Abstract
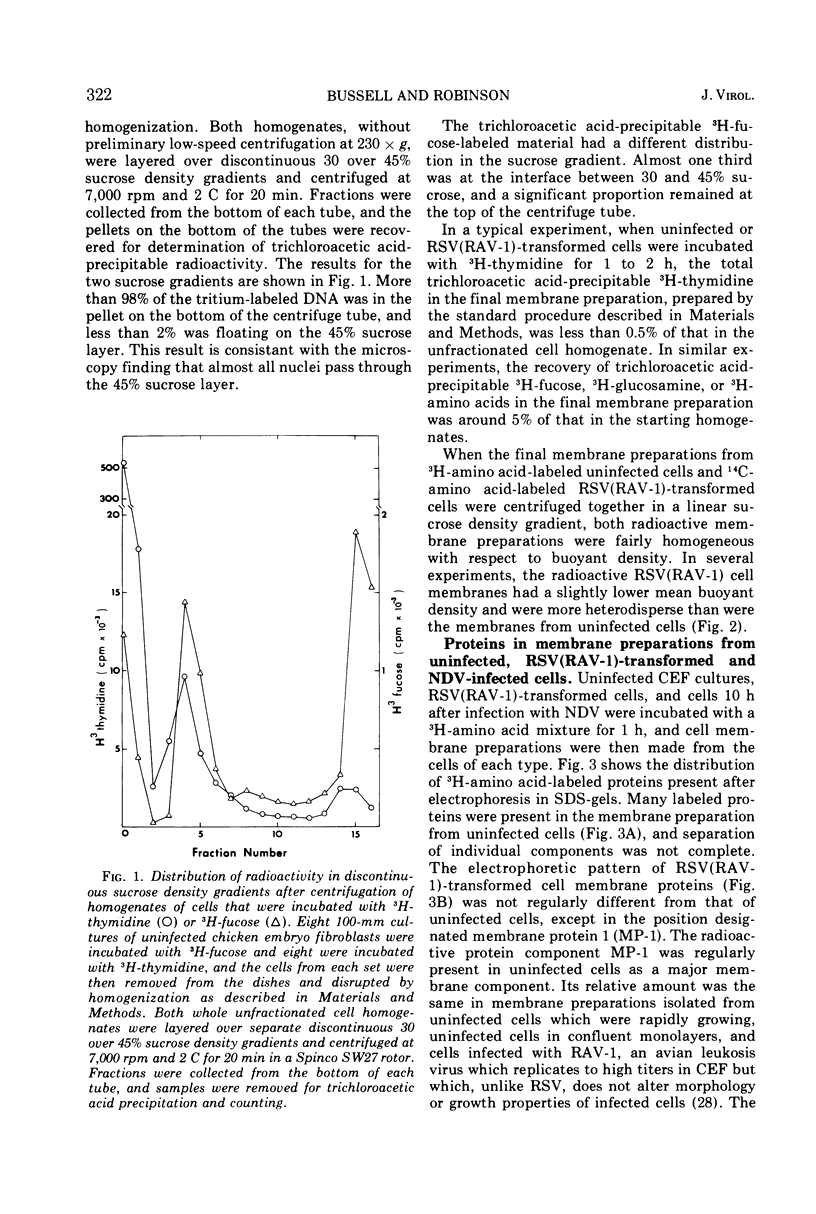
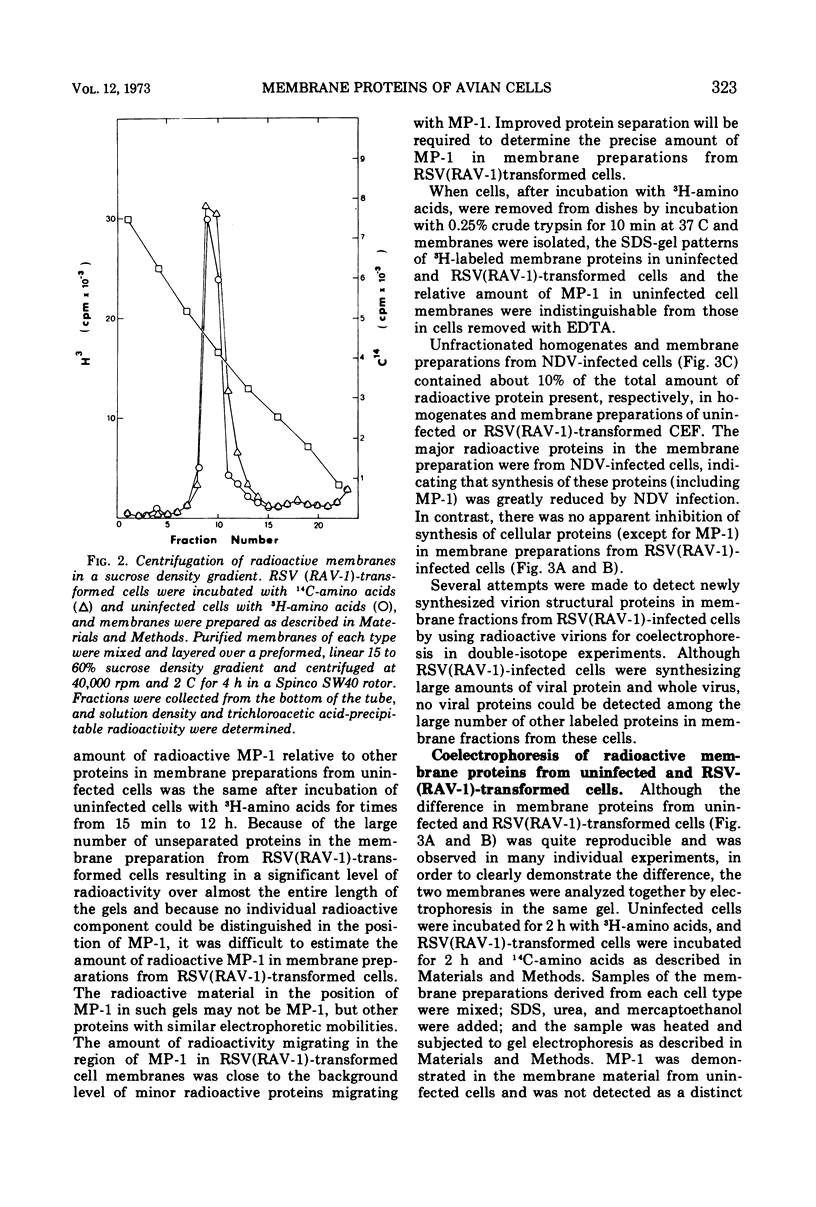
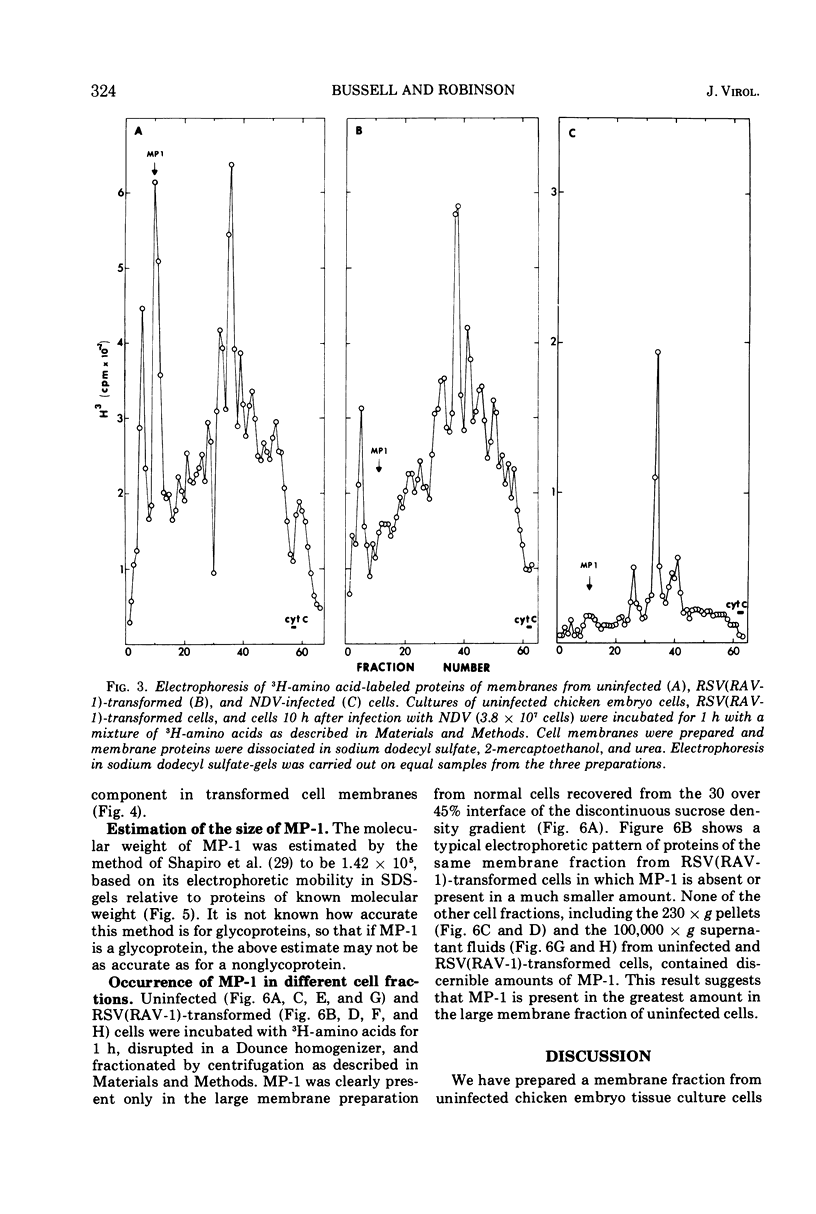
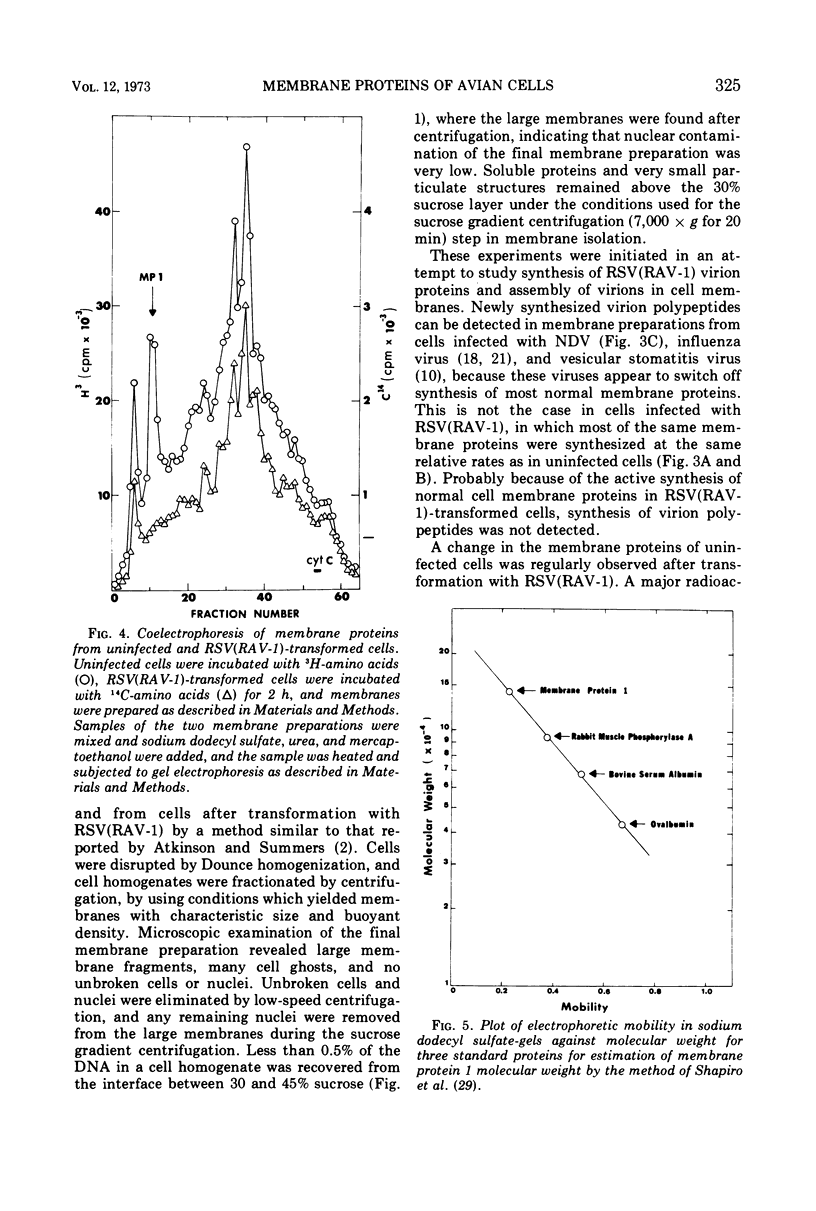
A method for preparing large membrane fragments and cell ghosts was developed for uninfected and Rous sarcoma virus-transformed chicken embryo fibroblasts in culture. Membrane proteins were analyzed by electrophoresis in acrylamide gels containing sodium dodecyl sulfate. A major amino-acid-containing component of uninfected cell membranes was greatly diminished in amount or absent in membranes of virus-transformed cells. This component, called MP-1, had an electrophoretic mobility in sodium dodecyl sulfate-containing gels similar to that of a protein of a mol wt of 1.42 × 105. MP-1 was not altered by changes in cell growth rate or in cells infected with the nontransforming virus RAV-1.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson P. H., Summers D. F. Purification and properties of HeLa cell plasma membranes. J Biol Chem. 1971 Aug 25;246(16):5162–5175. [PubMed] [Google Scholar]
- Buck C. A., Glick M. C., Warren L. A comparative study of glycoproteins from the surface of control and Rous sarcoma virus transformed hamster cells. Biochemistry. 1970 Nov 10;9(23):4567–4576. doi: 10.1021/bi00825a016. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Glick M. C., Warren L. Glycopeptides from the surface of control and virus-transformed cells. Science. 1971 Apr 9;172(3979):169–171. doi: 10.1126/science.172.3979.169. [DOI] [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M., Goldberg A. R. Identification of a tumor-specific determinant on neoplastic cell surfaces. Proc Natl Acad Sci U S A. 1967 Feb;57(2):359–366. doi: 10.1073/pnas.57.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M., Martin G. S. Agglutination of cells transformed by Rous sarcoma virus by wheat germ agglutinin and concanavalin A. Nat New Biol. 1972 May 3;237(70):9–12. doi: 10.1038/newbio237009a0. [DOI] [PubMed] [Google Scholar]
- Chiarugi V. P., Urbano P. Electrophoretic analysis of membrane glycoproteins in normal and polyoma virus transformed BHK 21 cells. J Gen Virol. 1972 Feb;14(2):133–140. doi: 10.1099/0022-1317-14-2-133. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Atkinson P. H., Summers D. F. Interactions of vesicular stomatitis virus structural proteins with HeLa plasma membranes. Nat New Biol. 1971 May 26;231(21):121–123. doi: 10.1038/newbio231121a0. [DOI] [PubMed] [Google Scholar]
- David A. E. Lipid composition of Sindbis virus. Virology. 1971 Dec;46(3):711–720. doi: 10.1016/0042-6822(71)90073-0. [DOI] [PubMed] [Google Scholar]
- FORRESTER J. A., AMBROSE E. J., MACPHERSON J. A. Electrophoretic investigations of a clone of hamster fibroblasts and polyoma-transformed cells from the same population. Nature. 1962 Dec 15;196:1068–1070. doi: 10.1038/1961068a0. [DOI] [PubMed] [Google Scholar]
- FORRESTER J. A., AMBROSE E. J., STOKER M. G. MICROELECTROPHORESIS OF NORMAL AND TRANSFORMED CLONES OF HAMSTER KIDNEY FIBROBLASTS. Nature. 1964 Feb 29;201:945–946. doi: 10.1038/201945a0. [DOI] [PubMed] [Google Scholar]
- Hakomori S. I., Murakami W. T. Glycolipids of hamster fibroblasts and derived malignant-transformed cell lines. Proc Natl Acad Sci U S A. 1968 Jan;59(1):254–261. doi: 10.1073/pnas.59.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M., Hanafusa H. Analysis of a functional change in membrane in the process of cell transformation by Rous sarcoma virus; alteration in the characteristics of sugar transport. Virology. 1970 Aug;41(4):647–652. doi: 10.1016/0042-6822(70)90429-0. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Huebner R. J., Gilden R. V. Alterations in the characteristics of sugar uptake by mouse cells transformed by murine sarcoma viruses. J Natl Cancer Inst. 1969 Nov;43(5):1091–1096. [PubMed] [Google Scholar]
- Hobom-Schnegg B., Robinson H. L., Robinson W. S. Replication of Rous sarcoma virus in synchronized cells. J Gen Virol. 1970;7(2):85–93. doi: 10.1099/0022-1317-7-2-85. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Kiehn E. D. Influenza virus effects on cell membrane proteins. Science. 1970 Jan 9;167(3915):202–205. doi: 10.1126/science.167.3915.202. [DOI] [PubMed] [Google Scholar]
- Hung P. P., Robinson H. L., Robinson W. S. Isolation and characterization of proteins from Rous sarcoma virus. Virology. 1971 Jan;43(1):251–266. doi: 10.1016/0042-6822(71)90243-1. [DOI] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Interaction of the carbohydrate-binding protein concanavalin A with normal and transformed cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1418–1425. doi: 10.1073/pnas.63.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowitz S. G., Compans R. W., Choppin P. W. Influenza virus structural and nonstructural proteins in infected cells and their plasma membranes. Virology. 1971 Dec;46(3):830–843. doi: 10.1016/0042-6822(71)90084-5. [DOI] [PubMed] [Google Scholar]
- Martin G. S., Venuta S., Weber M., Rubin H. Temperature-dependent alterations in sugar transport in cells infected by a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2739–2741. doi: 10.1073/pnas.68.11.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meezan E., Wu H. C., Black P. H., Robbins P. W. Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. II. Separation of glycoproteins and glycopeptides by sephadex chromatography. Biochemistry. 1969 Jun;8(6):2518–2524. doi: 10.1021/bi00834a039. [DOI] [PubMed] [Google Scholar]
- Mora P. T., Brady R. O., Bradley R. M., McFarland V. W. Gangliosides in DNA virus-transformed and spontaneously transformed tumorigenic mouse cell lines. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1290–1296. doi: 10.1073/pnas.63.4.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue J. F., Kletzien R., Miller K. The isolation and characterization of plasma membrane from cultured cells. I. The chemical composition of membrane isolated from uninfected and oncogenic RNA virus-converted chick embryo fibroblasts. Biochim Biophys Acta. 1971 Dec 3;249(2):419–434. doi: 10.1016/0005-2736(71)90120-9. [DOI] [PubMed] [Google Scholar]
- Perdue J. F., Kletzien R., Wray V. L. The isolation and characterization of plasma membrane from cultured cells. IV. The carbohydrate composition of membranes isolated from oncogenic RNA virus-converted chick embryo fibroblasts. Biochim Biophys Acta. 1972 May 9;266(2):505–510. doi: 10.1016/0005-2736(72)90106-x. [DOI] [PubMed] [Google Scholar]
- RUBIN H., VOGT P. K. An avian leukosis virus associated with stocks of Rous sarcoma virus. Virology. 1962 May;17:184–194. doi: 10.1016/0042-6822(62)90096-x. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Robinson W. S. Inhibition of growth of uninfected and rous sarcoma virus-infected chick-embryo fibroblasts by rifampicin. J Natl Cancer Inst. 1971 Apr;46(4):785–788. [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Stoker M. G., Rubin H. Density dependent inhibition of cell growth in culture. Nature. 1967 Jul 8;215(5097):171–172. doi: 10.1038/215171a0. [DOI] [PubMed] [Google Scholar]



