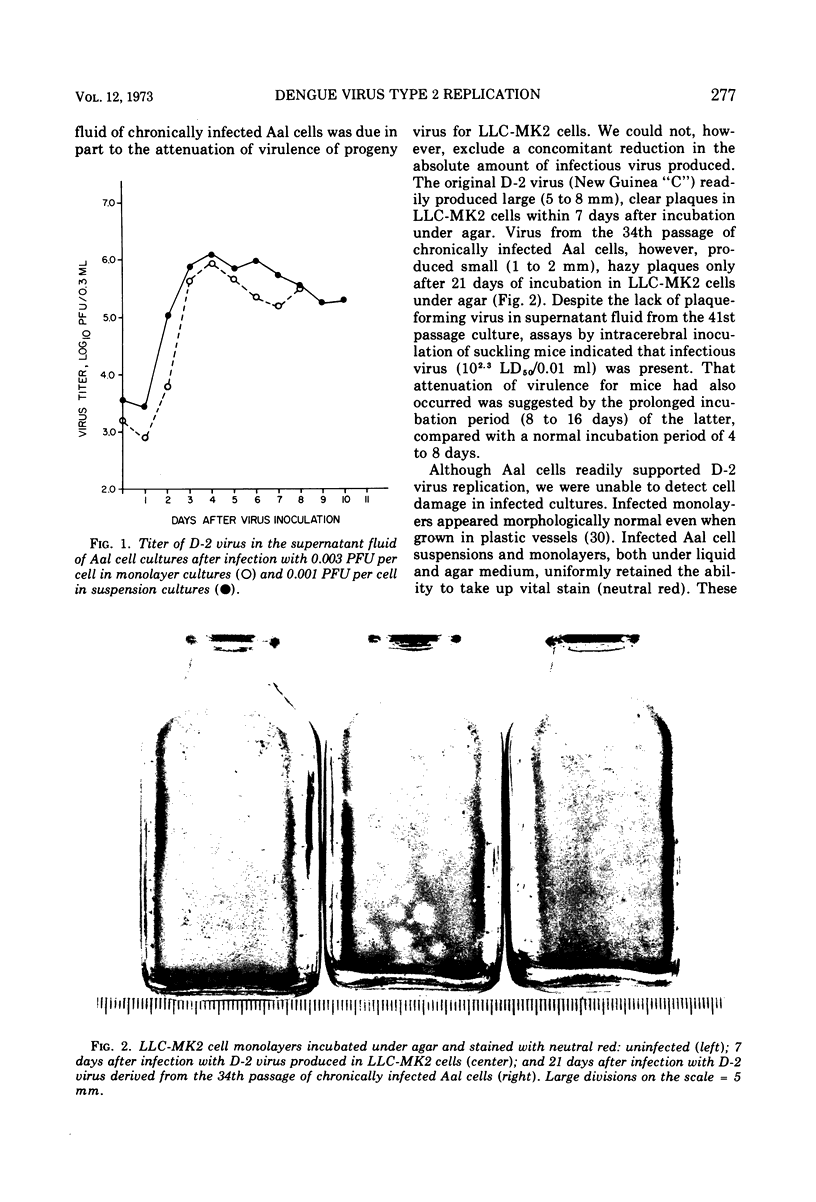
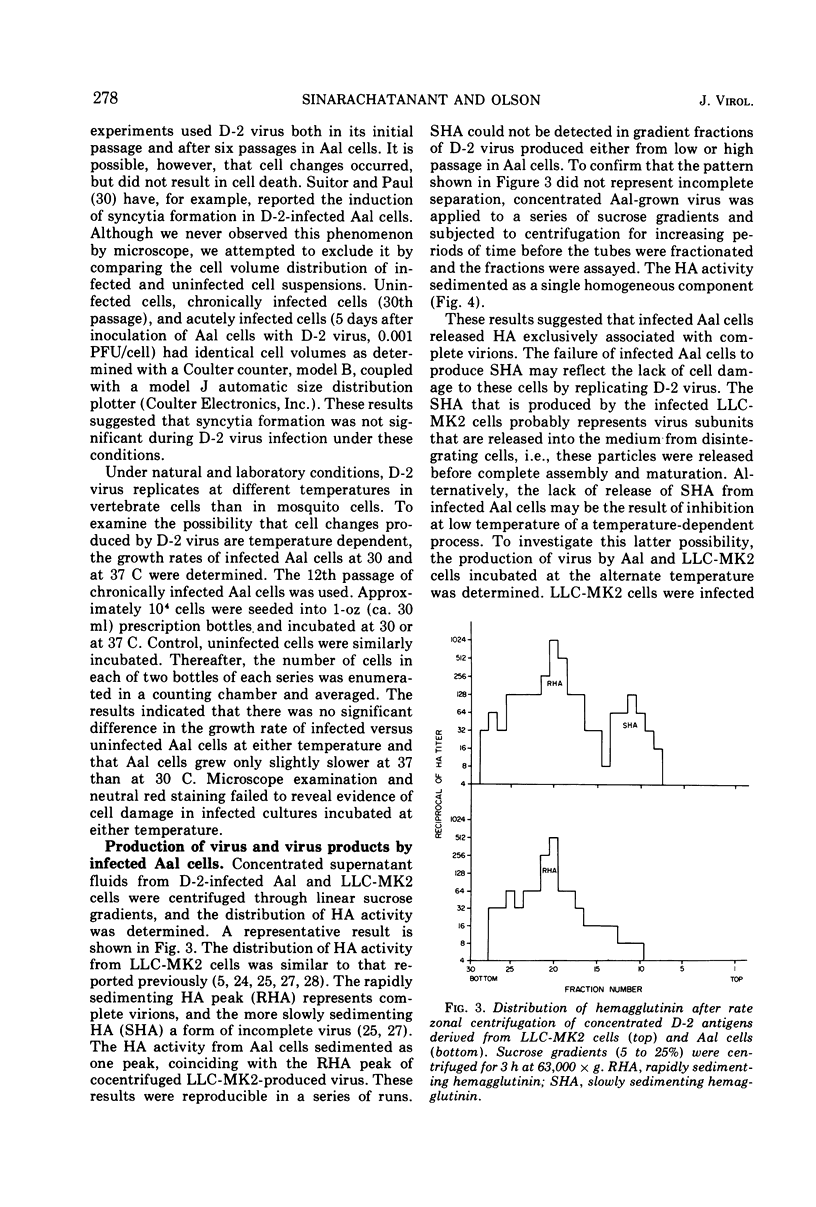
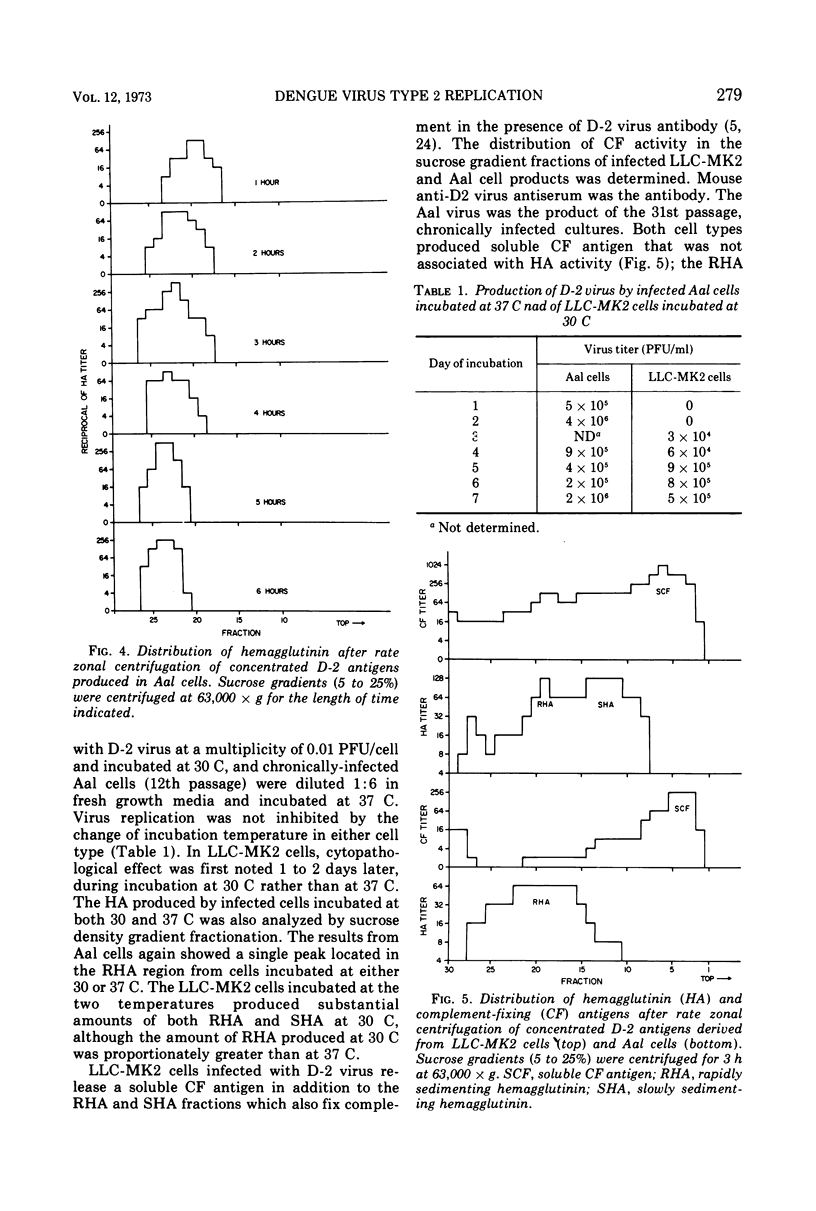
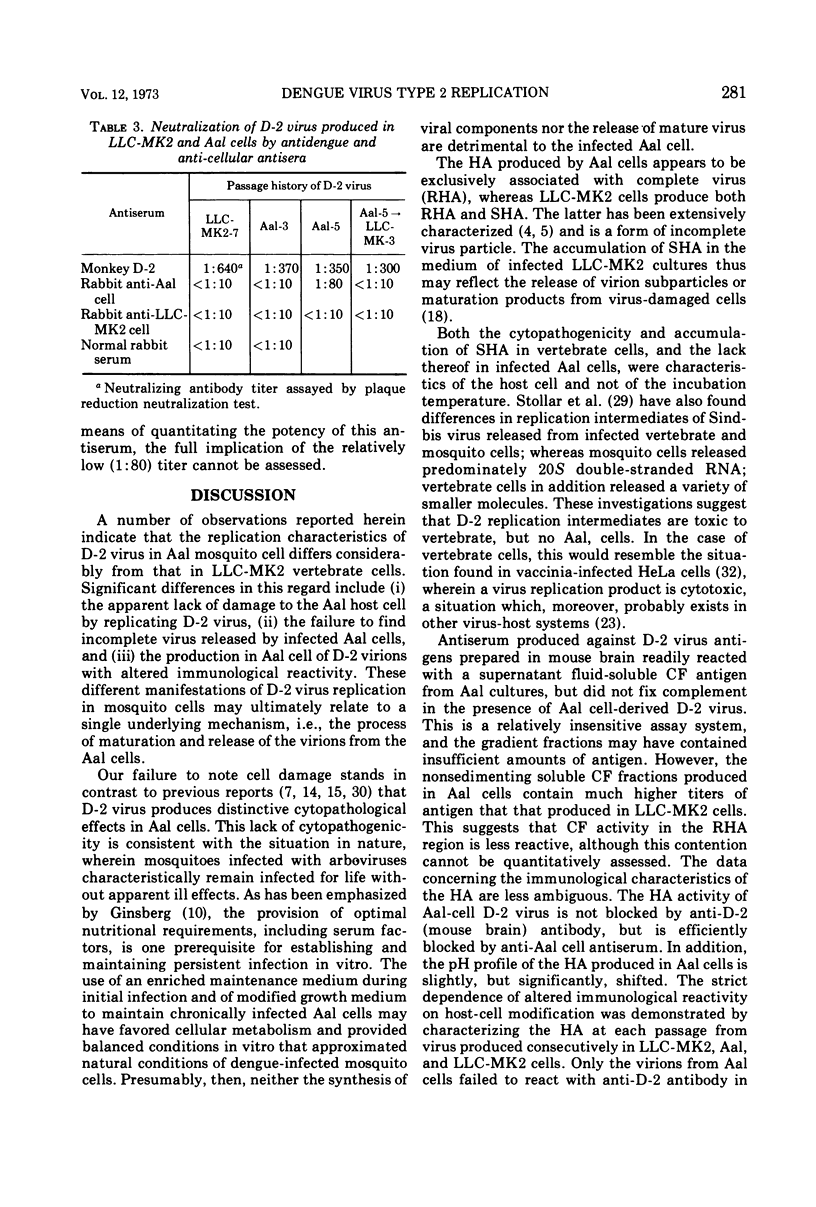
Abstract
The replication of type 2 dengue (D-2) virus in Aedes albopictus (Aal) mosquito cell cultures differed from that in vertebrate (LLC-MK2) rhesus monkey kidney cells. Virus readily replicated in Aal cells at either 30 or 37 C, but had no apparent effect on the host cell. Persistent infection was established with continual virus production for at least 6 months, although the virulence of progeny virus for both suckling mice and LLC-MK2 cells became attenuated. Density gradient analysis of infected Aal cell supernatant products indicated that only complete virus was released, in contrast to infected LLC-MK2 cells which also released incomplete virus. The surface antigens of the virus produced in Aal cells appeared to be considerably modified in that antiserum to vertebrate cell-produced D-2 virus did not block hemagglutination, whereas anti-Aal cell antiserum did. Virus infectivity could be neutralized by the antiserum to D-2 virus grown in vertebrate cells, however. Virus produced in LLC-MK2 cells did not demonstrate a similar host-cell modification. These results may reflect a difference in the mechanism by which D-2 virus matures in Aal cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger N. E., Harant J. A., Willis L. C., Jorgensen G. M. Sporozoite and normal salivary gland induced immunity in malaria. Nature. 1972 Aug 11;238(5363):341–341. doi: 10.1038/238341a0. [DOI] [PubMed] [Google Scholar]
- Banerjee K., Singh K. R. Establishment of carrier cultures of Aedes albopictur cell line infected with arboviruses. Indian J Med Res. 1968 Jun;56(6):812–814. [PubMed] [Google Scholar]
- Brandt W. E., Cardiff R. D., Russell P. K. Dengue virions and antigens in brain and serum of infected mice. J Virol. 1970 Oct;6(4):500–506. doi: 10.1128/jvi.6.4.500-506.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- CUTCHINS E., WARREN J., JONES W. P. The antibody response to smallpox vaccination as measured by a tissue culture plaque method. J Immunol. 1960 Sep;85:275–283. [PubMed] [Google Scholar]
- Cardiff R. D., Brandt W. E., McCloud T. G., Shapiro D., Russell P. K. Immunological and biophysical separation of dengue-2 antigens. J Virol. 1971 Jan;7(1):15–23. doi: 10.1128/jvi.7.1.15-23.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Brown F. Glycolipid nature of the complement-fixing host cell antigen of vesicular stomatitis virus. J Gen Virol. 1972 Jun;15(3):243–245. doi: 10.1099/0022-1317-15-3-243. [DOI] [PubMed] [Google Scholar]
- Chappell W. A., Calisher C. H., Toole R. F., Maness K. C., Sasso D. R., Henderson B. E. Comparison of three methods used to isolate dengue virus type 2. Appl Microbiol. 1971 Dec;22(6):1100–1103. doi: 10.1128/am.22.6.1100-1103.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBERG H. S. The significance of the viral carrier state in tissue culture systems. Prog Med Virol. 1958;1:36–58. [PubMed] [Google Scholar]
- Keller J. M., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. 3. Viruses differing in their effects on the social behavior of infected cells specify different membrane glycoproteins. Proc Natl Acad Sci U S A. 1970 Apr;65(4):865–871. doi: 10.1073/pnas.65.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloud T. G., Brandt W. E., Russell P. K. Molecular size and charge relationships of the soluble complement-fixing antigens of dengue viruses. Virology. 1970 Jul;41(3):569–572. doi: 10.1016/0042-6822(70)90180-7. [DOI] [PubMed] [Google Scholar]
- Paul S. D., Singh K. R., Bhat U. K. A study on the cytopathic effect of arboviruses on cultures from Aedes albopictus cell line. Indian J Med Res. 1969 Feb;57(2):339–348. [PubMed] [Google Scholar]
- Russell P. K., Nisalak A., Sukhavachana P., Vivona S. A plaque reduction test for dengue virus neutralizing antibodies. J Immunol. 1967 Aug;99(2):285–290. [PubMed] [Google Scholar]
- Shapiro D., Brandt W. E., Russell P. K. Change involving a viral membrane glycoprotein during morphogenesis of group B arboviruses. Virology. 1972 Dec;50(3):906–911. doi: 10.1016/0042-6822(72)90445-x. [DOI] [PubMed] [Google Scholar]
- Singh K. R., Paul S. D. Isolation of Dengue viruses in Aedes albopictus cell cultures. Bull World Health Organ. 1969;40(6):982–983. [PMC free article] [PubMed] [Google Scholar]
- Singh K. R., Paul S. D. Susceptibility of Aedes albopictus and Aedes aegypti cell lines to infection by Arbo and other viruses. Indian J Med Res. 1968 Jun;56(6):815–820. [PubMed] [Google Scholar]
- Smith T. J., Brandt W. E., Swanson J. L., McCown J. M., Buescher E. L. Physical and biological properties of dengue-2 virus and associated antigens. J Virol. 1970 Apr;5(4):524–532. doi: 10.1128/jvi.5.4.524-532.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T. M. Arbovirus replication in mosquito cell lines (Singh) grown in monolayer or suspension culture. Proc Soc Exp Biol Med. 1970 May;134(1):356–361. doi: 10.3181/00379727-134-34793. [DOI] [PubMed] [Google Scholar]
- Stevens T. M., Schlesinger R. W. Studies on the nature of dengue viruses. I. Correlation of particle density, infectivity, and RNA content of type 2 virus. Virology. 1965 Sep;27(1):103–112. doi: 10.1016/0042-6822(65)90147-9. [DOI] [PubMed] [Google Scholar]
- Stollar V., Shenk T. E., Stollar B. D. Double-stranded RNA in hamster, chick, and mosquito cells infected with Sindbis virus. Virology. 1972 Jan;47(1):122–132. doi: 10.1016/0042-6822(72)90245-0. [DOI] [PubMed] [Google Scholar]
- Stollar V., Stevens T. M., Schlesinger R. W. Studies on the nature of dengue viruses. II. Characterization of viral RNA and effects of inhibitors of RNA synthesis. Virology. 1966 Oct;30(2):303–312. doi: 10.1016/0042-6822(66)90105-x. [DOI] [PubMed] [Google Scholar]
- Stollar V. Studies on the nature of dengue viruses. IV. The structural proteins of type 2 dengue virus. Virology. 1969 Nov;39(3):426–438. doi: 10.1016/0042-6822(69)90091-9. [DOI] [PubMed] [Google Scholar]
- Suitor E. C., Jr, Paul F. J. Syncytia formation of mosquito cell cultures mediated by type 2 dengue virus. Virology. 1969 Jul;38(3):482–485. doi: 10.1016/0042-6822(69)90162-7. [DOI] [PubMed] [Google Scholar]
- Whitehead R. H., Chaicumpa V., Olson L. C., Russell P. K. Sequential dengue virus infections in the white-handed gibbon (Hylobates lar). Am J Trop Med Hyg. 1970 Jan;19(1):94–102. doi: 10.4269/ajtmh.1970.19.94. [DOI] [PubMed] [Google Scholar]
- Yunker C. E. Arthropod tissue culture in the study of arboviruses and rickettsiae: a review. Curr Top Microbiol Immunol. 1971;55:113–126. doi: 10.1007/978-3-642-65224-0_19. [DOI] [PubMed] [Google Scholar]



