Abstract
Cigarette smoking is highly prevalent among people living with HIV/AIDS and poses unique health risks. Smoking cessation programs tailored to this population have documented improved smoking outcomes with nicotine replacement therapy (NRT). The current study examined 6-month abstinence rates from a randomized clinical trial targeting 412 HIV-positive adult current smokers (51% European American, 19% African American, and 17% Hispanic American) and tested whether psychosocial variables, such as self-efficacy and decisional balance, mediated the relationship between NRT and long-term abstinence. Meeting criteria for complete mediation, 6-month smoking abstinence rates improved significantly with increases in these mediators, and the association of NRT and smoking abstinence was no longer significant once changes in self-efficacy and decisional balance were taken into account. Failure to translate gains in self-efficacy among African Americans into improved abstinence rates accounted for racial/ ethnic differences among participants. Specific psychosocial factors, such as self-efficacy, may be particularly amenable to change in cessation interventions and should be addressed with greater awareness of how cultural and social contextual factors impact treatment response among people living with HIV/AIDS.
Tobacco use is highly prevalent among people living with HIV/AIDS. Estimates of smoking prevalence among people living with HIV/AIDS range from 47% to 70% (Burkhalter, Springer, Chhabra, Ostroff, & Rapkin, 2005; Gritz, Vidrine, Lazev, Amick, & Arduino, 2004; Niaura et al., 2000), which is more than two to three times the smoking prevalence rates in the general U.S. population (Centers for Disease Control and Prevention [CDC], 2007). Evidence suggests that in addition to the well-established health risks of tobacco use, HIV-positive smokers also incur additional health risks for bacterial pneumonia (Gordin et al., 2008; Kohli et al., 2006) and AIDS related pneumothorax (Metersky, Colt, Olson, & Shanks, 1995). In light of the high prevalence of tobacco use and the specific risks that tobacco use poses for people living with HIV/AIDS, tobacco cessation interventions have the potential to vastly improve the health, well-being, and quality of life of individuals living with HIV/AIDS (Niaura et al., 2000).
Nicotine replacement therapy (NRT) is one of the most common smoking cessation interventions available to smokers seeking to quit. NRT has been found to increase the rate of cessation by 50% to 70% in a meta-analysis of 132 NRT trials (Stead, Perera, Bullen, Mant, & Lancaster, 2008). Despite the efficacy and availability of NRT, to date there have only been three studies involving NRT with HIV-positive populations (Ingersoll, Cropsey, & Heckman, 2007; Lloyd-Richardson et al., in press; Vidrine, Arduino, Lazev, & Gritz, 2006a). Ingersoll, Cropsey, and Heckman (2007) conducted a small randomized trial of 40 HIV-positive smokers involved in one of two treatment conditions: (a) motivational interviewing plus NRT or (b) self-guided reading plus NRT. In this study, treatment group differences did not emerge with respect to 3-month biochemically verified 7-day point prevalence rates. Vidrine and colleagues (2006b) tested a cellular telephone behavioral intervention versus standard care among 95 HIV-positive smokers receiving NRT. In this study, the cellular telephone group was found to have a significantly higher rate of biochemically verified 24-hour point prevalence abstinence rate at 3-month follow-up as compared with the standard care group (10.3%). However, treatment group differences in 7-day point prevalence rate were not significant. Furthermore, as in the Ingersoll and colleagues (2007) study, 6- and 12-month follow-up assessments were not conducted.
The largest trial to date, Positive PATHS (“Motivation and Patch Treatment for HIV-Positive Smokers”; Lloyd-Richardson et al., in press) randomized 444 HIV-positive smokers who received physician advice and the option of NRT to either a brief behavioral intervention (standard care, or SC) or a more intensive motivational-counseling intervention (motivational enhancement, or ME). Biochemically verified 7-day abstinence rates at 2-, 4-, and 6-month follow-ups were 12%, 9%, and 9%, respectively, in the ME condition, and 13%, 10%, and 10%, respectively, in the SC condition. Although no between-group differences were noted at any follow-ups, differences in six-month abstinence rates were detected by race/ethnicity; with Hispanic Americans more likely to be abstinent than other racial/ethnic groups (Hispanic Americans, 19%; European Americans, 8%; African Americans, 5%; other, 8%). Furthermore, failure to use the nicotine patch during the study was a perfect predictor of smoking at 6-month follow-up (Lloyd-Richardson et al., in press).
Demonstrating that nicotine patch use improves smoking outcomes among smokers living with HIV/AIDS is of considerable interest in itself. However, the public health significance of these findings would be enhanced through a greater understanding of the mediating mechanisms through which patch use improves abstinence (MacKinnon & Luecken, 2008). One possible mediator of this relationship is self-efficacy. Smoking related self-efficacy has been defined as an individual’s perceived ability to resist smoking across various tempting situations (Prochaska, DiClemente, Velicer, Ginpil, & Norcross, 1985). Studies have found self-efficacy to be a strong predictor of successful tobacco cessation (Boardman, Catley, Mayo, & Ahluwalia, 2005; Friend & Pagano, 2007). Vidrine, Arduino, & Gritz (2006a) conducted a follow-up study to the above described clinical trial and also found self-efficacy to be a significant mediator of the relationship between 3-month abstinence and treatment group status.
Beyond self-efficacy, a number of studies have identified other psychosocial variables that are associated with smoking cessation; motivation to quit smoking (McCarthy et al., 2008); decisional balance, or perception of the pros versus cons of smoking (Carlson et al., 2003), depression (Perez, Nicolau, Romano, & Laranjeira, 2008), perceived vulnerability to smoking related diseases (Kaufert, Rabkin, Syrotuik, Boyko, & Shane, 1986), stress (Tsourtos, & O’Dwyer, 2008), substance use (Agrawal, Sartor, Pergadia, Huizink, & Lynskey, 2008), social networks (Christakis & Fowler, 2008), and various demographic characteristics (e.g., age, gender, and race/ethnicity) (Ames et al., 2008; Cokkinides, Halpern, Barbeau, Ward, & Thun, 2008; D’Angelo, Reid, Brown, & Pipe, 2001) have all been found to be important constructs associated with smoking cessation.
Although the impact of treatment mediators on smoking cessation outcomes has received a significant amount of focus in the general population, these relationships have yet to be extensively explored among people living with HIV/AIDS. Considering the unique psychosocial characteristics of smokers living with HIV/ AIDS (Lloyd-Richardson et al., 2008), identifying such mediating relationships can provide valuable information for further developing and tailoring effective smoking cessation interventions for this population. The current study examined 6-month abstinence rates from the Positive PATHS trial and the psychosocial constructs targeted in the intervention. Moreover, we tested those variables that significantly predicted abstinence and examined whether they mediated the relationship between NRT and long-term abstinence.
METHODS
PARTICIPANTS
Patients were eligible for participation in Positive PATHS if they (a) were sero-positive for HIV; (b) were 18 years of age or older; (c) were current, regular smokers (≥5 cigarettes/day for the past 3 months); (d) spoke English or Spanish; and (e) agreed to be available over the next 6 months (the length of study follow-up). Participants were not required to quit smoking or to use the nicotine patch. Participants were excluded if they (a) suffered from any unstable medical condition precluding use of the nicotine patch (e.g., uncontrolled hypertension) or an active skin condition (e.g., psoriasis, eczema); (b) were currently using smokeless tobacco, NRT or other smoking cessation treatment, or (c) were pregnant or nursing. Participants who met criteria were offered monetary compensation for intervention and follow-up visits. All materials and interventions were available in English and Spanish. The study protocol was approved by the institutional review boards of participating hospitals. Baseline data were collected between February 2000 and June 2004.
PROCEDURE
The study was performed at six outpatient HIV clinics and two primary care medical offices in southeastern New England. Study physicians were trained to ask all patients about their smoking status, provide brief cessation advice to smokers, and refer those willing to speak with a health educator (HE) about their smoking to the study. After providing informed consent, participants were administered the baseline assessment via laptop computer over the course of two sessions. Participants were then randomly assigned to the two treatment arms (SC or ME) within strata homogeneous with respect to both gender and level of motivation to quit smoking as measured by the Contemplation Ladder, a continuum of 10 attitudes regarding quitting smoking (Biener & Abrams, 1991). Participants in both intervention arms who were willing to set a quit date were generally provided with 8 weeks of NRT. Follow-up assessments, administered by research staff blinded to participant intervention assignment, consisted of a questionnaire battery along with biochemical verification of self-reported tobacco use via an expired carbon monoxide (CO) test.
SC participants participated in two study sessions, consisting largely of baseline assessments, randomization, and brief assessment of quitting plans. Those willing to set a target quit date (TQD) received self-help quitting materials (U.S. Department of Health and Human Services, 2003) and NRT (i.e., nicotine patches). Participants returned to the clinic biweekly for distribution of additional patches, allowing the HE to briefly (≤ 5 minutes) reinforce quitting efforts, check on patch side effects, and answer questions. Thus, a typical participant receiving SC treatment who opted to use the full eight-week course of NRT participated in two brief intervention contacts and four NRT contacts with their HE. Those participants not willing to set a quit date were instructed to contact the HE when they were ready (anytime within the next 6 months). This brief treatment reflects the minimum standard of care recommended by the Agency for Health Research and Quality panel convened to address smoking cessation treatment (Fiore, 2000).
In the ME intervention, effort was made to tailor smoking materials to the needs of individuals living with HIV/AIDS (e.g., emphasis on improved immune functioning, prevention of infections, improvement in HIV-related side-effects, such as cardiovascular disease, fatigue). In addition to the above-described SC components, ME participants received motivational counseling and a quit-day counseling call. Sessions included CO breath tests and discussion of results, as well as discussions of the pros and cons of quitting smoking, barriers to cessation, and goal setting to enhance motivation and preparedness for quitting. Nicotine patches were distributed to interested participants on a bi-weekly schedule for the duration of use. Participants not willing to set a quit date were engaged in discussion of “quitting as a process” and the barriers to quitting.
MEASURES
Assessment measures have been previously described in detail (Lloyd-Richard-son et al., 2008) and are briefly summarized herein. Sociodemographic information was obtained from participants on age, sex, sexual orientation, marital status, race and ethnicity, employment, education, and living situation. All participants that self-identified as Hispanic were treated as such, irrespective of race. Remaining participants were classified according to their racial origin.
Outcome Measure—Abstinence
The primary outcome variable in this study was 7-day point prevalence abstinence (PPA) rates assessed at 6-month follow-up. Those participants reporting any amount of smoking in the past 7 days were considered smokers. Participants reporting 7-day abstinence were verified with a 24-hour biochemical measure of abstinence using CMD/CO Carbon Monoxide Monitors (Spirometrics, Inc., Auburn, ME). In those cases where participant self-report was discrepant with a cutoff of CO<10 ppm, participants were considered smokers.
NRT Treatment Dose
HEs were trained to document the duration and nature of each patient care activity, including preparation of session materials, counseling intervention contact with patient, and NRT-related contact. Importantly, NRT-related contacts were tracked separately from counseling intervention contacts. The “dose” of delivery of these elements included number of counseling intervention contacts, number of NRT-related contacts, minutes of counseling contacts, and minutes of NRT-related contacts.
Psychological Mediators
The Readiness to Quit Ladder was used to assess motivation to quit smoking along 10 “rungs” (Biener & Abrams, 1991), with higher scores suggesting a greater willingness to quit; the six-item Smoking Decisional Balance Scale (Velicer, DiClemente, Prochaska, & Brandenburg, 1985), used to assess the pros and cons of smoking, with higher scores indicating greater importance of pros/ cons for the individual; the 9-item Smoking Self-Efficacy Scale (Velicer, Diclemente, Rossi, & Prochaska, 1990), used to assess the degree of self-efficacy in resisting temptations to smoke in various situations, with the standard scoring scheme reversed, so that higher scores indicate higher self-efficacy; and a measure of Perceived Vulnerability and Response Efficacy (Lee, 1989), with subscales assessing perceptions of the probability of acquiring a smoking-related illness if continued smoking versus if quit smoking, with higher scores indicating greater perceived vulnerability and efficacy, respectively. The Center for Epidemiologic Studies Depression Scale (CES-D; Kohout, Berkman, Evans, & Cornoni-Huntley, 1993; Radloff, 1977) was used to assess depressive symptoms (scores ≥ 10 suggestive of elevated clinical depressive symptoms) and the Perceived Stress Scale (PSS; Cohen, Karmack, & Mermelstein, 1983) was used to assess participant perceived stress level, with higher scores indicating greater perceived stress. In addition to information on the number of smokers in the household, patients provided information on the important people in their lives using a modified version of the Important People and Activities Instrument (IPA; Longabaugh, 2001), designed to assess involvement in and support of the social network and activities in the person’s smoking and abstinence. Two composite variables were calculated: proportion of social network who smoke and proportion of network who are supportive of quitting.
Other Substance Use
Past 30-day alcohol and other substance use was assessed using a 30-day time line follow-back (TLFB; Sobell & Sobell, 1995), retrospectively assessing estimates of the number of days in which alcohol or other drugs were used.
DATA ANALYSIS PLAN
Our primary aim was to examine whether changes from baseline to 6-month follow-up in psychological variables targeted by the intervention acted as potential mediators of the effect of NRT on smoking abstinence, controlling for differences at baseline. For these analyses, an inclusive approach to model building was implemented: 6-month change scores of all psychosocial variables listed in Table 1 were initially evaluated on an individual basis according to the Baron and Kenny (1986) framework using a fairly liberal alpha = 0.10 level of significance. All variables that satisfied single mediation criteria, were subsequently evaluated jointly in a multiple mediation setting, with regression models for the abstinence outcomes simplified using backward elimination at the more conventional alpha = 0.05 level of significance. Testing was hierarchical, in the sense that if the 6-month change scores were significantly related to outcome, baseline values were retained in the model as well, irrespective of their statistical significance. Because the higher rungs of the Readiness to Quit Ladder are defined by self-reported abstinence, this psychological measure of motivation was excluded from the analyses.
TABLE 1.
Baseline Demographic, Smoking, and Psychosocial Characteristics (N = 412).
| Variable | N | Overall |
|---|---|---|
| Age | 412 | 42.04 (7.53) |
| Gender | ||
| Male | 260 | 63.11% |
| Female | 152 | 36.89% |
| Race/Ethnicity | ||
| European American | 212 | 51.46% |
| Hispanic American | 70 | 16.99% |
| African American | 78 | 18.93% |
| Other | 52 | 12.62% |
| Employment | ||
| Yes — Full time | 54 | 13.14% |
| Yes — Part time | 31 | 7.54% |
| No | 326 | 79.32% |
| Cigarettes per Day | 363 | 18.47 (9.87) |
| Motivation (Ladder) | ||
| Less Motivated to Quit | 84 | 20.39% |
| More Motivated to Quit | 328 | 79.61% |
| Smoking decisional balance | 412 | 0.09 (0.99) |
| Smoking self-efficacy | 412 | 3.80 (0.66) |
| Perceived vulnerability score | 412 | 83.33 (23.82) |
| Response efficacy score | 412 | 36.71 (24.67) |
| CESD total score | 409 | 12.01 (6.61) |
| Cohen PSS total score | 408 | 6.67 (3.30) |
| Proportion of network smokers | 377 | 0.47 (0.25) |
| Proportion of network supports | 377 | 0.81 (0.24) |
| Alcohol use past 30 days | 363 | 3.14 (6.98) |
| Marijuana use past 30 days | 363 | 2.56 (7.34) |
| Other substance use past 30 days | 363 | 0.88 (3.78) |
Note. CES-D = Center for Epidemiological Studies Depression Rating Scale; PSS = Perceived Stress Scale. Mean (SD) for continuous variable; percentages for categorical variables.
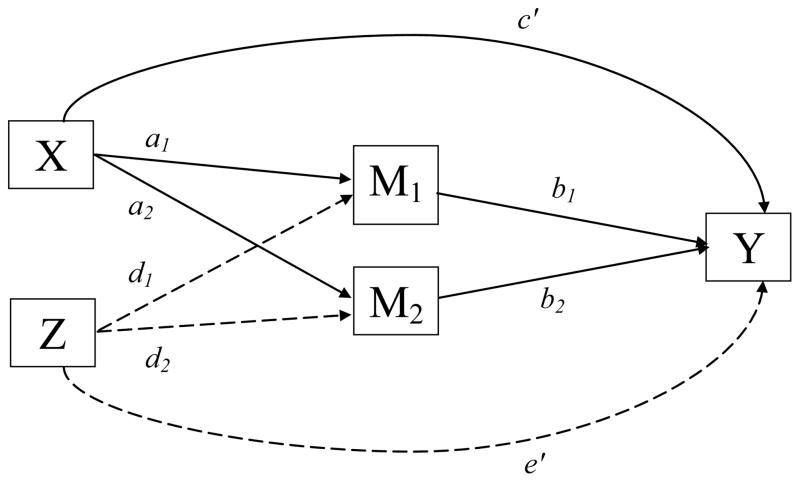
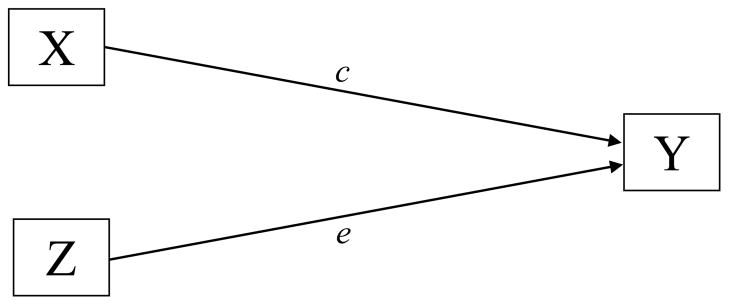
The mediation framework established by Baron and Kenny (1986) and elaborated further by Judd, Kenny, and McClelland (2001) does not explicitly mention how to handle multiple mediators or potential confounders of the treatment-outcome relationship (e.g. race). We describe such model extensions using two path analytic diagrams. Figure 1 depicts a scenario in which mediators have not yet been explicitly modeled, but confounders are present. Here the overall effect of NRT (X) on 6-month smoking abstinence (Y) adjusted for potential confounders (Z), race/ ethnicity, is given by the coefficient c of the path from X to Y. In Figure 2, the model is elaborated further by the introduction of two mediators of interest, e.g., smoking self-efficacy ( M1 ), and decisional balance ( M2 ). In this multiple mediation scenario, the total indirect effect of X on Y is calculated by adding the indirect effects over all possible mediation paths. Each of these mediator-specific effects is given by the product a b i i of the coefficients along the path from X to Y via Mi, where ai represents the direct effect of X on Mi partialling out Z, and bi represents the direct effect of Mi on Y partialling out both X and Z. Hence, the overall treatment effect c depicted in Figure 1 can be decomposed in Figure 2 into:
the sum of the direct effect of X on Y and of the total indirect effect of X on Y through the mediators of interest. Complete mediation occurs when c– = 0, that is, when the direct effect of NRT-related contacts in achieving and maintaining smoking abstinence vanishes when controlling for changes in smoking self-efficacy and decisional balance. Although a similar decomposition is available for the effect of confounders Z on outcome Y, it is of not theoretical interest in the current investigation and thus not pursued further.
FIGURE 1.
No Mediation.
FIGURE 2.
Multiple Mediation.
Further model elaboration is possible when the confounders Z are allowed to moderate both the direct and indirect paths from X to Y (Muller, Judd, & Yzerbyt, 2005). In this particular setting, a comprehensive moderated-mediation model would involve testing moderation by race/ethnicity of the effect of NRT-related contacts on changes in self-efficacy ( a1 ), decisional balance ( a2 ), and smoking abstinence (c– ), as well as of the effect of changes in self-efficacy ( b1 ) and decisional balance ( b2 ) upon smoking abstinence. All five of these interactions were explored in the models described below.
RESULTS
Treatment dose data were available for 412 of the 444 participants enrolled in the original study. Table 1 describes the sample and provides means and standard deviations for the demographic, smoking, and psychosocial variables of interest. Detailed descriptions of the full sample’s baseline characteristics and how psychosocial variables differed by racial/ethnic group have been reported in earlier publications (Lloyd-Richardson et al., 2008; Lloyd-Richardson et al., in press).
Self-reported 7-day abstinence rates were verified with a 24-hour biochemical measure of abstinence. The biserial correlation between self-reported abstinence and CO levels was .46 overall (N = 412), although the accuracy of self-report appeared to vary by racial/ethnic group (European Americans, .41; African Americans, .39; Hispanic Americans, .79, other, .75) (Lloyd-Richardson et al., in press). Participants with CO < 10 ppm were considered smokers. Because of significant racial/ethnic differences in biochemically verified smoking abstinence rates (European Americans, 8.49%; African Americans, 1.28%; Hispanic Americans, 18.57%; other, 9.61%; , p = .004), all analyses controlled for race/ethnicity.
Consistent with reports in Lloyd-Richardson et al. (in press), the mean number of total NRT-related contacts per patient did not differ significantly between the SC and ME groups (2.20 for ME vs. 2.42 for SC; p = .110). Given our prior findings that assignment to the ME intervention was not associated with smoking abstinence at the 6-month follow-up, these findings are consistent with our previous reports that the two treatment arms had very similar outcomes (Lloyd-Richardson et al.,). Therefore, we conducted all forthcoming analyses on the combined sample (regardless of treatment arm) and focused on a proxy of patch use, as measured by number of biweekly NRT-related contacts.
In Tables 2–5, NRT-related contacts have been centered at the sample mean of 2.31 contacts per subject, while self-efficacy and decisional balance were centered to 0 mean and unit standard deviation at baseline before calculating change scores from baseline to 6-month follow-up. Therefore, a common scaling is used for these variables across time points.
TABLE 2.
Predictors of 6-month Smoking Abstinence Excluding Change in Mediators (N = 412).
| Adjusted Odds Ratios | Value | LCL | UCL | p |
|---|---|---|---|---|
| Intercepta | 0.08 | 0.05 | 0.14 | < .000 |
| Hispanic American versus European American | 2.84 | 1.27 | 6.34 | .010 |
| African American versus European American | 0.13 | 0.02 | 1.01 | .051 |
| Other versus European-American | 0.99 | 0.34 | 2.90 | .990 |
| Baseline self-efficacy | 1.50 | 1.04 | 2.17 | .029 |
| Baseline decisional Balance | 0.88 | 0.60 | 1.28 | .500 |
| NRT-related Contacts | 1.35 | 1.03 | 1.76 | .028 |
Note. LCL = 95% lower confidence limit; UCL = 95% upper confidence limit; NRT = nicotine replacement therapy.
Intercept gives the baseline odds for European Americans.
TABLE 5.
Predictors of 6-month Smoking Abstinence Including Change in Mediators (N = 412).
| Adjusted Odds Ratios | Value | LCL | UCL | p |
|---|---|---|---|---|
| Intercepta | 0.01 | 0.00 | 0.04 | < .000 |
| Hispanic American versus European American | 0.76 | 0.15 | 3.89 | .743 |
| African American versus European American | 0.11 | 0.00 | 5.98 | .275 |
| Other versus European American | 0.39 | 0.06 | 2.69 | .342 |
| Baseline self-efficacy | 4.96 | 2.25 | 10.93 | .001 |
| Change in self-efficacy | 9.77 | 4.68 | 20.37 | < .001 |
| Change in self-efficacy | ||||
| African-American versus European American | 0.01 | 0.00 | 0.21 | .003 |
| Baseline decisional balance | 1.30 | 0.63 | 2.66 | .477 |
| Change in decisional balance | 2.80 | 1.41 | 5.55 | .003 |
| NRT-related contacts | 0.84 | 0.49 | 1.46 | .541 |
Note. Intercept = Baseline Odds for European-Americans; LCL = 95% Lower Confidence Limit; UCL = 95% Upper Confidence Limit; NRT = nicotine replacement therapy.
Intercept is the baseline odds for European Americans.
Mediation Analyses
Our mediation analyses followed the Baron and Kenny (1986) recommendations and were thus conducted in steps aimed at evaluating the following four mediation criteria. Because of space constraints we do not present single mediator models but note that of the psychosocial variables listed in Table 1, changes in smoking self-efficacy, smoking decisional balance, and proportion of network support were the only ones to satisfy individual mediation criteria. When considered jointly with self-efficacy and decisional balance, proportion of network support dropped out of the multiple-mediation model. Hence, all subsequent analyses pertain to a model in which changes in self-efficacy and decisional balance are jointly evaluated as mediators of the effect of NRT on 6-month smoking abstinence, adjusting for their own values at baseline as well as racial/ethnic differences.
Criterion 1 requires that NRT-related contacts improve smoking abstinence at 6-month follow-up, controlling for race and baseline values of both putative mediators (self-efficacy, decisional balance) that were identified in the multivariate analyses. By fitting the model depicted in Figure 1, we were able to indeed establish a statistically significant benefit of increased number of contacts (p = .028): exponentiation of the c path coefficient in a logistic regression model aimed at capturing the total effect of NRT-related contacts on outcome suggested an increase in the regression-adjusted odds of smoking abstinence by 35% per additional contact, as indicated by an adjusted odds ratio (AOR) of 1.35 (95% confidence interval [CI] = 1.03–1.76). Results for the remaining covariates are shown in Table 2, with European Americans as the reference group. Controlling for baseline differences in self-efficacy and decisional balance, racial/ethnic differences in the effect of NRT contacts were not significant ( , p = .775) and were dropped from the model. Very highly significant overall differences were observed in the model intercept ( , p < .001), with Hispanic Americans almost three times as likely to be abstinent as European Americans (AOR = 2.84, 95% CI = 1.27–6.34) at any given level of NRT-related contacts. In contrast, African Americans where only an eighth as likely to be abstinent (AOR = .13, 95% CI = 0.02–1.01) as European Americans.
Criterion 2 requires that increases in NRT-related contacts produce statistically significant increases in both putative mediator variables from baseline to 6-month follow-up. To this effect, we estimated normal regression models with baseline to 6-month follow-up mediator change scores as the dependent variables, and NRT-related contacts as the independent variable. We additionally controlled for the effects of race and baseline values of both mediators, effects summarily captured by the e– path coefficients in Figure 2. Examination of the a path coefficients in this figure showed this criterion was satisfied as well, with significant direct effect of NRT-related contacts on both mediators: smoking self-efficacy (p < .001) and smoking decisional balance (p = .017). As seen from Tables 3 and 4, smoking self-efficacy increased by 0.19 baseline standard deviations per contact (95% CI = 0.10–0.28), while decisional balance increased by 0.08 baseline standard deviations per contact (95% CI = 0.01–0.15). Controlling for baseline differences in self-efficacy and decisional balance, racial/ethnic differences in the effect of NRT contacts were not significant (self-efficacy: F(3,397) = 0.485, p = .693; decisional balance: F(3,392) = 1.05, p = .371), and were dropped from the model.
TABLE 3.
Predictors of 6-Month Change in Self-Efficacy (N = 412)
| Regression Coefficient | Value | LCL | UCL | p |
|---|---|---|---|---|
| Intercepta | 0.49 | 0.31 | 0.66 | < .001 |
| Hispanic American versus European American | 0.35 | −0.01 | 0.71 | .058 |
| African American versus European American | 0.35 | 0.01 | 0.69 | .045 |
| Other versus European American | 0.02 | −0.38 | 0.42 | .933 |
| Baseline self-efficacy | −0.26 | −0.40 | −0.13 | .001 |
| Baseline decisional balance | 0.05 | −0.08 | 0.19 | .428 |
| NRT-related contacts | 0.19 | 0.10 | 0.28 | < .001 |
Note. LCL = 95% lower confidence limit; UCL = 95% upper confidence limit; NRT = nicotine replacement therapy.
Intercept gives the baseline odds for European Americans.
TABLE 4.
Predictors of 6-month Change in Decisional Balance (N = 412)
| Regression Coefficient | Value | LCL | UCL | p |
|---|---|---|---|---|
| Intercepta | −0.03 | −0.16 | 0.09 | .592 |
| Hispanic American versus European American | 0.09 | −0.16 | 0.35 | .474 |
| African American versus European American | 0.01 | −0.23 | 0.26 | .924 |
| Other versus European American | −0.22 | −0.51 | 0.06 | .131 |
| Baseline self-efficacy | 0.03 | 0.06 | 0.12 | .539 |
| Baseline decisional balance | −0.61 | −0.70 | −0.53 | < .001 |
| NRT-related contacts | 0.08 | 0.01 | 0.15 | .017 |
Note. LCL = 95% Lower Confidence Limit; UCL = 95% Upper Confidence Limit. NRT = nicotine replacement therapy.
Intercept is the baseline odds for European Americans.
Criterion 3 requires that the observed increases in the putative mediator variables from baseline to 6-month follow-up produced statistically significant increases in the odds of smoking abstinence over the same time frame, controlling for any other effects owing to NRT-related contacts. Hence, we fit a logistic regression model with 6-month smoking outcome as the outcome, and estimated both the direct effects of NRT-related contacts on outcome, denoted by the c′ path coefficient of Figure 2, as well as the direct effects of mediators on outcome, denoted by the b path coefficients in the same figure. Just as in step 2, we additionally controlled for the direct effect of race and baseline values of both mediators; these effects were summarily denoted by a single e′ path coefficient in Figure 2. As seen from Table 5, the b path coefficients were very highly significant for both change in self-efficacy (p < .001), and change in decisional balance (p = 0.003). Converted from the log-odds to the odds scale, the b path coefficient for increases in smoking self-efficacy translates into a 54% increase in the adjusted odds of smoking abstinence (AOR = 1.54, 95% CI = 1.34–1.77) for a 0.19 baseline standard deviation increase in this measure, while increases in decisional balance produce a 9% increase in the adjusted odds of smoking abstinence (AOR = 1.09, 95% CI = 1.03–1.15) for a 0.07 baseline standard deviation increase in the measure. In both cases, results assume that all other independent variables in the model are held constant, including change in the other mediator. In the above, we purposefully chose to calculate the AOR in terms of the expected change in the mediators per additional contact as estimated in step 2; this is a more interpretable metric that can be compared across mediators and gives us the indirect effect of NRT-related contacts through a particular mediation path corresponding to approximately 2 weeks of patch use. AORs in which the effect of both baseline values of each mediator and of mediator change from baseline to follow-up are expressed in baseline standard deviation units of the mediators are presented in Table 5 and are much stronger than the ones reported above, since they capture changes associated with much longer periods of patch use. Controlling for baseline differences in self-efficacy and decisional balance, no improvement in model fit resulted from controlling for racial/ethnic differences in the effect on smoking abstinence of either change in smoking decisional balance ( , p = .851) or that of NRT contacts ( , p = .328). However, the effect of changes in self-efficacy on smoking abstinence did vary by race/ethnicity ( , p = .004), Examination of individual contrasts identified African Americans as the group solely responsible for this moderation effect ( , p = .003). As seen from the reduced model presented in Table 5, increases in self-efficacy among African Americans failed to translate into improved odds of smoking abstinence, with the impact of a standard unit increase in self-efficacy among African Americans only one one-hundredth as large as among European Americans (AOR = .01, 95% CI = 0.00–0.21).
Finally, Criterion 4 requires that the effect of NRT be attenuated after accounting for its indirect effects via the mediators. Examination of the direct effect of NRT-relate contacts on outcome reported in Table 5, shows a 16% reduction (AOR = 0.84, 95% CI = 0.49–1.46) in the odds of smoking abstinence per additional contact, which is no longer statistically significant (p = 0.541), indicating a complete mediation scenario. Since Criteria 1–4 are satisfied for both change in self-efficacy and change in decisional balance, these two psychological variables appear to be bonafide mediators of NRT effects.
DISCUSSION
To date, smoking cessation studies tailored to individuals living with HIV/AIDS have been scarce (Ingersolll et al., 2007; Lloyd-Richardson et al., 2008; Vidrine et al., 2006b). The only study of mediators among HIV-positive smokers (N = 90) found changes in depression, anxiety, and self-efficacy weakened the association between a cell-phone intervention that included NRT and 24-hour abstinence at 3-months follow-up (Vidrine, Arduino, and Gritz, 2006a). To our knowledge, no studies have evaluated mediators of the long-term effectiveness of cessation intervention components, such as NRT. Even in the general population, there are many gaps in what is known regarding the mechanism by which NRT supports behavior change that sustains abstinence.
Multivariate analyses identified self-efficacy to refuse cigarettes and decisional balance (beliefs about smoking) as predictors of 6-month abstinence and therefore potential mediators of the NRT treatment effect in our sample. Contrary to Vidrine and colleagues’ (2006a) results, no evidence was found for an association between symptoms of distress (depression, stress, substance use) and smoking abstinence. Formal mediation models established that patch use (NRT-related sessions largely reflecting visits for additional patches) in our sample significantly predicted the odds of 6-month abstinence. This alone informs intervention efforts of the importance of maintaining patient contact to improve compliance and increase weeks of patch use. Further, NRT compliance was found to predict changes from baseline to 6-month follow-up in self-efficacy and decisional balance. When change in these psychological variables was entered into the model, the effect of NRT was no longer significant, and there was a strong increase in the odds of abstinence predicted by increased self-efficacy. A weaker, but statistically significant, increase in abstinence odds resulted from increases in decisional balance as well. On balance, mediation from these psychological variables appears to be dominated by changes in the individual’s confidence that they can resist temptations to smoke (self-efficacy).
One can hypothesize that longer periods of patch compliance reduce withdrawal and craving symptoms that, in turn, impact the individual’s sense of self-efficacy to resist temptations and prevent relapse. NRT has been shown to improve smoking treatment outcome (Stead et al., 2008). However, less is known regarding the major mechanisms of this improvement in outcome. Ferguson, Shiffman, & Gwaltney (2006) were the first to test whether NRT’s reduction of real-time craving and withdrawal symptoms mediates its effects on smoking abstinence. These authors found that NRT treatment did significantly decrease withdrawal and craving severity; however, these reductions only partially accounted for NRT’s impact on time to first lapse and suggest other mechanisms for the effectiveness of NRT have yet to be identified. Although we did not directly include craving or withdrawal in our models, our results suggest that psychological variables, such as self-efficacy, may play a critical role in preventing relapse in this patient sample. Perhaps it is not just the lessening of withdrawal symptoms, but that success experiences (supported by increased time on the patch) contribute to a sense of confidence that translates to behavior change (the ability to resist temptations and avoid relapse). In other words, self-efficacy is a cognitive mediator that is impacted by the reduction of withdrawal symptoms that help an individual achieve the initial quit attempt. This cognition of growing confidence, in turn, may help the individual to increase behaviors that reduce relapse.
Surprisingly, providing patches along with minimal support (e.g., reinforcing the quitting efforts, monitoring patch side effects, and answering questions) was equally effective as a motivational interviewing counseling approach that is intended to increase self-efficacy and motivation to quit (Lloyd-Richardson et al., in press). These counterintuitive findings, suggest that counseling specifically to increase motivation and self-efficacy was not as effective as the impact that longer periods of NRT had on those same variables. Providing patients with success experiences may do more to improve their confidence than talking about it. These compelling findings speak to the importance of pharmacotherapy, and the need for interventions that can connect with motivated patients and increase compliance to pharmacological support.
One cannot interpret our results without specific attention to racial/ethnic differences that have been revealed in all of our analyses with this patient sample. Hispanic Americans were almost three times as likely to be abstinent compared with European American participants, which was due in part to larger gains in self-efficacy from baseline to 6-month follow-up. Once changes in self-efficacy were controlled for, their smoking abstinence rates no longer differed from those of the European American reference group. This finding highlights the important role of self-efficacy among Hispanic American smokers seeking to quit. Regardless of assigned treatment, Hispanic Americans reported fewer smokers in the home at baseline and larger improvements in self-efficacy to quit, compared with other groups. Perhaps protective baseline social support led to these improvements in self-efficacy.
African American participants in our study were only an eighth as likely to be abstinent at 6-month follow-up compared with European Americans. With HIV cases among African Americans on the rise (CDC, 2008), the public health implications of African Americans’ poor abstinence rates are significant. The role of self-efficacy as a mediator of cessation success has been documented in Vidrine and colleagues’ (2006a) study of a low-income, largely African American population of smokers living with HIV/AIDS. In our sample, there were improvements in African Americans’ self-efficacy to quit (of comparable magnitude to Hispanics); however, those improvements did not translate into improved abstinence rates for African Americans, unlike for all other groups. African Americans presented with fewer previous quit attempts at baseline and more smokers in the home, perhaps making it more difficult for them to remain compliant with patch use and translate any gains in their cognitive confidence to success in their actual ability to resist smoking. This suggests that different psychosocial risk and protective factors may have had an impact on how certain groups respond to interventions. The ability of participants to cope with situational temptations to smoke (self-efficacy) cannot be underestimated, making this a natural target of future smoking cessation treatment among people living with HIV. Notably, African Americans comprise the only group in the original PATHS study (Lloyd-Richardson et al., in press) whose abstinence trends over time appeared to benefit from the motivationally enhanced intervention (9% 6-month quit in ME vs. 0% in SC). However these trends were not statistically significant, and small sample sizes prohibited further exploration or interpretation. Clearly larger studies with this population are needed to recruit and retain individuals from different backgrounds and elucidate intervention techniques that can more effectively reach and connect to vulnerable subpopulations.
Although the proportion of participants’ social network supportive of quitting was associated with abstinence, it lost significance in multivariate analyses when self-efficacy and decisional balance were entered in the model. This suggests that the protective effect of psychological variables overlaps with influences from the social environment. In fact, the self-efficacy measure used in this study asks about confidence to resist smoking in social situations. If one’s social network is unsupportive of quitting, then indeed the impact on these psychological factors may be quite powerful. Future smoking cessation interventions may have a greater impact if they focus on behavioral strategies that are amenable to treatment, such as increasing support for abstinence in an individual’s social network, which may in turn provide more opportunities to increase confidence in resisting temptations.
Findings of this study provide evidence that improving self-efficacy to resist smoking temptations may be a psychological mechanism that results from the success experiences boosted by NRT treatments. Our ability to directly test whether patch compliance reduces withdrawal symptoms that in turn impact self-efficacy to quit is limited by our lack of a measure of withdrawal/craving. Moreover, one’s confidence that he/she can resist temptations to smoke may be confounded with level of nicotine dependence. Self-efficacy and nicotine dependence were indeed found to be associated at baseline (Lloyd-Richardson et al., 2008). Additionally, this sample of individuals living with HIV/AIDS showed high baseline levels of motivation to quit, as has been reported in other HIV smoker samples (Burkhalter et al., 2005; Gritz et al., 2004), and participants received brief advice from their physician. Readiness to quit and motivation may have been elevated and thus limits generalizability to other samples.
In sum, our analysis found the efficacy of NRT compliance on 6-month quit rates to be mediated by positive changes in self-efficacy to resist temptations to smoke and, to a lesser extent, changes in beliefs about the pros and cons of quitting smoking. This was the case for all ethnic-racial groups, with the exception of Afri-can Americans who were not able to translate increased self-efficacy to abstinence. Importantly, brief and frequent contacts focused on NRT were more effective at yielding improved cessation outcomes than a time-intensive motivational counseling approach. Specific psychosocial factors, such as self-efficacy, may be particularly amenable to change, and should be addressed with greater awareness of how cultural and social contextual factors impact treatment response.
Acknowledgments
This research was supported by Grant R01-DA12344-06 from the National Institute of Drug Abuse (R. Niaura, PhD), grant K07-CA95623 from the National Cancer Institute (C. Stanton, Principal Investigator), Grant K23-HL069987 from the National Heart, Lung, and Blood Institute (E. Lloyd-Richard-son, PhD), National Institutes of Health (NIH)-funded Transdisciplinary Tobacco Use Research Center Award (P50 CA084719), an NIH-funded Lifespan/Tufts/Brown Center for AIDS Research Award (P30 AI42853), and by the Robert Wood Johnson Foundation.
Contributor Information
Cassandra A. Stanton, Department of Psychiatry and Human Behavior, the Warren Alpert Medical School of Brown University, Transdisciplinary Research Group, Butler Hospital, Providence, RI
Elizabeth E. Lloyd-Richardson, Department of Psychology, University of Massachusetts, Dartmouth, and the Department of Psychiatry and Human Behavior, the Warren Alpert Medical School of Brown University, Centers for Behavioral and Preventive Medicine, the Miriam Hospital, Providence, RI
George D. Papandonatos, Center for Statistical Sciences, Brown University, Providence, RI
Marcel A. de Dios, Department of Psychiatry and Human Behavior, the Warren Alpert Medical School of Brown University, Centers for Behavioral and Preventive Medicine, the Miriam Hospital, Providence, RI
Raymond Niaura, Department of Psychiatry and Human Behavior, the Warren Alpert Medical School of Brown University, Transdisciplinary Research Group, Butler Hospital, Providence, RI.
References
- Ames SC, Croghan IT, Clark NM, Patten CA, Stevens SR, Schroeder DR, et al. Change in perceived stress, partner support, decisional balance, and self-efficacy following residential nicotine dependence treatment. Journal of Addictive Disorders. 2008;27(1):73–82. doi: 10.1300/J069v27n01_08. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Sartor C, Pergadia ML, Huizink AC, Lynskey MT. Correlates of smoking cessation in a nationally representative sample of U.S. adults. Addictive Behaviors. 2008;33(9):1223–1226. doi: 10.1016/j.addbeh.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of Personality & Social Psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Biener L, Abrams DB. The Contemplation Ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology. 1991;10(5):360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- Boardman T, Catley D, Mayo MS, Ahluwalia JS. Self-efficacy and motivation to quit during participation in a smoking cessation program. International Journal of Behavioral Medicine. 2005;12(4):266–272. doi: 10.1207/s15327558ijbm1204_7. [DOI] [PubMed] [Google Scholar]
- Burkhalter JE, Springer CM, Chhabra R, Ostroff JS, Rapkin BD. Tobacco use and readiness to quit smoking in low-income HIV-infected persons. Nicotine and Tobacco Research. 2005;7(4):511–522. doi: 10.1080/14622200500186064. [DOI] [PubMed] [Google Scholar]
- Burns DN, Hillman D, Neaton JD, Sherer R, Mitchell T, Capps L, et al. Cigarette smoking, bacterial pneumonia, and other clinical outcomes in HIV-1 infection. Terry Beirn Community Programs for Clinical Research on AIDS. Journal of Acquired Immune Deficiency Syndrome Human Retrovirol. 1996;13(4):374–383. doi: 10.1097/00042560-199612010-00012. [DOI] [PubMed] [Google Scholar]
- Carlson LE, Taenzer P, Koopmans J, Casebeer A. Predictive value of aspects of the Transtheoretical Model on smoking cessation in a community-based, large-group cognitive behavioral program. Addictive Behaviors. 2003;28(4):725–740. doi: 10.1016/s0306-4603(01)00268-4. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cigarette smoking among adults—United States. Mortality and Morbidity Weekly Report. 2007;56(44):1157–1151. [PubMed] [Google Scholar]
- Christakes NA, Fowler JH. The collective dynamics of smoking in a large social networks. New England Journal of Medicine. 2008;358(21):2284–2286. doi: 10.1056/NEJMsa0706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Cokkinides VE, Halpern MT, Barbeau EM, Ward E, Thun MJ. Racial and ethnic disparities in smoking-cessation interventions: Analysis of the 2005 National Health Interview Survey. American Journal of Preventive Medicine. 2008;34(5):404–412. doi: 10.1016/j.amepre.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Conley LJ, Bush TJ, Buchbinder SP, Penley KA, Judson FN, Holmberg SD. The association between cigarette smoking and selected HIV-related medical conditions. AIDS. 1996;10(10):1121–1126. [PubMed] [Google Scholar]
- D’Angelo ME, Reid RD, Brown KS, Pipe AL. Gender differences in predictors for long-term smoking cessation following physician advice and nicotine replacement therapy. Canadian Journal of Public Health. 2001;92(6):418–422. doi: 10.1007/BF03404531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiClemente CC, Prochaska JO, Fairhurst SK, Velicer WF, Velasquez MM, Rossi JS. The process of smoking cessation: An analysis of precontemplation, contemplation, and preparation stages of change. Journal of Consulting and Clinical Psychology. 1991;59(2):295–304. doi: 10.1037//0022-006x.59.2.295. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S, Gwaltney CJ. Does reducing withdrawal severity mediate nicotine patch efficacy? A randomized clinical trial. Journal of Consulting and Clinical Psychology. 2006;74(6):1153–1161. doi: 10.1037/0022-006X.74.6.1153. [DOI] [PubMed] [Google Scholar]
- Fiore MC. Treating tobacco use and dependence: An introduction to the US Public Health Service Clinical Practice Guideline. Respiratory Care. 2000;45(10):1196–1199. [PubMed] [Google Scholar]
- Friend KB, Pagano ME. Time-varying predictors of smoking cessation among individuals in treatment for alcohol abuse and dependence: Findings from Project MATCH. Alcohol and Alcoholism. 2007;42(3):234–240. doi: 10.1093/alcalc/agm026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordin FM, Roediger MP, Girard PM, Lundgren JD, Miro JM, Palfreeman A, et al. Pneumonia in HIV-infected persons: increased risk with cigarette smoking and treatment interruption. American Journal of Critical Care Medicine. 2008;178(6):630–636. doi: 10.1164/rccm.200804-617OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritz ER, Vidrine DJ, Lazev AB, Amick BC, Arduino RC. Smoking behavior in a low-income multiethnic HIV/ AIDS population. Nicotine and Tobacco Research. 2004;6(1):71–77. doi: 10.1080/14622200310001656885. [DOI] [PubMed] [Google Scholar]
- Ingersoll KS, Cropsey KL, Heckman CJ. A test of motivational plus nicotine replacement interventions for HIV positive smokers. AIDS Behavior. 2007 doi: 10.1007/s10461-007-9334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd CM, Kenny DA, McClelland GH. Estimating and testing mediation and moderation in within-subject designs. Psychological Methods. 2001;6(2):115–134. doi: 10.1037/1082-989x.6.2.115. [DOI] [PubMed] [Google Scholar]
- Kaufert JM, Rabkin SW, Syrotuik J, Boyko E, Shane F. Health beliefs as predictors of success of alternate modalities of smoking cessation: Results of a controlled trial. Journal of Behavioral Medicine. 1986;9(5):475–489. doi: 10.1007/BF00845134. [DOI] [PubMed] [Google Scholar]
- Kohli R, Lo Y, Homel P, Flanigan TP, Garnder LI, Howard AA, et al. Bacterial pneumonia, HIV therapy, and disease progression among HIV-infected women in the HIV epidemiologic research (HER) study. Clinical Infectious Disease. 2006;43(1):90–98. doi: 10.1086/504871. [DOI] [PubMed] [Google Scholar]
- Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. Journal of Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- Lee C. Perceptions of immunity to disease in adult smokers. Journal of Behavioral Medicine. 1989;12:267–277. doi: 10.1007/BF00844871. [DOI] [PubMed] [Google Scholar]
- Lloyd-Richardson EE, Stanton CA, Papandonatos GD, Betancourt RM, Stein M, Tashima K, et al. HIV-positive smokers considering quitting: Differences by race/ethnicity. American Journal of Health Behavior. 2008;32(1):3–15. doi: 10.5555/ajhb.2008.32.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Richardson EE, Stanton CA, Papandonatos GD, Shadel WG, Stein M, Tashima K, et al. Motivation and patch treatment for HIV-positive smokers: A randomized controlled trail. doi: 10.1111/j.1360-0443.2009.02623.x. in press. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longabaugh R. Manual for the administration of the Important People Instrument adapted for use for BST Decision Trees. Providence, RI: Brown University Center for Alcohol and Addiction Studies; 2001. [Google Scholar]
- MacKinnon DP, Luecken LJ. How and for whom? Mediation and moderation in health psychology. Health Psychology. 2008;27(2):S99–S100. doi: 10.1037/0278-6133.27.2(Suppl.).S99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Baker TB. Psychological mediators of Bupropi-on sustained-release treatment for smoking cessation. Addiction. 2008;103(9):1521–1533. doi: 10.1111/j.1360-0443.2008.02275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metersky ML, Colt HG, Olson LK, Shanks TG. AIDS-related spontaneous pneumothorax: Risk factors and treatment. Chest. 1995;108(4):946–951. doi: 10.1378/chest.108.4.946. [DOI] [PubMed] [Google Scholar]
- Muller D, Judd CM, Yzerbyt VY. When moderation is mediated and mediation is moderated. Journal of Personality and Social Psychology. 2005;89(6):852–863. doi: 10.1037/0022-3514.89.6.852. [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Morrow K, Tashima K, Flanigan T, Abrams DB. Human immunodeficiency virus infection, AIDS, and smoking cessation: The time is now. Clinical Infectious Diseases. 2000;31(3):808–812. doi: 10.1086/314048. [DOI] [PubMed] [Google Scholar]
- Perez GH, Nicolau JC, Romano BW, Laranjeira R. Depression: A predictor of smoking relapse in a 6-month follow-up after hospitalization for acute coronary syndrome. European Journal of Cardiovascular Prevention and Rehabilitation. 2008;15(1):89–94. doi: 10.1097/HJR.0b013e3282f4b212. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC, Velicer WF, Ginpil S, Norcross JC. Predicting change in smoking status for self-changers. Addictive Behavior. 1985;10(4):395–406. doi: 10.1016/0306-4603(85)90036-x. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, Velicer WF, Rossi JS, Gold-stein MG, Marcus BH, Rakowski W, et al. Stages of change and decisional balance for 12 problem behaviors. Health Psychology. 1994;13(1):39–46. doi: 10.1037//0278-6133.13.1.39. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depressive scale for research in the general population. Journal of Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Shepard D, Neighbors C, Lloyd-Richardson EE, Beaston-Blaakman A, Farrell N, Niaura R. Assessing costs and time of counseling services for HIV-positive patients. Paper presented at the 14th International AIDS Conference; Barcelona, Spain. 2002. Jul, [Google Scholar]
- Sobell LC, Sobell MB. Alcohol consumption measures. In: Allen JP, Columbus M, editors. Assessing alcohol problems: A guide for clinicians and researchers. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 1995. pp. 55–73. [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Review. 2008;(1):CD000146. doi: 10.1002/14651858.CD000146.pub3. [DOI] [PubMed] [Google Scholar]
- Tsourtos G, O’Dwyer L. Stress, stress management, smoking prevalence and quit rates in a disadvantaged area: Has anything changed? Health Promotion Journal of Australia. 2008;19(1):40–44. doi: 10.1071/he08040. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Clearing the air: Quit smoking today. (NIH Publication No. 03–1647) Washington, DC: National Institutes of Health/ National Cancer Institute; 2003. [Google Scholar]
- Velicer WF, DiClemente CC, Prochaska JO, Brandenburg N. Decisional balance measure for assessing and predicting smoking status. Journal of Personality and Social Psychology. 1985;48(5):1279–1289. doi: 10.1037//0022-3514.48.5.1279. [DOI] [PubMed] [Google Scholar]
- Velicer WF, Diclemente CC, Rossi JS, Prochaska JO. Relapse situations and self-efficacy: An integrative model. Addictive Behavior. 1990;15(3):271–283. doi: 10.1016/0306-4603(90)90070-e. [DOI] [PubMed] [Google Scholar]
- Vidrine DJ, Arduino RC, Gritz ER. Impact of a cell phone intervention on mediating mechanisms of smoking cessation in individuals living with HIV/AIDS. Nicotine & Tobacco Research. 2006;8(1):S103–S108. doi: 10.1080/14622200601039451. [DOI] [PubMed] [Google Scholar]
- Vidrine DJ, Arduino RC, Lazev AB, Gritz ER. A randomized trial of a pro-active cellular telephone intervention for smokers living with HIV/AIDS. AIDS. 2006;20:253–260. doi: 10.1097/01.aids.0000198094.23691.58. [DOI] [PubMed] [Google Scholar]
- Williams GC, Gagne M, Ryan RM, Deci EL. Facilitating autonomous motivation for smoking cessation. Health Psychology. 2002;21(1):40–50. [PubMed] [Google Scholar]