Abstract
The first experimental artificial red blood cells have all three major functions of red blood cells (rbc). However, the first practical one is a simple polyhemoglobin (PolyHb) that only has an oxygen-carrying function. This is now in routine clinical use in South Africa and Russia. An oxygen carrier with antioxidant functions, PolyHb-catalase-superoxide dismutase, can fulfill two of the three functions of rbc. Even more complete is one with all three functions of rbc in the form of PolyHb-catalase-superoxide dismutase-carbonic anhydrase. The most advanced ones are nanodimension artificial rbc with either PEG-lipid membrane or PEG-PLA polymermembrane. Extensions in to oxygen therapeutics include a PolyHb-tyrosinase that suppresses the growth of melanoma in a mice model. Another is a PolyHb-fibrinogen that is an oxygen carrier with platelet-like function. Research has now extended well beyond the original research on artificial rbc into many areas of artificial cells. These include nanoparticles, nanotubules, lipid vesicles, liposomes, polymer-tethered lipid vesicles, polymersomes, microcapsules, bioencapsulation, nanocapules, macroencapsulation, synthetic cells, and others. These are being used in nanotechnology, nanomedicine, regenerative medicine, enzyme/gene therapy, cell/stem cell therapy, biotechnology, drug delivery, hemoperfusion, nanosensers, and even by some groups in agriculture, industry, aquatic culture, nanocomputers, and nanorobotics.
Keywords: Blood substitutes, polyhemoglobin, oxygen carrier, artificial cells, carbon dioxide, oxygen radicals, Nanomedicine
Artificial red blood cells
The first artificial red blood cells fulfilled the following three major functions of red blood cells: (1) oxygen transport (Chang 1957); (2) carbon dioxide transport (Chang 1964); and (3) antioxidant functions (Chang and Poznanski 1968). However, serious interest in this area did not start until the HIV-contaminated-donor blood crisis. By then, there was no time to carry out the much-needed basic research.
Hemoglobin based oxygen carriers (HBOC)
The urgency led to the development of different hemoglobin based oxygen carriers (HBOC) that have only one of the functions of red blood cells. One of these is based on the basic research on glutaraldehyde crosslinked polyhemoglobin (Chang 1971). The group from Northfield has independently developed glutaraldehyde crosslinked human polyhemoglobin and carried out very extensive clinical trials (Moore et al. 2009). The group from Biopure has independently developed glutaraldehyde crosslinked bovine polyhemoglobin and has also carried out very extensive clinical trials (Jahr et al. 2008). South Africa has approved the routine clinical uses of polyhemoglobin for a number of years and Russia has recently approved the routine clinical uses of polyhemoglobin. Another HBOC in the form of conjugated hemoglobin can be formed by the basic principle of crosslinking hemoglobin to polymer (Chang 1964, 1972). The one in development is a PEG conjugated hemglobin (Liiu and Xiu 2008, Winslow 2006).
HBOC only has one of the three major functions of red blood cells. However, the risk/benefit ratios of polyhemoglobin have already been shown in situations where rbc is not available (Moore et al. 2009, Jahr et al. 2008 ). Furthermore, polyhemoglobin can be sterilized to be free from HIV or other infective agents and it can also be stored at room temperature for more than one year. This is compared to donor red blood cells that require storage at 4 °C for up to only 42 days. Furthermore, there are questions of whether this length of storage of donor blood would lead to adverse effects (Roa et al. 2005).
Oxygen carriers with antioxidant functions
In the meantime, studies are being carried out towards oxygen carriers with antioxidant functions to fulfill two of the three functions of red blood cells (D’Agnillo and Chang 1998, Powanda and Chang 2002). This is important for conditions with potential for ischemia-reperfusion injury (Alayash et al. 2007). One approach is in the form of PolyHb-catalase-superoxide dismutase (D’Agnillo and Chang 1998) that is effective in preventing ischemia-reperfusion inury in a hemorrhagic shock-stroke rat model (Powanda and Chang 2002). Hsia’s group has extended this using hemoglobin with synthetic antioxidant enzymes (Buehler et al 2004). There is also important recent research to resolve the effect of HBOC on nitric oxide in those conditions with endothelial dysfunction (Yu et al. 2010).
Blood substitute with enhanced antioxidant functions that can transport both oxygen and carbon dioxide
Sims et al. (2001) carried out studies in animal using tissue CO2 microelectrodes. They show that tissue CO2 is not reflected by blood PCO2. Furthermore, tissue CO2 increases with severity of hemorrhagic shock and is correlated with survival.
We have therefore carried out research on polyhemoglobin-catalase-supoxide dismutase-carbonic anhydrase (Bian et al. 2011). This fulfills all three major functions of red blood cells in acting as O2 and CO2 carrier with enhanced antioxidant properties.
Complete nanodimension artificial red blood cells
The original complete polymer membrane artificial red blood cells (Chang 1964, 1972) had very short circulation time. A study was carried out to prepare artificial cells with lipid membrane in the form of lipidprotein membrane and lipidpolymer membrane (Chang 1972). Djordjevich and Miller (1980) prepared submicron 0.2 micron-diameter artificial RBC using lipid membrane vesicles to encapsulate Hb. This increased the circulation time significantly. Philips’ group (1992) markedly improve the circulation time by incorporating polyethylene-glycol (PEG) into the lipid membrane. The submicron hemoglobin lipid vesicle hemoglobin approach is being extensively developed by a group in Japan towards clinical trial (Tsuchida et al. 2006). Lipid vesicles would be useful for conditions that do not require a large volume of blood substitutes. Since the smaller the diameter the larger would be the surface-to-volume relationship, these 200 nanometer lipid vesicles have a total surface area and therefore lipid that is about 10 times that of seven micron red blood cells. A large amount of lipid can cause the saturation of the reticuloendothelial system. We therefore used biodegradable polymer membranes to form complete nanodimension artificial red blood cells (Chang et al. 2003). These nanoartificial RBCs of 80 to 150 nanometers contained all the red blood cell enzymes. Using a polyethylene-glycol-polylactide copolymer membrane, we were able to increase the circulation time of these nanoartificial red blood cells to double that of PolyHb (Chang et al. 2003). Further studies in rats by Liu and Chang (2008) show that one infusion with 1/3 the total blood volume did not result in any adverse effects or changes in the histology or blood biochemistries when followed on days 1, 7, and 21 after infusion.
Oxygen therapeutics
An oxygen carrier with enhanced antioxidants is not only for rbc replacement. It can be prepared with much higher antioxidant activities so that it can be used as oxygen therapeutic in conditions of severe ischemia-reperfusion, as in severe sustained hemorrhagic shock, myocardial infarction, stroke, or organ transplantation. An oxygen carrier with NO transport is another example of oxygen therapeutic, as is an oxygen carrier with platelet activity (Wong and Chang 2007). In high blood volume loss, replacement with a large volume of oxygen carrier or red blood cell alone would not replace platelets and coagulation factors. We have prepared a polyhemoglobin-fibrinogen that is effective as an oxygen carrier with platelet-like properties in rats with 98% volume exchange (Wong and Chang 2007). An oxygen carrier with anti-tumour activity in the form of PolyHb-tyrosinase has the combined function of increasing oxygen tension to sensitize the melanoma to therapy and lowering systemic tyrosine to retard the growth of this fatal skin cancer (Yu and Chang 2004).
Beyond oxygen carriers, blood substitutes, and oxygen therapeutics to artificial cells
The general principle of artificial cells can form the basis of a large number of artificial systems (Chang 2005, 2007) (Figure 1). In addition to being of cellular dimensions in the micron range, they can also be in the macro range, in the nano range, or in the molecular range. Furthermore, the membrane material includes polymer, biodegradable polymer, lipid, crosslinked protein, lipid-polymer complex, lipid-protein complex, and membrane with transport carriers. The artificial cells can contain an unlimited variety of material individually or in combinations (Figure 1). These include cells, stem cells, enzymes, multienzyme systems, hemoglobin, magnetic materials, microorganism, vaccines, genes for gene therapy, genetically engineered cells, adsorbents, drugs, hormones, peptides, proteins, and others. There have been increasing and explosive interest and research activities around the world on artificial cells, especially in fields related to biotechnology, nanomedicine, nanoscience, bio-encapsulation, cell therapy, blood substitutes, advanced drug delivery systems, and even nanoscale robotics and others (Chang 2007). Some of these “artificial cells” are disguised under other terminologies such as liposomes, polymersomes, nanoparticles, microcapsules, blood substitutes, bioencapsulation, and so on.
Figure 1.
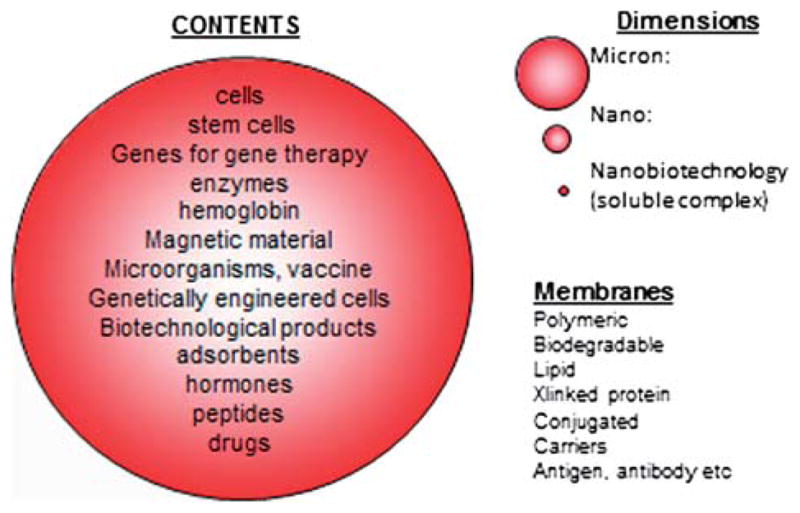
Possible variations in dimensions, membrane mateerials, and contents of artificial cells (from Chang 2007, with permission from World Science Publisher).
Worldwide poll and artificial cells
In a recent worldwide poll by McGill University, the inventor of artificial cells was voted the Greatest McGillian in the university’s 190-year history (www.artcell.mcgill.ca). This has less to do with the individual but is more related to the potential of artificial cells, including blood substitutes, that are being extensively developed by many groups around the world (Chang 2007, Liu and Xiu 2008, Mozzarelli 2010, Zapol 2012).
Acknowledgments
The operating term grant of the Canadian Institute of Health Research to T. M. S. Chang is gratefully acknowledged. This paper has been posted previously on www.artcell.mcgill.ca.
Footnotes
Declaration of interest
The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- Alayash AI, D’Agnillo F, Buehler PW. Expert Opin Biol Ther. 2007;7(5):665–675. doi: 10.1517/14712598.7.5.665. [DOI] [PubMed] [Google Scholar]
- Bian Y, Rong Z, Chang TMS. Artificial Cells, Blood Substitutes & Biotechnology. 2011;39:127–136. doi: 10.3109/10731199.2011.581052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehler PW, Haney CR, Gulati A, Ma L, Hsia CJ. Free Radical Biology & Medicine. 2004;37(1):124–35. doi: 10.1016/j.freeradbiomed.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Chang TMS. Hemoglobin corpuscles. Report of a research project for Honours Physiology, Medical Library, McGill University. Reprinted 1988 in J Biomaterials. Artificial Cells & Artificial Organs. 1957;16:1–9. [PubMed] [Google Scholar]
- Chang TMS. Science. 1964;146(3643):524. doi: 10.1126/science.146.3643.524. [DOI] [PubMed] [Google Scholar]
- Chang TMS, Poznanski M. Nature. 1968;218(5138):242–45. [Google Scholar]
- Chang TMS. Biochem Biophys Res Common. 1971;44(6):1531–1536. doi: 10.1016/s0006-291x(71)80260-7. [DOI] [PubMed] [Google Scholar]
- Chang TMS. Artificial Cells. Springfield, IL: Charles C. Thomas; 1972. (available for access at www.artcell.mcgill.ca) [Google Scholar]
- Chang TMS, Powanda D, Yu WP. Artificial Cells, Blood Substitutes and Biotechnology. 2003;31:231–248. doi: 10.1081/bio-120023155. [DOI] [PubMed] [Google Scholar]
- Chang TMS. Nature Review: Drug Discovery. 2005;4:221–235. doi: 10.1038/nrd1659. [DOI] [PubMed] [Google Scholar]
- Chang TMS. Artificial Cells: Biotechnology, Nanotechnology, Blood Substitutes, Regenerative Medicine, Bioencapsulation, Cell/Stem Cell Therapy. World Science Publisher/Imperial College Press; Singapore/London: 2007. pp. 1–452. (available for free access on www.art-cell.mcgill.ca) [Google Scholar]
- D’Agnillo F, Chang TMS. Nature Biotechnology. 1998;16(7):667–671. doi: 10.1038/nbt0798-667. [DOI] [PubMed] [Google Scholar]
- Djordjevich L, Miller IF. Synthetic erythrocytes from lipid encapsulated hemoglobin. Exp Hematol. 1980;8:584. [PubMed] [Google Scholar]
- Jahr JS, Mackenzie C, Pearce LB, Pitman A, Greenburg AG. J Trauma. 2008;64:1484–97. doi: 10.1097/TA.0b013e318173a93f. [DOI] [PubMed] [Google Scholar]
- Liu Q, Xiu RJ. XI ISBS symposium proceeding. Artificial Cells, Blood Substitutes and Biotechnology. 2008;36(3):169–293. [Google Scholar]
- Liu ZC, Chang TMS. Long term effects on the histology and function of livers and spleens in rats after 33% toploading of PEG-PLA-nano artificial red blood cells. Artificial Cells, Blood Substitutes & Biotechnology. 2008;36:513–524. doi: 10.1080/10731190802554224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EE, Moore FA, Fabian TC, Bernard AC, Fulda GJ, Hoyt DB, Duane TM, Weireter Jr LJ, Gomez GA, Cipolle MD, Rodman Jr GH, Malangoni MA, Hides GA, Omert LA, Gould SA. J Am Coll Surg. 2009;208:1–13. doi: 10.1016/j.jamcollsurg.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Mozzarelli A. XII ISBS symposium proceeding. Artificial Cells, Blood Substitutes and Biotechnology. 2010;38(6):295–342. doi: 10.3109/10731199.2010.525808. [DOI] [PubMed] [Google Scholar]
- Phillips WT, Rudolph AS, Klipper R. Biodistribution studies of liposome encapsulated hemoglobin (LEH) studied with a newly developed 99m_technetium liposome label. Biomaterials, Artificial Cells and Immobilization Biotechnology. 1992;20:757–760. doi: 10.3109/10731199209119715. [DOI] [PubMed] [Google Scholar]
- Powanda D, Chang TMS. Artificial Cells, Blood Substitutes & Biotechnology. 2002;30:25–42. doi: 10.1081/bio-120002725. [DOI] [PubMed] [Google Scholar]
- Rao S, Harrington R, Califf R, Stamler J. J Am Med Assoc. 2005;293:673–674. [Google Scholar]
- Sims C, Seigne P, Menconi M, Monarca J, Barlow C, Pettit J, Puyana J. J Trauma: Injury, Infection, and Critical Care. 2001;51(6):1137–1146. doi: 10.1097/00005373-200112000-00020. [DOI] [PubMed] [Google Scholar]
- Tsuchida E, Sakai H, Horinouchi H, Kobayashi K. Artificial Cells, Blood Substitutes and Biotechnology. 2006;34:581–588. doi: 10.1080/10731190600973907. [DOI] [PubMed] [Google Scholar]
- Winslow RM, editor. Blood Substitutes. Amsterdam: Academic Press; 2006. [Google Scholar]
- Wong N, Chang TMS. Artificial Cells, Blood Substitutes and Biotechnology. 2007;35:481–489. doi: 10.1080/10731190701586210. [DOI] [PubMed] [Google Scholar]
- Yu BL, Chang TMS. Melanoma Research. 2004;14:197–202. doi: 10.1097/01.cmr.0000131013.71638.c0. [DOI] [PubMed] [Google Scholar]
- Yu B, Shahid M, Egorina EM, Sovershaev MA, Raher MJ, Lei C, Wu MX, Bloch KD, Zapol WM. Anesthesiology. 2010;112:586–594. doi: 10.1097/ALN.0b013e3181cd7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapol W. Artificial Cells, Blood Substitutes and Biotechnology; XIII ISBS symposium proceeding; 2012. p. 40. [Google Scholar]


