Abstract
Based on regional-scale studies, aboveground production and litter decomposition are thought to positively covary, because they are driven by shared biotic and climatic factors. Until now we have been unable to test whether production and decomposition are generally coupled across climatically dissimilar regions, because we lacked replicated data collected within a single vegetation type across multiple regions, obfuscating the drivers and generality of the association between production and decomposition. Furthermore, our understanding of the relationships between production and decomposition rests heavily on separate meta-analyses of each response, because no studies have simultaneously measured production and the accumulation or decomposition of litter using consistent methods at globally relevant scales. Here, we use a multi-country grassland dataset collected using a standardized protocol to show that live plant biomass (an estimate of aboveground net primary production) and litter disappearance (represented by mass loss of aboveground litter) do not strongly covary. Live biomass and litter disappearance varied at different spatial scales. There was substantial variation in live biomass among continents, sites and plots whereas among continent differences accounted for most of the variation in litter disappearance rates. Although there were strong associations among aboveground biomass, litter disappearance and climatic factors in some regions (e.g. U.S. Great Plains), these relationships were inconsistent within and among the regions represented by this study. These results highlight the importance of replication among regions and continents when characterizing the correlations between ecosystem processes and interpreting their global-scale implications for carbon flux. We must exercise caution in parameterizing litter decomposition and aboveground production in future regional and global carbon models as their relationship is complex.
Introduction
It is a long-held tenet of ecosystem ecology that regional (i.e., areas bounded by sub-continental scale geographic features) variation in production and decomposition processes are positively correlated with both temperature and precipitation and hence, production and decomposition processes should be coupled at regional scales, e.g. [1]–[3]. This assumption is supported by recent meta-analyses and models that suggest climate strongly influences plant production and decomposition rates of terrestrial foliage [4]–[7]. Carbon cycling models (e.g., CENTURY model [8], [9]), motivated by such results, assume a coupling between net primary production (NPP) and litter loss, driven by parallel responses to temperature and precipitation. Given predicted scenarios of climate change, these carbon models predict significant changes to the way that biological systems influence atmospheric carbon dioxide concentrations [10], [11]. The degree of coupling will be particularly important for regions where live biomass and litter accumulation are not in equilibrium.
A challenge to understanding and quantifying the production-decomposition relationship is considering the covarying influence of other regulatory factors. Biotic drivers such as vegetation type, vegetation chemistry, and trophic interactions can also significantly affect rates of plant growth or organic matter decay, even within the same climatic region (e.g., [4], [12], [13], [6], [14], [15]. Because production and decomposition are rarely measured concurrently, and because these processes are often characterized across large spatial scales where vegetative type covaries with climate, the relative effects of biotic and climate drivers can be difficult to untangle [16], [17]. Further, abiotic drivers other than temperature and precipitation also influence plant growth and litter decomposition, including nutrient limitation [18]–[20] and UV degradation in semi-arid environments [21]. The net result is that climate impacts on production and decomposition, rather than being universal, could vary regionally depending on the relative strength of these other factors. Testing for regional variation in the relationship between production and decomposition is crucial to climate change research globally because it may require revisions to ecosystem response projections that inform Earth system models.
Here we test whether climate factors (precipitation, temperature, radiation), elevation, and latitude predict concurrent aboveground biomass (as an estimate of aboveground net primary production) and litter disappearance (as an estimate of litter decomposition) in grassland ecosystems worldwide. Recent global syntheses have shown that plant functional traits play a major role in influencing decomposition rates [6], so we examine drivers of aboveground biomass and litter disappearance within ecosystems dominated by herbaceous species (mainly members of the Poaceae family) to control for functional composition. We also focus on this biome because grasslands are globally important in terms of carbon pools, species diversity, and human livelihood. Grasslands cover approximately 30% of the Earth’s ice-free surface and are critical for supporting livestock and maintaining biodiversity [22]. Further, the relative rates of production and decomposition in this biome control soil carbon pools, and govern whether these systems are a carbon source or sink [23]–[26]. Thus, accurately parameterized models of grassland production and decomposition using such data will be useful in predicting potential feedbacks in grasslands under future climate scenarios.
Grassland aboveground biomass and litter loss may vary at small spatial scales (<1 km) due to species interactions such as plant species competition for resources, interactions with the microbial community, herbivore density, or soil and plant chemistry. These processes also may vary at larger regional or continental scales due to climatic and/or environmental factors. We hypothesized that aboveground biomass and litter disappearance are positively correlated at smaller plot and site scales because of similarities in species pools and abiotic conditions. Factors that could limit the amount of biomass production, e.g., low temperatures, radiation and precipitation, will also limit amount of loss through decomposition, thus making them positively correlated. Likewise, sites that have high biomass production should have high rates of loss. We also hypothesized that the greatest amount of variation in aboveground biomass and loss should be found at the regional or continental scale due to differences in climate.
Methods
Site Selection
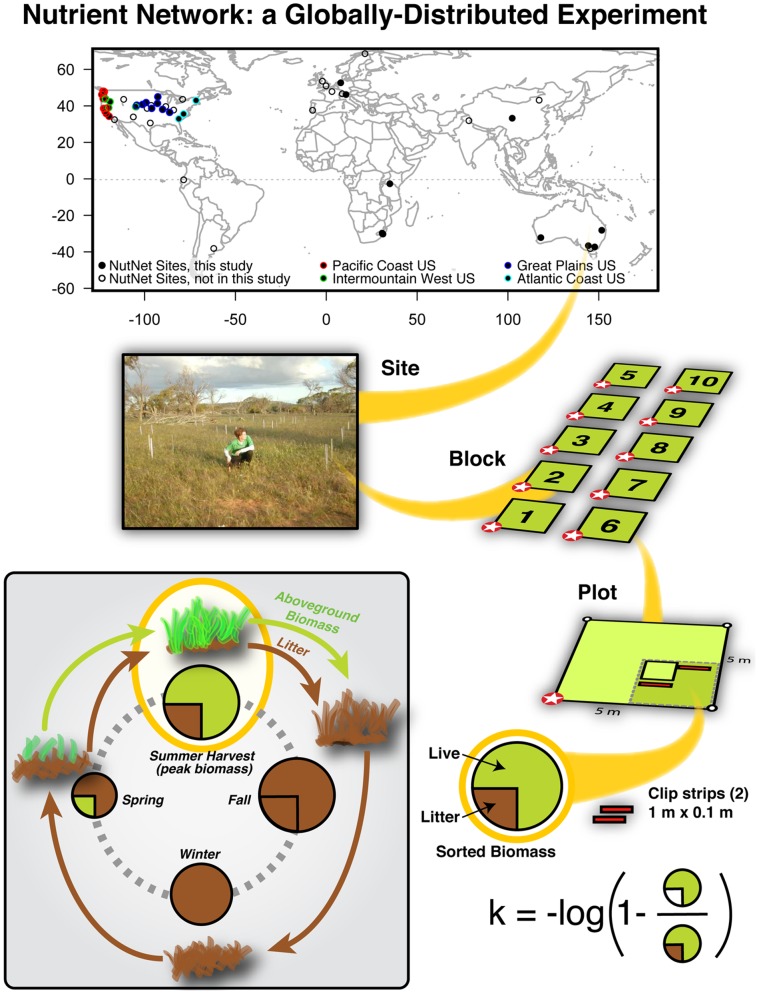
Our study included data from 39 sites that are part of the globally-distributed Nutrient Network (http://nutnet.org/). Access to study areas was negotiated by the lead scientist at each site. All sites are dominated by low-statured, primarily grassland vegetation. Each site selected for the study is relatively homogeneous (i.e., not encompassing large or obvious environmental gradients) and dominated by herbaceous vegetation, primarily Poaceae. Sites actively grazed by livestock or burned for management purposes were excluded from this study. Most sites sampled vegetation in 2007, but a subset sampled in 2008. The sites in this study range from 37.81°S to 53.99°N latitude, 250 to 2314 mm year−1 mean annual precipitation, 0 to 22.1°C mean annual temperature and 0.5 to 3500 m in elevation. Sites were located in Australia, Canada, China, Germany, South Africa, Switzerland, Tanzania and the United States (Table 1). We included some anthropogenic grassland sites (i.e. historically altered by humans via fire or clearing to create grass dominance), given the increasing prevalence of these grasslands globally [27]. There were no statistical differences between natural and anthropogenic grasslands for any of our measures (results not shown), so we include all sites as one dataset.
Table 1. Nutrient Network experimental sites.
| Site | Country | State | Region | Latitude | Longitude | Elevation (m) | MAP (mm) | MAT (C) |
| American Camp | USA | Washington | Pacific Coast | 48.47 | –123.01 | 41 | 672.4 | 9.8 |
| Azi | China | Gansu | Eurasia | 33.58 | 101.53 | 3500 | 620.0 | 0 |
| Barta Brothers | USA | Nebraska | Great Plains | 42.24 | −99.65 | 767 | 568.0 | 8.7 |
| Bogong | Australia | Victoria | Australia | −36.87 | 147.25 | 1760 | 1217.0 | 5.7 |
| Boulder | USA | Colorado | Great Plains | 39.97 | −105.23 | 1633 | 482.0 | 9.7 |
| Bunchgrass LTER | USA | Oregon | IM West | 44.28 | −122.26 | 1318 | 2160.0 | 5.5 |
| Burrawan | Australia | Queensland | Australia | 27.73 | 151.14 | 425 | 600.0 | 18.4 |
| Buttercup LTER | USA | Oregon | IM West | 44.28 | −121.96 | 1500 | 2160.0 | 5 |
| Cedar Creek LTER | USA | Minnesota | Great Plains | 45.40 | −93.20 | 270 | 800.0 | 6.3 |
| Cedar Point | USA | Nebraska | Great Plains | 41.20 | −101.63 | 965 | 470.0 | 9.3 |
| Chichaqua Bottoms | USA | Iowa | Great Plains | 41.79 | −93.39 | 275 | 891.0 | 9 |
| Cowichan | Canada | British Columbia | Pacific Coast | 48.46 | 123.38 | 50 | 1038.6 | 9.8 |
| Finley | USA | Oregon | Pacific Coast | 44.41 | −123.28 | 68 | 1200.0 | 11.3 |
| Glacial Heritage | USA | Washington | Pacific Coast | 46.87 | −123.03 | 33 | 1299.8 | 10.5 |
| Hall’s Prairie | USA | Kentucky | Great Plains | 36.96 | −86.73 | 194 | 1282.0 | 13.6 |
| Hanover | USA | New Hampshire | Atlantic Coast | 43.42 | −72.14 | 271 | 919.5 | 6.4 |
| Hart Mountain | USA | Oregon | IM West | 42.72 | −119.50 | 1508 | 304.8 | 7.4 |
| Hastings | USA | California | Pacific Coast | 36.20 | −121.55 | 750 | 550.0 | 10.9 |
| Hopland | USA | California | Pacific Coast | 39.00 | −123.07 | 417 | 939.8 | 12.3 |
| Jasper Ridge | USA | California | Pacific Coast | 37.41 | −122.24 | 120 | 655.0 | 13.8 |
| Kinypanial | Australia | Victoria | Australia | −36.20 | 143.75 | 90 | 395.0 | 15.5 |
| Konza Prairie | USA | Kansas | Great Plains | 39.08 | −96.58 | 440 | 835.0 | 12 |
| Leadbetter | USA | Washington | Pacific Coast | 46.61 | −124.05 | 2 | 2044.2 | 9.9 |
| Lookout LTER | USA | Oregon | IM West | 44.21 | −122.26 | 1500 | 2314.0 | 4.8 |
| Mclaughlin UCNRS | USA | California | Pacific Coast | 38.87 | −122.40 | 550 | 650.0 | 13.5 |
| Mount Caroline | Australia | W. Australia | Australia | −31.78 | 117.61 | 285 | 352.0 | 17.3 |
| Niwot LTER | USA | Colorado | IM West | 39.99 | −105.38 | 3050 | 930.0 | 6.4 |
| Papenburg | Germany | Lower Saxony | Europe | 53.09 | 7.47 | 0.5 | 850.1 | 8.9 |
| Sagehen Creek UCNRS | USA | California | IM West | 39.43 | −120.24 | 1920 | 850.0 | 5.7 |
| Savannah | USA | South Carolina | Atlantic Coast | 33.34 | 81.65 | 71 | 1000.0 | 17.3 |
| Sedgewick UCNRS | USA | California | Pacific Coast | 34.70 | −120.02 | 550 | 380.0 | 15 |
| Serengeti | Tanzania | NA | Africa | −2.25 | 34.51 | 1536 | 789.0 | 22.1 |
| Short−Grass LTER | USA | Colorado | Great Plains | 40.82 | −104.77 | 1650 | 341.7 | 8.4 |
| Sierra Foothills | USA | California | Pacific Coast | 39.29 | −121.34 | 333 | 711.2 | 15.6 |
| Smith Prairie | USA | Washington | Pacific Coast | 48.21 | −122.62 | 62 | 549.9 | 9.8 |
| Tyson | USA | Missouri | Great Plains | 38.52 | 90.56 | 169 | 1090.0 | 12.5 |
| Ukulinga | South Africa | KwaZulu-Natal | Africa | −29.67 | 30.4 | 843 | 838.0 | 18.1 |
| UNC-Duke | USA | North Carolina | Atlantic Coast | 35.91 | −79.06 | 141 | 1210.0 | 14.7 |
| Val Mustair | Switzerland | NA | Europe | 46.63 | 10.37 | 2329 | 950.0 | 0.3 |
Note: IM West = Intermountain West. Complete site names can be found at: www.nutnet.umn.edu/field_sites.
Aboveground Biomass and Litter
The standard Nutrient Network sampling protocol was followed at all sites. Plots were 5×5 m. The majority (33 of 39) of sites sampled 3 blocks of 10 plots per block; although 1 site had 1 block, 1 had 2, 1 had 4, 2 had 5, and 1 had 6. There was a 1 m buffer between each plot. Aboveground live biomass and litter were collected in each plot from a randomly selected 0.2 m2 (10×200 cm) strip at peak biomass (Figure 1). For sites exhibiting biphasic seasonal growth patterns, biomass was collected and summed for both peak periods. Aboveground live biomass of individual plants rooted within the strip was clipped at ground level, and all litter standing stock also was collected. For plots with shrubs and subshrubs rooted within the strip, leaves and current year’s woody growth were collected. All biomass was dried to a constant mass at 60°C and weighed to the nearest 0.01 g. In these herbaceous ecosystems with minimal perennial aboveground organs, aboveground biomass provides an estimate of aboveground net primary production (ANPP), although the estimate may be slightly lower than the true value of ANPP because of tissue turnover during the growing season [28].
Figure 1. The Nutrient Network is a globally-distributed experiment testing top-down and bottom-up controls over grassland diversity and ecosystem function.
Our nested hierarchical analysis quantified variability for aboveground biomass and litter disappearance for 39 sites among continents, regions (i.e., among sites in the continental US, shown as filled points with colored circles), sites, blocks within sites (each with 1–6 blocks of 8–10 plots per block), and plots within blocks. Aboveground biomass was sampled using identical protocols within a subplot of each plot and sorted to live (current year’s production) and litter (previous years’ production). Litter disappearance represents an estimate of the log-transformed fraction of the previous year’s total above ground biomass (live plus dead) that is remaining at the end of the subsequent growing season (litter biomass divided by total biomass) using Olson’s equation. The inset figure illustrates the fate of biomass over one growing season: Current year’s production (green) at end of growing season (Fall) senesces and combines with previous years’ production (brown); total litter biomass decays over time (indicated by decreasing size of circle); new production (green) in Spring increases while remaining litter continues to decrease; peak biomass along with remaining litter is harvested at the end of Summer and used to estimate litter disappearance rate (k = −log(litter/total) ).
Litter disappearance is a metric used to estimate the amount of litter lost via decomposition and herbivory among growing seasons. This metric is a commonly used tool in estimating loss [29] in grassland studies [30], [31]. Because it derives from the sampling of aboveground biomass, it is a relatively easy measure allowing for high replication not possible with litter bags. It also captures the potential influence of UV-mediated decomposition on aboveground litter that is increasingly recognized as an important factor in grasslands but cannot be accurately measured by litter bags (bag material shields litter from direct radiation).
Litter disappearance estimates (k) were calculated using an equation derived from Olson [32] for deciduous forest decay rates:
where live biomass is the standing stock during peak season and total biomass is live biomass plus litter collected at the same time (Figure 1). Although our experimental system is not a forested system as modeled in Olson’s paper, both are deciduous with annual biomass contributions to the litter pool.
Temperature, Precipitation and Radiation Estimates
Precipitation and temperature data were generated from the WorldClim database [33]. We used four measures for each site (1 km2 scale resolution): mean annual temperature (MAT), mean annual precipitation (MAP), maximum summer temperature, and minimum winter temperature. The last two measures provide an estimate of temperature range at each site, given that both mean and variation in climate are known to affect growth and decomposition [34]. It is difficult to assess causation in observational data when there is strong covariance among the explanatory variables. In our case, climate variables were only weakly covarying with the exception of MAT and the derived minimum winter temperature where some degree of relationship would be expected. We derived a coefficient of variation from 10 years of precipitation data. Without commensurate biomass data, however, the analysis of interannual variability relationships was not possible.
Radiation data were generated from the NASA surface meteorology and solar energy release 6.0 data set (http://eosweb.larc.nasa.gov/sse/). A mean annual radiation was calculated for each site by integrating daily surface measurements (kWh/m2/day) over a 20-year period on a 1×1 degree grid.
Statistical Methods
The relationship between aboveground biomass and litter disappearance was analyzed using a linear regression analysis both at the plot and site scale. We quantified variability for aboveground biomass and litter disappearance using variance component analyses in which continent, region, site, block, and plot were considered as nested random effects [35], [36]. We used a multiple linear regression to analyze the relationship between dependent (aboveground biomass, litter and litter disappearance) and independent variables (latitude, elevation, radiation, mean annual precipitation, mean annual temperature, mean minimum winter temperature and mean maximum summer temperature) at the site level. First order interactions between terms were also analyzed but no significance was found and interactions are not included in the results. A suite of non-linear relationships between independent and dependent variables were also explored using Eureqa [37] but no significant relationships were found and were not included in the results. In addition to the site-wide comparisons, the North American sites were divided into four regions based on the location of large mountain ranges (Pacific Coast, Intermountain West, Central, and Atlantic Coast). We also examined these relationships within three regions of the United States with sufficient replication for comparisons. All analyses were conducted using R version 2.8.0 [38].
Results
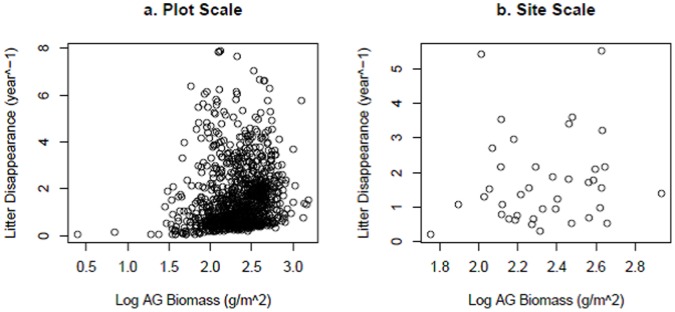
Site scale biomass ranged from 61.5 g/m2 (Savannah River, Georgia, USA) to 917.8 g/m2 (Papenburg, Germany), and standing litter between sites ranged from 0.7 g/m2 (Mt. Caroline, Australia) to 689.6 g/m2 (Leadbetter, Washington, USA). Site scale litter disappearance ranged from 0.19 yr−1 (Savannah River, Georgia, USA) to 5.52 yr−1 (Ukulinga, South Africa), representing a larger range than for decomposition in North American grasslands (0.28 yr−1 to 1.73 yr−1 [39]). Aboveground biomass and litter disappearance showed a very weak positive relationship at the plot scale (p<0.0001, r2 = 0.02; Figure 2a) but were not related when compared at the site scale (p = 0.61, r2 = 0.01; Figure 2b).
Figure 2. Aboveground (AG) biomass and litter disappearance were weakly correlated at the plot scale (a; p<0.0001, r2 = 0.02) but not correlated at the site scale (b; p = 0.61, r2 = 0.01).
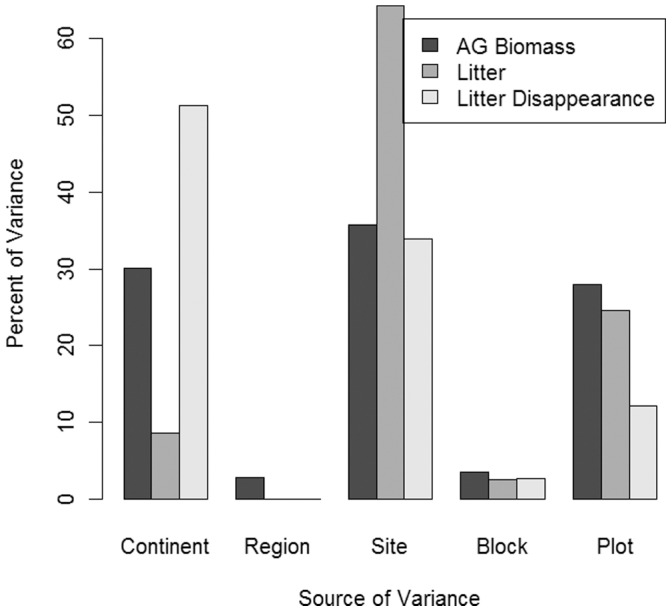
Counter to our expectations, there were no strong correlations between site-level averages of aboveground biomass, litter, or litter disappearance and most climate variables (Table 2) at the site scale. Although there were some significant relationships (live biomass with radiation and latitude), the correlation coefficients were small, suggesting that climate variables are relatively poor predictors of aboveground biomass and loss across global scales. For example, radiation and latitude were correlated with biomass production across sites but were not correlated with litter or litter disappearance (Table 2). Litter disappearance and aboveground biomass also varied at different spatial scales (Figure 3); litter disappearance was strongly variable among continents, whereas variation in aboveground biomass was more evenly distributed across plots, sites and continents.
Table 2. Backwards selected multiple linear regression results for site-level live biomass model (R2 = 0.34, p<0.01).
| Variable | Coefficient | Error | t | p |
| Radiation | −0.298 | 0.103 | −2.89 | 0.01 |
| Latitude | −0.022 | 0.010 | −2.26 | 0.03 |
| Elevation | – | – | – | – |
| Max. High 1 | – | – | – | – |
| Min. Low 2 | – | – | – | – |
| MAT 3 | – | – | – | – |
| MAP 4 | – | – | – | – |
Maximum high temperature,
Minimum low temperature,
Mean annual temperature,
Mean annual precipitation.
– indicates non-significant terms and thus are not included in the final model or reported here. Note: Multiple linear regression analyses for litter and decomposition with climate variables were insignificant and not included in table.
Figure 3. Variance components for site scale aboveground biomass, litter stocks, and litter disappearance.
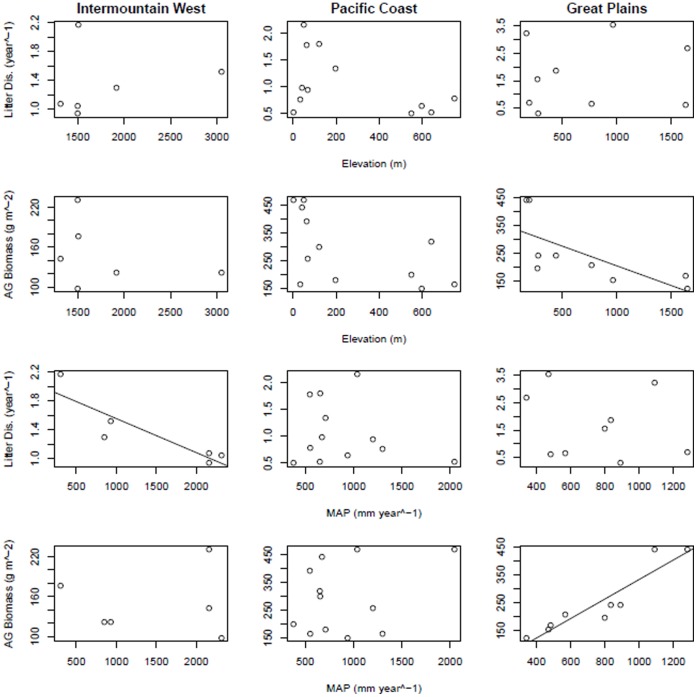
Previous studies have found strong relationships among productivity, decomposition, and biophysical factors (e.g., precipitation, soil chemistry) within regions (e.g., U.S. Great Plains [40], [41]), so we examined relationships among productivity, litter and climate factors within three regions with sufficient replication in the U.S., Pacific Coast (n = 12), Intermountain West (n = 6), and Great Plains (n = 9). We found a significant negative correlation between litter disappearance and mean annual precipitation (r2 = 0.71, p = 0.01) for the Intermountain West region (Figure 4). Sites in the Great Plains showed a positive relationship between aboveground biomass and precipitation (r2 = 0.85, p<0.001) and a negative relationship between aboveground biomass and elevation (r2 = 0.40, p = 0.02), although the strength of the latter relationship was much weaker (Figure 4).
Figure 4. Site scale correlations between litter disappearance (Litter Dis.), aboveground biomass (AG Biomass), and physical variables (elevation and mean annual precipitation (MAP)) within three U.S. regions, Intermountain West, Pacific Coast, and Great Plains.
Significant relationships are depicted by correlation lines; Intermountain West litter disappearance and precipitation (p = 0.02, r2 = 0.74), Great Plains aboveground biomass and elevation (p = 0.03, r2 = 0.44) and Great Plains aboveground biomass and mean annual precipitation (p<0.001, r2 = 0.84).
Discussion
In contrast to more commonly held perspectives that aboveground biomass production and decomposition processes should be positively correlated [3], we found inconsistent site-scale correlations between aboveground biomass and loss. Aboveground biomass, litter stocks, and litter disappearance varied depending on spatial scales, with aboveground biomass varying similarly at plot, site and continent scales, litter varying strongly among sites and litter disappearance varying strongly among continents. These results do not call into question the fundamental importance of temperature and precipitation for primary production or microbial decomposition, but rather indicate that their relative influences may vary, possibly due to differences in seasonality (e.g., temperate vs. Mediterranean), interannual variability, and the strength of feedbacks between climate and factors including vegetation quality (e.g., [4]. [6]), herbivory (e.g., [14]), UV degradation (e.g., [40], [21]), or nutrient cycling (e.g., [42], [18], [20]).
Regional-scale analyses of grassland processes have found strong relationships between productivity, decomposition, and climatic variables (e.g., [40], [41], [43]), but we found the relative intensity of these relationships can vary across grassland biomes. These previous studies were concentrated in the Great Plains region of the United States, and have served as the basis for assumptions of the generality of regional-scale coupling among these factors (e.g., [44], [3]). Our data from this same region confirm a strong, positive relationship between aboveground biomass and mean annual precipitation. In other regions of the planet, however, there were substantial deviations. Similar regional-scale discrepancies have been reported previously in research on climate influences on net primary production. Knapp and Smith [34] reported no generalizable trend between variability in rainfall and production in 11 LTER sites in North America, but a broad-scale analysis of the same relationship in China found these factors to be tightly linked [45]. Our results demonstrate that aboveground biomass and litter disappearance do not necessarily covary nor are they always similarly controlled by climatic influences. Our results underscore the need for replication among regions and continents when characterizing live biomass-litter relationships, including their implication for global-scale carbon flux models.
While aboveground biomass and litter disappearance both varied at the site scale, the spatial scale of their variation was uncoupled at larger (e.g., continent) and smaller (e.g., plot) spatial scales. Further, while litter disappearance varied among sites and continents, it was not well-predicted by climate variables, suggesting that across widely distributed sites, neither process can be accurately predicted by regional climate. This is in contrast to the relationships found in previous studies between biomass production or decomposition rates (k values) and geographic and climatic factors, a discrepancy explained by the wider scope of our study and our simultaneous measurement of both factors (e.g., [40], [46], [47], [7], [49], [48], [41]). One implication is that, at a global scale, temperature alone may not always accelerate the release of litter carbon to the atmosphere via decomposition, which has been a predicted effect of global warming [50]. Again, this does not contradict the fundamental importance of temperature in influencing decomposition, but suggests the impact of global temperature increases may vary regionally depending on the relative importance of other factors.
Radiation and latitude appear to influence the amount of biomass production at the site scale but were not related to the amount of litter or decomposition. This decoupling between production and decomposition processes is reinforced by the difference in spatial scales at which each process varies, pointing to likely drivers. The large-scale variation of decomposition is concordant with previous work showing decomposition as a function of temperature (although effects of temperature on organic matter can vary depending on quality, microbial community and enzymatic influences [51], soil moisture [52], leaf litter chemistry [53], [28], [29], [5], [6], actual evapotranspiration [1], leaf litter lignin [31] and microbial activity [54], all of which vary strongly among regions and continents. Because we focus on aboveground litter disappearance as a measure of decomposition, the relevance of these findings to belowground processes remains to be tested. In general, consistency in rates of decomposition between roots and shoots tends to depend on relative levels of recalcitrant carbon compounds and/or nutrients in the two tissue types; in some cases they are concordant [55], [5], whereas in other cases, roots tend to be more decay-resistant [4], [56]. For aboveground biomass, variability was evident among plots, sites and continents. This suggests that, in some regions, local factors such as small-scale variation in water or nutrient variability, species composition, herbivory or diversity [57]–[59] may constrain biomass production more than climatic factors.
There is increasing need for effective predictions of carbon cycle responses in grasslands, as mediated by production and decomposition, because of the importance of this biome to carbon pools, species diversity, and human livelihood. This is challenging because of the regional variation in projected shifts in temperature and precipitation associated with climate change [60]. Although carbon cycling models (e.g., CENTURY model [8], [9]) assume that net primary production and decomposition are coupled via parallel responses to climatic factors, our results demonstrate that the relationship of these processes with climate can differ by region, and the dominant spatial scales of variation differ for grassland production and decomposition. While the CENTURY model was developed for the US Great Plains [8], [9], our empirical results suggest that effective long-term predictions of carbon flux will require a careful consideration of production and decomposition and should be applied with caution to other areas of the globe. In particular, carbon flux models that are regionally parameterized with flexible terms describing the independent strength and direction of production and decomposition with temperature and precipitation are likely to improve predictions of carbon dynamics in this globally important ecosystem.
Our study provides a succinct comparison of important herbaceous ecosystem functions: biomass production and litter loss across many geographical regions. Provided sufficient funding and spatial replication between sites, future studies over multiple growing seasons will contribute to this growing understanding of global divers in these systems. Future data from multiple years will allow us to capture interannual variability, an important component of herbaceous system carbon dynamics, not reflected in this dataset.Furthermore, a more comprehensive examination of nutrient and light availability and use in the context of biomass and litter measurements across grasslands worldwide will further explain global patterns in grassland carbon dynamics.
Acknowledgments
This work was generated using data from the Nutrient Network collaborative experiment. We thank the Minnesota Supercomputer Institute for hosting project data and the Institute on the Environment for hosting Network meetings. The authors also thank Dr. Eric Lind (University of Minnesota) for his valuable work on data synthesis.
Funding Statement
This work was generated using data from the Nutrient Network collaborative experiment, funded at the site-scale by individual researchers and coordinated through Research Coordination Network funding from National Science Foundation (NSF) to ETB and EWS (NSF-DEB-1042132). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Meentemeyer V (1978) Macroclimate and lignin control of litter decomposition rates. Ecology 59: 465–472. [Google Scholar]
- 2.Swift MJ, Heal OW, Anderson JM (1979) Decomposition in Terrestrial Ecosystems. University of California Press, Berkeley, California, USA.
- 3.Chapin III, FS, Matson P, Mooney HA (2002) Principles of Terrestrial Ecosystem Ecology. Springer-Verlag, New York, USA.
- 4. Gholz HL, Wedin DA, Smitherman SM, Harmon ME, Parton WJ (2000) Long-term dynamics of pine and hardwood litter decomposition in contrasting environments: toward a global model of decomposition. Global Change Biology 6: 751–765. [Google Scholar]
- 5. Adair EC, Parton WJ, Del Grosso SJ, Silver WL, Harmon ME, et al. (2008) A simple three pool model accurately describes patterns of long-term litter decomposition in diverse climates. Global Change Biology 14: 2636–2660. [Google Scholar]
- 6. Cornwell WK, Cornelissen JHC, Amatangelo, Dorrepaal EK, Eviner VT, et al. (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecology Letters 11: 1065–1071. [DOI] [PubMed] [Google Scholar]
- 7. Del Grosso SJ, Parton WJ, Stohlgren T, Zheng D, Bachelet D, et al. (2008) Global Net Primary Production Predicted from Vegetation Class, Precipitation, and Temperature. Ecology 89: 2117–2126. [DOI] [PubMed] [Google Scholar]
- 8. Parton WJ, Schimel DS, Cole CV, Ojima DS (1987) Analysis of factors controlling soil organic matter levels in Great Plains grasslands. Soil Science Society of America Journal 51: 1173–1179. [Google Scholar]
- 9.Metherall AK, Harding LA, Cole CV, Parton WJ (1993) CENTURY Soil Organic Matter Model Environment Technical Documentation, Agroecosystem Version 4.0, Great Plains System Research Unit, Technical Report No. 4. USDA-ARS, Ft. Collins, Colorado, USA.
- 10. Kirschbaum MU (1995) The temperature dependence of soil organic matter decomposition and the effect of global warming on soil organic C storage. Soil Biology and Biochemistry 27: 753–760. [Google Scholar]
- 11. Aerts R (2006) The freezer defrosting: global warming and litter decomposition rates in cold biomes. Journal of Ecology 94: 713–724. [Google Scholar]
- 12. Frank DA, Kuns MM, Guido DR (2002) Consumer control of grassland plant production. Ecology 83: 602–606. [Google Scholar]
- 13. Derner JD, Schuman GE (2007) Carbon sequestration and rangelands: A synthesis of land management and precipitation effects. Journal of Soil and Water Conservation 62: 77–85. [Google Scholar]
- 14. Bagchi S, Ritchie ME (2010) Herbivore effects on above- and belowground plant production and soil nitrogen availability in the Trans-Himalayan shrub-steppes. Oecologia 164: 1075–1082. [DOI] [PubMed] [Google Scholar]
- 15.Ziter C, MacDougall AS (2013) Nutrients and defoliation increase soil carbon inputs in grassland. Ecology http://dx.doi.org/10.1890/11-2070.1. In press. [DOI] [PubMed]
- 16. Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79: 439–449. [Google Scholar]
- 17. Silver WL, Miya RK (2001) Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129: 407–419. [DOI] [PubMed] [Google Scholar]
- 18. Hobbie SE, Vitousek PM (2000) Nutrient limitation of decomposition in Hawaiian forests. Ecology 81: 1867–1877. [Google Scholar]
- 19. Parton W, Silver WL, Burke IC, Grassens L, Harmon M, et al. (2007) Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315: 361–364. [DOI] [PubMed] [Google Scholar]
- 20. LeBauer DS, Treseder KK (2008) Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89: 371–379. [DOI] [PubMed] [Google Scholar]
- 21. Austin A, Vivanco L (2006) Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 442: 555–558. [DOI] [PubMed] [Google Scholar]
- 22.White R, Muray S, Rohweder M (2000) Pilot Analysis of Global Ecosystems: Grassland Ecosystems Technical Report. World Resources Institute, Washington, DC.
- 23. Kern JS, Johnson MG (1993) Conservation tillage impacts on national soil and atmospheric carbon levels. Soil Science Society of America Journal 53: 200–210. [Google Scholar]
- 24. Conant RT, Paustian K, Elliott ET (2001) Grassland management and conversion into grassland: Effects on soil carbon. Ecological Applications 11: 343–355. [Google Scholar]
- 25. Guo L, Gifford R (2002) Soil carbon stocks and land use change: a meta analysis. Global Change Biology 8: 345–360. [Google Scholar]
- 26. Lal R (2004) Soil carbon sequestration impacts on global climate change and food security. Science 304: 1623–1627. [DOI] [PubMed] [Google Scholar]
- 27. Hobbs RJ, Arico S, Aronson J, Baron JS, Bridgewater P, et al. (2006) Novel ecosystems: theoretical and management aspects of the new ecological world order. Global Ecology and Biogeography 15: 1–7. [Google Scholar]
- 28. Scurlock JMO, Johnson K, Olson RJ (2002) Estimating net primary productivity from grassland biomass dynamics measurements. Global Change Biology 8: 736–753. [Google Scholar]
- 29. Holdsworth A, Frelich LE, Reich PB (2012) Leaf litter disappearance in earthworm-invaded northern hardwood forests: role of tree species and the chemistry and diversity of litter. Ecosystems 15(6): 913–926. [Google Scholar]
- 30.McLauchlan KK, Hobbie SE, Post WM (2006) Conversion from agriculture to grassland builds soil organic matter on decadal timescales. Ecological Applications16: 1, 143–153. [DOI] [PubMed]
- 31. Fortunel C, Garnier E, Joffre R, Kazakou E, Quested H, et al. (2009) Leaf traits capture the effects of land use changes and climate on litter decomposability of grasslands across Europe. Ecology 90: 598–611. [DOI] [PubMed] [Google Scholar]
- 32. Olson J (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44: 322–331. [Google Scholar]
- 33. Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965–1978. [Google Scholar]
- 34. Knapp AK, Smith MD (2001) Variation among biomes in temporal dynamics of aboveground primary production. Science 291: 481–484. [DOI] [PubMed] [Google Scholar]
- 35.Pinheiro JC, Bates DM (2000) Mixed Effects Models in S and S-Plus. Springer-Verlag New York Inc., New York, USA.
- 36.Crawley M (2007) The R Book. John Wiley & Sons Ltd, West Sussex, England.
- 37. Schmidt M, Lipson H (2009) Distilling free-form natural laws from experimental data. Science 324: 81–85. [DOI] [PubMed] [Google Scholar]
- 38.R Development Core Team. (2010) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3–900051–07–0, URL http://www.R-project.org.
- 39.Risser PG, Birnsy EC, Blocker HD, May SW, Parton WS, et al. (1981) The True Prairie Ecosystem. Hutchinson Ross Publishing Company, Stroudsburg, Pennsylvania, USA.
- 40. Sala OE, Parton WJ, Joyce LA, Lauenroth WK (1988) Primary production of the central grassland region of the United States. Ecology 69: 40–45. [Google Scholar]
- 41. Epstein H, Buke IC, Lauenroth WK (2002) Regional patterns of decomposition and primary production rates in the U.S. Great Plains. Ecology 83: 320–327. [Google Scholar]
- 42. Parton WJ, Scurlock JMO, Ojima DS, Gilmanov TG, Scholes RJ, et al. (1993) Observations and modeling of biomass and soil organic matter dynamics for the grassland biome worldwide. Global Biogeochemical Cycles7: 785–809. [Google Scholar]
- 43. McCulley RL, Burke IC, Nelson JA, Lauenroth WK, Knapp AK, et al. (2005) Regional patterns in carbon cycling across the Great Plains of North America. Ecosystems 8: 106–121. [Google Scholar]
- 44.Burke IC, Lauenroth WK, Milchunas D (1997) Biogeochemisty of managed grasslands in Central North America. In: Paul EA, Paustian K, Elliott ET and Cole CV (eds.) Soil organic matter in temperate agroecosystems: long-term experiments in North America, 85–102. CRC Press, Boca Raton, Florida, USA.
- 45. Fang J, Piao S, Tang Z, Peng C, Ji W (2001) Interannual variability in net primary production and precipitation. Science 293: 1723. [DOI] [PubMed] [Google Scholar]
- 46. Noy-Meir I (1973) Desert ecosystems: environment and producers. Annual Review of Ecology and Systematics 4: 23–51. [Google Scholar]
- 47.Lauenroth WK (1979) Grassland primary production: North American grasslands in perspective. In: French NR (ed.) Perspectives in grassland ecology. Ecological studies, 3–24.Springer-Verlag, New York, NY, USA.
- 48. Zhang D, Hui D, Luo Y, Zhou G (2008) Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. Journal of Plant Ecology 1: 85–93. [Google Scholar]
- 49. Wolkovich EM, Lipson DA, Virginia RA, Cottingham KL, Bolger DT (2009) Grass invasion causes rapid increases in ecosystem carbon and nitrogen storage in a semiarid shrubland. Global Change Biology 16: 1351–1365. [Google Scholar]
- 50. Niklinska M, Maryanski M, Laskowski R (1999) Effect of temperature on humus respiration rate and nitrogen mineralization: Implication for global climate change. Biochemistry 44: 239–257. [Google Scholar]
- 51. Conant RT, Ryan MG, Ågren GI, Birge HE, Davidson EA, et al. (2011) Temperature and soil organic matter decomposition rates – synthesis of current knowledge and a way forward. Global Change Biology 17: 3392–3404. [Google Scholar]
- 52. Liski J, Nissinen A, Erhard M, Taskinen O (2003) Climatic effects on litter decomposition from arctic tundra to tropical forest. Global Change Biology 9: 575–584. [Google Scholar]
- 53. Hobbie SE (1996) Temperature and plant species control over litter decomposition in Alaskan tundra. Ecological Monographs 66: 503–522. [Google Scholar]
- 54.Dormaar JF (1992) Decomposition as a process in natural grasslands. In: Coupland RT (ed) Ecosystems of the world: natural grasslands: introduction and western hemisphere, 121–136. Elsevier, Amsterdam.
- 55. Chen H, Harmon ME, Griffiths RP (2001) Decomposition and nitrogen release from decomposing woody roots in coniferous forests of the Pacific Northwest: a chronosequence approach. Canadian Journal of Forest Research 31: 246–253. [Google Scholar]
- 56. Hobbie SE, Eissenstat D, Oleksyn J, Reich PB (2010) Fine root decomposition rates do not mirror those of leaf litter among temperate tree species. Oecologia 162: 505–513. [DOI] [PubMed] [Google Scholar]
- 57. Harpole WS, Tilman D (2007) Grassland species loss due to reduced niche dimension. Nature 446: 791–793. [DOI] [PubMed] [Google Scholar]
- 58. Cardinale BJ, Hillebrand H, Harpole WS, Gross K, Ptacnik R (2009) Separating the influence of resource ‘availability’ from resource; ‘imbalance’ on productivity-diversity relationships. Ecology Letters 12: 475–487. [DOI] [PubMed] [Google Scholar]
- 59. Harpole S, Ngai JT, Cleland EE, Seabloom EW, Borer ET, et al. (2011) Nutrient co-limitation of primary producer communities. Ecology Letters 14: 852–862. [DOI] [PubMed] [Google Scholar]
- 60.Christensen JH, Hewitson B, Busuioc A, Chen A, Gao X, et al. (2007) Regional Climate Projections In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M and Miller HL (eds) Climate change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.