Abstract
Background
Non-adherence with immunosuppressive medications can result in allograft rejection and eventually allograft loss.
Methods
In a racially diverse population, we utilized microelectronic cap monitors to determine the association of adherence with a single immunosuppressive medication and kidney allograft outcomes post-transplantation. This prospective cohort study enrolled 243 patients from eight transplant centers to provide adherence and kidney allograft outcomes data. To determine the association of adherence with change in estimated glomerular filtration rate (eGFR), we fit mixed effects models with the outcome being change in eGFR over time. We also fit Cox proportional hazards models to determine the association of adherence with time to persistent 25% and 50% decline in eGFR.
Results
The distribution of adherence post-transplant was as follows: 164 (68%), 49 (20%) and 30 (12%) had >85–100%, 50–85% and <50% adherence, respectively. 79 (33%) and 36 (15%) of the subjects experienced a persistent 25% decline in eGFR or allograft loss and 50% decline in eGFR or allograft loss during follow-up. Adherence was not associated with acute rejection or 25% decline or 50% decline in eGFR. In the adjusted and unadjusted model, adherence and black race were not associated with change in eGFR over time.
Conclusions
Non-adherence with a single immunosuppressive medication, was not associated with kidney allograft outcomes.
Keywords: kidney transplant, adherence, renal function
Introduction
Non-adherence to immunosuppressive medications results in premature allograft rejection and poor kidney function among kidney transplant recipients. Up to 50% of kidney transplant recipients are non-adherent. (1–4) This non-adherence can range from an occasional missed dose to discontinuation of all immunosuppressive medications for an extended period of time. Some prior studies (2, 5–8) including our own (9), have reported an association between Black race and lower levels of medication adherence. However, socioeconomic factors and other factors surrounding delivery of care may explain this association. (6, 7)
Currently, microelectronic event monitoring is considered an excellent method for measuring adherence to drug therapies. It is a valid, reliable and sensitive method for measuring adherence. (10–14). Microelectronic event monitoring entails using a microprocessor embedded in the cap of a medication bottle. This microprocessor records every opening and closing of the cap, and each such opening and closing is presumed to represent an ingestion of the monitored medication. A record of each cap opening and closing permits counting of missed doses and provides data on deviations from the prescribed schedules for medication ingestion. (10).
In our previously published work, we utilized microelectronic monitoring for a prospective cohort study of recipients of deceased donor kidney transplants.(9) This work showed that the following factors were associated with adherence: Black race, transplant center, and prescribed frequency of the monitored drug. An increased frequency of the monitored drug was associated with decreased adherence. After accounting for the transplant center and prescribed frequency of the monitored drug, the association of Black race with lower levels of adherence was attenuated and was no longer statistically significant. We now present the results of a follow-up study of this cohort (1) to assess the association of adherence on kidney function following transplantation, and (2) to determine whether adherence explains the disparity in outcomes seen between black and non-black transplant recipients.
Materials and Methods
Study Design and Procedures
The study enrolled recipients of deceased donor kidneys into a cohort between April 1998 until November 2001 and detailed explanation of the study procedures have been published previously.(9) These recipients were recruited at the time of transplantation from eight transplant centers served by the Gift of Life Donor Program in eastern Pennsylvania. These centers included: Albert Einstein Medical Center, Hahnemann University Hospital, the Hospital of the University of Pennsylvania, and Thomas Jefferson University Hospital (Philadelphia, PA); Geisinger Medical Center (Danville, PA); Hershey Medical Center (Hershey, PA); Lehigh Valley Hospital Center (Allentown, PA); and Lankenau Hospital (Wynnewood, PA). All deceased donor kidney recipients at these centers who were 18 years or older were eligible for enrollment. The human subjects Institutional Review Board at Minneapolis Medical Research Foundation approved the study.
While the patients were hospitalized for their transplantation, they were approached to obtain informed consent and were interviewed regarding their demographic (including race), socioeconomic, and medical characteristics. Enrolled patients underwent telephone interviews after discharge and every six months post-transplant up to 36 months post-transplant or until July 2004. We examined these patients’ inpatient and outpatient medical records every six months for up to 36 months after transplantation or until July 2004, whichever occurred first. Referring community nephrologists were contacted to obtain data on kidney allograft function for those patients who did not return to their transplant center for routine visits. Kidney function was calculated using the four- variable Modified Diet in Renal Disease formula (15) to generate an estimated glomerular filtration rate (eGFR). Baseline eGFR was established between 90 and 180 days post-transplant. Relative to this baseline, time to persistent 25% decline and 50% decline in eGFR were calculated. Acute rejection was defined as a clinical rejection event requiring use of intravenous steroids and/or antibody therapy during the first year post-transplant. Delayed allograft function was defined as needing dialysis in the first week post-transplant. Allograft loss was defined as return to dialysis or retransplantation.
Patients’ immunosuppressive regimens and medical management were determined by their individual medical providers. The study data regarding adherence to immunosuppressive medication were not shared with the medical providers or the patient.
Measurement of Adherence
Adherence was measured using the electronic Drug Exposure Monitor (eDEM) cap (AARDEX, Ltd; Zug, Switzerland). This electronic cap monitor records the date and the time of opening and closing of the medication bottle. In each patient, we monitored only one immunosuppressive medication at a time. The choice of monitored medication, in order of preference, included mycophenolate mofetil (MMF) (82.7% of initially monitored drug), tacrolimus (7.9%), prednisone (8.3%), azathioprine (0.7%), and sirolimus (0.4%). The most common dosing frequency of the initial drug monitored was two times per day (70.5% of the initially monitored drug) followed by three-or-four times per day (19.4%) and one time per day (10.1%). (9)
After the patient was discharged shortly from the initial transplant hospitalization, the eDEM cap was mailed to the patient. Patients were then instructed to return the caps to the study coordinator after six months, and a follow-up telephone interview was conducted. The cap opening and closing data were downloaded from the caps and the caps were then returned to the patients for continuation of monitoring. In cases where the prescribing provider discontinued the monitored medication, we instructed patients to utilize the eDEM cap for another immunosuppressive medication, from the list above.
Definition and Calculation of Adherence
The data from the microelectronic cap was used from either the second day of cap use or the day after the initial telephone interview, whichever occurred last. We excluded patients who had fewer than 14 days of usable data from the microelectronic cap. Microelectronic data were utilized for the remaining patients to assess adherence until either six months post-transplant, patient death, or patient withdrawal from the study, as described in detail previously. (9) For every patient that was included in the analysis, we calculated the percentage of prescribed doses that were taken by a patient for each day. Then for each patient’s eligible days, the mean daily percent adherence was calculated and this mean adherence was the primary exposure.
Statistical Analysis
Owing to its skewed distribution, study participants were grouped into three ordered categories of mean adherence over the six months post-transplant; 0 to 50%, >50 to 85%, >85 to 100%, before linking with renal function outcome data. In our previous publication (9), we had four categories of adherence; however, due to the smaller number of subjects with follow-up data, we created only these 3 categories of mean adherence. To determine the association of adherence with change in eGFR, we fit a mixed effects model with the outcome being eGFR. This model also included fixed effects for timing of eGFR measurement after transplant in months, mean adherence, and a term for the interaction between these two variables. The interaction term provides a method to interpret the relationship between mean adherence and change in eGFR. This interaction term, if significant, would indicate that mean adherence was associated with change in eGFR over time. Other fixed effects, including recipient Black race, gender, transplant center, donor age, and delayed allograft function, were also considered for association with change in eGFR over time using a similar interaction term.
We also assessed the association of adherence with a persistent 25% decline in eGFR using a Cox proportional hazards model. A persistent decline in eGFR was defined by allograft loss or at least two consecutive eGFR readings, at least one month apart, that were 25% or more below the baseline eGFR level. The baseline was established at 90–180 days post-transplant, a priori, based on the nadir of the serum creatinine values post-transplantation. A similar composite outcome was constructed for 50% decline in eGFR also using allograft loss or at least two consecutive eGFR readings, at least one month apart, that were 50% or more below the baseline eGFR level. We also used a Cox proportional hazards model to test for association of adherence with acute rejection in the first year post-transplant. Socio-economic factors (employment status at the time of transplant, household annual income, education level) and other factors such as Black race, gender, transplant center, donor age and delayed allograft function, were considered in these models. Each of these variables was considered in a bivariate model that included adherence, and only those variables in the model that were significant with likelihood ratio test (p≤0.10) or variables with p-value <0.10, were included in the multivariate model.
Analyses were performed using SAS statistical software, version 8.1 (SAS Institute, Cary, NC) and Stata 9.0 (College Station, Texas). All two-sided P-values less than 0.05 were considered statistically significant.
Results
Patient Sample
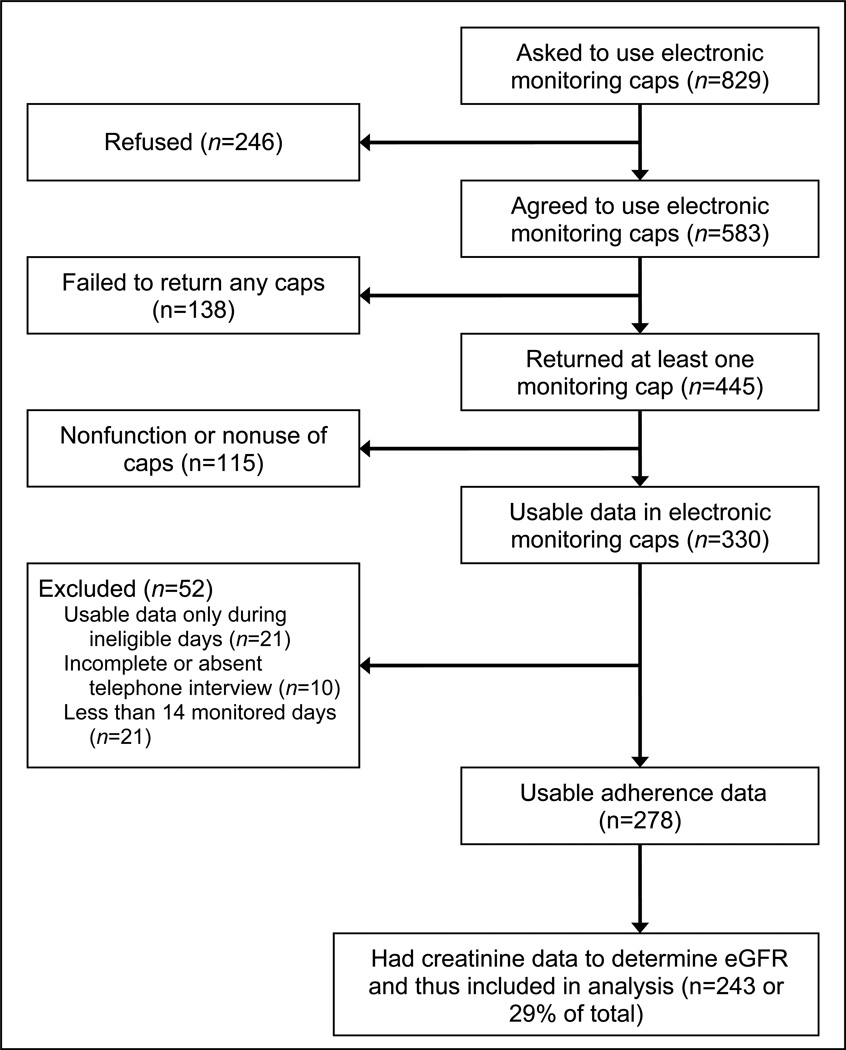
As previously reported, of the 829 transplant recipients approached by the study, 278 subjects consented and provided usable adherence data. The specific reasons for exclusions are shown in Figure 1. Fewer recipients with the following characteristics consented to participate and provide adherence data: Black, maintenance dialysis before transplantation, delayed allograft function, hypertension as cause of ESRD, less than college education, on cyclosporine as the initial immunosuppressive agent.(9) Of the 278 recipients of deceased donor kidney transplants enrolled in the study, 243 had follow-up serum creatinine data available. Therefore, the analysis of the association between adherence and outcome was conducted among these 243 patients. Their baseline characteristics and post-transplant outcomes, including episodes of rejection, are described in Table 1. The 243 participants had a median follow-up of 876 days (range, 19 to 1,094 days). The distribution of mean adherence over the first six months post-transplant was as follows: 164 (68%), 49 (20%) and 30 (12%) had >85–100%, 50–85% and <50% adherence, respectively.
Figure 1.
Enrollment and flow of participants through the study
Table 1.
Baseline Characteristics at the time of Transplantation, n=243. (Number in parenthesis is %, unless otherwise noted)
| Recipient Race | |
|---|---|
| Black | 63 (26 %) |
| Caucasian | 176 (72 %) |
| Other | 4 (2 %) |
| Recipient Sex | |
| Male | 153 (63 %) |
| Recipient Age at time of transplant (years± S.D.) | 47.7 ± 11.9 |
| Dialysis Pre-transplant | |
| Yes | 209 (86 %) |
| No | 34 (14 %) |
| Cause of ESRD | |
| Hypertension | 81 (33 %) |
| Diabetes | 77 (32 %) |
| Other | 74 (30 %) |
| Unknown | 11 (5 %) |
| Previous Transplant | 45 (19 %) |
| Recent Panel Reactive Antigen (PR A) | |
| >20 % | 28 (12 %) |
| 1–20 % | 19 (8 %) |
| < 1 % | 176 (72 %) |
| Unknown | 20 (8 %) |
| Cold Ischemia Time | |
| >24 hours | 46 (19 %) |
| 12–24 hours | 162 (67 %) |
| < 12 hours | 35 (14 %) |
| Donor Age (years years± S.D.) | 37.0 ± 18.1 |
| Donor Cause of Death | |
| Cardiovascular | 42 (18 %) |
| Intracranial Hemmorhage | 59 (25 %) |
| Trauma | 117 (49 %) |
| Other | 20 (8 %) |
| Dialysis in Week One Post-transplant | 75 (31 %) |
| In-hospital rejection, prior to discharge | 15 (6 %) |
| Acute Rejection in first year (excluding in-hospital rejection) | 25 (10 %) |
| Allograft Loss* | 15 (6%) |
| Patient Death | 4 (2 %) |
Allograft loss defined as return to dialysis or re-transplantation.
Adherence and Outcomes in Blacks versus non-Blacks
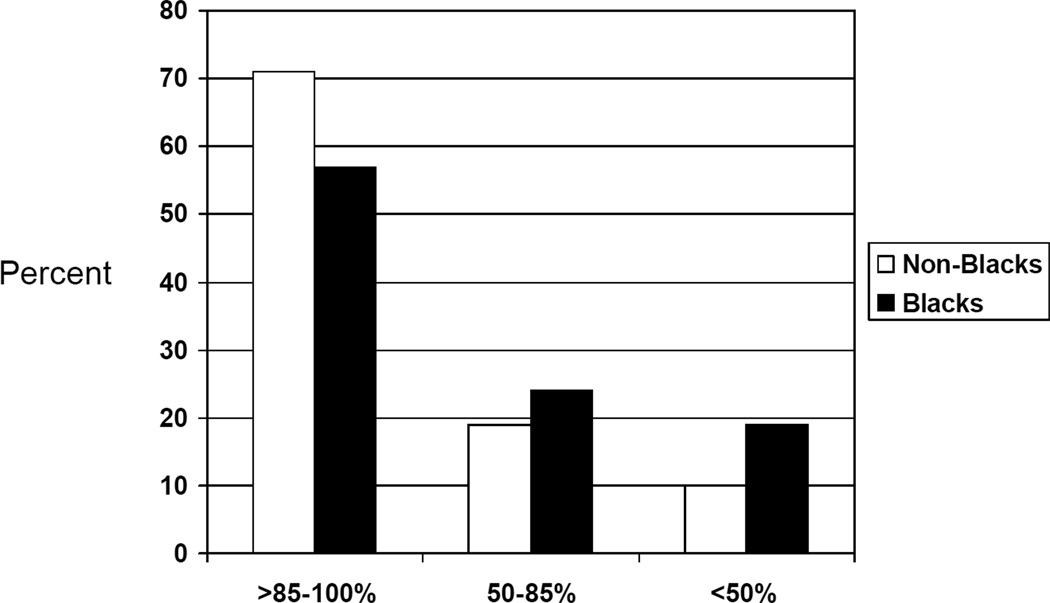
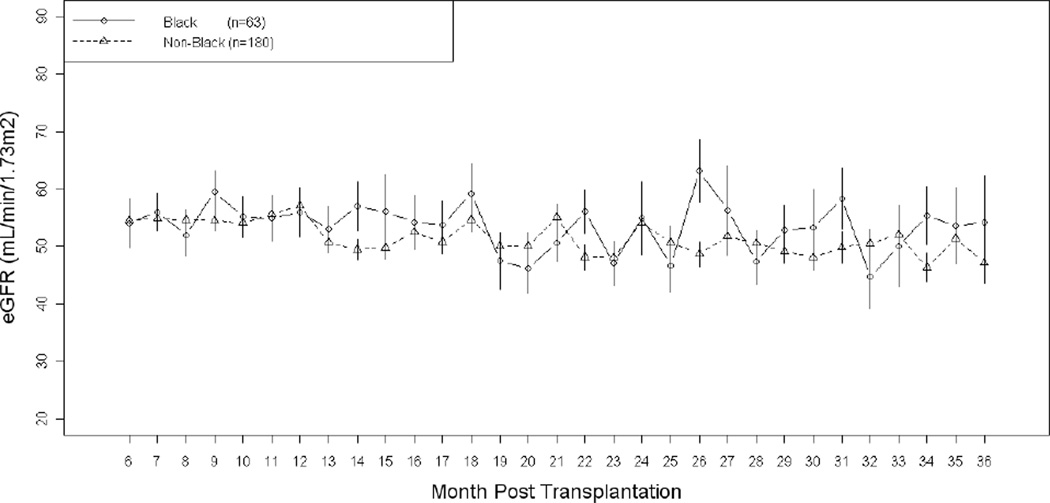
For Blacks, the distribution of mean adherence over the first six months post-transplant is shown in Figure 2. There were significant differences in distribution of adherence by race, with Blacks exhibiting lower levels of drug adherence (t-test, p=0.0061), consistent with our previous finding.(9) The eGFR for the study patients, stratified by Black race, is shown in Figure 3. Compared to non-Blacks, Blacks were more likely to experience a 25% decline in eGFR [HR=2.20, 95% C.I. (1.14–4.28), p=0.019 for 25% decline in eGFR]. Compared to non-Blacks, the association of Black race with 50% decline in eGFR was not statistically significant [HR= 1.51, 95% C.I. (0.94–2.41), p=0.087] Black race was not associated with increased risk of acute rejection in the first year post-transplant [HR=0.89, 95% C.I. (0.36–2.23), p=0.80]. However, among Blacks there were only 6 (10%) acute rejections. Blacks had 7 (11%) allograft loss and 3 (5%) patient death events.
Figure 2.
Distribution of Adherence for Blacks (n= 63 ) versus Non-Blacks (n= 180).
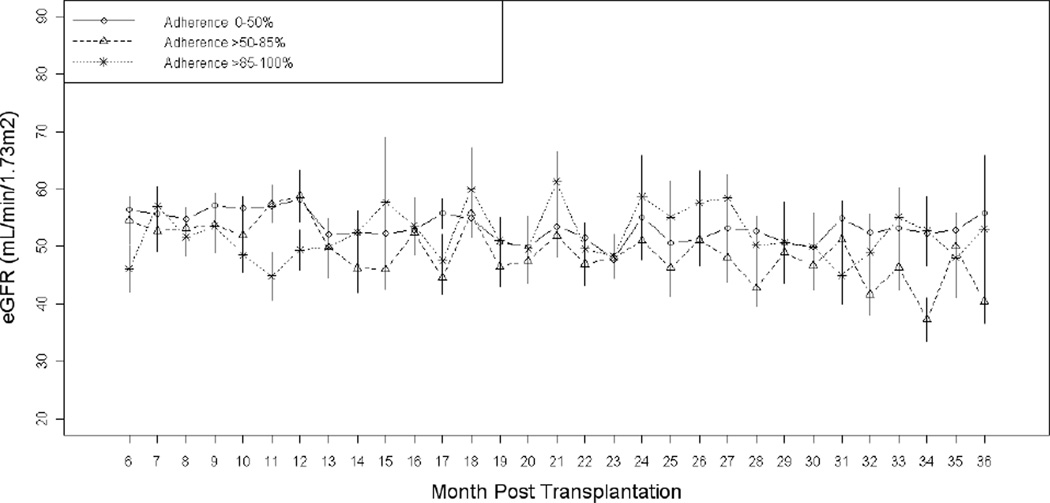
Figure 3.
Comparison of eGFR over time by Adherence Group (n=243)
Adherence, Acute Rejection, and Kidney Function
Acute rejections occurred in 25 (10%) of the subjects. Adherence was not associated with risk of acute rejection over the first year. (Table 2). The distributions of eGFR over time for recipients in the three adherence groups is shown in Figure 4. 79 (33 %) of the subjects experienced a persistent 25% decline in eGFR and 36 (15 %) of the subjects experienced a persistent 50% decline in eGFR during follow-up. 15 subjects (6%) lost their allograft during the study period. Adherence was not associated with persistent 25% or 50% decline in eGFR. (Table 2)
Table 2.
A. Association of adherence with acute rejection and renal function outcomes, unadjusted analysis.*
| Adherence Level | Acute Rejection HR (95% C.I.) |
25 % decline in eGFR or allograft loss HR (95% C.I.) |
50 % decline in eGFR or allograft loss HR (95% C.I.) |
|---|---|---|---|
| Greater than 85%–100% | 1.0 (Reference) |
1.0 (Reference) |
1.0 (Reference) |
| 85% – 59% | 1.47 (0.56 – 3.82) |
1.32 (0.78 – 2.24) |
1.31 (0.61 – 2.84) |
| Less than 50% | 2.06 (0.74 – 5.73) |
1.12 (0.57 – 2.21) |
0.93 (0.32 – 2.68) |
| B Association of adherence with acute rejection and renal function outcomes, adjusted analysis.* Adjusted for recipient race and in-hospital rejection that occurred prior to discharge post-transplantation. | |||
|---|---|---|---|
| Adherence Level | Acute Rejection HR (95% C.I.) |
25 % decline in eGFR or allograft loss HR (95% C.I.) |
50 % decline in eGFR or allograft loss HR (95% C.I.) |
| Greater than 85%–100% | 1.0 (Reference) |
1.0 (Reference) |
1.0 (Reference) |
| 85% – 59% | 1.80 (0.60 – 5.42) |
1.24 (0.73 – 2.12) |
1.09 (0.50 – 2.41) |
| Less than 50% | 5.34 (1.68 – 16.95) |
1.03 (0.52 – 2.05) |
0.79 (0.27 – 2.30) |
| Black (vs. Non-Black) | 0.58 (0.20 – 1.66) |
1.46 (0.90–2.35) |
2.14# (1.10– 4.19) |
| In-hospital Rejection |
**# |
1.86 (0.89–3.89) |
3.26# (1.33–7.96) |
Analysis conducting using a Cox proportional hazards model which shows the association of lower adherence levels with renal function outcomes, using the highest adherence category as the reference category.
Analysis conducting using a Cox proportional hazards model which shows the association of lower adherence levels with renal function outcomes, using the highest adherence category as the reference category.
All 15 subjects with in-hospital rejection, experienced acute rejection, post-discharge. Due to this collinearity in the small number of subjects, the HR was large with wide confidence interval that did not cross 1.
p<0.05
Figure 4.
Comparison of eGFR over Time by Race in the Adherence Study (n=243)
In the adjusted and unadjusted model, adherence was not associated with change in eGFR over time. (Table 3) Adherence was not associated with change in eGFR over time as assessed by the interaction term between the variables for change in eGFR over time and adherence (Table 3). In a similar model, Blacks, dosing frequency, and transplant center were not significantly associated with change in eGFR over time. (data not shown)
Table 3.
Association of Adherence with change in eGFR over time*.
| Variable | Unadjusted Coefficient ± for change in eGFR over time ± Std Error (p-value) |
Adjusted Coefficient± for change in eGFR over time Std Error (p-value) |
|---|---|---|
| Interaction between change in eGFR over time and adherence over 12 months |
−0.24 ± 0.31 (p=0.45) |
−0.20 ± 0.26 (p=0.43) |
| Time (in months) | 0.049 ± 0.27 (p=0.85) |
0.029 ± 0.23 (p=0.90) |
| Adherence over 6 months | 8.48 ± 7.02 (p=0.23) |
−0.73 ± 5.93 (p=0.90) |
| Constant Term | 49.70 ± 6.06 (p<0.001) |
73.39 ± 5.83 (p<0.0001) |
| Recipient gender: female vs male | −4.09 ± 1.43 (p=0.005) |
|
| Donor age (per year) | −0.44 ± 0.05 (p<0.0001) |
|
| Donor Cause of Death (vs Other) Cardiovascular |
6.93 ± 3.24 (p=0.03) |
|
| Intracranial Hemmorhage | 1.22 ± 3.04 (p=0.69) |
|
| Trauma | 9.33 ± 2.63 (p=0.0005) |
|
| Delayed allograft function | −7.02 ± 1.51 (p<0.0001) |
The interaction term between adherence and change in eGFR describes the association of adherence with change in eGFR over time. The adjusted model was also adjusted for transplant center.
Co-efficients were determined using a mixed effects model with the outcome being eGFR. This model also included fixed effects such as date of eGFR measurement in terms of month after transplant, mean adherence over six months and an interaction term between these two variables.
Discussion
In this prospective, multicenter cohort study, we monitored adherence to a single immunosuppressive medication during the first six months post-transplant and were unable to detect an association between adherence and kidney function during the first three years following transplantation. Adherence was not associated with time to 25% decline in eGFR and acute rejection. The association of adherence with change in eGFR over time was tested using an interaction term between eGFR over time and adherence, and this interaction term did not achieve statistical significance. Factors that influenced adherence, such as race, dosing frequency, and transplant center, were not associated with a change in eGFR over time.
In this and other studies, electronically monitored medication intake has shown that kidney transplant recipients are non-adherent to immunosuppressive therapy in 3–7% of monitored days.(4, 16, 17). Utilizing electronic monitoring in kidney transplant recipients, Nevins et al. (16) studied the impact of adherence on allograft outcomes. They showed an association between non-adherence and outcomes such as acute rejection and allograft loss. In contrast to our current study, Nevins et al. reported a much higher rate of acute rejection of 39% in their overall population, with 60% of these rejections occurring within the first 90 days after transplantation. (16) Their study also recruited some pediatric patients, who are generally at higher risk for acute rejections than adults. In contrast to the present study, Nevins et al. had a lower rate of non-participation and lack of usable data at 50 % (the present study with 71% as shown in Figure 1), and the baseline risk factors were similar between patients participating and those who declined to participate or provided unusable data. Unlike the present study, their study only enrolled patients from a single transplant center. Lastly, they had a longer average follow-up for allograft loss of 50 months compared to our maximum follow-up of 36 months.
Our results are somewhat similar to the results of a previous study by Nevins et. al. that also described the association between electronically monitored adherence and allograft outcomes. Both studies showed that patients with poor compliance in the current immunosuppression era do not always experience overt rejection. Nevins et al. noted that the majority (>70%) of patients in the worst category of adherence did not experience overt rejection. (16) Another recent, cross-sectional study, that utilized electronically monitored adherence and other measurement adherence measurement techniques, was not able to detect an association between adherence 1 year after transplantation and allograft outcomes.(18) Those prior results study, in conjunction with our findings are somewhat counterintuitive. They,suggest that moderate non-adherence with newer immunosuppression, especially when prescribed as a three-drug regimen along with antibody induction, may not necessarily cause poor allograft outcomes.
Non-adherence, measured using techniques besides electronic monitoring, has been associated with late acute rejections and allograft loss (16, 19–22). In contrast to prior published findings, the present study did not detect an association between non-adherence and either decline in eGFR or occurrence of acute rejection. There are several potential explanations for our findings. During the first six months post-transplant, most patients are more closely followed in clinic than later post-transplant, and this may result in better adherence with the other non-monitored medications. This difference in adherence between early and late post-transplant has been described by other studies as well. For example, Hilbrands et al. noted an average adherence rate of 100% in the first year post-transplant utilizing pill counts. Another study using electronic monitoring reported 98% adherence with drug ingestion during the first year post-transplant. (23) In contrast, after the first year post-transplant, studies have found a 73% rate of non-adherence using standardized patient interviews. (18) Also, it is possible that non-adherence during the first year post-transplant is not as detrimental as non-adherence after the first year post-transplant. After the first year post-transplant, patients are generally on fewer and lower doses of immunosuppression medications. During the first 6 months post-transplant, residual immunosuppression from antibody induction therapies may allowing for more non-adherence without worse allograft outcomes. Future studies should consider measuring adherence after the first year post-transplant and monitoring adherence with all, not just one, immunosuppressive drugs being prescribed, to better characterize the risks of non-adherence.
Several studies have found that adherence does not explain the worse outcomes seen in Black transplant recipients. In addition to adherence with immunosuppression medications, other factors such as transplant center, stress, education level, and economic status may play a role in explaining the worse outcomes seen in Blacks. (6, 7, 9, 24) In our study, the lower levels of adherence did not explain the worse outcomes seen in Blacks. We may have had insufficient power to detect an association owing to the small size of our Black participant group that included only 63 individuals. This highlights the challenges of enrolling sufficient number of patients in adherence studies that utilize microelectronic cap monitors, given our recruitment efforts that approached 829 patients for enrollment.
Our study had several limitations. First, we did not corroborate the microelectronic data with other adherence measures, such as medication levels, pill counts, or prescription refill rates. Unfortunately, at the time of the study the transplant centers involved were not measuring MMF levels and MMF was the predominant drug that was monitored. We only measured adherence to one immunosuppressive medication while patients were on several immunosuppressive medications. Second, we estimated GFR using the four-variable MDRD formula, instead of measuring GFR utilizing a gold standard test such as iothalamate. Third, we monitored adherence only during the first six months post-transplant. Adherence during the first six months after transplantation may not be consistent with adherence patterns in the subsequent time period post-transplant. Monitoring adherence for six months may have introduced measurement error and exposure misclassification, likely by underestimating the amount of non-adherence in study patients. This study limitation may have contributed to the lack of association between adherence, as measured in our study, and subsequent decrements in eGFR. Fourth, we were underpowered to detect an association between adherence and outcomes such as kidney function and acute rejection among Blacks. Lastly, the study participants were not a random subset of the initial target population. Though, our diverse, multicenter cohort represents well the national kidney transplant population.
In summary, this was the first multi-center study to measure adherence using electronic monitoring and assess its association with kidney allograft outcomes. We did not detect an association between adherence to a single immunosuppressive medication during the first 6 months post-transplant and subsequent kidney allograft function. Future studies will need to consider monitoring all, not just one, immunosuppressive medications prescribed to a patient beyond the first year post-transplant.
Acknowledgements
Supported in part by grants K23-DK062829 (A.K.I.), K24-DK002651 (H.I.F.), and R01-AI043295 (H.I.F.) from the National Institutes of Health. Dr. Feldman was an Established Investigator of the American Heart Association during the conduct of part of this study and A.K.I. is funded by the Robert Wood Johnson Physician Faculty Scholars Program. We thank the staff and patients at the participating transplant centers. Thanks to Dr. Thomas Nevins for his constructive comments on the manuscript.
Footnotes
Author Contribution: A.I.: Participation in research design, writing of manuscript, performance of research, contributed analytic tools, data analysis; F.W.: Participation in research design, writing of manuscript, performance of research,; M.J: Participation in research design, contributed analytic tools, data analysis; M.K.: Participation in research design, writing of manuscript, performance of research; H.F.: Participation in research design, writing of manuscript, performance of research, contributed analytic tools.
References
- 1.Butler JARP, Mullee M, Mason JC, Peveler RC. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: A systematic review. Transplantation. 2004;77:769. doi: 10.1097/01.tp.0000110408.83054.88. [DOI] [PubMed] [Google Scholar]
- 2.Kiley DJLC, Pollak R. A study of treatment compliance following kidney transplantation. Transplantation. 1993;55:51. doi: 10.1097/00007890-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Chisholm MAVL, Mulloy LL, Jagadeesan M, Wynn JJRH, Wade WE, DiPiro JT. Renal transplant patient compliance with free immunosuppressive medications. Transplantation. 2000;70:1240. doi: 10.1097/00007890-200010270-00020. [DOI] [PubMed] [Google Scholar]
- 4.Frazier PA, Davis-Ali SH, Dahl KE. Correlates of noncompliance among renal transplant recipients. Clin Transplant. 1994;8:550. [PubMed] [Google Scholar]
- 5.Gaston RSHS, Ward M, Jones P, Macon R. Late renal allograft loss: Noncompliance masquerading as chronic rejection. Transplant Proc. 1999;31:21S–23S. doi: 10.1016/s0041-1345(99)00118-9. [DOI] [PubMed] [Google Scholar]
- 6.Schweizer R, Rovelli M, Palmeri D, Vossler E, Hull D, Bartus S. Noncompliance in organ transplant recipients. Transplanation. 1990;49:374. doi: 10.1097/00007890-199002000-00029. [DOI] [PubMed] [Google Scholar]
- 7.Rovelli MP D, Vossler E, Bartus S, Hull D, Schweizer R. Noncompliance in renal transplant recipients: evaluation by socioeconomic groups. Transplant Proc. 1989;21:3979. [PubMed] [Google Scholar]
- 8.Didlake RHDK, Kerman RH, Van Buren CT, Kahan BD. Patient noncompliance: A major cause of late graft failure in cyclosporine-treated renal transplants. Transplant Proc. 1988;20 [PubMed] [Google Scholar]
- 9.Weng F, Israni A, Joffe M, Hoy T, Gaughan C, Newman M, et al. Race and electronically-measured adherence to immunosuppressive medications after deceased donor renal transplantation. JASN. 2005;16(6):1839. doi: 10.1681/ASN.2004121059. [DOI] [PubMed] [Google Scholar]
- 10.De Geest S, Vanhaecke J. Methodological issues in transplant compliance research. Transplant Proc. 1999;31:81S. doi: 10.1016/s0041-1345(99)00137-2. [DOI] [PubMed] [Google Scholar]
- 11.De Geest SAI, Dunbar-Jacob J. Measuring transplant patients’ compliance with immunosuppressive therapy. West J Nurs Res. 1996;18:595. doi: 10.1177/019394599601800509. [DOI] [PubMed] [Google Scholar]
- 12.Butler JAPR, Roderick P, Horne R, Mason JC. Measuring compliance with drug regimens after renal transplantation: Comparison of self-report and clinician rating with electronic monitoring. Transplantation. 2004;77:786. doi: 10.1097/01.tp.0000110412.20050.36. [DOI] [PubMed] [Google Scholar]
- 13.Cramer JA. Microelectronic systems for monitoring and enhancing patient compliance with medication regimens. Drugs. 1995;49:321. doi: 10.2165/00003495-199549030-00001. [DOI] [PubMed] [Google Scholar]
- 14.Feldman HIHM, Bilker W, Strom BL. Potential utility of electronic drug compliance monitoring in measures of adverse outcomes associated with immunosuppressive agents. Pharmacoepidemiol Drug Saf. 1999;8:1. doi: 10.1002/(SICI)1099-1557(199901/02)8:1<1::AID-PDS382>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate.[see comment] Annals of Internal Medicine. 2006;145(4):247. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 16.Nevins TE, Kruse L, Skeans MA, Thomas W. The natural history of azathioprine compliance after renal transplantation. Kidney Int. 2001;60:1565. doi: 10.1046/j.1523-1755.2001.00961.x. [DOI] [PubMed] [Google Scholar]
- 17.De Geest S, Borgermans L, Gemoets H, Abraham I, Vlaminck H, Evers G, et al. Incidence, determinants, and consequences of subclinical noncompliance with immunosuppressive therapy in renal transplant recipients. Transplantation. 1995;59:340. [PubMed] [Google Scholar]
- 18.Denhaerynck K, Burkhalter F, Schafer-Keller P, Steiger J, Bock A, De Geest S. Clinical consequences of non adherence to immunosuppressive medication in kidney transplant patients. Transplant International. 2009;22:441. doi: 10.1111/j.1432-2277.2008.00820.x. [DOI] [PubMed] [Google Scholar]
- 19.Vlaminck H, Maes B, Evers G, et al. Prospective study on late consequences of subclinical non-compliance with immunosuppressive therapy in renal transplant patients. Am J Transplant. 2004;4:1509. doi: 10.1111/j.1600-6143.2004.00537.x. [DOI] [PubMed] [Google Scholar]
- 20.Hilbrands LB, Hoitsma AJ, Koene RA. Medication compliance after renal transplantation. Transplantation. 1995;60:914. [PubMed] [Google Scholar]
- 21.Takemoto SK, Pinsky BW, Schnitzler MA, Lentine KL, Willoughby LM, Burroughs TE, et al. A retrospective analysis of immunosuppression compliance, dose reduction and discontinuation in kidney transplant recipients. American Journal of Transplantation. 2007;7:2704. doi: 10.1111/j.1600-6143.2007.01966.x. [DOI] [PubMed] [Google Scholar]
- 22.Pinsky BW, Takemoto SK, Lentine KL, Burroughs TE, Schnitzler MA, Salvalaggio PR. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. American Journal of Transplantation. 2009;9(11):2597. doi: 10.1111/j.1600-6143.2009.02798.x. [DOI] [PubMed] [Google Scholar]
- 23.Denhaerynck K, Steiger J, Bock A, Schafer-Keller P, Kofer S, Thannberger N, et al. Prevalence and risk factors of non-adherence with immunosuppresssive medication in kidney transplant patients. American Journal of Transplantation. 2006;7:108. doi: 10.1111/j.1600-6143.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- 24.Chakkera H, O'Hare A, Johansen K, Hynes D, Stroupe K, Colin P, et al. Influence of race on kidney transplant outcomes within and outside the department of veterans affairs. Journal of American Society of Nephrology. 2005;16:269. doi: 10.1681/ASN.2004040333. [DOI] [PubMed] [Google Scholar]