Abstract
Rationale
Mitochondrial [Ca2+] ([Ca2+]mito) regulates mitochondrial energy production, provides transient Ca2+buffering under stress and can be involved in cell death. Mitochondria are near the sarcoplasmic reticulum (SR) in cardiac myocytes and evidence for crosstalk exists. However, quantitative measurements of [Ca2+]mito are limited and spatial [Ca2+]mito gradients have not been directly measured.
Objective
To directly measure local [Ca2+]mito during normal SR Ca release in intact myocytes, and evaluate potential subsarcomeric spatial [Ca2+]mito gradients.
Methods and Results
We used in-situ calibration of the mitochondrially targeted inverse pericam indicator Mitycam and directly measured [Ca2+]mito during SR Ca2+ release in intact rabbit ventricular myocytes by confocal microscopy. During steady state pacing Δ[Ca2+]mito amplitude was 29 ± 3 nM, rising rapidly (similar to cytosolic [Ca2+]i) but declining much more slowly. Taking advantage of the structural periodicity of cardiac sarcomeres, we found that [Ca2+]mito near SR Ca2+ release sites (Z-lines) vs. mid sarcomere (M-line) reached a higher peak amplitude (37 ± 4 vs. 26 ± 4 nM, respectively P < 0.05) which occurred earlier in time. This difference was attributed to ends of mitochondria being physically closer to SR Ca2+ release sites, because the mitochondrial Ca2+ uniporter was homogeneously distributed and elevated [Ca2+] applied laterally did not produce longitudinal [Ca2+]mito gradients.
Conclusions
We developed methods to measure spatiotemporal [Ca2+]mito gradients quantitatively during excitation-contraction coupling. The amplitude and kinetics of [Ca2+]mito transients differ significantly from those in the cytosol and are higher and faster near the Z- vs. M-line. This approach will help clarify SR-mitochondrial Ca2+ signaling.
Keywords: Mitochondria, cardiac myocytes, calcium, SR Ca release
INTRODUCTION
Sarcoplasmic reticulum (SR) and mitochondria are both essential for cardiac myocyte function. The SR is the site for Ca2+ storage and release that drives contraction during each heartbeat. Cytosolic Ca2+ influences many other targets, including ion channels and transporters, signaling cascades, gene transcription and mitochondrial ATP production.1,2 Ca2+ pumping by the SR Ca-ATPase (SERCA) and out of the cell via Na+/Ca2+ exchange allow diastolic relaxation and cardiac refilling between beats. Excitation-contraction coupling (ECC) consumes much ATP, which is generated mainly in mitochondria by oxidative phosphorylation, regulated in part via Ca2+-dependent dehydrogenases.2–4 Hence, mitochondrial Ca2+ participates in “excitation-metabolism coupling” (EMC) critical to ATP availability. EMC may be facilitated by structural interactions in cardiomycytes where mitochondria are surrounded by an SR network.4–6 At least one end of most mitochondria is in close proximity to SR Ca2+ release sites, and physical transorganellar tethers connecting SR/ER and mitochondria have been identified.7 The low affinity mitochondrial Ca2+ uniporter (MCU; K0.5 ~10–20 µM Ca2+)8 could also preferentially take up Ca2+ at the high local [Ca2+]i expected near the release sites. In other words, local Ca2+ signal transmission may efficiently alter cellular energy supply to match demand, and consequently also shape cytosolic Ca2+ dynamics.9–11 The concept of mitochondrial-SR Ca2+ microdomains is timely,9,10,12 however, whether local spatial and temporal gradients of intra-mitochondrial free [Ca2+] ([Ca2+]mito) occur near SR Ca release sites and what the absolute kinetics of [Ca2+]mito signals are in adult ventricular myocytes has not been well defined. Moreover, it is controversial whether [Ca2+]mito signals are dominated by rapid changes parallel to the cytosolic Ca2+ transients or more slow, integrating responses during ECC and what the functional implications this has in cardiac myocytes.13–15 Furthermore, under pathological condition, mitochondrial–SR crosstalk also contributes to programmed cardiac myocyte death via opening Ca2+-sensitive mitochondrial permeability transition pore (MPTP) which permits unrestricted proton flow out of the mitochondrial matrix that interrupts the respiratory electron transport chain. This allows the release of cytochome c into the cytosol, and then initiates necrotic cell death or apoptosis.16,17 However, limitations exist in the measurement of mitochondrial [Ca2+] in intact myocytes. Moreover, subsarcomeric [Ca2+]mito microdomains have not been assessed functionally in intact adult cardiac myocytes. Here we detect subsarcomeric spatiotemporal gradients of [Ca2+]mito using a genetically encoded and mitochondrially targeted Ca2+ sensor, Mitycam.18
While [Ca2+]mito transients are known to occur during SR Ca release and reuptake in cardiac myocytes,14,18–20 calibrations and exclusion of contamination by cytosolic signals are challenging for [Ca2+]mito measurements in nanomolar values. Understanding how local and global Ca2+ transients influence [Ca2+]mito is controversial15 and quantitative data is essential to deepen our understanding of these issues. We provide here quantitative estimates of [Ca2+]mito transients in intact ventricular myocytes, and demonstrate detectable subsarcomeric spatial [Ca2+]mito gradients during normal Ca transients in adult ventricular myocytes for the first time. The methods we use to detect the spatial [Ca2+]mito established here could be useful approach to investigate mitochondrial Ca2+ handling in many conditions.
METHODS
Additional information is in the Online Supplement at http://circres.ahajournals.org.
Myocyte isolation & viral transfection
All protocols involving animals were performed in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the University of California, Davis Institutional Animal Care and Use Committee. Adult rabbit ventricular cardiomyocytes were isolated from New Zealand White rabbits by standard enzymatic dissociation as described previously.21 Freshly isolated cells were plated on laminin-coated glass cover slips for 45 min before the transfection. Adenoviral-deiated Mitycam gene transduction was carried out at a multiplicity of infection (MOI) of 500 virus particles per cell (vp/cell).18 Infected cells were cultured in serum-free PC-1 medium (Lonza) for 36 hours, with 1 final replacement of fresh medium 1 hour before experiments. Lower MOI or shorter duration in culture often provided poorer signal resolution.
Fluorescence microscopy
Mitycam fluorescence was measured with excitation at 488nm (emission between 530 ± 15 nm) for 2D imaging (Zeiss, LSM5 Pascal) and line scan imaging (Bio-rad Radiance). Mitochondria were localized by 1 µM MitoTracker Red (Invitrogen Ltd) using 543 nm excitation. For some experiments, cells were field-stimulated and Mitycam and Di-8 ANEPPS (488 nm excitation; >600 nm emission) were recorded by line scan imaging. Image-J software was used for image analysis. Cytosolic Ca2+ transients were detected in myocytes loaded with Fluo-4 (Invitrogen).
Chemicals and solutions
For permeabilized myocyte experiments a highly Ca2+-buffered, Na+-free internal solution was used containing (in mM): EGTA 5, HEPES 20, K-aspartate 100, KCl 40, MgCl2 1, maleic acid 2, glutamic acid 2, pyruvic acid 5, KH2PO4 0.5, pH 8.0 adjusted with Trizma base. To control [Ca2+]i, 100 mM CaCl2 solution (Thermo) was added as calculated using the program MaxChelator (http://www.stanford.edu/~cpatton/maxc.html). For intact myocyte experiments, cells were superfused with normal Tyrode’s (NT) solution containing (in mM) 140 NaCl, 4 KCl, 1 MgCl2, 1.8 CaCl2, 10 glucose, and 5 HEPES, pH 7.4. To detect intra-mitochondrial distribution of MCU, anti-MCU antibody (Sigma-Aldrich) was used at 1:500 dilution. The secondary antibody carried FITC derivative (Alexa Flour 488; Molecular Probes) and was used at a 1:1000 dilution.
Statistics
Pooled data are represented as the mean ± SEM. Statistical comparisons were made using unpaired or paired Student’s t tests where applicable (P < 0.05 was considered significant).
RESULTS
Mitycam targets to Mitochondria and provides [Ca2+]mito signals
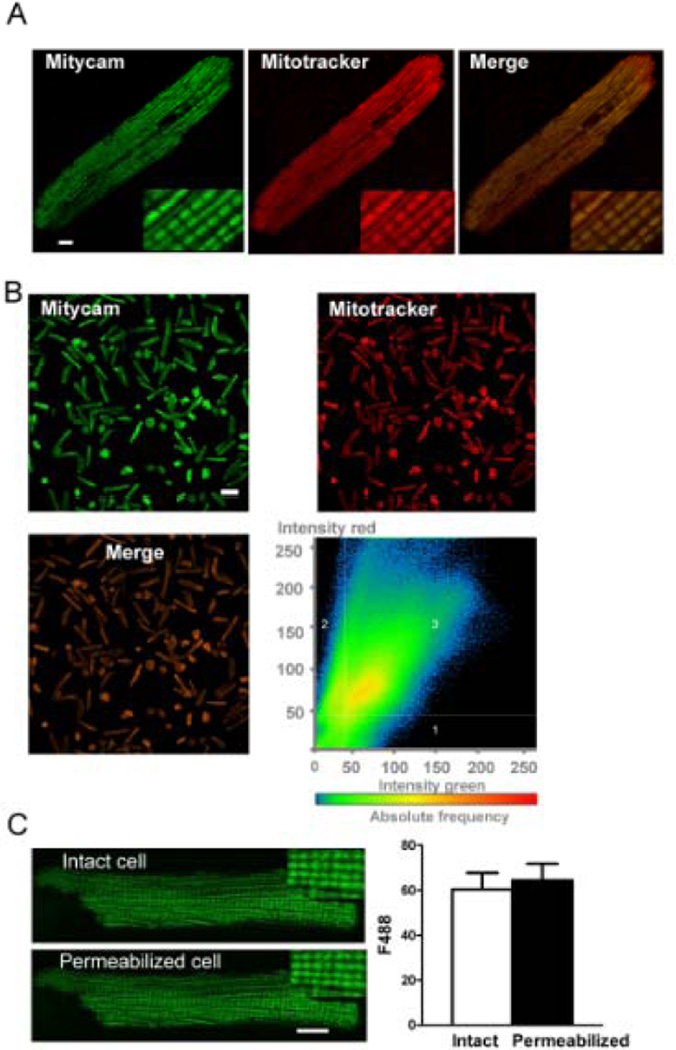
Figure 1A shows confocal images of a typical adult rabbit cardiomyocyte expressing Mitycam. Mitycam fluorescence (488 nm excitation) shows specific mitochondrial expression, overlaping completely with the mitochondrial marker MitoTracker Red (543 nm excitation). Mitycam was expressed in virtually all myocytes, as indicated by the low magnification images in Figure 1B, and again was well matched with MitoTracker Red imaging. Moreover, quantitative pixel by pixel analysis of many cells showed strong correlation between Mitycam (green) and MitoTracker Red signals (Fig 1B, bottom right). The overlap coefficient of Mitycam and MitoTracker was 0.843 ± 0.007 (r =∑(ch1i)(ch2i)/(∑(ch1i)2 × ∑(ch2i)2)1/2), where 0 implies no colocalization and 1 implies perfect colocalization). Importantly, upon myocyte permeabilization by saponin in an intracellular solution (with physiological [Ca2+] and [Na+]), there was no significant change of Mitycam fluorescence (Fig 1C). This indicates that virtually all Mitycam is targeted inside mitochondria, with no appreciable cytosolic Mitycam (which would have been lost upon permeabilization). Thus, Mitycam can provide truly mitochondrial-specific signals.
Figure 1. Mitycam in adult rabbit ventricular myocytes.
(A) Mitycam, MitoTracker Red and merged signals exhibit a mitochondrial pattern (scale bar 8 µm), especially apparent in enlarged insets. (B) Lower magnification image of myocyte (scale bar, 100 µm). Scatter 2D plots of pixel intensities in red (MitoTracker) and green (Mityam) channels (right-bottom). Intensity thresholds were automatically determined by excluding dark pixels via algorithm in image analysis software, ZEN (Zeiss). Region 1 and 2 pixels represent signal in channel 1 or 2 only, respectively; region 3 represents colocalized pixels. (C) Mitycam signal is unaltered by saponin-permeabilization (typical images (left), average fluorescence before and after at (right); n=10; scale bar, 8 µm).
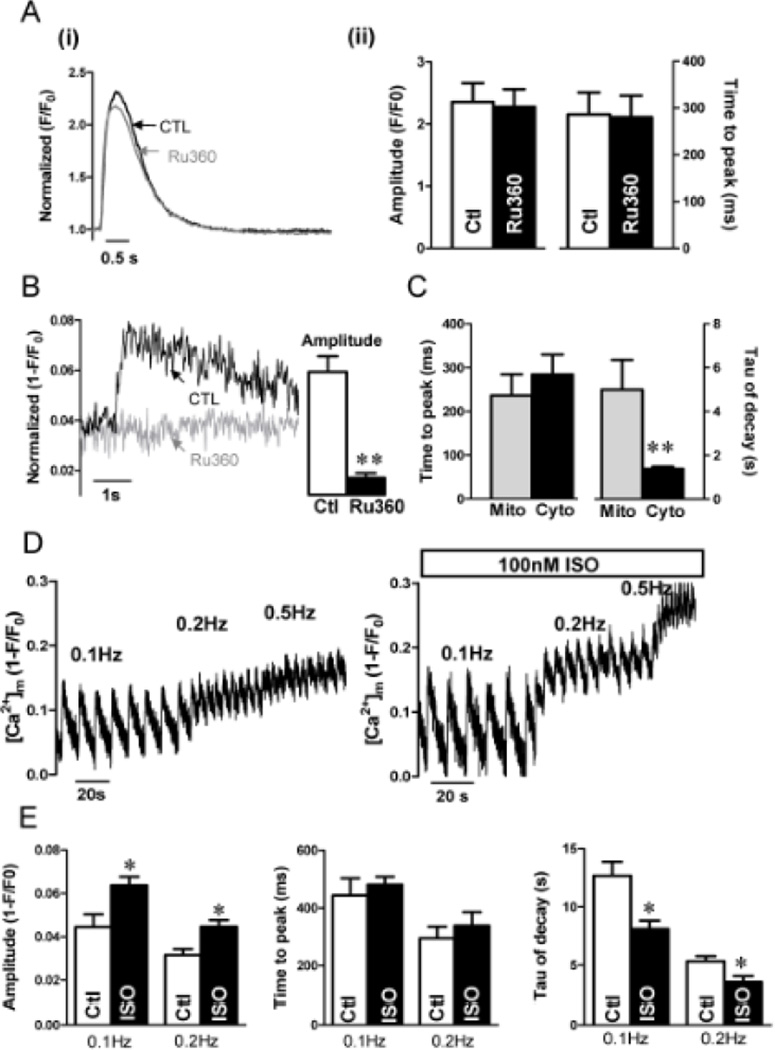
Cytoplasmic Ca2+ and mitochondrial Ca2+ transients were recorded at 0.2 Hz with and without the MCU inhibitor 1 µM Ru360 (Fig 2), a concentration that does not alter SR Ca uptake or release, Ca current, Na/Ca exchange, Ca transients, contraction or myofilament Ca sensitivity.22 Figure 2A shows that neither the amplitude nor kinetics of [Ca2+]i transients were altered significantly by Ru360. In contrast, the [Ca2+]mito signal was virtually abolished by Ru360 (Fig 2B). This confirms that the Mitycam signal is due to [Ca2+]mito changes and is prevented by MCU block. The time to peak of [Ca2+]mito and [Ca2+]cyto was not significantly different, but the decay time constant (τ) of [Ca2+]mito was much slower (5 s) than that for [Ca2+]cyto (Fig 2C).
Figure 2. Kinetics of [Ca2+]mito in adult rabbit ventricular myocytes.
(A) Kinetics of [Ca2+]cyto with and without 1 µM Ru360 during 0.2 Hz stimulation with 1.8 mM Ca (i) and mean Δ[Ca] and time to peak (ii). (B) [Ca2+]mito transient and mean Δ[Ca2+]mito with and without 1 µM Ru360. (C) Time to peak and decline tau of [Ca2+]mito and [Ca2+]i. (D) Influence of pacing frequency (as indicated above traces) on [Ca2+]mito signal with (right) and without 100 nM isoproterenol (left). (E) Amplitude and kinetics of [Ca2+]mito at different frequencies (±ISO). (n = 6, *P<0.05, ***P< 0.001).
As the frequency of stimulation increases the diastolic [Ca2+]mito increases progressively, but the amplitude of [Ca2+]mito transients decreases, which leads to progressive fusion of [Ca2+]mito transients, consistent with the slow kinetics of [Ca2+]mito decline (Fig 2D). For this cell, the signal (1-F/Fo) is saturated (Fmin) at 0.624, indicating that Mitycam is far from saturation at 0.5 Hz. Furthermore, to stress the myocyte we measured [Ca2+]mito transients at different pacing frequencies in the absence and presence of the β-adrenergic agonist isoproterenol (ISO). Figure 2D–E shows that with ISO there is an increase in the amplitude of [Ca2+]mito transients during individual beats (0.1Hz: 0.044 ± 0.006 vs. 0.063 ± 0.004; 0.2Hz: 0.031± 0.0028 vs.0.045± 0.003, p < 0.05) and a higher steady state [Ca2+]mito at higher frequency. The time to peak [Ca2+]mito was not altered by ISO, but [Ca2+]mito decay was faster with ISO (Fig 2E). To focus on larger amplitude [Ca2+]mito signals, further studies focused on control conditions at 0.2 Hz.
Calibration of [Ca2+]mito signals during Ca transients
To calibrate [Ca2+]mito signals in myocytes, we first assessed the affinity of Mitycam for Ca2+ in cardiac mitochondria in situ. Saponin-permeabilized myocytes expressing Mitycam were pretreated with 5 µM thapsigargin to block SR Ca uptake and release and then equilibrated with internal solution of different [Ca2+] containing 5 µM ionomycin (Ca2+ ionophore) for 20–30 min. The solution included 5 µM FCCP and 1 µM oligomycin to dissipate mitochondrial membrane potential and was at pH=8 (to mimic mitochondrial pH). The in situ Kd was 197 ±11 nM (Fig 3A).
Figure 3. Calibration and [Ca2+]mito transients in cardiomyocytes.
(A) In situ Mitycam calibration in myocytes (n=8). (B) Average [Ca2+]mito transients, diastolic [Ca2+]mito and amplitude of transient during 0.2 Hz stimulation.
After measuring beat-to-beat [Ca2+]mito transients we assessed Fmax and Fmin in the same myocyte (maximum and minimum fluorescence in low and high [Ca2+], respectively). First cells were saponin-permeabilized and the same type of calibration solutions as above were used for Fmax and Fmin conditions. [Ca2+]mito was calculated using the relationship F = (Fmin-Fmax)/ (1+(Kd/[Ca2+]))+Fmax). Mean diastolic [Ca2+]mito was 146 ± 9 nM and a mean transient amplitude of 29±3 nM (Fig 3B).
[Ca2+]mito gradients along the sarcomere during [Ca2+]i transients
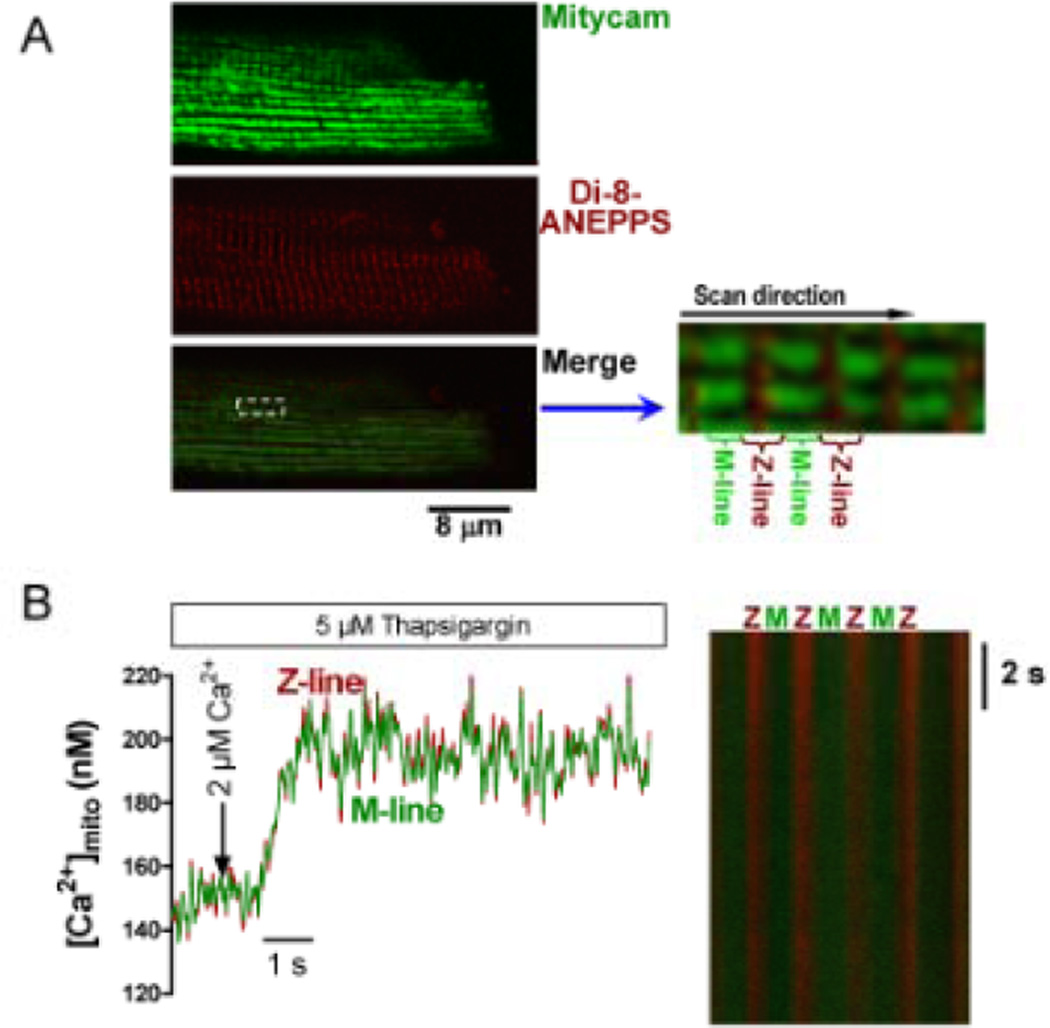
We took advantage of the periodic sarcomeric structure in ventricular myocytes. Mitochondria in cardiac myocytes are oriented in longitudinal rows (between myofibrils; Fig 4A) that are perpendicular to the transverse tubules (T-tubules, where Ca2+ is released from the junctional SR)2 and the sarcomeric Z-line, which both create a physical boundary to mitochondria. T-tubule membranes are identified by transverse Di-8-ANEPPS striations (Fig 4A) that exist at Z-lines. Thus, the Mitycam signal from the ends of mitochondria nearest Z-lines are close to SR Ca release sites. Using longitudinal line scans (in the direction shown in Fig 4A inset) we pooled Mitycam signals from within 0.5 µm of the center of the T-tubule, and from 6–8 junctions in series, and refer to that as the Z-line signal (near SR Ca2+ release sites). The signal from the remainder of the sarcomere (~1 µm centered on the M-line) we call the M-line signal, coming from sites which are furthest from SR Ca release sites. This signal averaging allows us to assess whether there are detectable spatial [Ca2+]mito gradients in confocal line-scan mode. Control experiments (Fig 4B) were done in saponin-permeabilized myocytes (pretreated with 5 µM thapsigargin) exposed to a rapid global [Ca2+]i elevation from 50 nM to 2 µM with solution flow from the lateral side. No detectable [Ca2+]mito gradient was detected.
Figure 4. Spatial [Ca2+]mito signals at Z- and M-line regions.
(A) Localization of T-tubule/Z-line (Di-Anepps, middle) and Mitycam. Merged image (with enlarged region) shows how Z- and M-line Mitycam signals are obtained by separating signals spatially. (B) Direct lateral application of 2 µM Ca2+ internal solution to permeabilized myocyte (pretreated with 5 µM thapsigargin and 40 µM cytochalasin D) and line scan image (right).
Intact myocytes with functioning SR during normal Ca2+ transients exhibited differences in the kinetics and amplitude of the [Ca2+]mito transient between Z- and M-line (Fig 5). The [Ca2+]mito amplitude was significantly higher at the Z-line vs. the M-line (37 ± 4 nM vs 26 ± 5 nM, P < 0.05), and peaked earlier at the Z-line site (0.24 ± 0.05 vs. 0.57 ± 0.17 s, P <0.05). The time constant of [Ca2+]mito decline was similar between M-line and Z-line regions (τ ~5 s), suggesting that the slow [Ca2+]mito decline is less influenced by location and that [Ca2+]mito gradients dissipate during [Ca2+]mito decline. During rapid caffeine application (10 mM) there was still a detectably higher peak [Ca2+]mito near Z-lines (driven by RyR-mediated Ca2+ release from SR) vs. M-line sites (Online Figure I; 62 ± 2 vs 49 ± 2 nM, P < 0.05). The higher amplitude is consistent with a higher fractional SR Ca release with caffeine and less competition by SR Ca uptake.
Figure 5. [Ca2+]mito transients at Z-line and M-line.
Averaged [Ca2+]mito transients (A), diastolic and amplitude (B), time to peak (C) and decay time constant (τ; D). (E) Isochronal dependence of [Ca2+]mito on distance from Z-line at 50 ms before peak in the mean Z-line signal (horizontal line and large symbol indicate mean values for 0–0.5 and 0.5–1 µm as in A). (n = 6, *P < 0.05).
Because the average [Ca2+]mito within 0.5 µm of the Z-line may underestimate the maximal [Ca2+]mito in the region closest to the Z-line, we also examined the spatial profile of [Ca2+]mito as [Ca2+]Mito approaches its peak. Figure 5E shows isochronic [Ca2+]mito signals 50 ms before the peak of the Z-line signal. Within 0.1 µm [Ca2+]mito is significantly higher than the mean Z-line peak value from Fig 5A (horizontal line in Fig 5E). The [Ca2+]mito declines with increasing distance from Z-line regions (Fig 5E). Thus, during SR Ca2+ release, [Ca2+]mito may reach as high as 226 ± 13 nM at the regions nearest to Z-line, although the average signal from M-line regions is approximately 152 nM.
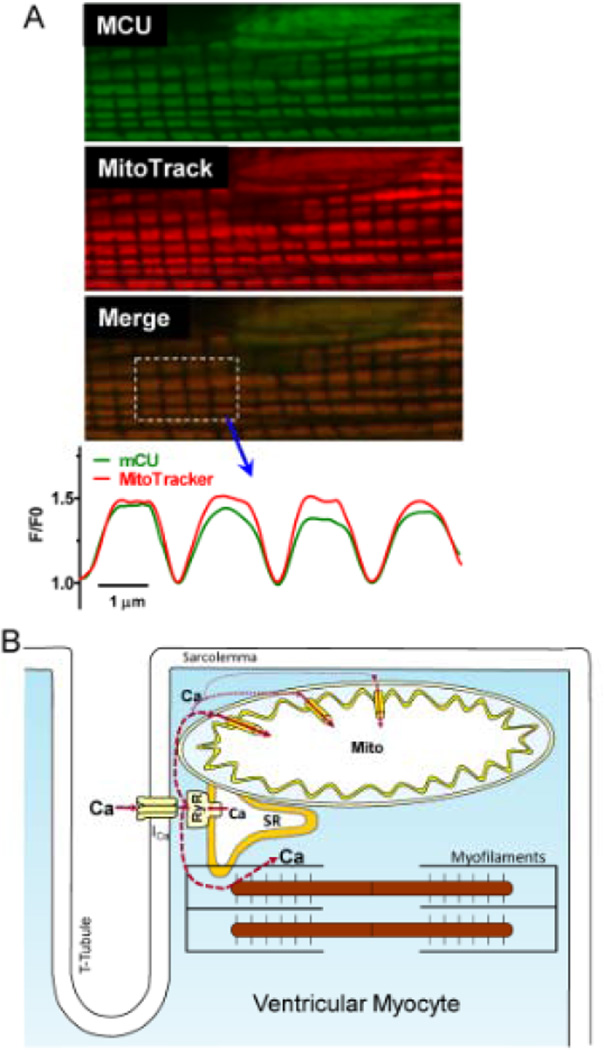
It is possible that the higher and faster [Ca2+]mito rise near the Z-line could also be due to a higher localization of MCU channels near Z-line vs. M-line. Indeed, MCU channel density at the inner mitochondrial membrane was estimated to be 10–40 µm−2, and inhomogeneous distribution could also cause spatial [Ca2+]mito gradients.23 To test for differential sarcomeric distribution of MCU channels, we used a MCU-specific antibody for immunolocalization. The longitudinal sarcomeric distribution of MCU-related fluorescence was very similar to that of Mito tracker (Fig 6A) and Mitycam (Online Fig II). This suggests relatively uniform MCU distribution over the mitochondrion and is consistent with the lack of [Ca2+]mito gradients seen upon global (vs. local) [Ca]i elevation (Fig 4B).
Figure 6. Sarcomeric expression of MCU.
(A) MCU immunofluorescence, MitoTracker Red fluorescence, merged image and plot profiles (normalized to Z-line minimum). (B) Scheme of SR Ca2+ release and mitochondrial Ca2+ uptake.
DISCUSSION
Cardiac mitochondrial Ca2+ uptake is important in maintaining cellular ATP, protecting myocytes from transient Ca2+ overload and in mediating cell death pathways.1,2,16,,24 However, the amplitude, kinetics and time-dependent integration of mitochondrial Ca2+ uptake during ECC in adult ventricular myocytes are controversial.13,15 It seem clear now that [Ca2+]mito transients can occur with each cytosolic Ca2+ transient and that diastolic [Ca2+]mito increases progressively at increased frequency or cellular Ca2+ loading.14,18,20 However, obtaining calibrated [Ca2+]mito signals has been difficult because of potential contamination by cytosolic fluorescent indicator (for membrane permeable esters), complications of using Mn2+ (to quench cytosolic Ca2+ indicator) and for mitochondrial targeted aequorin calibrations are highly non-linear and require correction for indicator consumption. Here, we take advantage of work using carefully calibrated [Ca2+]mito measurements in permeabilized myocytes (using fura-2 and rhod-2)20 and the genetically encoded mitochondrially targeted Ca2+ sensor Mitycam18 to assess [Ca2+]mito in intact adult rabbit ventricular myocytes during ECC.
We calibrated Mitycam in situ in cardiomyocyte mitochondria, obtaining a Kd value (~200 nM) similar to that in Hela cells18 and comparable to that of organic Ca2+ indicators used to measure [Ca2+]mito transients in myocytes.14 We used Fmax and Fmin from each myocyte to directly infer [Ca2+]mito values. Diastolic [Ca2+]mito was ~150 nM, with a ~30 nM Δ[Ca2+]mito during individual Ca2+ transients at 0.2 Hz pacing frequency. This Δ[Ca2+]mito amplitude is larger than in our previous study20 in permeabilized cardiomyocytes during spontaneous SR Ca2+ release waves with internal [Ca2+] of 150 nM. Differences could be due to the intracellular buffer, SR Ca2+ content, lower frequency and unsynchronized nature of Ca2+ waves in permeabilized myocytes. The rapid intra-mitochondrial Ca2+ buffering power in these intact myocytes is unknown, but a value of 33–100 (Δbound/Δfree; similar to the ~100 in cytosol) would imply a total mitochondrial Ca2+ uptake during a twitch of 0.5–1.6 µmol/l cytosol. That is consistent with quantitative analysis of Ca2+ transport rates during normal Ca2+ transients in rabbit ventricular myocytes by SR Ca-ATPase, Na/Ca exchanger, mitochondrial uptake and plasma membrane Ca-ATPase (54, 21, 0.8 and 0.8 µmol/l cytosol, respectively).25,26 Thus mitochondrial uptake is ~1% of the total Ca2+ removed from the cytosol at each beat. That also agrees with the lack of significant impact of Ru360 on the amplitude and kinetics of [Ca2+]i transients here (Fig 2A). If mitochondrial uptake rate and buffering are upregulated at higher cellular Ca2+ loads, the impact could be increased.14,27
The time to peak [Ca2+]mito (236 ± 48 ms) and slow kinetics of [Ca2+]mito decline (τ~5 s) are consistent with our previous data,20,28 but much faster [Ca2+]mito declines have also been reported,14,18 especially with stronger Ca2+ loading conditions (isoproterenol and/or elevated extracellular [Ca2+]). We think that our slow rates of [Ca2+]mito decline are consistent with the known slow Ca2+ extrusion rate via mitochondrial Na/Ca exchanger, but we suspect that faster reported rates might also involve increased intramitochondrial Ca2+ buffering at higher Ca2+ loads.27,29
We found that ISO increased the [Ca2+]mito transient amplitude, which may be driven by the larger SR Ca2+ content and Ca transients induced by β-adrenergic signaling, resulting in higher local [Ca2+]i and promoting Ca2+ influx into mitochondria. ISO also accelerated [Ca2+]mito decline, consistent with data from other groups.14,18 Twitch [Ca2+]i decline is accelerated by ISO because of PKA-dependent phosphorlamban phosphorylation and accelerated SR Ca-ATPase activity.26 However, it is not clear that that effect would suffice to hasten [Ca2+]mito decline because [Ca2+]i is already near diastolic levels during most of the [Ca2+]mito decay time. Since [Ca2+]mito decline relies mainly on mitochondrial Na/Ca exchange (mNCX), slower decline could result from elevated [Na+]i. However, PKA-dependent phospholemman phosphorylation and Na/K-ATPase stimulation limit the rise in [Na+]i expected from other effects of ISO,30 making that explanation unlikely. It is possible that ISO could result in activation of the mNCX, but such regulation has not been described.
We focused on a low stimulation frequency here for two reasons. First, this allows [Ca2+]mito to largely recover between beats and attain steady state. Second, at higher frequency the amplitude of [Ca2+]mito transients becomes smaller as [Ca2+]mito accumulates. Moreover, as [Ca2+]mito rises it may begin to approach saturation for Mitycam. While we did not approach that limit here, lower affinity mitochondrial Ca2+ sensors might be valuable to examine the full physiological range of [Ca2+]mito at high Ca2+ loading conditions and under pathological conditions. These local [Ca2+]mito gradients detected may produce local increases in mitochondrial dehydrogenase activity and ATP production, because the [Ca2+]mito levels are in the range where these enzymes are Ca2+-sensitive.1 In that sense the subcellular regions where Ca2+ transients are highest may have enhanced ATP production, matching supply and demand. We suggest that at more physiological heart rates and temperature that [Ca2+]mito will be somewhat higher than values reported here, but also that the phasic [Ca2+]mito signals and associated spatiotemporal [Ca2+]mito gradients will be more limited. Thus, the true physiological impact of these [Ca2+]mito gradients on cardiac energy balance will require further study.
A central aim here was to assess whether spatial [Ca2+]mito gradients could be detected during cardiac Ca2+ transients. Ultrastructural evidence exists for proximity and even explicit tethering of mitochondria to the SR membrane, suggesting that diffusional distance from SR junctional couplings is 37–270 nm from the end of a nearby mitochondrion.4–6,31,32 Indeed, in permeabilized cells SR Ca2+ release drives mitochondrial Ca2+ uptake that is less sensitive to cytosolic Ca2+ buffers than is global [Ca2+]i suggesting some preferential local mitochondrial Ca2+ uptake.31,33
The discernible spatiotemporal [Ca2+]mito gradient between Z- and M-lines is consistent with the idea that the part of a mitochondrion nearest the SR Ca2+ release sites exhibits preferential Ca2+ uptake (Fig 6B) despite relatively uniform sarcomeric [Ca2+]i during ECC (Online Fig III). Of course, much of the total mitochondrial surface (e.g. that near M-lines) is far from the SR Ca2+ release sites and is expected to sense a similar local [Ca2+]i as that sensed by the myofilaments. These subsarcomeric [Ca2+]mito gradients are near the limit of spatial resolution, and the true [Ca2+]mito gradient seems to be larger and dissipates over ~0.5 µm (Fig 5E). We also used the same analytical methods to see whether similar cytosolic spatial gradients would be detectable along the sarcomere (using Fluo-4 AM as Ca2+ indicator, and Di-8ANNEPS for Z-line; Online Fig I). We could not readily detect such gradients. That is not particularly surprising because either isolated local release events (Ca sparks) or special conditions are required (combining EGTA with low affinity indicators) to detect local high [Ca2+]i near release sites.34,35 Likewise, gradients of free intra-SR [Ca2+] are not normally discernible during normal ECC, even using methods like those used here,36 although such local gradients in both [Ca2+]i and SR [Ca2+] are readily detected during isolated local release events (Ca sparks).
Our results here on local and calibrated [Ca2+]mito place new explicit spatiotemporal constraints on models of Ca2+ uptake and extrusion from mitochondria. Detailed diffusional-flux models will be required to determine whether these [Ca2+]mito gradients could be a simple consequence of the geometric position of mitochondria with respect to junctions, or whether more specialized communication is necessary. The approach described here will be valuable for clarifying many aspects of mitochondrial Ca2+ regulation.
Supplementary Material
Novelty and Significance.
What Is Known?
Mitochondria are located close to cytosolic [Ca2+] ([Ca2+]i) release sites (i.e. ryanodine receptors of the sarcoplasmic reticulum, SR), and crosstalk may facilitate the excitation-metabolism coupling.
Mitochondria take up Ca2+ uptake via a low affinity Ca2+ uniporter (MCU). This requires a high local [Ca2+], which occurs near the release sites. However, intra- mitochondrial [Ca2+] gradients have not been measured and the kinetics of mitochondrial [Ca2+]i rise during normal Ca transients are debated (large phasic Ca2+ transients vs. slow integrating changes).
A genetically- encoded, Ca2+ sensor located in the mitochondria could measurr mitochondrial [Ca2+]mito in intact cardiac myocytes.
What New Information Does This Article Contribute?
In cardiomyocyte mitochondria in situ, the Ca2+ sensor Mitycam has a Kd value (~200 nM).
The measurement of Mitycam signal provides quantitative estimates of [Ca2+]mito transients in ventricular myocytes.
During normal calcium transients in adult ventricular myocytes, subsarcomeric spatial [Ca2+]mito gradients with ~50% larger amplitude reache an earlier peak near SR Ca2+ release sites (at the Z-line) than in the middle of the sarcomere (M-line).
Measurements of spatial [Ca2+]mito gradients using this approach could be useful for investigating mitochondrial Ca2+ handling in many conditions.
During excitation-contraction coupling (ECC) cardiac myocytes increases in [Ca2+]mito can enhance ATP synthesis via Ca2+-dependent dehydrogenases. The highly organized juxtaposition of sarcolemma, SR and mitochondria provides a possibility for their crosstalk. However, the kinetics and amplitude of [Ca2+]mito remain unknown and it is unclear whether spatiotemporal [Ca2+]mito gradients exist upon SR Ca2+ release during ECC. Here we report quantitative estimates of [Ca2+]mito transients in intact adult ventricular myocytes and measure subsarcomeric spatial [Ca2+]mito gradients during normal Ca transients (larger and faster near the Z- vs. M-line). The amplitude and kinetics of [Ca2+]mito transients rises quickly along with cytosolic [Ca2+], but are much smaller in amplitude and decay slowly, leading to slow progressive changes during repeated stimuli. This approach to measuring [Ca2+]mito will be useful to further our understanding of how mitochondria handle Ca2+ in spatiotemporal detail and also how [Ca2+]mito regulates mitochondrial function under various physiological and pathological conditions.
ACKNOWLEDGEMENTS
We thank Dr. Brian O’Rourke for comments on a previous version of the manuscript.
SOURCES OF FUNDING
National Institutes of Health grants P01-HL080101 and R01-HL101235.
Non-standard Abbreviations
- [Ca2+]
calcium concentration
- [Ca2+]i
cytosolic free Ca2+ concentration
- [Ca2+]mito
mitochondrial free Ca2+ concentration
- Δ[Ca2+]mito
concentration change of mitochondrial Ca2+
- ECC
excitation-contraction coupling
- EMC
excitation-metabolism coupling
- ISO
isoproterenol
- MCU
mitochondrial Ca2+ uniporter
- mNCX
mitochondrial Na/Ca exchange
- MOI
multiplicity of infection
- MPTP
mitochondrial permeability transition pore
- SERCA
SR Ca-ATPase
- SR
sarcoplasmic reticulum
- τ
time constant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None
REFERENCES
- 1.Denton RM, McCormack JG. Ca2+ as a second messenger within mitochondria of the heart and other tissues. Ann Rev Physiol. 1990;52:451–466. doi: 10.1146/annurev.ph.52.030190.002315. [DOI] [PubMed] [Google Scholar]
- 2.Bers DM. Calcium cycling and signaling in cardiac myocytes. Ann Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 3.Territo PR, Mootha VK, French SA, Balaban RS. Ca2+ activation of heart mitochondrial oxidative phosphorylation: role of the F0/F1-ATPase. Am J Physiol Cell Physiol. 2000;278:C423–C435. doi: 10.1152/ajpcell.2000.278.2.C423. [DOI] [PubMed] [Google Scholar]
- 4.Territo PR, French SA, Dunleavy MC, Evans FJ, Balaban RS. Calcium activation of heart mitochondrial oxidative phosphorylation: Rapid kinetics of mvo2, nadh, and light scattering. J Biol Chem. 2001;276:2586–2599. doi: 10.1074/jbc.M002923200. [DOI] [PubMed] [Google Scholar]
- 5.Yoshikane H, Nihei T, Moriyama K. Three-dimensional observation of intracellular membranous structures in dog heart muscle cells by scanning electron microscopy. J Submicrosc cytol. 1986;18:629–636. [PubMed] [Google Scholar]
- 6.Boncompagni S, Rossi AE, Micaroni M, Beznoussenko GV, Polishchuk RS, Dirksen RT, Protasi F. Mitochondria are linked to calcium stores in striated muscle by developmentally regulated tethering structures. Mol Biol Cell. 2009;20:1058–1067. doi: 10.1091/mbc.E08-07-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 8.Vinogradov A, Scarpa A. The initial velocities of calcium uptake by rat liver mitochondria. J Biol Chem. 1973;248:5527–5531. [PubMed] [Google Scholar]
- 9.Rizzuto R, Duchen MR, Pozzan T. Flirting in little space: the ER/mitochondria Ca2+ liaison. Sci STKE. 2004;2004:re1. doi: 10.1126/stke.2152004re1. [DOI] [PubMed] [Google Scholar]
- 10.Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: Molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 11.Drago I, De Stefani D, Rizzuto R, Pozzan T. Mitochondrial Ca2+ uptake contributes to buffering cytoplasmic Ca2+ peaks in cardiomyocytes. Proc Natl Acad Sci USA. 2012;109:12986–12991. doi: 10.1073/pnas.1210718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajnoczky G, Csordas G, Madesh M, Pacher P. The machinery of local Ca2+ signalling between sarco-endoplasmic reticulum and mitochondria. J Physiol. 2000;529(Pt 1):69–81. doi: 10.1111/j.1469-7793.2000.00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hüser J, Blatter LA, Sheu SS. Mitochondrial calcium in heart cells: beat-to-beat oscillations or slow integration of cytosolic transients? J Bioenerg Biomembr. 2000;32:27–33. doi: 10.1023/a:1005556227425. [DOI] [PubMed] [Google Scholar]
- 14.Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, O'Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res. 2006;99:172–182. doi: 10.1161/01.RES.0000232546.92777.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Rourke B, Blatter LA. Mitochondrial Ca2+ uptake: tortoise or hare? J Mol Cell Cardiol. 2009;46:767–774. doi: 10.1016/j.yjmcc.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 17.Kitsis RN, Molkentin JD. Apoptotic cell death "Nixed" by an ER-mitochondrial necrotic pathway. Proc Natl Acad Sci USA. 2010;107:9031–9032. doi: 10.1073/pnas.1003827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kettlewell S, Cabrero P, Nicklin SA, Dow JA, Davies S, Smith GL. Changes of intra-mitochondrial Ca2+ in adult ventricular cardiomyocytes examined using a novel fluorescent Ca2+ indicator targeted to mitochondria. J Mol Cell Cardiol. 2009;46:891–901. doi: 10.1016/j.yjmcc.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 19.Robert V, Gurlini P, Tosello V, Nagai T, Miyawaki A, Di Lisa F, Pozzan T. Beat-to-beat oscillations of mitochondrial [Ca2+] in cardiac cells. EMBO J. 2001;20:4998–5007. doi: 10.1093/emboj/20.17.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrienko TN, Picht E, Bers DM. Mitochondrial free calcium regulation during sarcoplasmic reticulum calcium release in rat cardiac myocytes. J Mol Cell Cardiol. 2009;46:1027–1036. doi: 10.1016/j.yjmcc.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassani RA, Bers DM. Na-Ca exchange is required for rest-decay but not for rest-potentiation of twitches in rabbit and rat ventricular myocytes. J Mol Cell Cardiol. 1994;26:1335–1347. doi: 10.1006/jmcc.1994.1152. [DOI] [PubMed] [Google Scholar]
- 22.Matlib MA, Zhou Z, Knight S, Ahmed S, Choi KM, Krause-Bauer J, Phillips R, Altschuld R, Katsube Y, Sperelakis N, Bers DM. Oxygen-bridged dinuclear ruthenium amine complex specifically inhibits Ca2+ uptake into mitochondria in vitro and in situ in single cardiac myocytes. J Biol Chem. 1998;273:10223–10231. doi: 10.1074/jbc.273.17.10223. [DOI] [PubMed] [Google Scholar]
- 23.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Z, Matlib MA, Bers DM. Cytosolic and mitochondrial Ca2+ signals in patch clamped ventricular myocytes. J. Physiol. 1998;507:379–403. doi: 10.1111/j.1469-7793.1998.379bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bassani JWM, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: Species-dependent differences in cellular mechanisms. J. Physiol. 1994;476:279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht, Netherlands: Kluwer Academic Press; 1991. p. 258. [Google Scholar]
- 27.Wei AC, Liu T, Winslow RL, O'Rourke B. Dynamics of matrix-free Ca2+ in cardiac mitochondria: Two components of Ca2+ uptake and role of phosphate buffering. J Gen Physiol. 2012;139:465–478. doi: 10.1085/jgp.201210784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bassani JW, Bassani RA, Bers DM. Ca2+ cycling between sarcoplasmic reticulum and mitochondria in rabbit cardiac myocytes. J Physiol. 1993;460:603–621. doi: 10.1113/jphysiol.1993.sp019489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J Biol Chem. 2003;278:19062–19070. doi: 10.1074/jbc.M212661200. [DOI] [PubMed] [Google Scholar]
- 30.Despa S, Bossuyt J, Han F, Ginsburg KS, Jia L, Kutchai HC, Tucker AL, Bers DM. Phospholemman phosphorylation mediates the β-adrenergic effects on Na/K pump function in cardiac myocytes. Circ. Res. 2005;97:252–259. doi: 10.1161/01.RES.0000176532.97731.e5. [DOI] [PubMed] [Google Scholar]
- 31.Sharma VK, Ramesh V, Franzini-Armstrong C, Sheu SS. Transport of Ca2+ from sarcoplasmic reticulum to mitochondria in rat ventricular myocytes. J Bioenerg Biomemb. 2000;32:97–104. doi: 10.1023/a:1005520714221. [DOI] [PubMed] [Google Scholar]
- 32.Ramesh V, Sharma VK, Sheu SS, Franzini-Armstrong C. Structural proximity of mitochondria to calcium release units in rat ventricular myocardium may suggest a role in Ca2+ sequestration. Ann NY Acad Sci. 1998;853:341–344. doi: 10.1111/j.1749-6632.1998.tb08295.x. [DOI] [PubMed] [Google Scholar]
- 33.Szalai G, Csordas G, Hantash BM, Thomas AP, Hajnoczky G. Calcium signal transmission between ryanodine receptors and mitochondria. J Biol Chem. 2000;275:15305–15313. doi: 10.1074/jbc.275.20.15305. [DOI] [PubMed] [Google Scholar]
- 34.Song LS, Sham JS, Stern MD, Lakatta EG, Cheng H. Direct measurement of SR release flux by tracking ‘Ca spikes’ in rat cardiac myocytes. J Physiol. 1998;512:677–691. doi: 10.1111/j.1469-7793.1998.677bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altamirano J, Bers DM. Voltage-dependence of cardiac excitation-contraction coupling: unitary current amplitude and open channel probability. Circ. Res. 2007;101:590–597. doi: 10.1161/CIRCRESAHA.107.152322. [DOI] [PubMed] [Google Scholar]
- 36.Picht E, Zima AV, Shannon TR, Duncan AM, Blatter LA, Bers DM. Dynamic calcium movement inside cardiac sarcoplasmic reticulum during release. Circ Res. 2011;108:847–856. doi: 10.1161/CIRCRESAHA.111.240234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.