Abstract
Curcumin, the main constituent of turmeric, is suspected to possess cancer chemopreventive properties. Pharmacokinetic and pharmacodynamic parameters have been reported, but few data exist describing whether methodologies are suitably robust for curcuminoid detection in colonic biopsy specimens. Information on the acceptability of prolonged administration of daily curcumin is not available. This is of vital importance to implement chemoprevention strategies. This study aimed to quantify levels of curcuminoids in colorectal mucosa of patients undergoing colorectal endoscopy or surgical resection and to obtain information on the acceptability and compliance with daily curcumin. Curcumin C3-complex (2.35 g) was administered to patients once daily for 14 days prior to endoscopic biopsy or colonic resection. Safety and tolerance were monitored. Analysis of curcuminoids in plasma, urine and colonic mucosa was performed by UPLC-UV with characterisation by tandem LC-MS/MS. Twenty four out of 26 patients commencing curcumin completed the course. Six patients reported mild gastro-intestinal adverse events. Curcuminoids were detectable in 9/24 plasma samples, 24/24 urine samples and in the colonic mucosa of all 23 biopsied participants. Mean tissue levels were 48.4 μg/g (127.8 nmol/g) of parent curcuminoids. The major conjugate, curcumin glucuronide, was detectable in 29/35 biopsies. High levels of topical curcumin persisted in the mucosa for up to 40 h post-administration. Sixteen participants (67%) stated that they would take curcumin long-term should it be of proven benefit. In summary, pharmacologically active levels of curcumin were recovered from colonic mucosa. The regimen used here appears safe, and patients support its use in long-term trials.
Keywords: Curcumin, colon cancer, colonic polyps, chemoprevention, clinical trial
INTRODUCTION
Curcumin, the major constituent of the spice turmeric, has been the subject of increasing interest as having the potential to prevent and treat colorectal cancer (1) and may be of benefit in cardiovascular (2) and Alzheimer’s disease (3). It has demonstrated chemopreventive activity in a variety of in vitro cell-based systems and in vivo pre-clinical models (4-6). In the ApcMin mouse model of inherited colorectal cancer (7), dietary curcumin reduced adenoma formation by 39-64% (8, 9), decreased expression of cyclo-oxygenase 2 and endogenous DNA damage in adenoma tissue (10) and ameliorated levels of inflammatory markers interleukin-1b and chemokine ligand 2 within intestinal polyps (11). In clinical studies curcumin has been administered safely at doses of up to 12 g daily over 3 months (12). Curcumin and related species (collectively known as curcuminoids) have been characterised and quantified in plasma as well as colorectal and liver tissues from cancer patients receiving 1.8 g of daily curcumin (1, 13-15) whilst analogous studies have been performed in healthy volunteers taking 12 g daily (16). The lower dose produced colonic tissue concentrations of curcumin of an order of magnitude associated with pharmacological effects, both in cells in vitro and in rodents in vivo (17). However, it is unclear from these investigations how much of the curcumin recovered from the gut mucosa reflects material loosely adherent to the mucosal surface, as compared to agent absorbed into the tissue, which may elicit pharmacological activity.
The ultimate goal of translational research using curcumin as a chemopreventive agent for colorectal cancer is to establish an optimal dose, schedule and formulation for its administration to individuals at high risk of developing this disease. Unfortunately, information relating dose, schedules and formulations to achieving relevant target tissue levels and clinical efficacy is currently incomplete. Thus, the aim of the study described here was to quantify levels of curcumin in normal colorectal mucosa in patients undergoing colorectal endoscopy or cancer resection. These individuals received the curcumin formulation ‘Curcumin C3 complex’ (Sabinsa), which contains small amounts (combined total ~20%) of desmethoxycurcumin (DMC) and bisdesmethoxycurcumin (BDMC) in addition to curcumin. The hypothesis tested was that most of the curcuminoids recovered from the gut tissue were absorbed into the mucosa, and thus capable of eliciting direct pharmacological effect. Curcuminoids were measured using a UPLC-UV method developed in our laboratory (18) with the identity of UV-detected curcuminoids confirmed with tandem MS/MS. Tissue levels could be compared with those known to elicit benefit in pre-clinical models.
Curcumin was chosen as having the potential to combine many of the suggested mechanisms of aspirin and non-steroidal anti-inflammatory agents used in chemoprevention (19) but to have the potential for better patient compliance as it appears to lack serious toxicity. Little is known about possible barriers to patient uptake and compliance in the context of chemoprevention, but this is a crucial issue when developing agents for clinical evaluation. Therefore an additional component of this trial was to assess in a preliminary fashion the attitudes of this patient population towards curcumin and the experience of its use through the use of a questionnaire.
MATERIALS AND METHODS
Investigational medicinal product
Standardised turmeric extract ‘Curcumin C3 complex’, was provided by Sabinsa Corporation (Utah, USA), and encapsulated into gelatine capsules (Nova Laboratories, Leicestershire, UK) under conditions of Good Manufacturing Practice. Each capsule contained 470 mg of curcumin C3 complex consisting of 80% curcumin and 20% DMC/BDMC.
Patients
This pilot study (EUDRACT-2007-001971-13) was sponsored by the University Hospitals of Leicester (UHL) Trust, Leicester, UK and conducted at UHL and St Mark’s Hospitals (SMH), Harrow, UK. The study was approved by the Northern and Yorkshire Research Ethics Committee (UK) and conducted in accordance with the Declaration of Helsinki and guidelines on Good Clinical Practices. Twenty eight patients (20 awaiting lower gastrointestinal endoscopy and 8 scheduled for colorectal resection of primary disease) meeting the following criteria were recruited: positive faecal occult blood as part of colorectal screening programme, awaiting diagnostic or surveillance endoscopy or diagnosis of colorectal cancer; age >18 years; provide informed consent and comply with the protocol; reliable contraception if premenopausal female; no history in the past year of gastro-duodenal ulcer; no significant medical or psychiatric problems; no use of investigational agents within the last 3 months; no prior pelvic radiation. NSAID use was recorded and three patients completing the study took these regularly. Two participants who routinely took 75 mg oral daily aspirin continued to take their prescription during the trial. A third patient stopped taking aspirin for two weeks in anticipation of major surgery.
Study Design
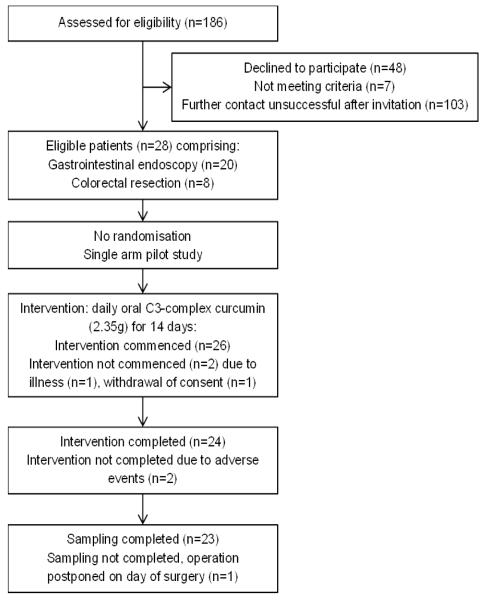
This was a pilot study administering 5 × 470 mg capsules (total 2.35 g curcuminoids) of daily oral Curcumin C3 complex for 14 days. This dose was chosen on the basis of the following three considerations: i. Previous trials at this centre and elsewhere demonstrated the safety and tolerability of this dose level when administered daily for up to 4 months. The dose was similar to that used in a study involving four colorectal cancer patients, in whom 1.8 g curcumin resulted in a measurable pharmacodynamic change, i.e. a reduction of malondialdehyde-DNA adduct levels in colorectal neoplastic tissue, compared to pre-administration levels in biopsy samples (14). ii. In a pre-clinical study in the ApcMin+ mouse model of gastro-intestinal carcinogenesis (9), the minimal (dietary) dose which decreased adenoma number was 0.2% in the diet, translating into approximately 1.8 g per 80 kg human per day, when extrapolated on the basis of body surface area. iii. The daily capsule number was compatible with patient acceptability. Capsules were taken once daily with food at the same time each day, with the final dose taken the day before the procedure. Plasma samples were obtained prior to curcumin ingestion and on the day of the procedure. A 24 h urine collection was obtained between the last two doses. Tissue was collected from endoscopy or surgery scheduled for the day after the final dose of curcumin. The summary of enrolment procedures and participant movement through the study is shown in the Consolidated Standards of Reporting Trials (CONSORT) chart in Figure 1. Participant compliance and the development of adverse events (AEs) were monitored using a daily diary which was supported by non-validated pre- and post-trial questionnaires that reported the thoughts and experiences of participants on curcumin. AEs were recorded according to Common Terminology Criteria Adverse Event reporting system version 3 (CTC-AE V3) (20). The primary objective was to determine levels of curcumin and its metabolites achieved in normal colorectal tissue of patients following a 14 day course. Secondary objectives were to assess the practicality, acceptability and safety of individuals taking curcumin.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) chart for this study.
Sample Collection
Plasma samples were obtained between 6.5 and 35.5 h post curcumin dose (median 24.5 h). Plasma and urine were obtained from 24 participants. Four biopsy samples were taken from healthy mucosa at endoscopy using cold forceps. For colorectal cancer patients, tissue that was surplus to diagnostic requirements was sampled. Complete bowel preparation with large volume osmotic laxative was used prior to colonoscopic examination and a phosphate enema was used before sigmoidoscopy. Pre-operative bowel preparation for patients undergoing left-sided resection consisted of a 72 h low-residue diet followed by a phosphate enema. Colonic mucosal biopsies were obtained from 23 participants (sampling time post dose: 14 – 39.5 h, median 26.5 h). All samples were stored at −80°C until analysis.
Materials and reagents
Acetonitrile, ammonium acetate, methanol, acetone and organo-phosphoric acid were purchased from Fisher Scientific (Loughborough, UK). Glacial acetic acid, dimethylsulphoxide, potassium chloride (KCl) and quercetin were from Sigma (Dorset, UK) and S9 human liver microsomes with UDPGA co-factor solutions A and B were from Becton Dickinson (Oxford, UK).
Curcuminoid sulphates were chemically synthesised in-house (21) using sulphur trioxide – dimethylformaldehyde (DMF) complex (Sigma, Dorset, UK). Curcumin C3-complex (200 mg) was dissolved in pyridine (1.25 mL) and DMF (3.75 mL) then sulphur trioxide - DMF complex (166 mg) was added to the solution which was incubated for 4 h at 37°C. Sodium bicarbonate (116 mg) was then added and thin layer chromatography (TLC) was performed for crude separation of new compounds supported by MS/MS. The reaction mixture was filtered and dried in vacuo. The crude reaction mixture was purified by flash column chromatography using silica (silica 60) and ethanol/ dichloromethane (1:9→4:6→7:3) as mobile phase. Pooled fractions were dried, redissolved in methanol and filtered prior to precise analysis by UPLC-UV and tandem LC-MS/MS by the method described below. The dominant curcumin monosulphates eluted after the parent compounds, at approximately 12 min (Figure 2a). The retention time of curcumin parent compounds ranged from 10.2 to 10.7 min.
Figure 2.
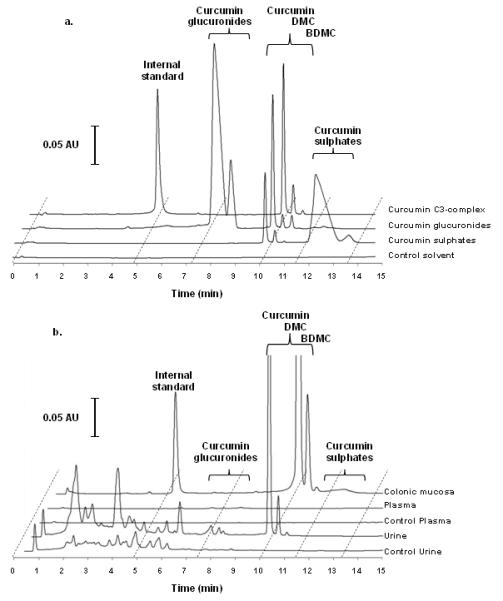
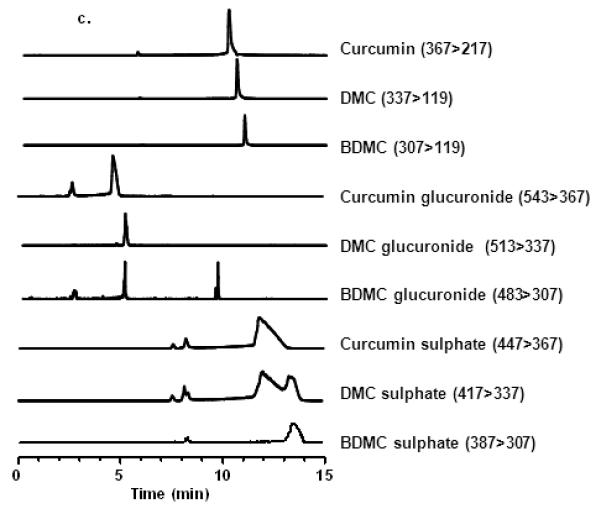
UPLC-UV chromatography of (a) standards of C3-complex curcumin, biosynthetic curcumin glucuronides, chemically synthesised curcumin sulphates and control solvent, and (b) extracts of colonic mucosa, plasma and urine from patients who had received oral C3-complex curcumin (2.35 g daily for 14 days) and plasma and urine from individuals who had yet to receive the agent (control biomatrix). Plasma and tissue bio-matrices were obtained approximately 24 h after the final dose of curcumin. Urine was collected continuously for the 24 h between the last two doses. (c) LC-MS/MS of curcuminoids present in curcumin and metabolite standards obtained in selected reaction monitoring (SRM) mode with the m/z transitions for each analyte in brackets.
Curcumin glucuronides were bio-synthesised in vitro following the previous method (18) with the following modifications: Curcumin C3-complex (2 μL of 2.5 mM stock=1.84 μg) was incubated for 30 min at 37°C with S9 human liver microsome preparation (12.5 μL of 20 mg/mL, BD Biosciences, Oxford, UK) and UDPGA cofactor solutions A (20 μL) and B (50 μL) made up to 250 μL with water. The reaction was stopped by the addition of ice-cold acetone (250 μL) and the mixture was incubated at -20 °C for 15 min. Protein was removed by centrifugation (6000 × g, 5 min) and the supernatant analysed as described below. The dominant curcumin monoglucuronides eluted before the parent compounds, at approximately 7.5 min (Figure 2a).
Sample preparation and analysis
Extractions were performed using methods previously validated in our laboratory (18) and adjusted to cater for minute mucosal biopsies and 1 mL plasma and urine samples. In brief, 1 mL of plasma or urine was diluted with 1 mL water and acidified with o-phosphoric acid (0.5 % final concentration). The acidified samples (2 mL) were loaded onto two 1 cc Oasis HLB cartridges (Waters, Elstree, UK), washed with acidified methanol (methanol:water:glacial acetic acid (25:25:1) preconditioned with 1 mL water and then 1 mL methanol and eluted with acidified methanol (2 % glacial acetic acid). The eluant was evaporated to dryness at 45 °C under nitrogen, and the combined residue re-suspended in 40 μL of methanol: glacial acetic acid (95:5). A final centrifugation was performed (16000 × g, 10 min) and the volume injected on to the column was 20 μL. The parent curcuminoids and curcuminoid glucuronides could be recovered by this method, with extraction efficiencies of >60 % and >25 % respectively, curcumin sulphates were recovered at only trace levels (<1 %) in the eluant obtained from HLB cartridges.
To quantify the levels of curcumin adherent to the mucosa, homogenisation was performed both with and without first extensively washing the biopsies. . The assay was validated with rodent and human colonic mucosa. Preliminary experiments using a small surplus of tissue demonstrated that surface levels of curcumin reached a steady state after seven separate washes, therefore, one of each set of biopsies was washed seven times in 1.15% KCl. All samples were then homogenised in 1.15% KCl, and quercetin (150 μg/mL) added as an internal standard prior to extraction with acidified acetone (10% glacial acetic acid). Solid matter was removed by centrifugation (16000 × g, 10 min), the supernatant evaporated to dryness at 45°C under nitrogen and the residue redissolved in 40 μL of methanol: glacial acetic acid (90:10). A final centrifugation was performed (16000 × g, 10 min). Washes obtained from the tissue samples were also processed by this extraction method
Curcuminoids were separated and quantified using an Acquity UPLC system (Waters, Elstree, UK) with a BEH RP18 (2.1 × 100 mm, 1.7 μm) column (Waters, Elstree, UK) at 35°C, as described previously (18). In brief, the mobile phase A consisted of 5 % aqueous acetonitrile adjusted to pH 3.5 with acetic acid whilst mobile phase B was 100 % acetonitrile. The elution gradient at 0 min was 15 % flow rate B at 0.54 mL/min; at 10 min, 45 % B at 0.6 mL/min; at 14 min, 42 % B at 0.6 mL/min; at 16 min, 100% B at 0.85 mL/min. The column was then washed with 100 % B for a further 2 min at 0.85 mL/min before re-equilibrating. The eluant was monitored at 426 nm. The limits of detection (LOD) and quantification (LOQ) for curcumin were consistent with previously published values of 0.5 ng and 1 ng on column. The LOD and LOQ for both plasma and urine were 1.25 ng/mL and 2.5 ng/mL and for tissue, 125 ng/g and 500 ng/g respectively.
Identification and quantification of curcumin metabolites in colonic tissue
LC-MS/MS characterisation was performed by coupling a Waters Xevo TQ to an Acquity UPLC using a 2:1 T-piece after the UV detector. LC-MS/MS was performed with negative polarity and conditions consisting of capillary voltage 3 kV, cone voltage 30 V, source temperature 150°C, desolvation temperature 500°C, desolvation gas flow 1200 L/h and collision energy 20 eV. Dried supernatant was re-dissolved in methanol: water (1:1).
Curcuminoid sulphates and glucuronides were analysed by UPLC-UV (Figure 2a and 2b) and tandem MS/MS (Figure 2c) using selected reaction monitoring (SRM), which enabled identification of the parent compounds and major metabolites based on characteristic m/z transitions employed previously (18). The m/z transitions for the dominant and most abundant compounds are shown on Figure 2c with the corresponding LC-MS/MS chromatogram. Levels of curcumin glucuronides and sulphates were estimated through the use of the curcumin standard curve.
Statistical analysis
Statistical analysis was performed with SPSS version 18, Chicago, USA (Windows XP). P values <0.05 were considered significant. Data are presented as means, range and with standard error of the mean (SEM). Comparison of paired post-intervention tissues was made by the non-parametric Wilcoxon-Mann-Whitney test because of the small sample size. Correlations within tissue sample sets were analysed using the Spearman coefficient with two-tailed significance testing.
RESULTS
Participants
Twenty eight patients were recruited into this study (for demographics see Table 1), with 18 recruited at the UHL and 10 at SMH. Of the 28 participants, 26 commenced curcumin, 24 completed the prescribed course and 23 met full sampling requirements. Two participants did not start curcumin, one withdrew consent and the other dropped out due to illness. Two participants withdrew after the course began due to adverse events. One participant completed the full course of curcumin, but the scheduled procedure was postponed and tissue was not obtained. Samples consisted of 12 pairs of matched left- and right-sided colonoscopic biopsies in duplicate, 6 quadruplicate left-sided sigmoidoscopic biopsies and 5 quadruplicate full-thickness surgical biopsies from 4 left-sided and 1 right-sided colonic resections.
Table 1.
Demographics for the 28 recruited participants. BMI: Body Mass Index; F: female; M: male. Values in brackets show range.
| Mean age (years) | 57.5 (35 – 82) |
| Gender | |
| Male | 13 |
| Female | 15 |
| Mean BMI (Kg/m2) | 31.2 (15.5 - 48.3) |
| WHO performance status | |
| 0 | 22 |
| 1 | 6 |
| Procedure (number completed/number planned) | |
| Sigmoidoscopy | 6/7 |
| Colonoscopy | 12/13 |
| Right hemicolectomy | 1/2 |
| Left hemicolectomy or rectal resection | 4/6 |
| Participants completing curcumin course | 24/28 |
| Participants completing all procedures | 23/24 |
Participant’s attitudes to curcumin
Pre-trial questionnaires assessing the patients’ knowledge of curcumin revealed that 21 (81%) participants had previously heard of turmeric, but only 14 (54%) had ever knowingly used it in their diet. Only one participant had previously used turmeric or curcumin as a health food supplement although 10 people reported using other food supplements. Most (85%) had planned when best to take the capsules and how to integrate them with existing medication, with the majority preferring a morning schedule. Three patients felt they may have problems remembering to take regular capsules, however upon conclusion of the trial none reported this concern. Thirty seven percent of responders felt they would not want to miss a dose of curcumin, and this number significantly increased (p=0.001) to 91% by the end of the study. Although 46% of patients agreed they were worried that curcumin may produce unpleasant side-effects at the start of the trial, 91% disagreed by the end. Upon completion of the course, 3 patients felt that 5 capsules may present a barrier to compliance, and 4 reported that the capsule size (00) used here may be problematic. Two patients commencing the course failed to finish it. Two-thirds of patients would recommend curcumin as a long-term chemopreventive agent if proven to be effective.
Safety of curcumin
In total, 13 AEs (NCI-CTC v3.0, grades 1 - 2) possibly or probably attributable to curcumin were recorded in 6 participants, all of which were gastrointestinal in nature (Table 2). Comparison of matched baseline (pre-curcumin) with post-trial questionnaires revealed a decrease in all self-reported gastrointestinal disturbances (constipation, diarrhoea, flatulence, incontinence and abdominal pain) following two weeks of curcumin administration (Table 3). There were no differences in the overall general health and activity of participants during the trial (data not shown). There were no serious adverse events (SAEs) attributable to curcumin. Of the 24 participants that completed the course, 16 (67%) stated that they would regularly take curcumin as an adjunct to current medication if it were shown to be of benefit. Importantly the number of patients stating the size or number of capsules to be swallowed may present a barrier to compliance before they started the course actually reduced slightly by the end of the trial.
Table 2.
Summary of adverse events (AE) deemed possibly or probably attributable to curcumin.
| Adverse event | Number of events | Grade | Number of patients |
|---|---|---|---|
| Total gastrointestinal events | 13 | 1, 2 | 6 |
| Abdominal pain | 3 | 1,2 | 3 |
| Bloating | 1 | 2 | 1 |
| Diarrhoea | 3 | 1 | 3 |
| Dyspepsia | 1 | 1 | 1 |
| Flatulence | 1 | 1 | 1 |
| Nausea | 2 | 1,2 | 2 |
| Vomiting | 2 | 1,2 | 2 |
Table 3.
Pre- and post-curcumin self-reported questionnaire assessments from 24 participants that completed the course of curcumin. Baseline responses refer to the period 3 months prior to commencing curcumin. Post-trial responses were completed at the end of the 2 week curcumin course and refer the period of administration.
| Responses (% participants) |
||
|---|---|---|
| Baseline | Post-trial | |
| Thought capsule size would be difficult to swallow | 26 | 17 |
| Thought 5 capsules at once would be difficult to swallow | 19 | 13 |
| Experienced constipation | 36 | 13 |
| Experienced diarrhoea | 52 | 38 |
| Experienced wind | 70 | 58 |
| Experienced abdominal pain | 37 | 21 |
| Experienced bowel incontinence | 22 | 12 |
Levels of curcuminoids in colonic mucosa
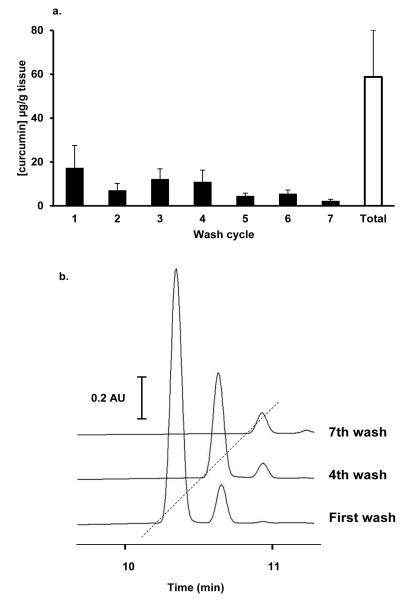
Curcuminoid concentrations were measured by UPLC-UV (Figure 2b) in colorectal tissue biopsies ranging in mass from 0.5 to 6.75 mg (median 2.9 ± 0.25 mg). Parent compounds, which eluted between 10.2 and 10.7 min, were present in the mucosa of every patient. In order to explore how much of the measured curcuminoids were loosely bound to the mucosa and how much had been absorbed into the tissue, volume-matched biopsy samples were subjected to a series of KCl washes. After seven washes, curcumin levels detected in the wash buffer were typically below the limit of quantification (Figure 3). Levels of curcuminoids remaining in washed tissues were between 17% (surgical) and 74% (endoscopic) of those measured in unwashed samples, with mean washed tissue levels of 18.85 ± 6.8 μg/g, 3.6 ± 1.4 μg/g and 1.6 ± 1.0 μg/g for curcumin, DMC and bDMC, respectively (Table 4). There was a relationship between the curcumin levels of unwashed left sided and right sided biopsies taken from the same patient (n=12 pairs, Spearman correlation coefficient 0.636, p=0.035). The level of curcumin measured in individual unwashed samples appeared to reflect the amount found in the tissue from the same patient after washing. (n=35 pairs, Spearman correlation coefficient 0.421, p=0.013). Unwashed tissue levels of curcumin ranged from 0.7 μg/g (from a left-sided colonoscopic biopsy) to 681 μg/g (from a flexible sigmoidoscopy biopsy), with a mean of 48.4 μg/g (Table 4). In paired right- and left-sided endoscopic biopsy samples, mean pre-wash curcumin levels were 35.4 and 9.0 μg/g respectively and significantly different (p=0.021). In contrast, there was no difference in levels after tissue had been washed (p=0.347). DMC and bDMC were detectable in 27 out of 35 mucosal samples (range 0.2 – 83.9 μg/g, mean 7.1 μg/g) and in 15 out of 35 (range 0.03 – 2.8 μg/g, mean 0.7 μg/g) mucosal samples, respectively. Curcumin glucuronides (eluting between 7.7 and 8.5 min), which constituted the most abundant conjugates, were detected in 19 out of 23 patients at levels which were approximately one tenth of those seen for curcumin (range 0.33 – 24.7 μg curcumin equivalents/g, mean 4.5 μg/g). Peaks with retention times corresponding to those of curcuminoid sulphates were identified in the mucosa of 14 out of 23 patients (range 0.11 – 31.1 μg/g, mean 6.1 μg/g).
Figure 3.
(a) Mean curcumin (expressed as μg/g of tissue) present in consecutive washes from 1 right-sided, 4 left-sided, 6 sigmoidoscopy, 12 paired right-sided and 12 paired left-sided biopsy samples. Black bars show mean data from each wash cycle. White bar shows mean cumulative curcumin removed during wash cycles (n=35), ± SEM. (b) UPLC-UV chromatography of extracts from serial washes (using 1.15% KCl) performed on colonic mucosa to remove surface C3-complex curcumin. Up to seven consecutive washes were performed until excess surface curcumin had been removed and surface levels were at a steady state.
Table 4.
Mucosal levels of parent compounds curcumin, desmethoxycurcumin and bisdesmethoxycurcumin (mean concentration μg/g, ± SEM) from colonic biopsies pre- and post-wash cycles, quantified by UPLC-UV. Values in brackets represent nmol/g ± SEM. There was a relationship between the curcumin levels of unwashed left sided and right sided biopsies taken from the same patient (n=12 pairs, Spearman correlation coefficient 0.636, p=0.035). The level of curcumin measured in each unwashed sample appeared to reflect the amount found in the tissue from the same patient after washing. (n=35 pairs, Spearman correlation coefficient 0.421, p=0.013).
| Curcumin | Desmethoxycurcumin | Bisdesmethoxycurcumin | |||||
|---|---|---|---|---|---|---|---|
| Sample group | n | unwashed | washed | unwashed | washed | unwashed | washed |
| All biopsies | 35 | 48.4±20.9 | 18.85±6.8 | 7.07±3.24 | 3.55±1.38 | 0.71±0.20 | 1.59±1.00 |
| Surgical biopsies | 5 | 75.32±39.71 | 12.50±3.07 | 10.42±6.52 | 1.27±0.42 | 1.18±0.15 | 0.21±0.04 |
| Endoscopy biopsies | 30 | 42.31±23.68 | 19.91±7.87 | 6.33±3.75 | 4.03±1.69 | 0.59±0.24 | 1.86±1.19 |
Levels of curcuminoids in plasma
Curcuminoids were identified in the plasma (Figure 2b) obtained from 9 out of 24 participants with parent compounds quantifiable in 4 participants (range for curcumin 8.1 – 13.9 ng/ml, mean 12.2 ng/ml) and present in a further 2 at trace levels below the LOQ. Curcumin glucuronides were quantifiable in 7 participants (range 0.33 – 29.4 ng curcumin equivalents/ml, mean 4.9 ng/ml) and were present in a further 8 at trace levels below the LOQ. In addition, analysis of plasma extracts from a further 15 participants produced peaks with retention times consistent with those of the parent compounds but levels were below the LOD.
Levels of curcuminoids in urine
Curcuminoids were identified in the 24 h urine collections (Figure 2b) obtained from all participants with parent compounds quantifiable in 12 participants (range for curcumin 0.67 – 503.3 ng/ml, mean 27.0 ng/ml) and present in a further 8 at trace levels below the LOQ. Analysis of urine extracts from 4 participants revealed peaks with retention times of the parent compounds but levels were below the LOD. Curcumin glucuronides were quantifiable in all urine samples (range 2.93 – 64.8 ng curcumin equivalents/ml, mean 17.6 ng/ml).
DISCUSSION
This is one of the first studies to report the analysis of tissue curcumin levels in patients receiving repeat daily dosing. It is the first to assess the patient attitudes towards taking diet-derived chemopreventive agents.
Curcumin-induced AEs observed in clinical trials in cancer patients have been, almost exclusively, mild and self-resolving effects on the gastrointestinal (GI) tract (22, 23). Tolerability and acceptability of curcumin seems to decrease with increasing dose, and also appears to be related to the number and size of capsules required to deliver high doses (22) although consistency of AE reporting varies considerably between countries (24). In the study described here, 24 out of 26 patients (92.3%) completed a 14 day course of daily oral curcuminoids (2.35 g). In contrast to the accrued AE data, only one participant felt that this regimen produced unpleasant side-effects. The symptoms reported as AEs, predominantly abdominal pain, bloating, nausea and diarrhoea may have been part of normal gastrointestinal behaviour within this patient cohort. It is therefore conceivable that attribution of GI AEs in patients to the doses of curcumin described in this, and several past studies of similar patient cohorts (1, 15), may not accurately reflect the side-effect profiles of curcumin. Moreover, comparison of pre- and post-trial questionnaire data actually suggests a reduction in patient reported bowel symptoms during the trial period. If these observations are confirmed in further trials, daily doses of up 2-3 g of curcumin may be considered safe and well tolerated even during prolonged administration.
The novel method of pharmacokinetic (PK) analysis used in this study measures tissue levels of curcumin both before and after tissue has been washed copiously with an aqueous salt solution. The majority of curcumin present in a tissue sample is probably adherent to the surface and not intracellular. Reported studies of a similar nature (1, 13) have not attempted to quantify curcumin which is bound to the surface, and details regarding sample cleaning processes prior to analysis are lacking. Direct comparison of human GI tissue levels of curcumin between studies is therefore difficult. Daily administration of curcumin (2 g) for 30 days (1) resulted in mean concentrations of 8.2 ± 2.9 μg/g curcumin in rectal mucosa in 5 of 21 patients and curcumin conjugates of 5.9 ± 2.6 μg/g in 13 patients implying that the levels of curcumin and curcumin metabolites in the remaining patients were below the limit of quantification. In our study, curcumin was detectable in the GI tract of all patients recruited after receiving a similar dose of curcumin daily for 14 days. Levels of the parent compound in tissue were 48.4 ± 20.9 μg/g without prior washing, which has been standard practice for both clinical and in vivo studies (9, 13, 25). Levels in unwashed tissue were 5.7-fold higher than those in the previous study (1), however washing the tissue reduced this difference to only 2-fold. Curcumin glucuronides, the major curcumin metabolites (25, 26) were semi-quantitated in our study whereas Carroll et al. (1) estimated total curcumin conjugates obtained by enzymatic hydrolysis in their study. Here the mean level of curcuminoid glucuronides, when quantifiable, was 8.4 nmol/g (4.54 μg curcumin equivalents/g) found in 27 of 35 (77%) samples. This value is similar to the amount of total curcumin conjugates reported by Carroll et al., where metabolites were not precisely characterised. Therefore, despite the differences in levels of parent compound, similar levels of conjugates would suggest that intracellular curcumin levels available for metabolism were of a similar order of magnitude.
A clear disparity in curcumin tissue levels between unwashed paired right and left-sided biopsies was apparent. A similar discrepancy in agent level has been observed in colorectal cancer patients who received daily oral resveratrol (21). Consistent with the explanation given for resveratrol (21), differences in gut content and faecal liquidity between the right and left sides may be responsible. The right-sided colonic mucosa is likely to be in contact with higher concentrations of faecal curcumin than the left. Curcumin is highly lipophilic and poorly soluble in aqueous media (6, 27) and appears to persist on the colon for a considerable length of time and may therefore provide a period of sustained action to the mucosa. Consecutive washes of mucosa in paired right-sided biopsy samples to remove loosely bound material resulted in a 74% decrease in tissue curcumin. Topical levels of curcumin were higher on the right but tissue levels did not significantly differ between the left and right sides after washing. This finding implies that curcumin is similarly absorbed on both sides of the colon which suggests that curcumin accesses gastrointestinal tissue mainly via the systemic circulation rather than the topical route. Bowel preparation techniques varied depending on the procedure being undertaken and this is likely to have had an effect on the levels of surface curcumin, particularly in unwashed mucosa. One participant undergoing right hemi-colectomy, and therefore receiving no bowel preparation of the biopsied area, had particularly high levels of tissue (228.3 μg/g) and surface curcumin (551.1 μg/g). Mucosal samples were biopsied up to 40 h post curcumin administration, indicating that despite iatrogenic bowel cleansing, high GI tissue levels are likely to persist for several days. This finding suggests that less than once daily dosing regimens might generate curcumin levels in the GI tract sufficient for activity in long-term polyp prevention trials. Dose scheduling has considerable implications in terms of participant compliance and study cost, with intermittent dosing schedules being associated with higher adherence than daily dosing regimens (28). Intermittent dose regimens may also decrease the incidence of AEs. However, it is increasingly likely that systemic rather than topical curcumin may well be the major source of intra-mucosal agent and prolonged availability of intraluminal curcumin is perhaps not so important, which is in contention with employing a less than once daily dose schedule.
PK studies with healthy volunteers (16, 22, 29) and colorectal cancer patients (13) reveal curcumin has a plasma Tmax between 1 and 4 h, with conjugates persisting for up to 36 h after 10 g of curcumin (16). In this study, plasma levels of parent curcumin were quantifiable in only 4 participants (mean 12.2 ng/mL) and glucuronides in 7 participants (mean 4.9 ng/mL). Detection of the parent compound would only be expected at trace levels because blood sampling was performed on average 24 h post-dose. This is important, because if curcumin is to be investigated in long-term polyp prevention regimens, evidence of systemic accumulation and rate of clearance should be considered when optimising prescription schedules. Carroll et al. (1) did not detect curcuminoids in plasma of patients receiving 2 g daily oral curcumin, however this disparity may be a result of the four-fold greater LOQ of their methodology. Detectable curcuminoid or curcuminoid conjugates were observed in the urine of all participants in this study, intimating that adherence monitoring for low dose curcumin in future trials could utilise urine sampling as suggested previously (29).
The questionnaire responses provide a valuable, albeit preliminary, insight into the patient perspective of long-term use of curcumin, which is vital for the optimal design of future trials. The use of dietary agents by patients is reported to be in excess of 50% (30) with a tendency to increase following a cancer diagnosis (31), although the majority of usage appears to be with vitamins and minerals rather than plant extracts (32). Forty percent of recruits to this study had used health food supplements, although only 20% had heard of curcumin as compared to 81% of the participants who were acquainted with the spice turmeric, and none of these had previously used it. It has been suggested that large or numerous capsules required to deliver higher doses may present problems with compliance (22). By the end of this trial, only 3 patients felt that 5 capsules may present a barrier to compliance and 4 reported that the capsule size (00) used here may be problematic. Only 2 patients commencing the course failed to finish it. It is promising that two-thirds of patients recommended curcumin as a long-term chemopreventive agent if proven to be effective.
We acknowledge there are limitations to this study which include the selection of a single dose instead of several dose levels, the timing of the tissue sample collection vis-à-vis the time of the last dose administered, the lack of a control arm, the heterogeneous nature of the patient population and the relatively short duration of the intervention. Several of these limitations are, at least to some extent, governed by aspects of clinical care pathways optimised for patients undergoing investigation or management of cancer and the availability of a sufficient number of participants with which to complete the study.
In summary, the analytical methodology described here provides robust and sensitive quantification of curcuminoids; such methods are vital in linking PK effects, on an individual basis, to target tissue concentrations in trials of chemopreventive agents. Pharmacologically active levels of curcumin were recovered from the bowel mucosa even after multiple tissue washes, and there was no systemic curcumin accumulation. These findings provide further support for the safety of curcumin and its potential usefulness in long-term colorectal cancer prevention strategies.
Precis: Pharmacologically active levels of curcumin were recovered from colonic mucosa, the regimen used here appears safe, and patients support its use in long-term trials.
Acknowledgements
The authors would like to thank the staff at the University Hospitals Leicester, the Comprehensive Local Research Network, and the National Cancer Research Network. Thank you specifically to Sarah Porter, Hadia Haque, Elizabeth Andrzejewski and Debbie Glancy.
Financial support: This work was funded by a Cancer Research UK CTAAC award [C325/A7255] and programme grant [C325/A6691] with additional support from the Leicester Experimental Cancer Medicine Centre [C325/A15575], which is funded by Cancer Research UK in conjunction with the UK Department of Health, and by the Royal College of Surgeons Augustus Newman Fellowship with support from Rosetree’s Trust.
Footnotes
Conflict of interest: None to declare
The authors disclose no potential conflicts of interest
References
- 1.Carroll RE, Benya RV, Turgeon DK, Vareed S, Neuman M, Rodriguez L, et al. Phase IIa Clinical Trial of Curcumin for the Prevention of Colorectal Neoplasia. Cancer Prev Res (Phila) 2011;4(3):354–64. doi: 10.1158/1940-6207.CAPR-10-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugawara J, Akazawa N, Miyaki A, Choi Y, Tanabe Y, Imai T, et al. Effect of endurance exercise training and curcumin intake on central arterial hemodynamics in postmenopausal women: pilot study. American journal of hypertension. 2012;25(6):651–6. doi: 10.1038/ajh.2012.24. [DOI] [PubMed] [Google Scholar]
- 3.Baum L, Lam CW, Cheung SK, Kwok T, Lui V, Tsoh J, et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol. 2008;28(1):110–3. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- 4.Epstein J, Sanderson IR, Macdonald TT. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. The British journal of nutrition. 2010;103(11):1545–57. doi: 10.1017/S0007114509993667. [DOI] [PubMed] [Google Scholar]
- 5.Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? The AAPS journal. 2009;11(3):495–510. doi: 10.1208/s12248-009-9128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer. 2005;41(13):1955–68. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247(4940):322–4. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 8.Mahmoud NN, Carothers AM, Grunberger D, Bilinski RT, Churchill MR, Martucci C, et al. Plant phenolics decrease intestinal tumors in an animal model of familial adenomatous polyposis. Carcinogenesis. 2000;21(5):921–7. doi: 10.1093/carcin/21.5.921. [DOI] [PubMed] [Google Scholar]
- 9.Perkins S, Verschoyle RD, Hill K, Parveen I, Threadgill MD, Sharma RA, et al. Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol Biomarkers Prev. 2002;11(6):535–40. [PubMed] [Google Scholar]
- 10.Tunstall RG, Sharma RA, Perkins S, Sale S, Singh R, Farmer PB, et al. Cyclooxygenase-2 expression and oxidative DNA adducts in murine intestinal adenomas: modification by dietary curcumin and implications for clinical trials. Eur J Cancer. 2006;42(3):415–21. doi: 10.1016/j.ejca.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 11.Murphy EA, Davis JM, McClellan JL, Gordon BT, Carmichael MD. Curcumin’s effect on intestinal inflammation and tumorigenesis in the ApcMin/+ mouse. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2011;31(2):219–26. doi: 10.1089/jir.2010.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irving GR, Karmokar A, Berry DP, Brown K, Steward WP. Curcumin: the potential for efficacy in gastrointestinal diseases. Best practice & research Clinical gastroenterology. 2011;25(4-5):519–34. doi: 10.1016/j.bpg.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Garcea G, Berry DP, Jones DJ, Singh R, Dennison AR, Farmer PB, et al. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev. 2005;14(1):120–5. [PubMed] [Google Scholar]
- 14.Garcea G, Jones DJ, Singh R, Dennison AR, Farmer PB, Sharma RA, et al. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer. 2004;90(5):1011–5. doi: 10.1038/sj.bjc.6601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, et al. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7(7):1894–900. [PubMed] [Google Scholar]
- 16.Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z, et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1411–7. doi: 10.1158/1055-9965.EPI-07-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howells LM, Moiseeva EP, Neal CP, Foreman BE, Andreadi CK, Sun YY, et al. Predicting the physiological relevance of in vitro cancer preventive activities of phytochemicals. Acta Pharmacol Sin. 2007;28(9):1274–304. doi: 10.1111/j.1745-7254.2007.00690.x. [DOI] [PubMed] [Google Scholar]
- 18.Marczylo TH, Steward WP, Gescher AJ. Rapid analysis of curcumin and curcumin metabolites in rat biomatrices using a novel ultraperformance liquid chromatography (UPLC) method. J Agric Food Chem. 2009;57(3):797–803. doi: 10.1021/jf803038f. [DOI] [PubMed] [Google Scholar]
- 19.Half E, Arber N. Colon cancer: preventive agents and the present status of chemoprevention. Expert opinion on pharmacotherapy. 2009;10(2):211–9. doi: 10.1517/14656560802560153. [DOI] [PubMed] [Google Scholar]
- 20.US Department of Health and Human Services NCI Common Terminology Criteria for Adverse Events (CTCAE) (Version 3.0) 2006 Available from: http://ctep.cancer.gov/
- 21.Patel KR, Brown VA, Jones DJ, Britton RG, Hemingway D, Miller AS, et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010;70(19):7392–9. doi: 10.1158/0008-5472.CAN-10-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21(4B):2895–900. [PubMed] [Google Scholar]
- 23.Epelbaum R, Schaffer M, Vizel B, Badmaev V, Bar-Sela G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr Cancer. 2010;62(8):1137–41. doi: 10.1080/01635581.2010.513802. [DOI] [PubMed] [Google Scholar]
- 24.Joelson S, Joelson IB, Wallander MA. Geographical variation in adverse event reporting rates in clinical trials. Pharmacoepidemiology and drug safety. 1997;6(Suppl 3):S31–5. doi: 10.1002/(sici)1099-1557(199710)6:3+<s31::aid-pds288>3.3.co;2-w. [DOI] [PubMed] [Google Scholar]
- 25.Ireson CR, Jones DJ, Orr S, Coughtrie MW, Boocock DJ, Williams ML, et al. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev. 2002;11(1):105–11. [PubMed] [Google Scholar]
- 26.Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, Luo JL, et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61(3):1058–64. [PubMed] [Google Scholar]
- 27.Tonnesen HH. Chemistry of curcumin and curcuminoids. In: Ho C-T, Lee CY, Huang M-T, editors. Phenolic compounds in food and their effects on health I. Merican Chemical Society; 1992. pp. 143–53. [Google Scholar]
- 28.Kruk ME, Schwalbe N. The relation between intermittent dosing and adherence: preliminary insights. Clinical therapeutics. 2006;28(12):1989–95. doi: 10.1016/j.clinthera.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10(20):6847–54. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 30.Block G, Jensen CD, Norkus EP, Dalvi TB, Wong LG, McManus JF, et al. Usage patterns, health, and nutritional status of long-term multiple dietary supplement users: a cross-sectional study. Nutr J. 2007;6:30. doi: 10.1186/1475-2891-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Velicer CM, Ulrich CM. Vitamin and mineral supplement use among US adults after cancer diagnosis: a systematic review. J Clin Oncol. 2008;26(4):665–73. doi: 10.1200/JCO.2007.13.5905. [DOI] [PubMed] [Google Scholar]
- 32.Miller MF, Bellizzi KM, Sufian M, Ambs AH, Goldstein MS, Ballard-Barbash R. Dietary supplement use in individuals living with cancer and other chronic conditions: a population-based study. Journal of the American Dietetic Association. 2008;108(3):483–94. doi: 10.1016/j.jada.2007.12.005. [DOI] [PubMed] [Google Scholar]