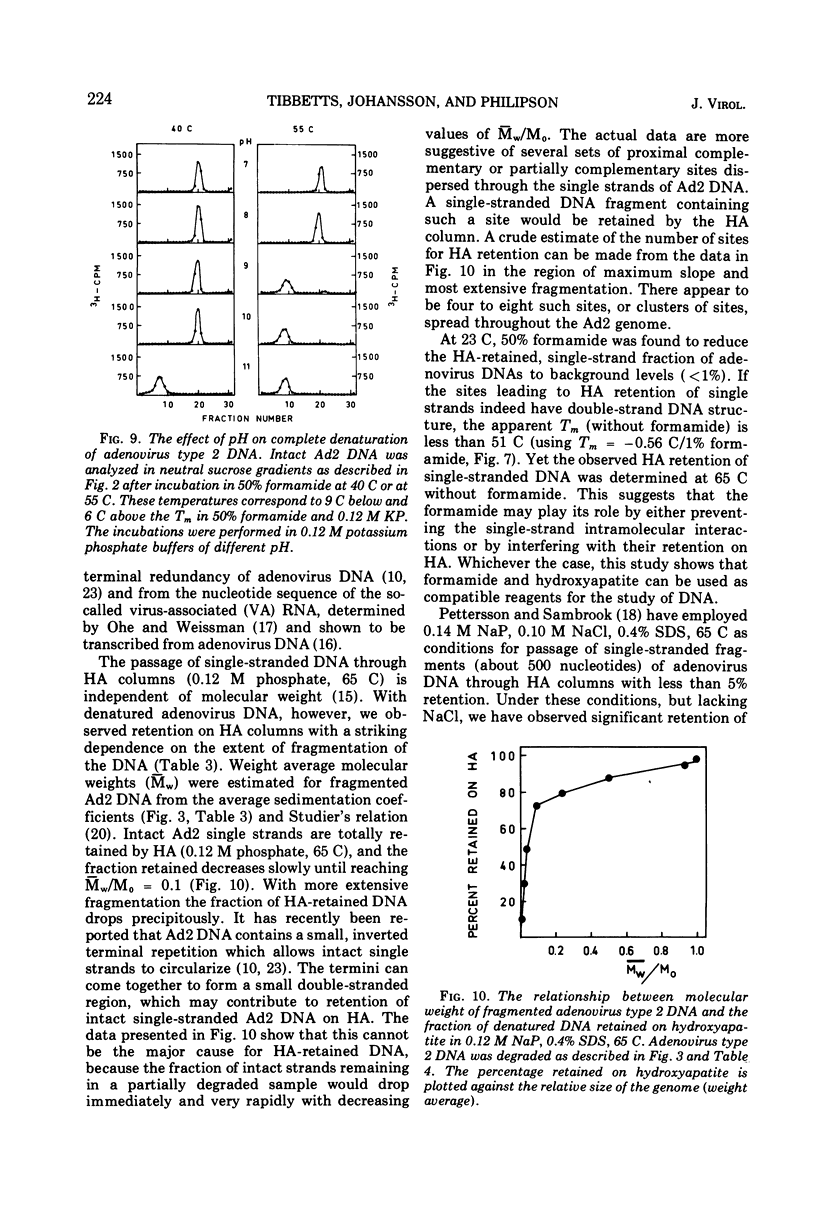
- Blüthmann H., Brück D., Hübner L., Schöffski A. Reassociation of nucleic acids in solutions containing formamide. Biochem Biophys Res Commun. 1973 Jan 4;50(1):91–97. doi: 10.1016/0006-291x(73)91068-1. [DOI] [PubMed] [Google Scholar]
- Bourgaux P., Bourgaux-Ramoisy D., Dulbecco R. The replication of the ring-shaped DNA of polyoma virus. I. Identification of the replicative intermediate. Proc Natl Acad Sci U S A. 1969 Oct;64(2):701–708. doi: 10.1073/pnas.64.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Chevallier M. R., Bernardi G. Residual transforming activity of denatured Haemophilus influenzae DNA. J Mol Biol. 1968 Mar 14;32(2):437–451. doi: 10.1016/0022-2836(68)90020-x. [DOI] [PubMed] [Google Scholar]
- Clayton D. A., Davis R. W., Vinograd J. Homology and structural relationships between the dimeric and monomeric circular forms of mitochondrial DNA from human leukemic leukocytes. J Mol Biol. 1970 Jan 28;47(2):137–153. doi: 10.1016/0022-2836(70)90335-9. [DOI] [PubMed] [Google Scholar]
- Garon C. F., Berry K. W., Rose J. A. A unique form of terminal redundancy in adenovirus DNA molecules. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2391–2395. doi: 10.1073/pnas.69.9.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R., Wensink P. C., MacHattie L. A., Thomas C. A., Jr Adenovirus DNA. I. Molecular weight and conformation. Proc Natl Acad Sci U S A. 1967 May;57(5):1302–1309. doi: 10.1073/pnas.57.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- MIYAZAWA Y., THOMAS C. A., Jr NUCLEOTIDE COMPOSITION OF SHORT SEGMENTS OF DNA MOLECULES. J Mol Biol. 1965 Feb;11:223–237. doi: 10.1016/s0022-2836(65)80053-5. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968 Sep;36(1):115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- McConaughy B. L., Laird C. D., McCarthy B. J. Nucleic acid reassociation in formamide. Biochemistry. 1969 Aug;8(8):3289–3295. doi: 10.1021/bi00836a024. [DOI] [PubMed] [Google Scholar]
- Ohe K. Virus-coded origin of a low molecular weight RNA from KB cells infected with adenovirus 2. Virology. 1972 Mar;47(3):726–733. doi: 10.1016/0042-6822(72)90562-4. [DOI] [PubMed] [Google Scholar]
- Ohe K., Weissman S. M. The nucleotide sequence of a low molecular weight ribonucleic acid from cells infected with adenovirus 2. J Biol Chem. 1971 Nov 25;246(22):6991–7009. [PubMed] [Google Scholar]
- Pettersson U., Sambrook J. Amount of viral DNA in the genome of cells transformed by adenovirus type 2. J Mol Biol. 1973 Jan;73(1):125–130. doi: 10.1016/0022-2836(73)90164-2. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Waring M., Britten R. J. Nucleotide sequence repetition: a rapidly reassociating fraction of mouse DNA. Science. 1966 Nov 11;154(3750):791–794. doi: 10.1126/science.154.3750.791. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Wolfson J., Dressler D. Adenovirus-2 DNA contains an inverted terminal repetition. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3054–3057. doi: 10.1073/pnas.69.10.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]


