Abstract
Ancient DNA (aDNA) provides powerful evidence for detecting the genetic basis for adaptation to environmental change in many taxa. Among the greatest of changes in our biosphere within the last century is rapid anthropogenic ocean warming. This phenomenon threatens corals with extinction, evidenced by the increasing observation of widespread mortality following mass bleaching events. There is some evidence and conjecture that coral-dinoflagellate symbioses change partnerships in response to changing external conditions over ecological and evolutionary timescales. Until now, we have been unable to ascertain the genetic identity of Symbiodinium hosted by corals prior to the rapid global change of the last century. Here, we show that Symbiodinium cells recovered from dry, century old specimens of 6 host species of octocorals contain sufficient DNA for amplification of the ITS2 subregion of the nuclear ribosomal DNA, commonly used for genotyping within this genus. Through comparisons with modern specimens sampled from similar locales we show that symbiotic associations among several species have been static over the last century, thereby suggesting that adaptive shifts to novel symbiont types is not common among these gorgonians, and perhaps, symbiotic corals in general.
Introduction
Many diverse cnidarians, including scleractinian corals and their alcyonarian and gorgonian soft coral relatives have evolved obligate, intra-cellular symbioses with dinoflagellate algae of the genus Symbiodinium. These algae are themselves diverse, representing at least 9 divergent lineages or “clades” [1], [2]. Some of these clades contain considerable diversity (‘types’), suggesting that species level boundaries may exist at sub-clade resolutions [3]. Currently, our understanding of the functional significance of symbiont diversity is based on zonation patterns of symbiont clades within and among corals distributed across the reefscape [4], [5], and carbon-centered metrics of photosynthetic performance under varied conditions [6], [7]. These physiological differences may be linked to a range of tolerances to environmental stressors including sedimentation, high irradiance, and most importantly, extreme temperatures among corals hosting distinct Symbiodinium clades [8]–[11]. Global observations of thermal tolerance and maintenance of symbiosis (bleaching resistance) exhibited by various clades and sub-clade types have led to the hypothesis that the process of bleaching may itself be an adaptive mechanism for acquiring novel, stress-tolerant symbionts, which could serve to increase the host’s survival during bouts of environmental change [12]–[13]. To test this hypothesis is to ask; have symbiont types changed since the onset of anthropogenic climate warming over the last century?
One way of answering this question is by identifying Symbiodinium from corals sampled prior to major anthropogenic global change. Sources of such materials can be found in museum archives [14]. Unfortunately, as scleractinian morphological taxonomy is based on skeletal structures, most museum held hard coral specimens collected over the last 200 years have been bleached to remove all organic tissues, while wet collections of hard corals are less common. Alternatively, gorgonian corals are often preserved dry with the outer tissues (coenenchyme), including the polyps and their Symbiodinium intact. The Smithsonian’s National Museum of Natural History contains over 6,000 specimens of gorgonian corals, with a large representation of dried specimens. Remarkably, the collection is not limited to individual type specimens and contains many lots of independent colonies contemporaneously collected from the same geographic areas thus providing important replication for genetic analyses.
Gorgonians are globally distributed but the majority of species hosting Symbiodinium spp. are found on shallow reefs in the Caribbean Sea. These octocorals exhibit different colony morphologies, (primarily rod, fan, and plume) as well as variation in polyp size which is hypothesized to be related to prey-capture and therefore, reflective of the relative reliance on auto- vs. heterotrophic nutrition [15]. Thus, gorgonian species may differ with respect to their reliance on autotrophic nutrition translocated from their symbionts. Moreover, current observations on the flexibility of gorgonian-algal symbioses suggests that they are more constrained than scleractinians ([16]; but see also [17]). Nearly 90% of Caribbean gorgonians are primarily found in symbiosis with clade B Symbiodinium [18], though there are some examples of clade flexibility. For instance, Plexaura homomalla and Eunicea tourneforti have been observed hosting multiple types from clade C as well as clade B Symbiodinium [14]. Furthermore, experimental infections of newly settled juveniles of Plexaura kuna and Pseudoplexaura porosa demonstrated flexibility in host associations with clades A, B, and C [19]. Like scleractinians, the diversity of gorgonian-symbiont associations may explain patterns of holobiont tolerance to environmental stressors.
Published studies on the bleaching response to thermal stress among gorgonians have shown that some species are highly resistant [20]–[22] while others are susceptible [23] and can subsequently succumb to mortality [24]–[26]. Following the mass bleaching of 2005 in Puerto Rico, Prada (2010) observed widespread bleaching (observed whitening) among many genera, including Muricea, Muriceopsis, Pseudoplexaura, Briarium, Pterogorgia and Plexaurella, while Gorgonia, Eunicea (with the exception of E. flexuosa) and Pseudopterogorgia did not visibly bleach. However, visible signs of bleaching may only manifest after substantial loss of symbiont cells has already occurred [27] and host-derived pigments, particularly carotenoids associated with gorgonian sclerites may confound visual assessments of bleaching in gorgonian corals [28]. Nevertheless we can conclude from these limited observations that many gorgonians bleach and there is substantial variation in bleaching susceptibility among various genera. Given that living gorgonians have displayed instances of flexible symbioses with clade B and C sub-types [29], and the observation that bleached gorgonians can acquire novel symbionts from the water column [30] there is a basis for questioning whether anthropogenic change over the last century is associated with a shift in the predominant symbiont clades hosted by modern gorgonian corals.
Whether or not the process of bleaching is an adaptive mechanism, it seems clear that Symbiodinium diversity is tied to a spectrum of environmental tolerances. Therefore, shifts to tolerant types via natural selection over time would be indicative of adaptation to environmental stress. Given the near +1.0°C warming of the surface ocean over the last 150 years has pushed corals to the limits of their thermal tolerance and increased the occurrence of mass bleaching events [31] the main goal of this study was to determine if shifts in dominant symbiont types hosted by several gorgonian species has occurred. We also tested the null hypothesis that past populations of gorgonians separated by distance host the same clades, and that all host species associated with the same symbiont types.
Methods
Sample Collection and Preparation
Historical gorgonian specimens (n = 82) were obtained from the dry collection of the Smithsonian National Museum of Natural History (NMNH; Table 1). Most of these specimens were small (<50 cm) and we estimate that these colonies were, on average, <20 years old at the age of collection based on size and band counts of basal cross-sections obtained from select specimens (data not shown). Thus, we estimate that the specimens sampled in this study likely represent up to 9 generations. We selected from the most abundant species in the NMNH collection, including the sea fans Gorgonia ventalina and G. flabellum, the sea plume Pseudopterogorgia acerosa, and the sea rod Eunicea flexuosa. These species have been found in association with several sub-clade types. Gorgonia and Pseudopterogorgia have been found in symbiosis with B1, and Eunicea flexuosa with B1, B1b, B2, and B8 [32].
Table 1. Summary of specimens.
| Catalog Number | Scientific Name | n (seq.) | Collector(s) | Year Collected | Country | Precise Locality |
| 59474 | Eunicea succinea | 1 (1) | J.E. Benedict | 1901 | USA | Caesar Creek, Florida |
| 14388 | Eunicea flexuosa | 15 (6) | W. Nye | 1886 | Bahamas | New Providence Island |
| no ID | 11 (8) | W.L. Schmitt | ∼1905 | USA | Dry Tortugas, Florida | |
| no ID | 5 (4) | unknown | 1925 | USA | Dry Tortugas, Florida | |
| na | 10 (5) | E. Bartels | 2007 | USA | Summerland Key, Florida | |
| 14766 | Gorgonia flabellum | 2 (1) | W. Nye | 1886 | Bahamas | Abaco Island |
| 54232 | 12 (8) | P. Bartsch | 1912 | Bahamas | Andros Island | |
| 14400 | 1 (1) | no data | 1886 | Bahamas | Watlings Island | |
| 14397 | Gorgonia ventalina | 4 (3) | W. Nye | 1886 | Bahamas | New Providence Island |
| 54232 | 6 (2) | P. Bartsch | 1912 | Bahamas | Andros Island | |
| 14400 | 2 (0) | unknown | 1886 | Bahamas | Watlings Island | |
| na | 5 (3) | D. Baker | 2010 | Bahamas | Lee Stocking Island | |
| 34779 | 3 (2) | Henderon and Barson | 1914 | Cuba | Santa Lucia Bay | |
| 8860 | 2 (2) | E. Palmer | 1884 | USA | Florida | |
| 8884 | 4 (1) | E. Palmer | 1884 | USA | Florida | |
| 95428 | 2 (0) | E. Palmer | 1884 | USA | Key West, Florida | |
| 33627 | 1 (1) | P. Bartsch* | 1912* | USA | Biscayne Bay, Florida | |
| 1625 | 2 (2) | C. Pickering | 1838–1842** | USA | Florida | |
| 54232 | 3 (1) | P. Bartsch | 1912 | USA | Biscayne Bay, Florida | |
| na | 7 (1) | E. Bartels | 2007 | USA | Summerland Key, Florida | |
| 8862 | Pseudopterogorgia acerosa | 6 (3) | E. Palmer | 1884 | USA | Carysfort Reef, Florida |
| 8866 | 1 (0) | E. Palmer | 1884 | USA | Salt Pond Key, Florida | |
| 33614 | 11 (8) | J.E. Benedict | 1901 | USA | Carysfort Reef, Florida | |
| 6913 | Pterogorgia anceps | 1 (1) | H. Hemphill | 1884 | USA | Tampa Bay, Florida |
n = total number of specimens sampled for this study.
(seq.) = total number of specimens yielding consensus sequences used in Fig. 1.
Estimated year: Charles Pickering was a crew member of the United States Exploring Expedition at this time.
Year estimated by collector/catalog number.
All museum specimens were collected from the Florida Keys and the Bahamas, though 3 specimens from Cuba were added to the Florida sample set. Each specimen or lot was accessed from storage cabinets organized by geographic origin and time of collection. Most specimens were enclosed in heavy plastic bags. For comparison, modern specimens of G. ventalina (n = 7) and E. flexuosa (n = 10) were collected offshore of Summerland Key, FL in 2007 and G. ventalina (n = 5) was collected from Lee Stocking Island, Bahamas in 2010 (Table 1). All modern samples were oven dried at 60°C to constant weight, ground into a powder, and stored in individual sealed tubes.
The storage and subsequent handling of both modern and museum specimens was conducted at separate locations throughout the duration of this study to eliminate the risk of cross-contamination between modern and historical specimens. Tissue grinding, DNA extraction, PCR, and cleanup were conducted in separate laboratories at different times using independent equipment and reagent kits up to the point of sequencing [33]. First, museum specimen work was conducted at the Laboratories of Analytical Biology at the Smithsonian’s Museum Support Center. Subsequently, all modern specimens were processed at the Geophysical Laboratory of the Carnegie Institution of Washington. Neither facility had conducted work on Symbiodinium DNA prior to this study. Approximately 3 g of tissue was removed by hand or using scissors and then homogenized using a mortar and pestle. To reduce cross contamination between samples, all equipment was washed with soap, rinsed with tap water, soaked in a bleach solution, and rinsed several times with deionized water followed by a final ethanol rinse to enhance drying. In between sets of samples from different species each mortar and pestle was autoclaved following the washing protocol.
Approximately 0.5–1 mL (vol.) of homogenized sample was placed in a 2.0 mL microcentrifuge tube, rehydrated with 1 mL of 0.2 µM sterile-filtered 0.5 mM EDTA buffer solution and vortexed. The contents were allowed to settle at 5°C, and the overlying liquid was decanted into a sterile tube. This step was repeated if further settlement of large particles was observed. The resulting liquids were spun at 10,000×g for 5 minutes, resulting in a pellet primarily composed of Symbiodinium cells and debris. This was followed by two spins at lower speed (500×g) to rinse the pellet and reduce host-derived material. While pellets from older specimens were visually similar to modern specimens in yield and color, microscopy revealed that older samples contained few intact theca, within which pigments were clearly degraded or absent.
DNA Extraction, Quantification, and Amplification
Genomic DNA was extracted from whole Symbiodinium pellets using a Mo Bio Power Soil DNA isolation kit (Mo Bio Laboratories, Inc. Carlsbad, CA, USA). Pellets were first resuspended in buffer, transferred to bead tubes and spun at 10,000×g following the wet soil sample protocol. The resulting extracts were screened using ethidium bromide gel electrophoresis, and DNA concentrations and estimates of purity were determined using a NanoDrop spectrophotometer. Amplification of the ITS2 sub-region was conducted using the primers ‘ITSintfor2’ (forward) and ‘ITS2CLAMP’ (reverse) following the ‘touchdown’ amplification protocol described in Lajeunesse et al. (2002) using a BIO-RAD T100 thermal cycler. Post-PCR screening for bands of ∼300 bp revealed amplification success in 63 of 89 museum samples (70.7%). Troubleshooting on failed PCR reactions using different PCR recipes and various DNA polymerases was rarely successful, thus we attributed a failed PCR reaction to low DNA concentrations and/or DNA fragmentation. All amplicons were cleaned using an Exo:SAP enzyme protocol. Following cleanup, 1 µL of the PCR product was cycle sequenced in both directions using Big Dye 3.1 (Applied Biosystems, Foster City, USA). The resulting product was filtered through a Sephadex column, dried at 95°C, and directly sequenced using a 3730×l DNA analyzer (Applied Biosystems/Hitachi).
Verification
A subset of museum specimens representing each host species (n = 14), and the additional species Eunicea succinea (n = 1) and Pterogorgia anceps (n = 1) were resampled to verify that our results could be repeated. All sample preparation, DNA extraction, PCR, and pre-sequencing reactions were conducted at a third, independent laboratory with no history of Symbiodinium research (EG3, NMNH). BLAST searches using these sequences aligned with Symbiodinum clades which corroborated our main conclusions. These data were included in the final analysis.
Sequence Analysis
Chromatograms were screened for quality, edited, and complementary sequences were aligned using Geneious Pro v.5.4.6 (Biomatters Ltd.) [34]. Consensus sequences representing replicate samples of each species from each region/time were assembled and BLAST searches yielded close matching with Symbiodinium clade B. Thus, unique sequence characters from a minority of individuals (24%) were ignored by our analysis if they differed from the consensus sequence. We then aligned our sequences with known sequences of Symbiodinium sub-types within clade B obtained from GeoSymbio [32]. A phylogenetic tree was created with a clade A sequence (A3; Geosymbio) was used as an outgroup using neighbor-joining with 1,000 bootstrapped iterations. All sequences resulting from this study are archived in NCBI’s GenBank with the following accession numbers: KC461830-KC461901. URL: http://www.ncbi.nlm.nih.gov/genbank/.
Results and Discussion
We have demonstrated that short genetic markers like the ITS2 sub-region of the nuclear rDNA are recoverable from Symbiodinium obtained from 6 species of dried gorgonian octocorals collected more than a century ago. Preliminary attempts at amplifying other common markers for genotyping, such as 18 s, were unsuccessful. Based on electrophoresis of genomic DNA extracts we observed high fragmentation, thus, given the relatively large size of 18 s (∼1800 bp) the probability of extracting a complete template was likely very low. However, amplification and sequencing of the smaller Symbiodinium ITS2 (∼200 bp) from dry-preserved living and historical octocorals specimens was successful and there was no apparent effect of preservation time on the percentage of samples that were successfully sequenced and aligned. Of the living specimens extracted, 52.2% resulted in high quality and aligned sequences compared to 65.8% of the museum specimens. This difference is probably due to experimental error in the process of extraction through sequencing and not a function of sample preservation. Indeed, DNA concentrations of modern sample extracts were as high as 38.6 ng µL−1 vs. 17.3 for museum specimens. However the mean DNA concentration obtained from living samples was no different than museum specimens (8.1 vs. 8.9 ng µL−1, Student’s t-test (two-tailed); t = 0.33, df = 42, p = 0.74). These findings illustrate that sufficient Symbiodinium aDNA for genotyping can be obtained from small quantities of dry-preserved coral tissues, and suggests that drying is a tractable option for DNA preservation when the use of liquid fixatives is not feasible.
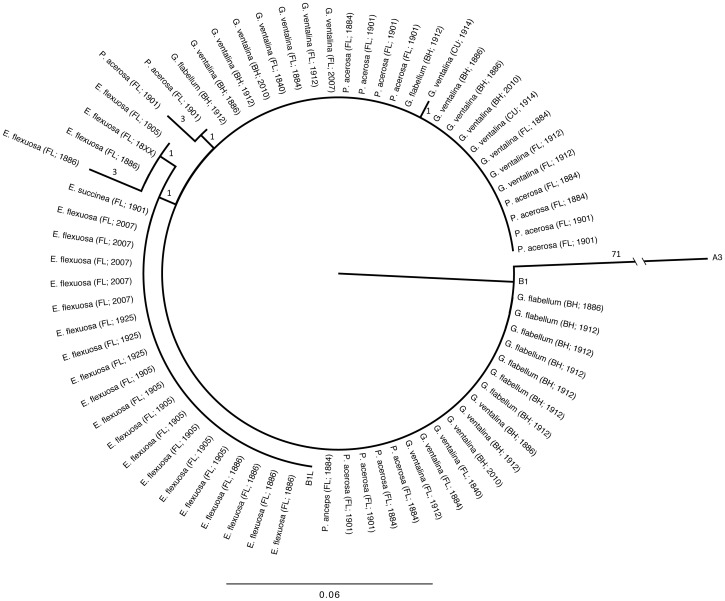
Our sequence data show that Symbiodinium types hosted by Caribbean octocorals collected between 98–172 years ago are indistinguishable from recently collected specimens. Both groups were found to contain representatives of clade B (Fig. 1). While our recent sample size was small, the results we obtained are in line with several previous works that have genotyped Symbiodinium from these host species [14], [18], [22], [32], [35]. Both recent and historical specimens of G. ventalina, and historical specimens of G. flabellum, and P. acerosa were 100% identical to GenBank sequences of Symbiodinium clade B1. E. flexuosa and E. succinea were found to host a different genotype, B1L, characterized by a substitution of cytosine for thymine at base pair 117, relative to B1 (Fig. 1). B1L has been previously described in symbiosis with Eunicea spp. [35] and other sea rods of the genus Pseudoplexaura and Plexaurella [36]. However, unique sequences that differed from the clade reference were found in 24% of the individuals. E. flexuosa had the highest occurrence of such characters with 4 out of 20 (5%) having 4 or fewer unique substitutions. One of these substitutions, a guanine for adenine substitution at base pair 189, was present in all four individuals which were all museum specimens collected at or before 1905. We found no similar SNP in GenBank or the Geosymbio database. While the sample size is low, we suggest that further sampling be conducted to determine if this is a new and/or extirpated Symbiodinium clade.
Figure 1. Phylogenetic tree of Symbiodinium extracted from various gorgonian host species based on ITS2 sequences from modern and museum-held specimens collected from Florida (FL), Cuba (CU), and the Bahamas (BH).
Year of collection is noted in each branch label. These groups are shown in comparison to reference sequences from GeoSymbio from common clade B sub-types as well as a clade A3 sequence as an outgroup. Tree construction was based on neighbor-joining. Branch labels indicate the number of unique substitutions per sequence and branches are scaled to average substitutions per site, with the exception of A3 which was cropped.
Overall, there was no apparent genetic structure or clade-specificity based on the geographic location of collection. Specimens collected from Florida and the Bahamas consistently grouped together (Fig. 1). This is not surprising given that the majority of Caribbean gorgonians associate with clade B Symbiodinium. However, using microsatellite markers, Andras et al. (2011) recently illustrated significant genetic structure among clade B1 symbionts hosted by G. ventalina from the Bahamas and the Florida Keys, possibly due to the strong Florida Current preventing mixing among these populations [37]. Moreover, Finney et al (2010) used a combined ITS2/microsatellite approach to reveal that diversity within clade B is high, reflecting divergence among lineages that are host- and habitat-specific [36]. Thus, a logical future step is to target microsatellite loci of Symbiodinium B1 obtained from museum specimens for comparison with modern populations as such markers may be better suited for quantifying change in symbiont populations and testing the hypothesis that population level shifts in genetic diversity have occurred in response to global change [37], [38].
Our results yielded no evidence that there have been shifts in symbiont type hosted by gorgonian corals since human-induced global change. This finding poses two important questions; 1) is the gorgonian-algal symbiosis static and inflexible, and 2) has the severity of global change not been sufficient to drive major shifts in symbiont types hosted by gorgonian corals?
First, there is evidence that some gorgonians possess flexible symbioses as has been observed in several species of hard corals [39]. Newly settled polyps are capable of acquiring multiple symbiont types during early ontogeny [30]. As adults, several species have been found in symbiosis with clade A and C Symbiodinium as well as clade B [14], [19] and early studies may have failed to describe the presence of cryptic clades [35]. Yet, these examples of flexibility are apparently rare. More common are examples of symbiont stability over space and time. Goulet and Coffroth (2003) monitored Symbiodinium within individual colonies of Plexaura kuna and saw no clade-level variation over a period of 10 years [40]. Similarly, LaJeunesse et al. (2004) revealed that symbiont identities among an introduced population of Fungia retained their Pacific Symbiodinium 35 years after introduction to the Caribbean [41]. These examples are supportive of the hypothesis that host-symbiont associations are highly specific, reflecting a long evolutionary history [42].
Yet, it remains to be tested whether or not different symbiont types confer thermal tolerance to gorgonian hosts. This is an interesting hypothesis to test as bleaching is not uncommon among gorgonians, and the severity of bleaching appears to be species-specific [20]. For example, long-term records of temperature and symbiont densities of the sea rod Plexaura kuna from the Bahamas suggested that this species resists bleaching whereas other sea rods like Plexaurella spp. appear to bleach readily during warm periods [20], [23]. The potential for certain sub-clade types to enhance tolerance to environmental stress has recently been illustrated in hard corals [43]. Although differential bleaching susceptibility among Pacific Alcyonaceans is apparently not explained by Symbiodinium identity, this has yet to be tested in gorgonian octocorals [44].
Second, the bleaching threshold for many coral species is near maximal summer temperatures, therefore apparently small increases in ocean temperature have large consequences for increasing mass coral bleaching events. Coincident with these events are observations of differential mortality [45] and to a lesser extent symbiont shuffling among scleractinian coral species, primarily to Symbiodinum types which have been found to be tolerant to high irradiance (e.g. A3) or sedimentation and thermal stress (e.g. clade D) [46,47]. If we assume that zooxanthellate octocoral symbioses are functionally analogous to scleractinians, we might expect to find similar shifts among gorgonian corals over time. The absence of genetic evidence in this study may indicate that gorgonians are inflexible with respect to their symbiotic partners as may be the case for most corals [48], or perhaps gorgonians are overall more resilient in the midst of ocean warming. We contend that neither hypothesis is parsimonious and posit that significant changes in symbiont genotypes among coral host populations are not likely to be ecologically significant under the punctuated stress of climate change over the last century. This reflects the evolution of the symbiosis between Caribbean gorgonians and clade B Symbiodinium since the Pleistocene [42].
Our successful demonstration of aDNA extraction and amplification of informative taxonomic markers from Symbiodinium holds great promise for future studies. It has been argued that scleractinian corals are more flexible in their symbiotic associations than gorgonians, particularly at the clade level [16], [17]. If this is true, aDNA studies of scleractinian corals is a high priority for future research, though a significant challenge exists in finding intact tissues or skeletal reservoirs of preserved Symbiodinium cells. We attempted DNA extraction from one museum specimen of Montastrea cavernosa (NMNH #255089), collected in 1864, which appeared to have some remaining surface tissues. Unfortunately, we recovered very small quantities of DNA and were unable to amplify ITS2. Even so, milling of subsurface skeletal materials from archived scleractinians, and perhaps even fossil and sub-fossil skeletons may yet yield preserved Symbiodinium containing sufficient DNA for genotyping and warrants further study.
Acknowledgments
We thank M. Halloran, J. Hunt, & A. Ormos of LAB, F. Dahlan of EG3, A. Steele, V. Starke, M. Glamoclija, K. Rogers, & I. Perez at CIW for laboratory support and training, S. Cairns and T. Coffer of NMNH for collections access and support and T. LaJeunesse for constructive feedback on the manuscript.
Funding Statement
This work was funded by the Smithsonian’s Marine Science Network Fellowship and the Laboratories of Analytical Biology. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Pochon X, Gates RD (2010) A new clade (Dinophyceae) from soritid foraminifera in Hawai’i. Mol Phylogenet Evol 56: 492–497. [DOI] [PubMed] [Google Scholar]
- 2.Baker AC (2003) Flexibility and specificity in coral-algal symbiosis: Diversity, ecology, and biogeography of Symbiodinium. Annu Rev Ecol Evol Syst 34, 661–689.
- 3.LaJeunesse TC, Parkinson JE (2012) A genetics-based description of Symbiodinium minutum sp. nov. and S. psygmophilum sp. nov. (Dinophyceae), two dinoflagellates symbiotic with Cnidaria. J Phycol doi: 10.1111/j.1529–8817.2012.01217.x. [DOI] [PubMed]
- 4. Rowan R (1997) Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 388: 265–268. [DOI] [PubMed] [Google Scholar]
- 5. Iglesias-Prieto R, Beltran VH, LaJeunesse TC, Reyes-Bonilla H, Thome PE (2004) Different algal symbionts explain the vertical distribution of dominant reef corals in the eastern Pacific. Proc Roy Soc B 271: 1757–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Warner M, LaJeunesse T, Robison J (2006) The ecological distribution and comparative photobiology of symbiotic dinoflagellates from reef corals in Belize: potential implications for coral bleaching. Limnol Oceanogr 51: 1887–1897. [Google Scholar]
- 7. Cantin NE, van Oppen MJH, Willis BL, Mieog JC, Negri AP (2009) Juvenile corals can acquire more carbon from high-performance algal symbionts. Coral Reefs 28: 405–414. [Google Scholar]
- 8. LaJeunesse TC, Pettay T, Sampayo EM, Phongsuwan N, Brown B, et al. (2010) Special Paper: Long-standing environmental conditions, geographic isolation and host-symbiont specificity influence the relative ecological dominance and genetic diversification of coral endosymbionts in the genus Symbiodinium. . J Biogeogr 37: 785–800. [Google Scholar]
- 9. Oliver T, Palumbi S (2011) Many corals host thermally resistant symbionts in high-temperature habitat. Coral Reefs 30: 241–250. [Google Scholar]
- 10. Toller WW, Rowan R, Knowlton N (2001) Repopulation of zooxanthellae in the Caribbean corals Montastraea annularis and M. faveolata following experimental and disease-associated bleaching. Biol Bull 201: 360–373. [DOI] [PubMed] [Google Scholar]
- 11. Garren M, Walsh SM, Caccone A, Knowlton N (2006) Patterns of association between Symbiodinium and members of the Montastrea annularis species complex on spatial scales ranging from within colonies to between geographic regions. Coral Reefs 25: 503–512. [Google Scholar]
- 12. Kinzie R III, Takayama M, Santos S (2001) The adaptive bleaching hypothesis: experimental tests of critical assumptions. Biol Bull 200: 51–58. [DOI] [PubMed] [Google Scholar]
- 13. Buddemeier RW, Fautin DG (1993) Coral bleaching as an adaptive mechanism. BioScience 43: 320–326. [Google Scholar]
- 14. van Oppen M, Mieog J, Sanchez C, Fabricius K (2005) Diversity of algal endosymbionts (zooxanthellae) in octocorals: the roles of geography and host relationships. Mol Ecol 14: 2403–2417. [DOI] [PubMed] [Google Scholar]
- 15. Porter J (1976) Autotrophy, heterotrophy, and resource partitioning in Caribbean reef-building corals. Am Nat 110: 731–742. [Google Scholar]
- 16. Baker A, Romanski A (2007) Multiple symbiotic partnerships are common in scleractinian corals, but not in octocorals: Comment on Goulet (2006). Mar Ecol Prog Ser 335: 237–242. [Google Scholar]
- 17. Goulet T (2007) Most scleractinian corals and octocorals host a single symbiotic zooxanthella clade. Mar Ecol Prog Ser 335: 243–248. [Google Scholar]
- 18. Goulet TL, Coffroth MA (2004) The genetic identity of dinoflagellate symbionts in Caribbean octocorals. Coral Reefs 23: 465–472. [Google Scholar]
- 19. Coffroth MA, Santos SR, Goulet TL (2001) Early ontogenetic expression of specificity in a cnidarian-algal symbiosis. Mar Ecol Prog Ser 222: 85–96. [Google Scholar]
- 20. Lasker H (2003) Zooxanthella densities within a Caribbean octocoral during bleaching and non-bleaching years. Coral Reefs 22: 23–26. [Google Scholar]
- 21. Hannes A, Barbeitos M (2009) Stability of symbiotic dinoflagellate type in the octocoral Briareum asbestinum . Mar Ecol Progr Ser 391: 65–72. [Google Scholar]
- 22. Kirk N, Ward J, Coffroth M (2005) Stable Symbiodinium composition in the sea fan Gorgonia ventalina during temperature and disease stress. Biol Bull 209: 227–234. [DOI] [PubMed] [Google Scholar]
- 23. Frenz-Ross J, Enticknap J (2008) The effect of bleaching on the terpene chemistry of Plexaurella fusifera: evidence that zooxanthellae are not responsible for sesquiterpene production. Mar Biotech 10: 572–578. [DOI] [PubMed] [Google Scholar]
- 24. Prada C, Weil E, Yoshioka P (2010) Octocoral bleaching during unusual thermal stress. Coral Reefs 29: 41–45. [Google Scholar]
- 25. Harvell D, Kim K, Quirolo C, Weir J, Smith G (2001) Coral bleaching and disease: contributors to 1998 mass mortality in Briareum asbestinum (Octocorallia, Gorgonacea). Hydrobiologia 460: 97–104. [Google Scholar]
- 26. Drohan AF, Thoney DA, Baker AC (2005) Synergistic effect of high temperature and ultraviolet-B radiation on the gorgonian Eunicea tourneforti (Octocorallia: Alcyonacea: Plexauridae). Bull Mar Sci 77: 257–266. [Google Scholar]
- 27. Siebeck U, Marshall N, Klüter A, Hoegh-Guldberg O (2006) Monitoring coral bleaching using a colour reference card. Coral Reefs 25: 453–460. [Google Scholar]
- 28. Leverette C, Warren M, Smith M, Smith G (2008) Determination of carotenoid as the purple pigment in Gorgonia ventalina sclerites using Raman microscopy. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 69: 1058–1061. [DOI] [PubMed] [Google Scholar]
- 29. Goulet T, Simmons C, Goulet D (2008) Worldwide biogeography of Symbiodinium in tropical octocorals. Mar Ecol Progr Ser 355: 45–58. [Google Scholar]
- 30. Lewis C, Coffroth M (2004) The acquisition of exogenous algal symbionts by an octocoral after bleaching. Science 304: 1490. [DOI] [PubMed] [Google Scholar]
- 31. Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, et al. (2007) Coral reefs under rapid climate change and ocean acidification. Science 318: 1737–1742. [DOI] [PubMed] [Google Scholar]
- 32.Franklin EC, Stat M, Pochon X, Putnam HM, Gates RD (2011) GeoSymbio: a hybrid, cloud‐based web application of global geospatial bioinformatics and ecoinformatics for Symbiodinium–host symbioses. Mol Ecol Res doi: 10.1111/j.1755-0998.2011.03081.x. [DOI] [PubMed]
- 33. Willerslev E, Cooper A (2005) Ancient DNA. Proc Biol Sci 272: 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drummond AJ, Ashton B, Buxton S, Cheung M, Cooper A, et al. (2010) Geneious v5.3, Available from http://www.geneious.com.Accessed 2012 May 29.
- 35. Santos SR, Shearer TL, Hannes AR, Coffroth MA (2004) Fine-scale diversity and specificity in the most prevalent lineage of symbiotic dinoflagellates (Symbiodinium, Dinophyceae) of the Caribbean. Mol Ecol 13: 459–469. [DOI] [PubMed] [Google Scholar]
- 36. Finney JC, Pettay DT, Sampayo EM, Warner ME, Oxenford HA, et al. (2010) The relative significance of host-habitat, depth, and geography on the ecology, endemism, and speciation of coral endosymbionts in the genus Symbiodinium . Microb Ecol 60: 250–263. [DOI] [PubMed] [Google Scholar]
- 37. Andras JP, Kirk NL, Harvell CD (2011) Range‐wide population genetic structure of Symbiodinium associated with the Caribbean sea fan coral, Gorgonia ventalina . Mol Ecol 20: 2525–2542. [DOI] [PubMed] [Google Scholar]
- 38. Pettay DT, Lajeunesse TC (2007) Microsatellites from clade B Symbiodinium spp. specialized for Caribbean corals in the genus Madracis . Mol Ecol Notes 7: 1271–1274. [Google Scholar]
- 39. Rowan R, Knowlton N (1995) Intraspecific diversity and ecological zonation in coral-algal symbiosis. P Natl Acad Sci 92: 2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goulet TL, Coffroth MA (2003) Stability of an octocoral-algal symbiosis over time and space. Mar Ecol Prog Ser 250: 117–124. [Google Scholar]
- 41. LaJeunesse TC, Lee S, Bush S, Bruno JF (2004) Persistence of non-Caribbean algal symbionts in Indo-Pacific mushroom corals released to Jamaica 35 years ago. Coral Reefs 24: 157–159. [Google Scholar]
- 42. LaJeunesse T (2004) “Species radiations of symbiotic dinoflagellates in the Atlantic and Indo-Pacific since the Miocene-Pliocene transition. Mol Biol Evol. 22: 570–581. [DOI] [PubMed] [Google Scholar]
- 43. Berkelmans R, van Oppen MJH (2006) The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proc Biol Sci 273: 2305–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goulet TL, LaJeunesse TC, Fabricius KE (2008) Symbiont specificity and bleaching susceptibility among soft corals in the 1998 Great Barrier Reef mass coral bleaching event. Mar Biol 154: 795–804. [Google Scholar]
- 45. Baker A (2001) Reef corals bleach to survive change. Nature 411: 765–766. [DOI] [PubMed] [Google Scholar]
- 46. LaJeunesse TC, Smith RT, Finney J, Oxenford H (2009) Outbreak and persistence of opportunistic symbiotic dinoflagellates during the 2005 Caribbean mass coral ‘bleaching’ event. Proc Roy Soc B 276: 4139–4148.47. Rowan R (2004) Coral bleaching: Thermal adaptation in reef coral symbionts. Nature 430: 742–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goulet TL (2006) Most corals may not change their symbionts. Mar Ecol Prog Ser 321: 1–7. [Google Scholar]