Abstract
Developing retinal neurons differentiate their distinctive dendritic morphologies through cell-intrinsic instructions and cellular interactions within the local environment. This review examines the contributions of interactions with afferents and with homotypic neighbors upon the dendritic morphogenesis of retinal bipolar cells in four different mouse models that modulate the frequency of these interactions. Comparisons with horizontal cell differentiation are discussed, and differences between the dendritic plasticity within the outer versus inner plexiform layers are highlighted. Finally, the developmental plasticity of the bipolar and horizontal cells is considered in light of the natural variation in afferent and target cell number, ensuring a uniformity of coverage and connectivity across the retinal surface.
Keywords: Bipolar cell, horizontal cell, photoreceptor, pedicle, outer plexiform layer, differentiation, morphogenesis, dendritic field, Nrl, Bax, Isl1, Lim1
Introduction
The morphogenesis of a nerve cell is controlled by a combination of cell-intrinsic instructions set in motion at the time of fate assignment interacting with environmental signals arising from other cells. Transcription factors coordinate the developmental expression of downstream genes that command the differentiation of a neuron into one particular class (Parrish et al., 2007; Jan and Jan, 2010), and features of the cellular morphology characteristic of this class can occasionally be observed during differentiation as single dissociated cells in vitro, removed from their normal environment (Bartlett and Banker, 1984; Powell et al., 1997; Dunn et al., 1998; Zmuda and Rivas, 1998). That neurons develop a polarized morphology in vitro indicates a cell-intrinsic capacity to do so, but within their normal environment, the increasingly complex apico-basal axis of the developing neural tube must provide a multitude of signals that serve to orient such polarized neurons and to target their axons and dendrites, particularly within layered structures such as the neocortex, hippocampus, cerebellum and retina (Randlett et al., 2010).
Within the retina, a combinatorial transcription factor code instructs newborn neurons to differentiate as one of the main classes of retinal nerve cell, being the ganglion cells, amacrine cells, bipolar cells, horizontal cells and photoreceptors (Ohsawa and Kageyama, 2008; Agathocleous and Harris, 2009). Each of those main classes can be subdivided into different types, readily discriminated on the basis of cellular morphology and occasionally by other features including the synthetic enzymes for neurotransmitter production and the receptors that render these neurons sensitive to afferent input (Masland, 2001; Wässle, 2004). These features are likewise assumed to be directed by distinct combinations of transcription factors expressed during their development. For example, retinal ganglion cells require Math5 for their formation (Brown et al., 2001; Wang et al., 2001), but further require Brn3a, Brn3b or Brn3c for discrete subtypes to differentiate (Badea and Nathans, 2010), while amacrine cells require the coordinated expression of NeuroD and Math3 in order to form (Inoue et al., 2002), yet their subsequent differentiation into unique morphological and neurotransmitter phenotypes requires the further expression of Barhl2, Isl1 or Bhlhb5 in order for glycinergic, cholinergic or GABAergic subtypes to differentiate (Mo et al., 2004; Feng et al., 2006; Elshatory et al., 2007b). The dendritic features characteristic of these subtypes have not been evidenced in dissociated cell cultures, and so it is unknown whether the morphological differences that characterize these subtypes emerge largely through cell-intrinsic mechanisms driven by such factors, or whether they are driven by interactions with environmental signals arising from other neurons. Indeed, it is easy to imagine how, once a nerve cell initiates its differentiation program, expressing cell surface receptors that guide migration, direct outgrowth and detect neurotransmitters, such cells become sensitive to environmental signals that instruct or constrain further morphological differentiation (Horch and Katz, 2002; Hoogenraad et al., 2005; Espinosa et al., 2009).
The mouse retina, like that of other mammals, contains at least ten different subtypes of bipolar cell, including nine cone bipolar cell types and one rod bipolar cell (Wässle et al., 2009). All of them are responsive to the neurotransmitter glutamate, with four of the former signaling decrements of light (OFF bipolar cells), while the remainder signal increments of light (ON bipolar cells), due to the different ionotropic and metabotropic receptors expressed on their dendritic endings, respectively (Brandstätter et al., 1998; Strettoi et al., 2010). Each of these bipolar cell subtypes is known to differentiate a terminal that stratifies at a characteristic depth within the inner plexiform layer (IPL), where these cells influence subsets of amacrine and ganglion cells with which they co-stratify and synapse upon (Ghosh et al., 2004; Pignatelli and Strettoi, 2004). This celltype-specific stratification in the IPL is readily observed when comparing the morphology of single cells in retinal section, but such radial sections through the retina do not permit much appreciation of how each of these subtypes differentiates a unique dendritic morphology. Figure 1 illustrates five of the ON-types of bipolar cell, the Types 6, 7, 8 and 9 cone bipolar cells, and the rod bipolar cell, viewed en face in retinal wholemounts. The five cells show conspicuous features that discriminate them from one another: the rod bipolar cell exhibits a dense distribution of dendritic terminals that receives innervation from the rod spherules, while the others are innervated primarily, if not exclusively, by cone pedicles (Mataruga et al., 2007; Tsukamoto et al., 2007; Haverkamp et al., 2008). Amongst the latter, the Type 9 cell is innervated exclusively by UV cone photoreceptors in the mouse retina (Haverkamp et al., 2005), while the others are either non-selective for M and UV cones or may selectively avoid the UV cones (Breuninger et al., 2011). Each of them differentiates a dendritic morphology distinct from one another, although appreciating the features that truly differentiate one type from another requires morphometric analysis of multiple cells of each type.
Figure 1.
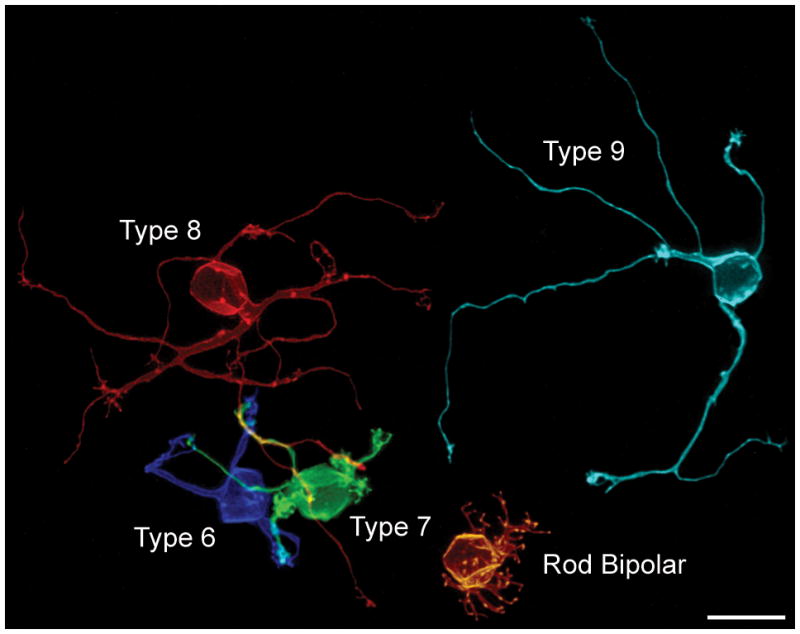
Five single ON-cone bipolar cells viewed en face in retinal wholemount preparations, illustrating the breadth of dendritic morphology in the outer plexiform layer (OPL). All but the Type 9 cell had been labeled in the same retina, and show their true spatial relationships to one another. The Type 9 cell is from another wholemounted retina, positioned aside these other four cells for direct comparison. Each cell had been labeled with DiI, reconstructed, and then pseudo-colored to discriminate them. Calibration bar = 10 μm.
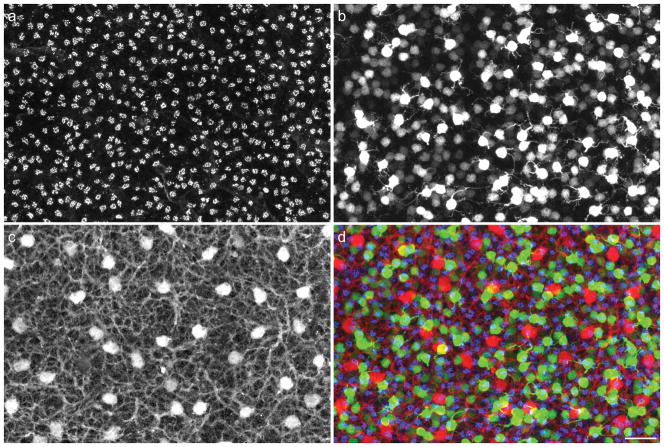
When viewing the detailed dendritic morphology of single labeled cells such as these, it is easy to lose sight of the other neurons within the local environment that may participate in their differentiation. Figure 2 intimates a degree of this complexity of the outer plexiform layer (OPL). Figure 2a shows the mosaic of cone pedicles within the OPL that synapse upon the dendrites of the cone bipolar cells. Each of these pedicles is believed to innervate every type of cone bipolar cell (Wässle et al., 2009), with the exception of the specificities mentioned above, and the image in figure 1 includes locations where the Types 6, 7 and 8 dendritic arbors coalesce upon what are presumed to be the sites of shared pedicles (unlabeled) in that sample. Figure 2b shows the population of one type of these cone bipolar cells, the Type 7 cone bipolar cells (those cells more intensely fluorescent for green fluorescent protein, GFP), along with the population of rod bipolar cells (being lightly labeled with GFP). This image makes clearer the fact that Type 7 cells such as that shown in figure 1 differentiate amongst many other Type 7 cells as they colonize the pedicles (shown together in figure 2d as bright green and blue, respectively), and the same must be true for each of the other bipolar cell subtypes. In addition, the one other class of post-synaptic neuron with connections in the OPL is shown in figure 2c, the horizontal cells, extending dendrites to every pedicle within their dendritic fields (Reese et al., 2005). Unlike the bipolar cell subtypes, that are each believed to “cover” the retina once (Wässle et al., 2009), the horizontal cells exhibit dendritic overlap, such that on average six neighboring cells have dendritic fields that form synapses with each pedicle (Reese et al., 2005). Each of these neurons, the afferents, the homotypic neighbors, and the other heterotypic neighbors, may participate in the differentiation of a particular type of bipolar cell, yet how much the features readily revealed in images such as that shown in figure 1, including the the size of the dendritic arbor, the branching pattern of the dendrites, and the number of its connections, depend upon these cellular interactions is unclear.
Figure 2.
a: Cone photoreceptors extend axons through the outer nuclear layer to reach the innermost stratum of the OPL where they form a periodic distribution of pedicles across the retina, labeled with PNA (blue in panel d), viewed here in a wholemount preparation. b: Type 7 cone bipolar cells, brightly labeled (green in panel d) due to a gustducin-GFP reporter transgene, extend their dendritic fields to contact the pedicles in the presence of numerous homotypic neighbors. Other heterotypic neighbors, including the rod bipolar cells (expressing the gustducin-GFP transgene weakly, and hence dimly labeled), also extend dendrites into the OPL, receiving innervation from the rod spherules (unlabeled herein) distributed through the full depth of the OPL. c: Each cone pedicle innervates nearly every type of cone bipolar cell, as well as one other post-receptoral neuron, the horizontal cell, the somata and processes of which are immunolabeled for calbindin (red in panel d). d: Superposition of all three labels: cone pedicles are shown in blue, bipolar cells in green, and horizontal cells in red. Calibration bar = 25 μm.
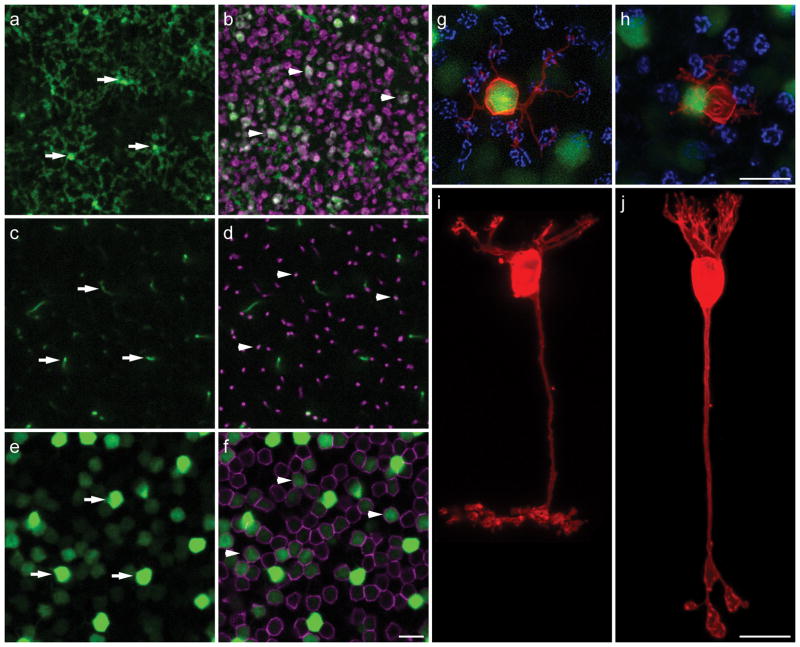
To address these issues, we have begun exploring the differentiation of particular types of bipolar cells within genetically modified retinas that alter the abundance of these populations. To study the detailed morphology of individual bipolar cells, we have used gustducin-GFP transgenic mice (Huang et al., 2003) to identify the axon terminals of single Type 7 cone bipolar cells (figure 3a) or rod bipolar cells (figure 3b), which can be traced back to their somata of origin in the inner nuclear layer (figure 3c and e, d and f, respectively). Once visualized in lightly fixed wholemount preparations, we impale the axon terminals in the IPL to deposit the lipophilic tracer, DiI, thereby producing enhanced filling of the entire cellular membrane, including the fine invaginating terminations of the dendrites in the OPL (figure 3g–j). Examination of single, DiI-labeled, bright versus dim GFP-positive cells confirms their identity as Type 7 cone versus rod bipolar cells, respectively, in both wholemount preparations as well as in sectioned retinas (Keeley and Reese, 2010b). We have crossed this gustducin-GFP reporter transgene onto several genetically modified lines and used this labeling approach to analyze the dendritic morphology of these cells under different conditions.
Figure 3.
a–f: Different depths of a wholemount retina showing GFP (all panels) along with PKC immunofluorescence (b, d, f) in the inner plexiform layer (IPL; ad) and inner nuclear layer (e, f) of a gustducin-GFP transgenic mouse. GFP-positive axon terminals of three Type 7 cone bipolar cells in stratum 4 of the IPL are indicated by arrows in a. Their axons can be traced through the confocal Z-stack reconstruction (in c) to intensely fluorescent Type 7 cone bipolar cells in e (arrows). GFP-positive axon terminals of three rod bipolar cells (double-labeled for PKC in magenta) in stratum 5 of the IPL of the same specimen are indicated by arrowheads in b. Their axons can likewise be traced (in d) to less intensely fluorescent rod bipolar somata, evidenced by their PKC labeling, in f (arrowheads). Note that not every rod bipolar cell shows detectable GFP fluorescence. g–j: When single GFP axon terminals are impaled with a pipette and injected with DiI (red), those labeled from stratum 4 are invariably associated with intensely fluorescent somata giving rise to dendritic labeling characteristic of the Type 7 cone bipolar cell morphology, shown in i, innervating individual cone pedicles (labeled with PNA in blue in g). Those labeled from stratum 5 show dendritic labeling characteristic of the rod bipolar cell morphology, shown in j, with barely detectable GFP-somal labeling subsequent to DiI and PNA labeling, bypassing the cone pedicles (shown in blue in h). A brightly GFP-labeled Type 7 cone bipolar cell is immediately to the left of this labeled rod bipolar cell. Panels h, i and j reproduced with permission, Society for Neuroscience. Calibration bars = 10 μm.
This review will first examine the role played by afferents in the differentiation of rod versus cone bipolar cells. Might those features that most distinguish the rod bipolar cell from cone bipolar cells reflect a specification carried by the afferents themselves? Bipolar cells have been shown to depend upon their afferents in order to maintain their characteristic morphologies in maturity (Marc et al., 2003), but whether they are critical for the development of that morphology has, until recently, been unexplored. Second, we will consider the role played by homotypic neighbors. Are bipolar cells sensitive to the presence of neighboring like-type cells? While such interactions are widely assumed to ensure a uniform coverage of the retinal surface, multiple recent examples within the retina have been shown to differentiate independent of homotypic influences (Farajian et al., 2004; Lin et al., 2004; Keeley et al., 2007; Keeley and Reese, 2010a). Finally, we will compare these results with those for retinal horizontal cells exposed to comparable manipulations, and consider the implications of these studies for understanding the natural variation in retinal nerve cell morphology.
The Role of Afferent Innervation
The rod bipolar cell shown in figure 1 differs characteristically from all types of cone bipolar cell, having a dense, bushy, dendritic field distributed through the depth of the OPL where, in the mouse retina, rod spherules are known to terminate. These are features that typify the rod bipolar cell in most other mammalian retinas (Greferath et al., 1990; Wässle et al., 1991), and might be expected to reflect a cell-intrinsic specification of dendritic morphogenesis. That this cell might be sensitive to afferent specification, however, comes from studies examining the morphology of this cell type wherein rods are a minority photoreceptor, in diurnal species such as the ground squirrel. Unlike the mouse, where the rods comprise roughly 97% of all photoreceptors (Young, 1985; Jeon et al., 1998), the ground squirrel has about 17% of its photoreceptor complement comprising rods, with a dorso-ventral gradient where the rods may be as sparse as only 5% within the visual streak (Kryger et al., 1998). The dendritic field of the rod bipolar cell in the ground squirrel retina is conspicuously sparse (Linberg et al., 1996), bearing no obvious resemblance to rod bipolar cells in other species with the exception of another diurnal mammal, the tree shrew (Müller and Peichl, 1989), but for being immunopositive for protein kinase C (PKC) and stratifying in the innermost stratum of the IPL (Cuenca et al., 2002). The cone bipolar cells in the mouse retina extend dendritic endings that are sparsely distributed, in accord with the lower density of cones therein (e.g. figure 3g). To ascertain whether the characteristic differences between the dendritic morphology of rod bipolar cells and cone bipolar cells in the mouse retina reflects afferent specification, we examined “coneless” transgenic mice, in which a diphtheria toxin transgene is expressed in differentiating cones (Wang et al., 1992; Soucy et al., 1998), killing them during the first postnatal week (Raven and Reese, 2003), prior to the differentiation of the bipolar cells (Morgan et al., 2006). We also compared these with Nrl knockout mice, in which all rods differentiate as cones, producing a “conefull” retina (Mears et al., 2001).
Figure 4a shows the dendritic morphology of single Type 7 cone bipolar cells in individual wildtype, coneless and conefull retinas, while figure 4b shows single rod bipolar cells, respectively. Despite the fact that the coneless retina has been depleted of roughly 97% of its cone afferents, while the conefull retina has undergone an approximately 20-fold increase in this same population, cone bipolar cell morphogenesis is largely unaltered, and by eye, these cells can hardly be discriminated from wildtype cells (figure 4a). Those in the coneless retina show no signs of stunted development, while those in the conefull retina are not obviously hypertrophic (Keeley and Reese, 2010b). Rod bipolar cells are also hardly altered in their dendritic morphology in these retinas (figure 4b): while there is little surprise that they are largely identical in the coneless retina, they show no hint of underdevelopment in the conefull retina, nor of any hint of a cone bipolar-like morphology (Keeley and Reese, 2010b), consistent with previous studies examining the morphology of PKC-immunoreactive cells in these conefull mice (Strettoi et al., 2004). Those results contrast with expectations from the above-mentioned studies from diurnal species in which rod bipolar cell dendritic morphology is conspicuously sparse in the presence of low densities of rod photoreceptors.
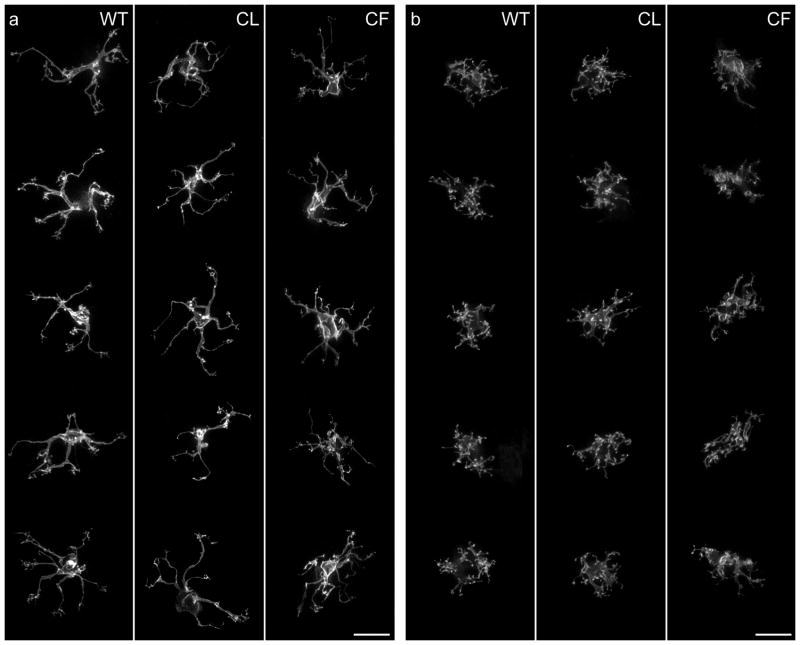
Figure 4.
a: Single DiI-labeled Type 7 cone bipolar cell dendritic arbors viewed en face in wildtype, coneless and conefull retinas. b: Single DiI-labeled rod bipolar cell dendritic arbors in wildtype, coneless and conefull retinas. Each cell is a Z-stack reconstruction through the OPL to include the full extent of the dendritic arbor. The gross morphogenesis of Type 7 cone or rod bipolar cells is largely unaffected in both conditions. Reproduced with permission, Society for Neuroscience. Calibration bar = 10 μm.
Closer examination of these labeled cells shows that finer features of their differentiation are in fact altered. The cone bipolar cells, absent a normal distribution of periodic pedicles in both the coneless and conefull retinas, fail to differentiate clustered terminations of dendritic endings across the dendritic field (Keeley and Reese, 2010b), instead having sparse terminal endings distributed more uniformly across the field area. The rod bipolar cells, apparently innervated directly by these genetically re-specified cones, exhibit longer and coarser dendritic stalks due to the increased thickness of the OPL in the conefull retinas, and position their dendritic endings less uniformly due to the crowding of re-specified pedicles therein (Keeley and Reese, 2010b). Despite these finer differences that implicate a role for normal afferent innervation upon the detailed differentiation of dendritic endings, the rod and Type 7 cone bipolar cells appear to differentiate their characteristic morphologies largely independent of afferent specification: rod bipolar cells retain their fundamental rod bipolar cell morphology when differentiating only in the presence of cones, while Type 7 cone bipolar cells do not lose their characteristic morphology in the entire absence of cones, nor when differentiating amongst an excess of them (figure 5).
Figure 5.
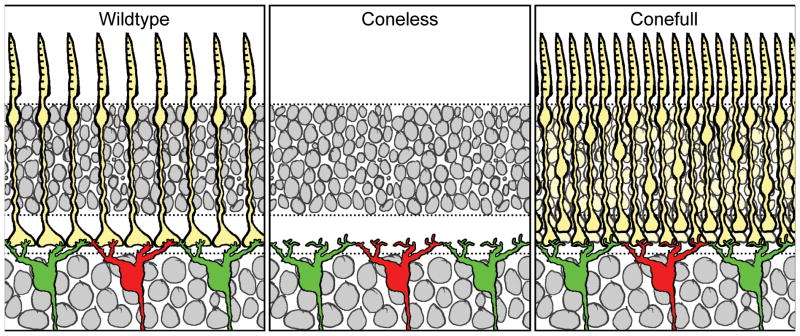
The fundamental dendritic organization of Type 7 cone bipolar cells is maintained despite large modulation of afferent density in these coneless and conefull retinas. The distribution of dendritic terminals is, however, afferent-dependent, portrayed here by the absence of discrete clusters of terminations in the OPL.
The Role of Homotypic Neighbors
Neither the rod bipolar cells nor the Type 7 cone bipolar cells showed any change in the areal size of their dendritic fields in the above coneless or conefull retinas (Keeley and Reese, 2010b), indicating that afferent innervation does not control this feature of dendritic morphology. Bipolar cells in the mouse retina (Wässle et al., 2009), like those in other species (Boycott and Wässle, 1999), and like some other types of retinal nerve cells (Dacey, 1993), differentiate dendritic fields that approximate a tiling of the retinal surface, producing a coverage factor around 1. Such tiling is generally assumed to reflect some form of signaling between like-type cells that constrains further dendritic growth, through contact-mediated inhibition (Reese, 2008b). Curiously, in two types of retinal ganglion cell with coverage factors close to 1, neither showed any evidence for homotypic regulation of dendritic field size when neighboring homotypic density was genetically reduced (Lin et al., 2004).
We are currently addressing this directly for the Type 7 cone bipolar cell by modulating the density of homotypic neighbors in knockout mice that have increased or decreased densities of inner, but not outer, retinal neurons. Specifically, we are examining Bax-KO retinas, in which loss of this pro-apoptotic gene leads to a doubling of the retinal ganglion cell population and thickness of the inner nuclear layer without affecting the size of the outer nuclear layer (Mosinger Ogilvie et al., 1999; Péquignot et al., 2003), and Isl1 conditional knockout (CKO) retinas, in which loss of this transcription factor results in a conspicuous reduction of inner, but not outer, retinal neurons (Elshatory et al., 2007b; Pan et al., 2008). We have confirmed that neither of these manipulations affects the size of the cone photoreceptor population, ensuring that afferent density for Type 7 cone bipolar cells is unaffected. We have also verified that Type 7 cone bipolar cell number is increased, and decreased, respectively, in these two modified retinas (Lee et al., in prep.).
Figure 6a–c shows an example of a Type 7 cone bipolar cell in each of the wildtype, Bax-KO, and Isl1-CKO retinas. Those in the Bax-KO retina are smaller than in littermate control retinas, while those in the Isl1-CKO are larger. Coincident with this modulation of dendritic field size in the presence of unchanging densities of cone afferents, the Type 7 cone bipolar cells in the Bax-KO retinas contact fewer pedicles, while those in the Isl1-CKO retinas contact more pedicles (labeled for PNA, in blue, in figure 6d–f). The average number of contacts per pedicle appears to remain constant across these conditions, suggesting that Type 7 cone bipolar cells are not cell-intrinsically constrained to specify the number of cone afferents with which they form connections, nor the total number of terminal endings a cell forms. While these results are preliminary (Lee et al., in prep.), they contrast with the above results from the conefull (Nrl-KO) retina (Keeley and Reese, 2010b), presumably because that retina fails to form normal pedicles altogether (Raven et al., 2007), although whether this is due simply to crowding of the pedicles that prohibits them from differentiating their normal structural features, or to a lack of complete re-specification as “cone”, remains unclear (Daniele et al., 2005). (Indeed, this latter possibility might account for the connectivity formed between rod bipolar cells and re-specified cones in the conefull retina). In short, these experiments suggest that homotypic density commands the size of the dendritic field of a Type 7 cone bipolar cell (figure 7), and that those dendritic fields in turn colonize the population of pedicles lying within it.
Figure 6.
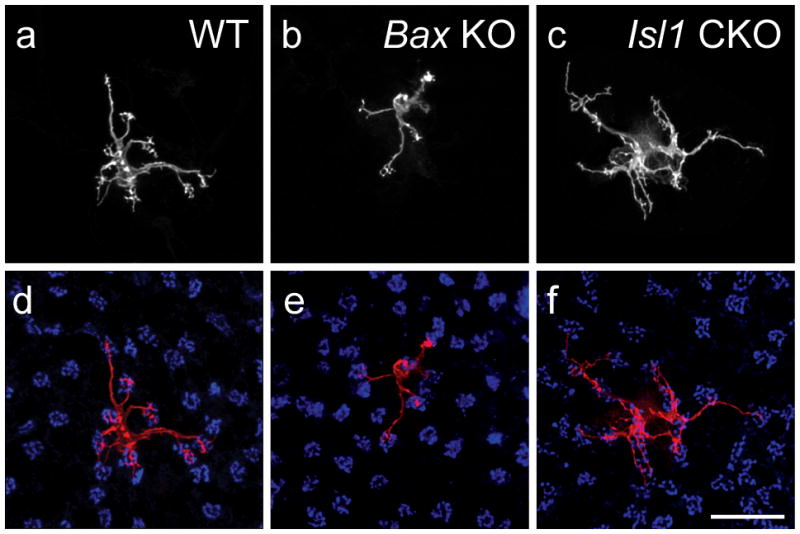
a–c: Single Type 7 cone bipolar cells in wildtype, Bax-KO, and Isl1-CKO retinas show a modulation of dendritic field area within the OPL. d–f: The number of pedicles contacted (blue) by these cells varies accordingly. Calibration bar = 20 μm.
Figure 7.
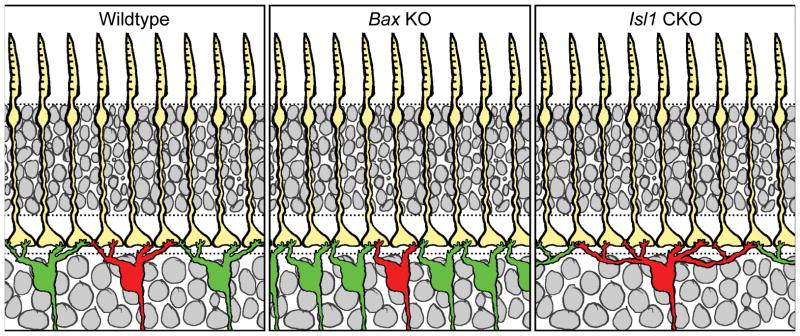
Type 7 cone bipolar cell dendritic fields decrease their dendritic field area in the presence of heightened densities of like-type neighbors, and increase them when like-type densities are reduced. Such modulation in dendritic field size yields a corresponding change in the convergence ratio of the cones upon these Type 7 cells.
The fact that Type 7 cone bipolar cells in the coneless retina differentiate comparable dendritic arbors in the absence of cone afferents (figure 4a) would suggest a degree of intrinsic specification of such dendritic patterning. Exactly how the pedicles interact with differentiating bipolar cells in this process is unclear. The occasionally abrupt turns of single dendrites to reach pedicles (Keeley and Reese, 2010b) might suggest a modulation of this cell-intrinsic program by the presence of pedicles detected locally as these dendrites grow out. An alternative interpretation, however, is that such turns do not reflect directional choices made during the process of differentiation, but rather are sculpted from an initially more complex set of outgrowing processes, as initial studies in our lab suggest. Detailed evaluation of differentiating bipolar cells in the absence of all cones should clarify the role played by the pedicles in this process.
Comparison with Horizontal Cells
The sensitivity to homotypic interactions evidenced by the Type 7 cone bipolar cell is similar to that described for retinal horizontal cells. As mentioned above, the horizontal cells have far more extensive dendritic arbors contacting many more pedicles that the bipolar cells (figure 8a) and which overlap the fields of five other homotypic neighbors. When homotypic density is reduced by greater than half, in Lim1-CKO mice, the remaining horizontal cells differentiate dendritic arbors nearly twice their normal area (Poché et al., 2008). As afferent density is unchanged in these mice, those horizontal cells may potentially receive innervation from a larger number of cone afferents, and consistent with this, they show a significant increase in the total number of terminal clusters per dendritic field, indicative of the sites of the pedicles innervating them (Poché et al., 2008). More recent studies have shown that Isl1-CKO retinas, while having reduced densities of bipolar cells, actually have increased densities of horizontal cells (Whitney et al., 2011b), and in accord with such heightened densities, those cells now have smaller than normal dendritic arbors contacting fewer total pedicles (figure 8b–d; S. Lee, unpubl. obs.). Such sensitivity to homotypic interactions within the outer retina is in marked contrast to what has been recently documented for neurons differentiating their dendritic fields in the IPL. There, excitotoxic reduction of the density of cholinergic amacrine cells by 40% produces no change in the remaining cholinergic dendritic fields (Farajian et al., 2004), despite the presence of synaptic connectivity between such like-type neighbors (Zhou and Lee, 2008). Genetic manipulations reducing the densities of melanopsin-positive and SM132-positive neurofilament-rich retinal ganglion cells by 80–95% also produce no compensatory changes in the dendritic fields of remaining like-type cells (Lin et al., 2004). And dopaminergic amacrine cells have also recently been shown to differentiate dendritic fields of a size and orientation that suggests an indifference to the presence of homotypic neighbors (Keeley and Reese, 2010a).
Figure 8.
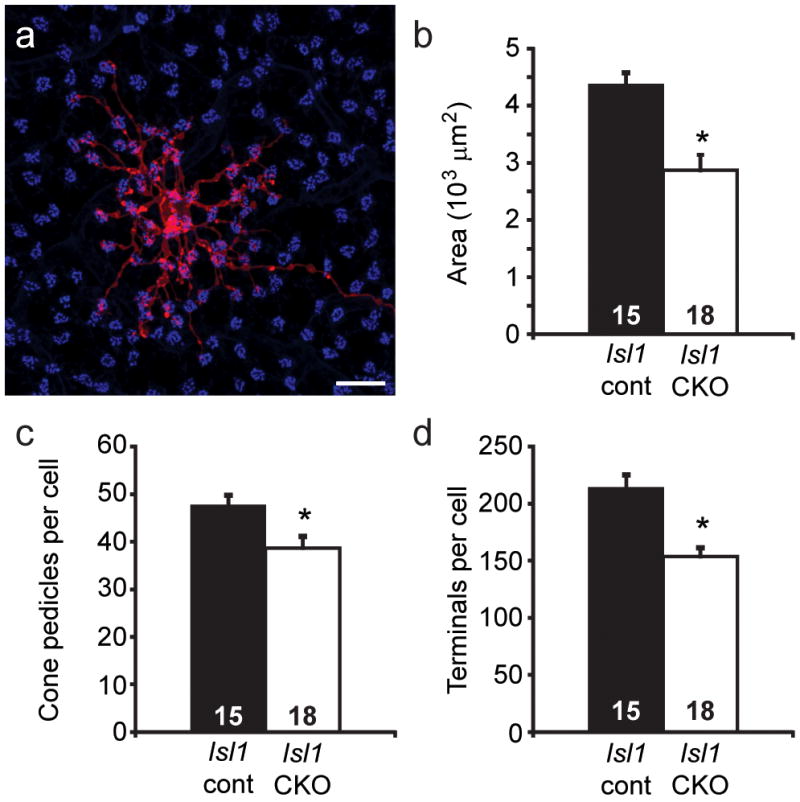
a: Horizontal cells have large dendritic fields (labeled with DiI, in red) that extend fine terminal endings clustering at the sites of individual pedicles in the OPL (labeled with PNA, in blue). Calibration bar = 20 μm. b–d: Dendritic field area is reduced in the Isl1-CKO retina (p = 0.0001, Student’s t-test), as is the number pedicles contacted (p = 0.009), along with the total number of terminations contacting pedicles per cell (p = 0.00008). The DiI labeling and morphometric procedures were identical to those described in Reese et al., 2005, while the PNA labeling procedures were identical to those described in Keeley and Reese, 2010a. Sampled cells came from comparable retinal eccentricities, ranging from 0.5 to 1.8 mm from the optic nerve head (p = 0.5). n equals the number of labeled cells measured.
This comparable developmental plasticity between the horizontal cells and the Type 7 cone bipolar cells in response to modulation of homotypic density is in marked contrast to their responses to modulation of afferent density in the above mouse models: whereas the morphogenesis of Type 7 cone bipolar cells was largely unaltered in the coneless and conefull retina, exhibiting only a loss of terminal clustering within an otherwise normal dendritic field, the horizontal cells undergo conspicuous changes in higher order dendritic branching and in the number of terminal endings in direct association with the variations in afferent density shown in these very same (coneless and conefull) mouse models (Reese et al., 2005; Raven et al., 2007). Indeed, the dendritic arbors of horizontal cells colonize the entire thickness of the OPL in the conefull retina, being hypertrophic in both density as well as depth, whereas the Type 7 cone bipolar cells refrain from extending into the outer extent of the OPL where the re-specified cones are positioned (Keeley and Reese, 2010b). As the Type 7 cone bipolar cell is normally innervated by both M cones as well as UV cones (Breuninger et al., 2011), their failure to extend into the outer parts of the OPL cannot be explained by the respecified photoreceptors being UV-sensitive (Daniele et al., 2005). Clearly, the Type 7 cone bipolar cell and the horizontal cell, each of which normally makes invaginating synaptic contacts with cone pedicles, must be differentially sensitive to some feature of the cone pedicle that is made apparent in the re-specified cones of the Nrl knockout mouse, but what this might be remains to be determined.
The Natural Variation in Afferent and Homotypic Cell Number
The modulation of dendritic field size by homotypic density, and the number of afferents accordingly connecting to them, brings to mind the possibility that midget bipolar cells subserving foveal vision in the primate retina differentiate their unique morphology under the constraints of local afferent and homotypic density rather than reflecting any developmental processes specific to the primate fovea. Indeed, similar intercellular determinants may shape the connectivity of midget-like bipolar cells in the ground squirrel retina (Puller et al., 2011). Midget bipolar cells in the primate retina increase their dendritic field size as their density declines with eccentricity, in turn contacting an increasing number of cone pedicles since the latter density does not decline as fast (Wassle et al., 1994; Telkes et al., 2008). This variation in natural density across eccentricity in the primate retina is not observed within the mouse retina, but different strains of mice exhibit a conspicuous natural variation in multiple types of retinal nerve cell number (Williams et al., 1998; Whitney et al., 2008;, 2009). While bipolar cell types have not yet been determined, the cones show a conspicuous variation in number: mice of the C57BL/6J strain have 70% more cones than do mice of the A/J strain (Whitney et al., 2011a). Horizontal cells show an even greater variation, exhibiting a nearly two-fold difference between these same strains (Raven et al., 2005a). That such natural variation in cone and horizontal cell density influences horizontal cell differentiation is suggested by the fact that dendritic field size is twice as large in the strain with half the density of horizontal cells, while the density of terminal clusters and higher order dendritic branches within the field matches the variation in afferent density (Reese et al., 2005), exactly as expected from genetic manipulations of afferent or homotypic densities (Raven et al., 2007; Poché et al., 2008; figure 8).
Such within-species variation in the numbers of interconnected nerve cells might be expected to co-vary as a consequence of allelic variants of genes that modulate proliferation and retinal size. Yet in the mouse retina, this variation is independent of retinal area (Raven et al., 2005a), and by examining different recombinant inbred strains derived from two parental C57BL/6J and A/J strains, the genetic variants that modulate cone cell number and horizontal cell number must be numerous and at least partially independent (figure 9). Each point in figure 9 indicates the estimated total number of cones (Whitney et al., 2011a) and horizontal cells (Whitney et al., 2011b) for each of 26 recombinant inbred strains of mice, as well as the two parental strains used to derive them. (Each recombinant inbred strain is a unique mix of the two parental haplotypes throughout the genome). That there is minimal correlation present across these strains (figure 9) indicates the two traits are largely controlled independently (Whitney et al., 2011a, b). The plasticity of post-receptoral neurons like the bipolar cells and horizontal cells, modulating their field size and connectivity in accord with the genetically-modified variation in the numbers of afferents and homotypic neighbors described above, would be well-suited to ensure uniformity of coverage and connectivity at this first synaptic layer in the retina in the presence of such natural variation in afferent and target cell numbers.
Figure 9.
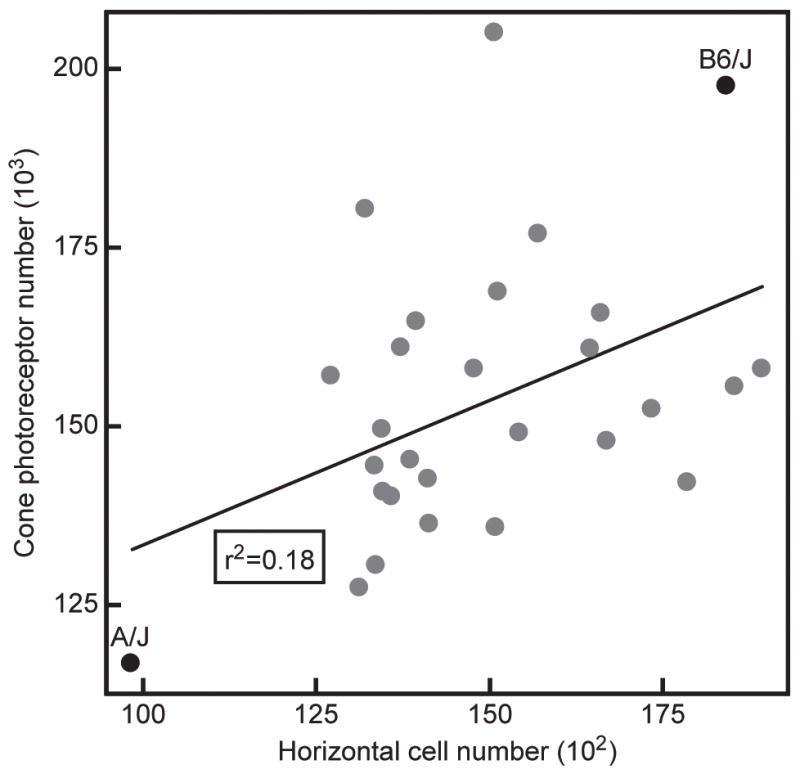
Variation in estimated total cone photoreceptor number and estimated total horizontal cell number in the C57BL/6J and A/J strains of mice, and in 26 recombinant inbred strains of mice derived from these same parental lines. While the A/J strain has the lowest number of both cones and horizontal cells, and C57BL/6J has 70% more cones and nearly 90% more horizontal cells, there is little correlation between them across the recombinant inbred strain-set. Both traits must be controlled by numerous genetic variants modulating developmental processes affecting cell number, and that do so for each population largely independently.
Conclusions
The present studies make clear that, while much of the morphogenesis of rod and Type 7 cone bipolar cells must be the product of cell-intrinsic signals that drive these features rather than arising through afferent specification of their distinctive forms, afferents and homotypic neighbors participate in defining the patterned distribution of dendritic terminations and in the size of the dendritic field itself. Indeed, the local density of homotypic neighbors is a major determinant of the connectivity associated with the cone bipolar cells, as its modulation has a direct effect upon the cone-to-bipolar cell convergence ratio, as observed for the horizontal cells as well. Through this plasticity, a uniformity in retinal coverage is ensured within the OPL. Ongoing studies are dissecting the role heterotypic interactions play in the colonization and maturation of the pedicle itself.
Acknowledgments
This research was supported by grants from the NIH (EY-11087; EY-19968; RR-22585). PWK is a NSF Graduate Research Fellow, while SCSL is a CJ Martin Fellow of the NH&MRC, Australia (567031).
References
- Agathocleous M, Harris WA. From progenitors to differentiated cells in the vertebrate retina. Ann Rev Cell Develop Biol. 2009;25:45–69. doi: 10.1146/annurev.cellbio.042308.113259. [DOI] [PubMed] [Google Scholar]
- Badea TC, Nathans J. Morphologies of mouse retinal ganglion cells expressing transcription factors Brn3a, Brn3b, and Brn3c: Analysis of wild type and mutant cells using genetically-directed sparse labeling. Vision Res. 2010 doi: 10.1016/j.visres.2010.08.039. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett WP, Banker GA. An electron microscopic study of the development of axons and dendrites by hippocampal neurons in culture. I Cells which develop without intercellular contacts. J Neurosci. 1984;4:1944–1978. doi: 10.1523/JNEUROSCI.04-08-01944.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott B, Wässle H. Parallel processing in the mammalian retina: the Proctor Lecture. Invest Ophthalmol Vis Sci. 1999;40:1313–1327. [PubMed] [Google Scholar]
- Brandstätter JH, Koulen P, Wässle H. Diversity of glutamate receptors in the mammalian retina. Vision Res. 1998;38:1385–1397. doi: 10.1016/s0042-6989(97)00176-4. [DOI] [PubMed] [Google Scholar]
- Breuninger T, Puller C, Haverkamp S, Euler T. Chromatic bipolar cell pathways in the mouse retina. J Neurosci. 2011;31:6504–6517. doi: 10.1523/JNEUROSCI.0616-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NL, Patel S, Brzezinski J, Glaser T. Math5 is required for retinal ganglion cell and optic nerve formation. Development. 2001;128:2497–2508. doi: 10.1242/dev.128.13.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca N, Deng P, Linberg KA, Lewis GP, Fisher SK, Kolb H. The neurons of the ground squirrel retina as revealed by immunostains for calcium binding proteins and neurotransmitters. J Neurocytol. 2002;31:649–666. doi: 10.1023/a:1025791512555. [DOI] [PubMed] [Google Scholar]
- Dacey DM. The mosaic of midget ganglion cells in the human retina. J Neurosci. 1993;13:5334–5355. doi: 10.1523/JNEUROSCI.13-12-05334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele LL, Lillo C, Lyubarsky AL, Nikonov SS, Philp N, Mears AJ, Swaroop A, Williams DS, Pugh EN., Jr Cone-like morphological, molecular, and electrophysiological features of the photoreceptors of the Nrl knockout mouse. Invest Ophthalmol Vis Sci. 2005;46:2156–2167. doi: 10.1167/iovs.04-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn ME, Schilling K, Mugnaini E. Development and fine structure of murine Purkinje cells in dissociated cerebellar cultures: dendritic differentiation, synaptic maturation, and formation of cell-class specific features. Anat Embryol. 1998;197:31–50. doi: 10.1007/s004290050118. [DOI] [PubMed] [Google Scholar]
- Elshatory Y, Everhart D, Deng M, Xie X, Barlow RB, Gan L. Islet-1 controls the differentiation of retinal bipolar and cholinergic amacrine cells. J Neurosci. 2007b;27:12707–12720. doi: 10.1523/JNEUROSCI.3951-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa JS, Wheeler DG, Tsien RW, Luo L. Uncoupling dendrite growth and patterning: single-cell knockout analysis of NMDA receptor 2B. Neuron. 2009;62:205–217. doi: 10.1016/j.neuron.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajian R, Raven MA, Cusato K, Reese BE. Cellular positioning and dendritic field size of cholinergic amacrine cells are impervious to early ablation of neighboring cells in the mouse retina. Vis Neurosci. 2004;21:13–22. doi: 10.1017/s0952523804041021. [DOI] [PubMed] [Google Scholar]
- Feng L, Xie X, Joshi PS, Yang Z, Shibasaki K, Chow RL, Gan L. Requirement for Bhlhb5 in the specification of amacrine and cone bipolar subtypes in mouse retina. Development. 2006;133:4815–4825. doi: 10.1242/dev.02664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wässle H. Types of bipolar cells in the mouse retina. J Comp Neurol. 2004;469:70–82. doi: 10.1002/cne.10985. [DOI] [PubMed] [Google Scholar]
- Greferath U, Grünert U, Wässle H. Rod bipolar cells in the mammalian retina show protein kinase C-like immunoreactivity. J Comp Neurol. 1990;301:433–442. doi: 10.1002/cne.903010308. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Specht D, Majumdar S, Zaidi NF, Brandstätter JH, Wasco W, Wässle H, Tom Dieck S. Type 4 OFF cone bipolar cells of the mouse retina express calsenilin and contact cones as well as rods. J Comp Neurol. 2008;507:1087–1101. doi: 10.1002/cne.21612. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Wässle H, Duebel J, Kuner T, Augustine GJ, Feng G, Euler T. The primordial, blue-cone color system of the mouse retina. J Neurosci. 2005;25:5438–5445. doi: 10.1523/JNEUROSCI.1117-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenraad CC, Milstein AD, Ethell IM, Henkemeyer M, Sheng M. GRIP1 controls dendrite morphogenesis by regulating EphB receptor trafficking. Nat Neurosci. 2005;8:906–908. doi: 10.1038/nn1487. [DOI] [PubMed] [Google Scholar]
- Horch HW, Katz LC. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nat Neurosci. 2002;5:1177–1178. doi: 10.1038/nn927. [DOI] [PubMed] [Google Scholar]
- Huang L, Max M, Margolskee RF, Su H, Masland RH, Euler T. The G protein subunit Gg13 is co-expressed with Gao and Gb3 in retinal On bipolar cells. J Comp Neurol. 2003;455:1–10. doi: 10.1002/cne.10396. [DOI] [PubMed] [Google Scholar]
- Inoue T, Hojo M, Bessho Y, Tano Y, Lee JE, Kageyama R. Math3 and NeuroD regulate amacrine cell fate specification in the retina. Development. 2002;129:831–842. doi: 10.1242/dev.129.4.831. [DOI] [PubMed] [Google Scholar]
- Jan YN, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci. 2010;11:316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon C-J, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–8946. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley PW, Reese BE. Morphology of dopaminergic amacrine cells in the mouse retina: Independence from homotypic interactions. J Comp Neurol. 2010a;518:1220–1231. doi: 10.1002/cne.22270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley PW, Reese BE. Role of afferents in the differentiation of bipolar cells in the mouse retina. J Neurosci. 2010b;30:1677–1685. doi: 10.1523/JNEUROSCI.5153-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley PW, Whitney IE, Raven MA, Reese BE. Dendritic spread and functional coverage of starburst amacrine cells. J Comp Neurol. 2007;505:539–546. doi: 10.1002/cne.21518. [DOI] [PubMed] [Google Scholar]
- Kryger Z, Galli-Resta L, Jacobs GH, Reese BE. The topography of rod and cone photoreceptors in the retina of the ground squirrel. Vis Neurosci. 1998;15:685–691. doi: 10.1017/s0952523898154081. [DOI] [PubMed] [Google Scholar]
- Lin B, Wang SW, Masland RH. Retinal ganglion cell type, size, and spacing can be specified independent of homotypic dendritic contacts. Neuron. 2004;43:475–485. doi: 10.1016/j.neuron.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Linberg KA, Suemune S, Fisher SK. Retinal neurons of the California ground squirrel, Spermophilus beecheyi: A Golgi study. J Comp Neurol. 1996;365:173–216. doi: 10.1002/(SICI)1096-9861(19960205)365:2<173::AID-CNE1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Watt CB, Strettoi E. Neural remodeling in retinal degeneration. Prog Ret Eye Res. 2003;22:607–655. doi: 10.1016/s1350-9462(03)00039-9. [DOI] [PubMed] [Google Scholar]
- Masland RH. Neuronal diversity in the retina. Curr Opin Neurobiol. 2001;11:431–436. doi: 10.1016/s0959-4388(00)00230-0. [DOI] [PubMed] [Google Scholar]
- Mataruga A, Kremmer E, Müller F. Type 3a and type 3b OFF cone bipolar cells provide for the alternative rod pathway in the mouse retina. J Comp Neurol. 2007;502:1123–1137. doi: 10.1002/cne.21367. [DOI] [PubMed] [Google Scholar]
- Mears AJ, Kondo M, Swain PK, Takada Y, Bush RA, Saunders TL, Sieving PA, Swaroop A. Nrl is required for rod photoreceptor development. Nat Gen. 2001;29:447–452. doi: 10.1038/ng774. [DOI] [PubMed] [Google Scholar]
- Mo Z, Li S, Yang X, Xiang M. Role of the Barhl2 homeobox gene in the specification of glycinergic amacrine cells. Development. 2004;131:1607–1618. doi: 10.1242/dev.01071. [DOI] [PubMed] [Google Scholar]
- Morgan JL, Dhingra A, Vardi N, Wong RO. Axons and dendrites originate from neuroepithelial-like processes of retinal bipolar cells. Nat Neurosci. 2006;9:85–92. doi: 10.1038/nn1615. [DOI] [PubMed] [Google Scholar]
- Mosinger Ogilvie J, Spreck JD, Lett JM, Fleming TT. A reliable method for organ culture of neonatal mouse retina with long-term survival. J Neurosci Meth. 1999;87:57–65. doi: 10.1016/s0165-0270(98)00157-5. [DOI] [PubMed] [Google Scholar]
- Müller B, Peichl L. Topography of cones and rods in the tree shrew retina. J Comp Neurol. 1989;282:581–594. doi: 10.1002/cne.902820409. [DOI] [PubMed] [Google Scholar]
- Ohsawa R, Kageyama R. Regulation of retinal cell fate specification by multiple transcription factors. Brain Res. 2008;1192:90–98. doi: 10.1016/j.brainres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Pan L, Deng M, Xie X, Gan L. ISL1 and BRN3B co-regulate the differentiation of murine retinal ganglion cells. Development. 2008;135:1981–1990. doi: 10.1242/dev.010751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish JZ, Emoto K, Kim MD, Jan YN. Mechanisms that regulate establishment, maintenance, and remodeling of dendritic fields. Ann Rev Neurosci. 2007;30:399–423. doi: 10.1146/annurev.neuro.29.051605.112907. [DOI] [PubMed] [Google Scholar]
- Péquignot MO, Provost AC, Sallé S, Taupin P, Sainton KM, Marchant D, Martinou JC, Ameisen JC, Jais J-P, Abitbol M. Major role of BAX in apoptosis during retinal development and in establishment of a functional postnatal retina. Develop Dyn. 2003;228:231–238. doi: 10.1002/dvdy.10376. [DOI] [PubMed] [Google Scholar]
- Pignatelli V, Strettoi E. Bipolar cells of the mouse retina: a gene gun, morphological study. J Comp Neurol. 2004;476:254–266. doi: 10.1002/cne.20207. [DOI] [PubMed] [Google Scholar]
- Poché RA, Raven MA, Kwan KM, Furuta Y, Behringer RR, Reese BE. Somal positioning and dendritic growth of horizontal cells are regulated by interactions with homotypic neighbors. Europ J Neurosci. 2008;27:1607–1614. doi: 10.1111/j.1460-9568.2008.06132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SK, Rivas RJ, Rodriguez-Boulan E, Hatten ME. Development of polarity in cerebellar granule neurons. J Neurobiol. 1997;32:223–236. doi: 10.1002/(sici)1097-4695(199702)32:2<223::aid-neu7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Puller C, Ondreka K, Haverkamp S. Bipolar cells of the ground squirrel retina. J Comp Neurol. 2011;519:759–774. doi: 10.1002/cne.22546. [DOI] [PubMed] [Google Scholar]
- Randlett O, Norden C, Harris WA. The vertebrate retina: A model for neuronal polarization in vivo. Dev Neurobiol. 2010 doi: 10.1002/dneu.20841. [DOI] [PubMed] [Google Scholar]
- Raven MA, Oh ECT, Swaroop A, Reese BE. Afferent control of horizontal cell morphology revealed by genetic re-specification of rods and cones. J Neurosci. 2007;27:3540–3547. doi: 10.1523/JNEUROSCI.0372-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven MA, Reese BE. Mosaic regularity of horizontal cells in the mouse retina is independent of cone photoreceptor innervation. Invest Ophthalmol Vis Sci. 2003;44:965–973. doi: 10.1167/iovs.02-0831. [DOI] [PubMed] [Google Scholar]
- Raven MA, Stagg SB, Reese BE. Regularity and packing of the horizontal cell mosaic in different strains of mice. Vis Neurosci. 2005a;22:461–468. doi: 10.1017/S0952523805224070. [DOI] [PubMed] [Google Scholar]
- Reese BE. Mosaic architecture of the mouse retina. In: Chalupa LM, Williams RW, Chalupa LM, Williams RWs, editors. Eye, Retina, and Visual Systems of the Mouse. Cambridge: MIT Press; 2008b. pp. 147–155. [Google Scholar]
- Reese BE, Raven MA, Stagg SB. Afferents and homotypic neighbors regulate horizontal cell morphology, connectivity and retinal coverage. J Neurosci. 2005;25:2167–2175. doi: 10.1523/JNEUROSCI.4876-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy E, Wang Y, Nirenberg S, Nathans J, Meister M. A novel signaling pathway from rod photoreceptors to ganglion cells in mammalian retina. Neuron. 1998;21:481–493. doi: 10.1016/s0896-6273(00)80560-7. [DOI] [PubMed] [Google Scholar]
- Strettoi E, Mears AJ, Swaroop A. Recruitment of the rod pathway by cones in the absence of rods. J Neurosci. 2004;24:7576–7582. doi: 10.1523/JNEUROSCI.2245-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Novelli E, Mazzoni F, Barone I, Damiani D. Complexity of retinal cone bipolar cells. Prog Ret Eye Res. 2010;29:272–283. doi: 10.1016/j.preteyeres.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telkes I, Lee SC, Jusuf PR, Grünert U. The midget-parvocellular pathway of marmoset retina: a quantitative light microscopic study. J Comp Neurol. 2008;510:539–549. doi: 10.1002/cne.21813. [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ishii M, Takao M, Iwatsuki K, Nakanishi S, Fukuda Y. A novel connection between rods and ON cone bipolar cells revealed by ectopic metabotropic glutamate receptor 7 (mGluR7) in mGluR6-deficient mouse retinas. J Neurosci. 2007;27:6261–6267. doi: 10.1523/JNEUROSCI.5646-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SW, Kim BS, Ding K, Wang H, Sun D, Johnson RL, Klein WH, Gan L. Requirement for math5 in the development of retinal ganglion cells. Genes Dev. 2001;15:24–29. doi: 10.1101/gad.855301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Macke JP, Merbs SL, Zack DJ, Klaunberg B, Bennett J, Gearhart J, Nathans J. A locus control region adjacent to the human red and green visual pigment genes. Neuron. 1992;9:429–440. doi: 10.1016/0896-6273(92)90181-c. [DOI] [PubMed] [Google Scholar]
- Wässle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Wassle H, Grunert U, Martin PR, Boycott BB. Immunocytochemical characterization and spatial distribution of midget bipolar cells in the macaque monkey retina. Vision Res. 1994;34:561–579. doi: 10.1016/0042-6989(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Wässle H, Puller C, Müller F, Haverkamp S. Cone contacts, mosaics and territories of bipolar cells in the mouse retina. J Neurosci. 2009;29:106–117. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, Yamashita M, Greferath U, Grünert U, Müller F. The rod bipolar cell of the mammalian retina. Vis Neurosci. 1991;7:99–112. doi: 10.1017/s095252380001097x. [DOI] [PubMed] [Google Scholar]
- Whitney IE, Raven MA, Ciobanu DC, Williams RW, Reese BE. Multiple genes on chromosome 7 regulate dopaminergic amacrine cell number in the mouse retina. Invest Ophthalmol Vis Sci. 2009;50:1996–2003. doi: 10.1167/iovs.08-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney IE, Raven MA, Keeley PW, Reese BE. Spatial patterning of cholinergic amacrine cells in the mouse retina. J Comp Neurol. 2008;508:1–12. doi: 10.1002/cne.21630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney IE, Raven MA, Lu L, Williams RW, Reese BE. A QTL on chromosome 10 modulates cone photoreceptor number in the mouse retina. Invest Ophthalmol Vis Sci. 2011a doi: 10.1167/iovs.10-6693. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney IE, Raven MA, Ciobanu DC, Poché RA, Ding Q, Elshatory Y, Gan L, Williams RW, Reese BE. Genetic modulation of horizontal cell number in the mouse retina. Proc Natl Acad Sci USA. 2011b doi: 10.1073/pnas.1103253108. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RW, Strom RC, Goldowitz D. Natural variation in neuron number in mice is linked to a major quantitative trait locus on Chr 11. J Neurosci. 1998;18:138–146. doi: 10.1523/JNEUROSCI.18-01-00138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- Zhou ZJ, Lee S. Synaptic physiology of direction selectivity in the retina. J Physiol. 2008;586:4371–4376. doi: 10.1113/jphysiol.2008.159020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmuda JF, Rivas RJ. The Golgi apparatus and the centrosome are localized to the sites of newly emerging axons in cerebellar granule neurons in vitro. Cell Motil Cytoskeleton. 1998;41:18–38. doi: 10.1002/(SICI)1097-0169(1998)41:1<18::AID-CM2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]