A provocative new study in this issue of Circulation Research by Ibarra et al.1 suggests that the insulin-like growth factor 1 receptors (IGF-1R), exist in plasma membrane invaginations that come in very close proximity to (or even invade) the nuclear membrane in cardiac myocytes to selectively raise local nuclear [Ca2+] ([Ca2+]Nuc). This paper raises an intriguing and novel mechanism by which plasma membrane receptors may have preferential local access to nuclear signaling, even ventricular myocytes which are large cells with central nuclei.
The IGF-1R is a tyrosine kinase growth factor receptor that connects with many downstream signaling cascades.2 The two best known pathways are Ras-Raf-mitogen-activated protein kinase and phosphatidylinositide 3 kinase (PI3K)-protein kinase B (PKB/Akt). However, Ibarra et al.1 focus on a less well studied pathway in which IGF-1induces Gi-dependent phospholipase C (PLC) activation3 and 1,4,5-inositol tris-phosphate (InsP3) that can release Ca2+ from intracellular InsP3 receptor (InsP3R) stores.4 They show that in cardiac myocytes IGF-1 triggers nuclear Ca2+ transients that precede cytosolic Ca2+ transients, and that blocking the nuclear Ca2+ transient prevents the cytosolic Ca2+ transient, but not vice-versa. Their data are consistent with the idea that a substantial fraction of plasma membrane IGF-R1 are in patches of plasma membrane invaginations that are extremely close to the nucleus, potentially as extensions of the transverse tubule (T-tubule) system. Further, they infer that these IGF-1R are Gi-coupled and that upon activation they produce PLC and InsP3-dependent nuclear Ca2+ transients that contributes to Ca-dependent regulation of gene transcription (Fig 1B).
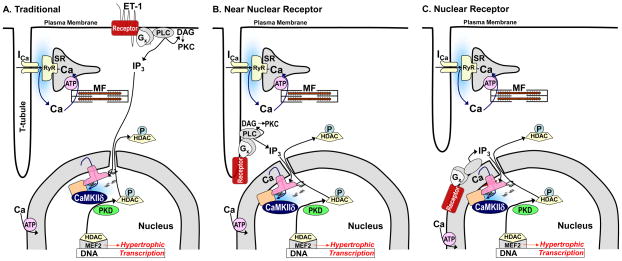
Figure 1. Three models of membrane receptor signaling to InsP3 dependent Ca2+ signals.
A) in the traditional model receptors (as for ET-1 in this example) activate InsP3 production at the cell periphery (or throughout the plasma membrane). But the InsP3 must diffuse a potentially long way to the nuclear InsP3R to cause local nuclear [Ca2+] elevation by release from the Ca2+ store in the nuclear envelope. B) The model shown by Ibarra et al.1 indicating the IGF-1R complex in plasma membrane invaginations, reducing the diffusional distance for InsP3 to reach the nucleus. C) A third model where functional G-protein coupled receptors can exist near or on the nuclear envelope. RyR, ryanodine receptor, ICa, Ca current, ATP, SR/ER Ca-ATPase; MF, myofilaments; P, phosphorylation, Cam, calmodulin, Gx, G-protein, DAG, diacylglycerol.
While many plasma membrane receptors signal to the nucleus, there are numerous potential pathways for this signaling. Even if we constrain ourselves to a narrow field involving InsP3-dependent nuclear Ca2+ signaling,5 which has been most extensively studied for Gq-coupled receptors (e.g. endothelin-1 (ET-1), α-adrenergic and angiotensin II receptors), there may be three different organizations to consider (Fig 1). In what might now be considered the classical or traditional model (Fig 1A), we showed that ET-1 triggered a rise in cytosolic [InsP3] in adult ventricular myocytes that precedes the rise of [InsP3] in the nucleus (using a FRET-based InsP3 sensor)6 and that most of the InsP3R in these myocytes is the type 2 InsP3R that is largely localized to the nuclear envelope and complexed with Ca-Calmodulin dependent protein kinase II (CaMKII).7 We showed evidence for the hypothesis (Fig 1A) that ET-1 activation produces InsP3 at the membrane which diffuses to nuclear InsP3R, elevating local [Ca2+] there to effectively activate CaMKII (and PKD) dependent phosphorylation of histone deacetylase 5 (HDAC5) to trigger HDAC5 nuclear export and de-repression of MEF2-dependent hypertrophic signaling.5 It was known already that InsP3 is better than Ca2+ at long-distance signaling in cells8 and that CaMKII and PKD are HDAC kinases which could drive HDAC nuclear export and thus activate MEF2-dependent transcription.9 Moreover, the high local [Ca] at the mouth of the InsP3R channel would facilitate CaMKII activation (which requires high local [Ca2+]) in the nuclear environment. This working model nicely connects Gq-coupled receptor activation to an important role for InsP3/Ca2+ signaling in cardiac myocytes aside from E-C coupling, and connects nuclear InsP3R and CaMKII to transcriptional regulation in the heart downstream of Gq-coupled receptor activation. Indeed, this allows Ca-dependent local signaling (around the InsP3R) to work in parallel to but independent of the global Ca2+ transients associated with regular E-C coupling.5
Ibarra et al.1 suggest an important extension to this concept (Fig 1B). They show evidence that an entire receptor (in this case IGF-1R), G-protein, PLC and InsP3 production complex is situated at the nucleus due to plasma membrane infoldings in direct apposition to the nuclear envelope. This would have the tremendous advantage of producing the InsP3 very close to the InsP3R at the nucleus. It would greatly reduce the amount of InsP3 production required to activate the local nuclear Ca transients. It also explains their observations of a faster rise in IGF-1-induced Ca transients in the nucleus vs. cytosol that was most apparent in cultured neonatal rat ventricular myocytes. Their nuclear vs. cytosolic difference in kinetics was not as clear in adult ventricular myocytes, but Ca2+ transients induced by IGF-1 were larger in the nucleus than cytosol, and that was opposite to what was seen with normal E-C coupling Ca2+ transients. Thus, IGF-1 and InsP3-driven transients initiate first and strongest in the nucleus, whereas the normal beat-to-beat Ca2+ transients initiate and are stronger in the cytosol. That is consistent with data suggesting that InsP3R activation promotes relatively stronger nuclear Ca2+ signals,10–12 although Ca2+ indicator calibrations in nucleus may differ from that in the cytosol.13 While it would be worthwhile to further test the model proposed by Ibarra, especially in adult ventricular myocytes, this is an intriguing structural organization.
There is also convincing evidence of a third organization of G-protein coupled receptor to InsP3/Ca2+ signaling in the nucleus (Fig 1C). That is, some fraction of Gq-coupled receptors appear to be functional, intracellular and localized at or near the nucleus.14–18 Indeed, there is evidence that many of the key molecules (G proteins, PLC and InsP3 metabolizing proteins) are also at the nuclear membrane. Since the SR/ER network throughout ventricular myocytes is continuous with the nuclear envelope membranes,19 the receptors and key signaling molecules could translocate to the nuclear envelope from ER without necessarily leaving that membrane. One issue for this organization is how the ligand (e.g. norepinephrine or Ang II) gets across the plasma membrane to gain access to the internal receptor. In some cases that might be mediated by membrane transporters (e.g. norepinephrine via extraneuronal monoamine transporter, EMT/OCT3),14 while in other cases it might represent an intracellular autocrine signaling pathway (e.g. in response to myocyte produced Ang II).15
It is quite plausible that all three of these receptor complex organizations coexist in cardiac myocytes, and maybe even for the same type of receptors. There are certainly details about each of these working models that need to be better worked out and validated. However, these models also raise intriguing questions regarding which pathway might be most important for a) a given receptor type, b) different types of signaling contexts, c) cross-talk with different signaling networks and d) different pathophysiological conditions or pharmacological responses. So, as in real estate, location again matters and receptor complexes may take up residence in different neighborhoods for reasons that we will still have to figure out.
Acknowledgments
Funding: NIH P01-HL080101, R37-HL030077.
Footnotes
Disclosures: None
References
- 1.Ibarra C, Vicencio JM, Estrada M, Lin Y, Rocco P, Rebellato P, Munoz JP, Garcia-Prieto J, Quest AFG, Chiong M, Davidson SM, Bulatovic I, Grinnemo K-H, Larsson O, Szabadkai G, Uhlen P, Jaimovich E, Lvandero S. Local control of nuclear Ca2+ signaling in cardiac myocytes by perinuclear sarcolemmal microdomains. Circ Res. 2012;112:xxx–xxx. doi: 10.1161/CIRCRESAHA.112.273839. (in this issue) [DOI] [PubMed] [Google Scholar]
- 2.Sachdev D, Yee D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol Cancer Ther. 2007;6:1–12. doi: 10.1158/1535-7163.MCT-06-0080. [DOI] [PubMed] [Google Scholar]
- 3.Luttrell LM, van Biesen T, Hawes BE, Koch WJ, Touhara K, Lefkowitz RJ. G beta gamma subunits mediate mitogen-activated protein kinase activation by the tyrosine kinase insulin-like growth factor 1 receptor. J Biol Chem. 1995;270:16495–8. doi: 10.1074/jbc.270.28.16495. [DOI] [PubMed] [Google Scholar]
- 4.Ibarra C, Estrada M, Carrasco L, Chiong M, Liberona JL, Cardenas C, Díaz-Araya G, Jaimovich E, Lavandero S. Insulin-like growth factor-1 induces an inositol 1,4,5-trisphosphate-dependent increase in nuclear and cytosolic calcium in cultured rat cardiac myocytes. J Biol Chem. 2004;279:7554–65. doi: 10.1074/jbc.M311604200. [DOI] [PubMed] [Google Scholar]
- 5.Wu X, Zhang T, Bossuyt J, Li X, McKinsey T, Dedman JR, Olson EN, Chen J, Brown JH, Bers DM. Local InsP3-dependent Perinuclear Ca Signaling in Cardiac Myocytes Excitation-Transcription Coupling. J Clin Invest. 2006;116:675–682. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bare DJ, Kettlun CS, Liang M, Bers DM, Mignery GA. cardiac type-2 Inositol tris phosphate receptor: interaction and modulation by CaMKII. J Biol Chem. 2005;280:15912–15920. doi: 10.1074/jbc.M414212200. [DOI] [PubMed] [Google Scholar]
- 7.Remus TP, Zima AV, Bossuyt J, Bare DJ, Martin JL, Blatter LA, Bers DM, Mignery GA. Biosensors to Measure InsP3 Concentration in Living Cells with Spatio-temporal Resolution. J Biol Chem. 2006;281:608–616. doi: 10.1074/jbc.M509645200. [DOI] [PubMed] [Google Scholar]
- 8.Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992;258:1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- 9.Passier R, Zeng H, Frey N, Naya FJ, Nicol RL, Mckinsey TA, Overbeek P, Richardson JA, Grant SR, Olson EN. Cam kinase signalling induces cardiac hypertrophy and activates the MEF2 transcription factor in vivo. J Clin Invest. 2000;105:1395–1406. doi: 10.1172/JCI8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipp P, Thomas D, Berridge MJ, Bootman MD. Nuclear Calcium Signalling By Individual. Cytoplasmic Calcium Puffs. EMBO J. 1997;16:7166–7173. doi: 10.1093/emboj/16.23.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zima AV, Bare DJ, Mignery GA, Blatter LA. IP3-dependent nuclear Ca2+ signalling in the mammalian heart. J Physiol. 2007;584:601–611. doi: 10.1113/jphysiol.2007.140731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kockskämper J, Seidlmayer L, Walther S, Hellenkamp K, Maier LS, Pieske B. Endothelin-1 enhances nuclear Ca2+ transients in atrial myocytes through Ins(1,4,5)P3-dependent Ca2+ release from perinuclear Ca2+ stores. J Cell Sci. 2008;121:186–95. doi: 10.1242/jcs.021386. [DOI] [PubMed] [Google Scholar]
- 13.Ljubojević S, Walther S, Asgarzoei M, Sedej S, Pieske B, Kockskämper J. In situ calibration of nucleoplasmic versus cytoplasmic Ca2+ concentration in adult cardiomyocytes. Biophys J. 2011;100:2356–66. doi: 10.1016/j.bpj.2011.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright CD, Chen Q, Baye NL, Huang Y, Healy CL, Kasinathan S, O’Connell TD. Nuclear alpha1-adrenergic receptors signal activated ERK localization to caveolae in adult cardiac myocytes. Circ Res. 2008;103:992–1000. doi: 10.1161/CIRCRESAHA.108.176024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tadevosyan A, Maguy A, Villeneuve LR, Babin J, Bonnefoy A, Allen BG, Nattel S. Nuclear-delimited angiotensin receptor-mediated signaling regulates cardiomyocyte gene expression. J Biol Chem. 2010;285:22338–49. doi: 10.1074/jbc.M110.121749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaniotis G, Allen BG, Hébert TE. Nuclear GPCRs in cardiomyocytes: an insider’s view of β-adrenergic receptor signaling. Am J Physiol Heart Circ Physiol. 2011;301:H1754–64. doi: 10.1152/ajpheart.00657.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tadevosyan A, Vaniotis G, Allen BG, Hébert TE, Nattel S. G protein-coupled receptor signalling in the cardiac nuclear membrane: evidence and possible roles in physiological and pathophysiological function. J Physiol. 2012;590:1313–30. doi: 10.1113/jphysiol.2011.222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright CD, Wu SC, Dahl EF, Sazama AJ, O’Connell TD. Nuclear localization drives α1-adrenergic receptor oligomerization and signaling in cardiac myocytes. Cell Signal. 2012;24:794–802. doi: 10.1016/j.cellsig.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X, Bers DM. Sarcoplasmic reticulum and nuclear envelope are one highly interconnected Ca2+ store throughout cardiac myocyte. Circ Res. 2006;99:283–291. doi: 10.1161/01.RES.0000233386.02708.72. [DOI] [PubMed] [Google Scholar]