Abstract
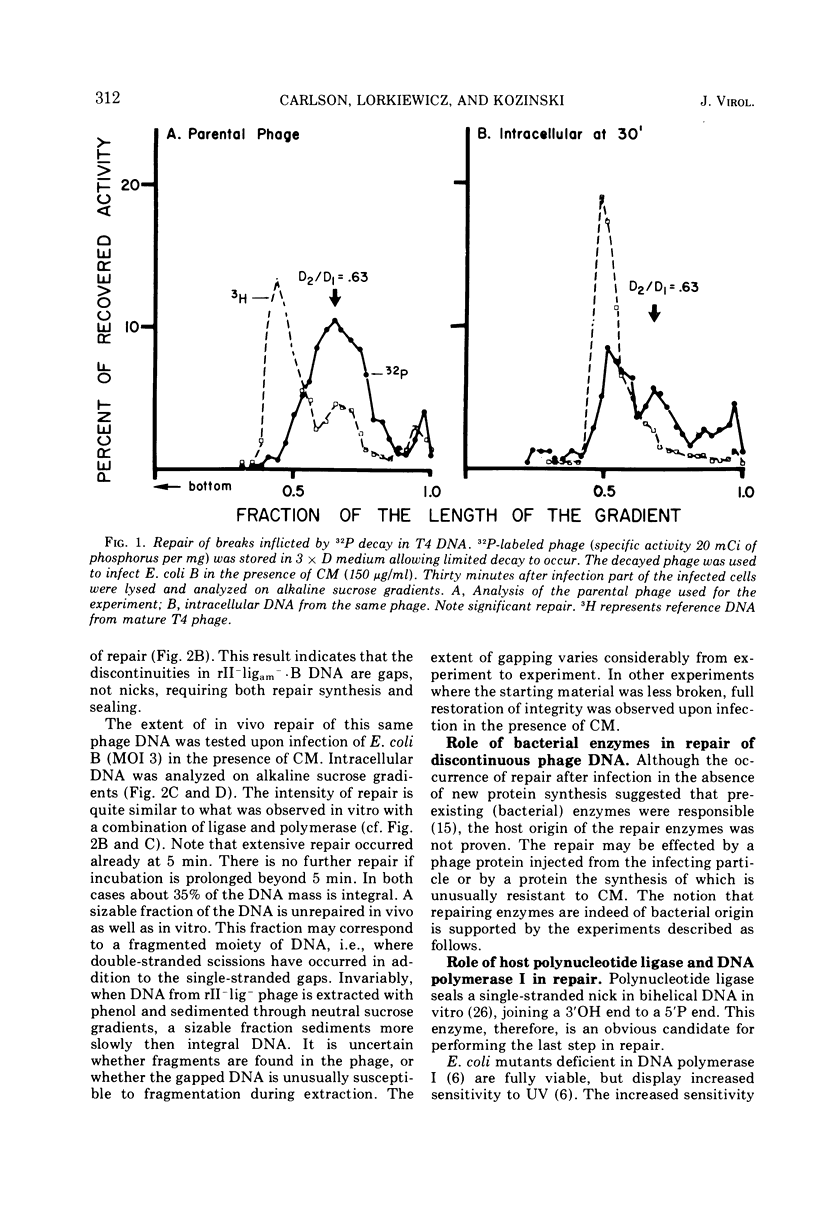
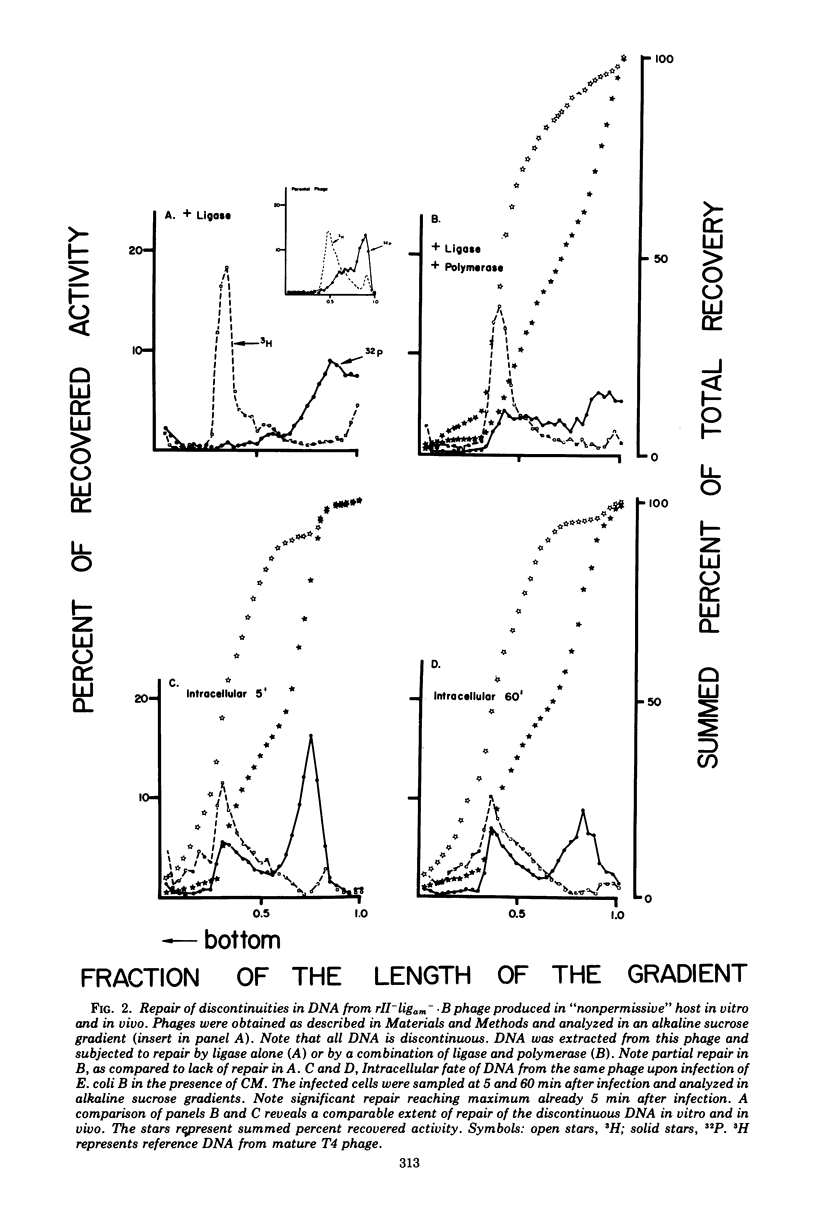
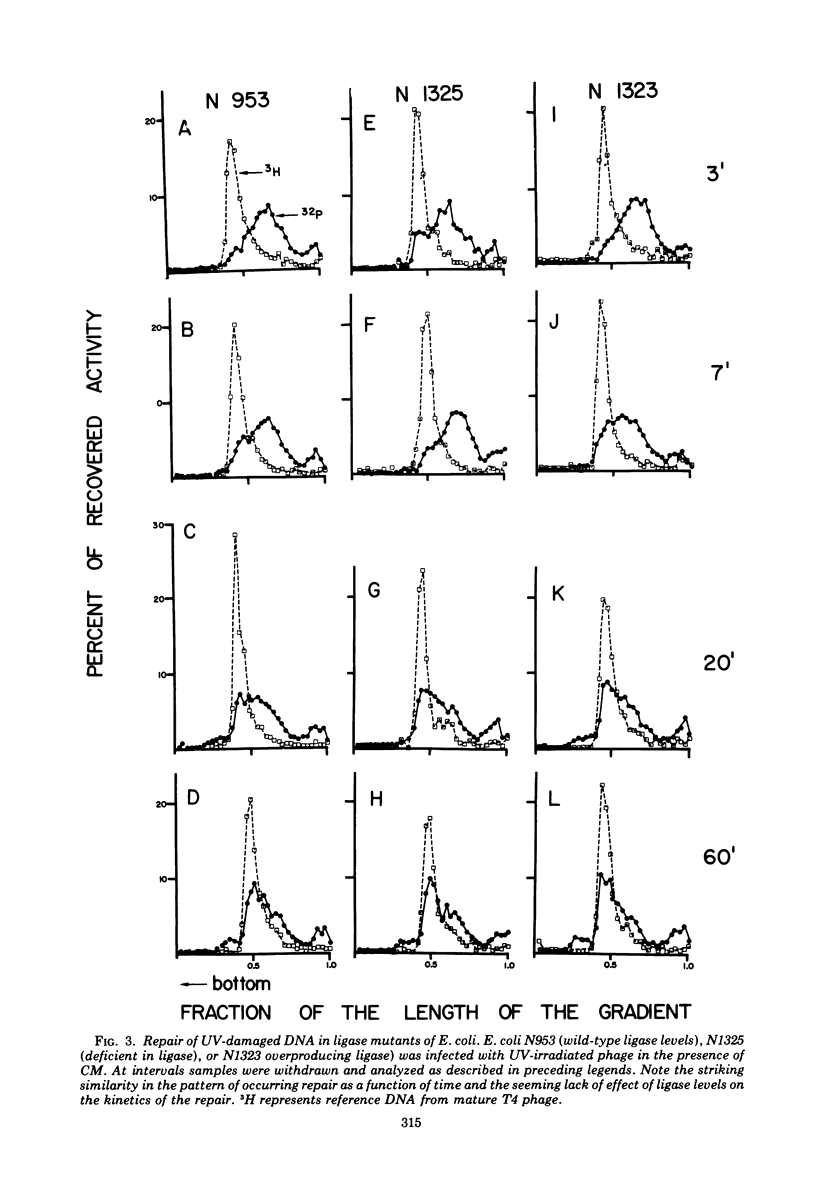
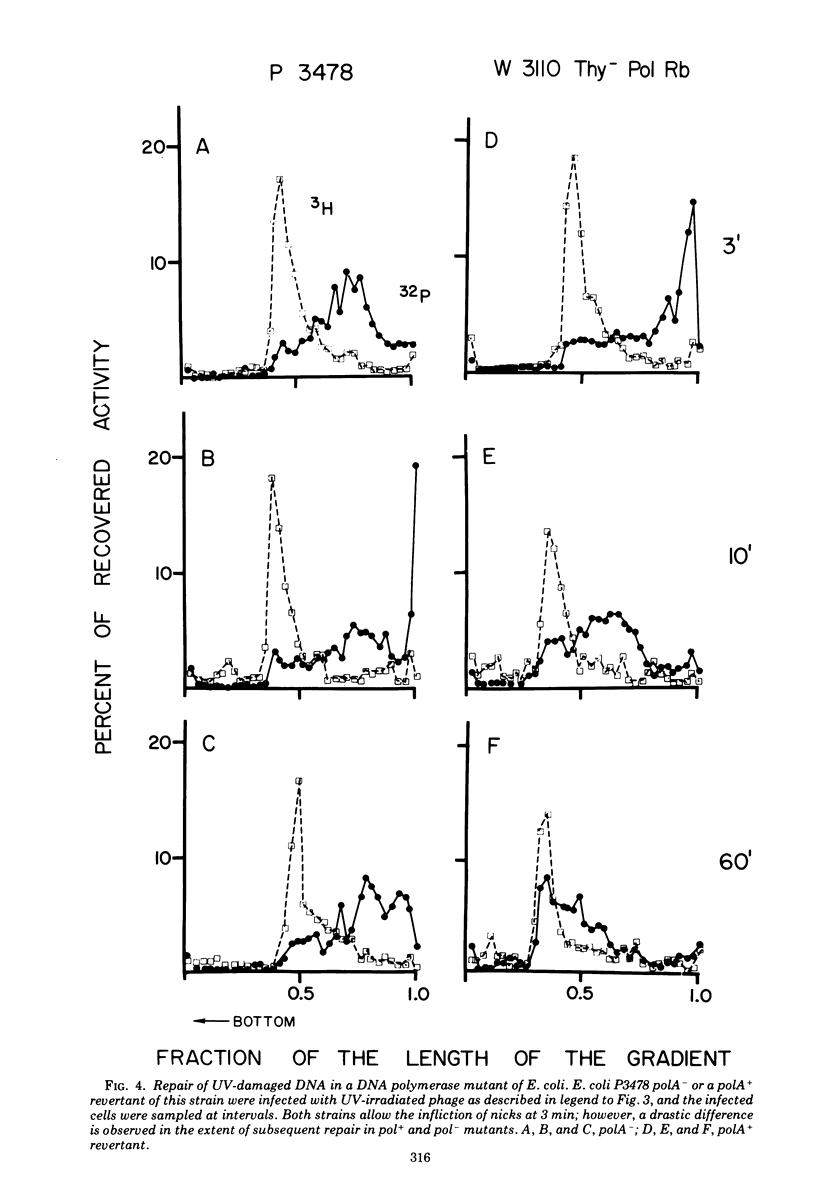
Discontinuities of T4 DNA which are caused by excision of UV-damaged areas, by decay of 32P atoms, or which are present in DNA from rII-ligam- phage produced in a host nonpermissive for amber mutants are all repaired by bacterial enzymes after infection in the presence of chloramphenicol. Escherichia coli DNA polymerase I participates in the host-mediated repair, but an approximately 20-fold variation in the levels of host polynucleotide ligase does not affect either the kinetics or the extent of repair observed. Upon removal of chloramphenicol, host-repaired DNA from UV-irradiated phage undergoes a secondary cycle of breakage, which ultimately results in solubilization of most of the phage DNA. If the cells are co-infected with nonirradiated helper phage, the secondary breaks are repaired and the continuity of the polynucleotide chain is restored. The close coincidence in the extent of primary and secondary breakage suggests that phage-coded enzymes recognize and excise areas improperly repaired by the host. In contrast to host-mediated repair, repair mediated by rescuing phage probably restored functionality to the damaged DNA.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H., Kozinski A. W. Suppression of T4D ligase mutations by rIIa and rIIb mutations. Proc Natl Acad Sci U S A. 1969 Nov;64(3):897–904. doi: 10.1073/pnas.64.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Ebisuzaki K., Campbell L. On the role of ligase in genetic recombination in bacteriophage T4. Virology. 1969 Aug;38(4):701–703. doi: 10.1016/0042-6822(69)90190-1. [DOI] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- Gellert M., Bullock M. L. DNA ligase mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1580–1587. doi: 10.1073/pnas.67.3.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. D., Grunstein J., Witkin E. M. Inviability of recA- derivatives of the DNA polymerase mutant of De Lucia and Cairns. J Mol Biol. 1971 Jun 14;58(2):631–634. doi: 10.1016/0022-2836(71)90377-9. [DOI] [PubMed] [Google Scholar]
- HARM W. Mutants of phage T4 with increased sensitivity to ultraviolet. Virology. 1963 Jan;19:66–71. doi: 10.1016/0042-6822(63)90025-4. [DOI] [PubMed] [Google Scholar]
- KOZINSKI A. W., SZYBALSKI W. Dispersive transfer of the parental DNA molecule to the progeny of phage phiX-174. Virology. 1959 Oct;9:260–274. doi: 10.1016/0042-6822(59)90119-9. [DOI] [PubMed] [Google Scholar]
- Kanner L., Hanawalt P. Repair deficiency in a bacterial mutant defective in DNA polymerase. Biochem Biophys Res Commun. 1970 Apr 8;39(1):149–155. doi: 10.1016/0006-291x(70)90770-9. [DOI] [PubMed] [Google Scholar]
- Karam J. D. DNA replication of phage T4 rII mutants without polynucleotide ligase (gene 30). Biochem Biophys Res Commun. 1969 Oct 22;37(3):416–422. doi: 10.1016/0006-291x(69)90931-0. [DOI] [PubMed] [Google Scholar]
- Kozinski A., Lorkiewicz Z. K. Early intracellular events in the replication of T4 phage DNA, IV. Host-mediated single-stranded breaks and repair in ultraviolet-damaged T4 DNA. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2109–2116. doi: 10.1073/pnas.58.5.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin S., Shahn E., Kozinski A. W. Interpretation of sucrose gradient sedimentation pattern of deoxyribonucleic acid fragments resulting from random breaks. J Virol. 1969 Jul;4(1):24–30. doi: 10.1128/jvi.4.1.24-30.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamune Y., Richardson C. C. Strand displacement during deoxyribonucleic acid synthesis at single strand breaks. J Biol Chem. 1971 Apr 25;246(8):2692–2701. [PubMed] [Google Scholar]
- Miller R. C., Jr, Kozinski A. W. Early intracellular events in the replication of bacteriophage T4 deoxyribonucleic acid. V. Further studies on the T4 protein-deoxyribonucleic acid complex. J Virol. 1970 Apr;5(4):490–501. doi: 10.1128/jvi.5.4.490-501.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosig G., Bowden D. W., Bock S. E. coli DNA polymerase I and other host functions participate in T4 DNA replication and recombination. Nat New Biol. 1972 Nov 1;240(96):12–16. doi: 10.1038/newbio240012a0. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Lehman I. R. Linkage of polynucleotides through phosphodiester bonds by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1967 May;57(5):1426–1433. doi: 10.1073/pnas.57.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi M., Yasuda S., Okubo S., Nakayama H., Shimada K., Takagi Y. Mechanism of repair of DNA in bacteriophage. I. Excision of pyrimidine dimers from ultraviolet-irradiated DNA by an extract of T4-infected cells. J Mol Biol. 1970 Jan 28;47(2):231–242. doi: 10.1016/0022-2836(70)90342-6. [DOI] [PubMed] [Google Scholar]
- Weiss B., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid, I. Repair of single-strand breaks in DNA by an enzyme system from Escherichia coli infected with T4 bacteriophage. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1021–1028. doi: 10.1073/pnas.57.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]


