Abstract
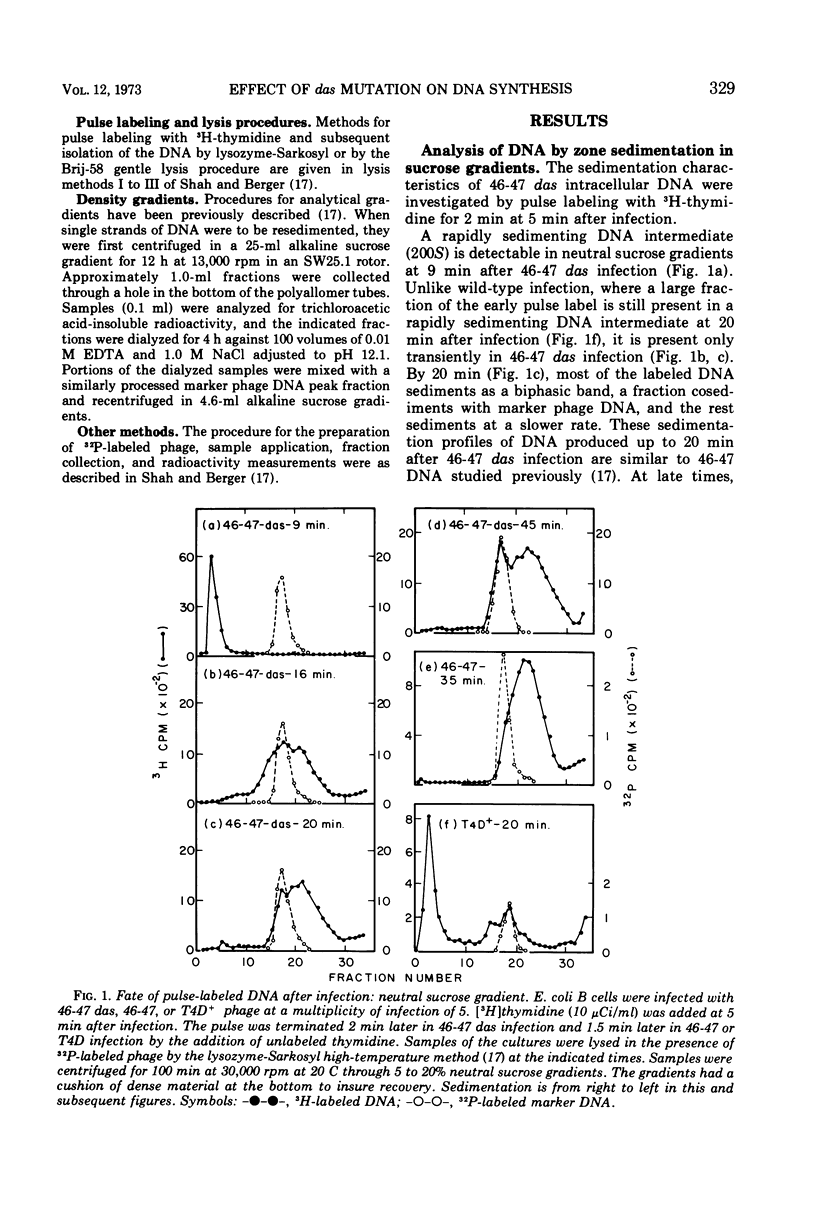
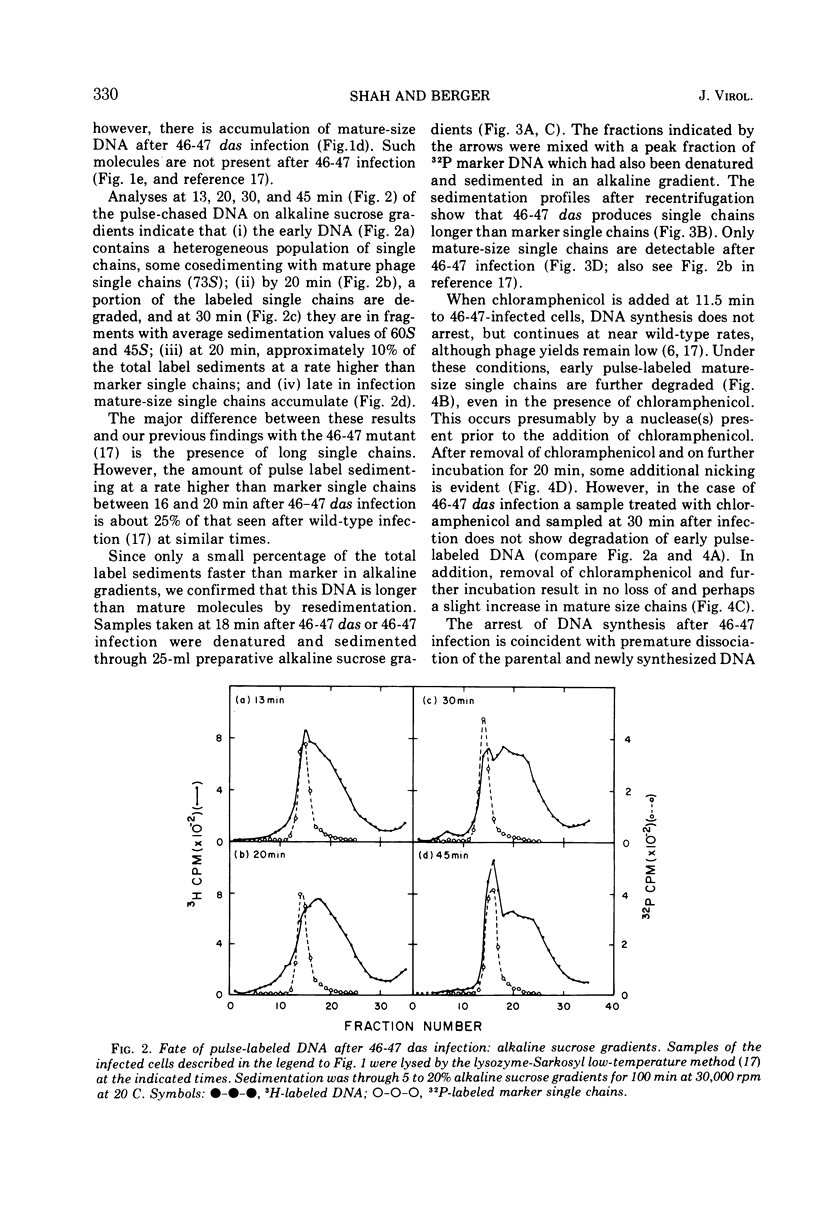
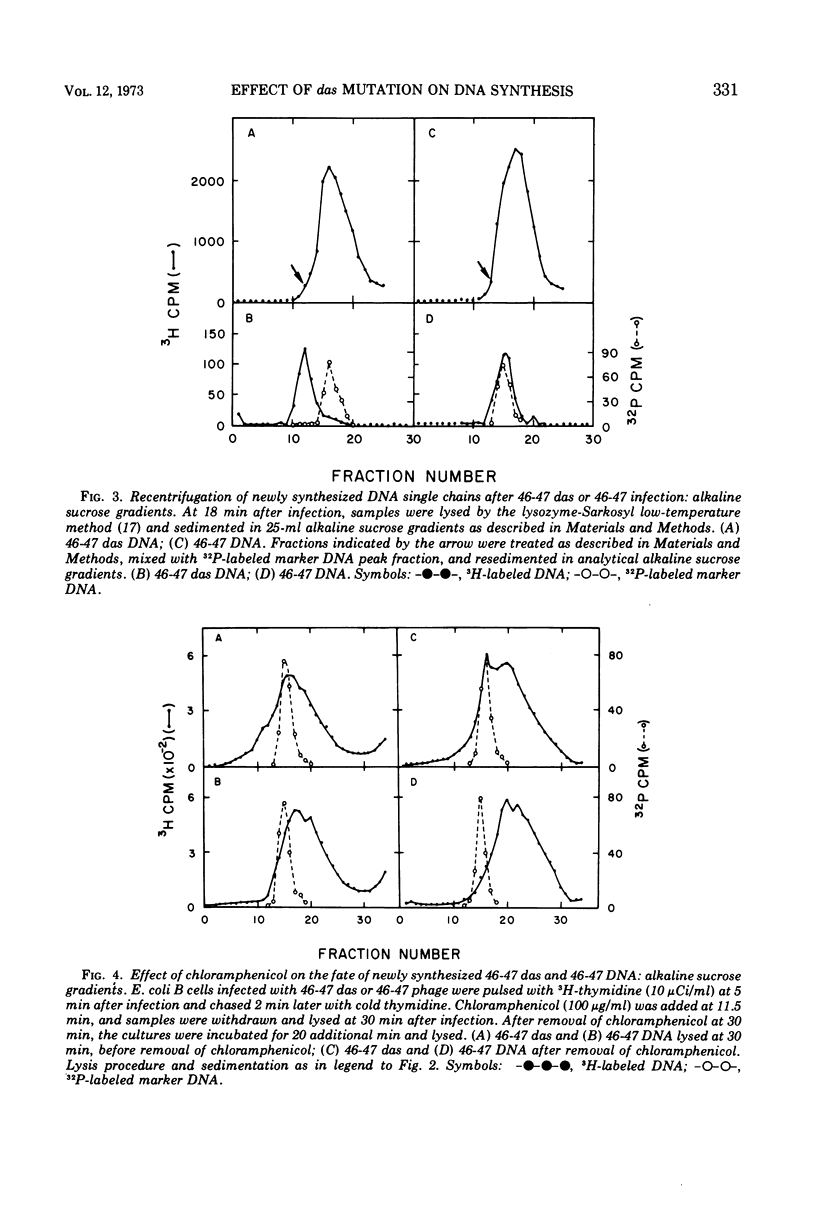
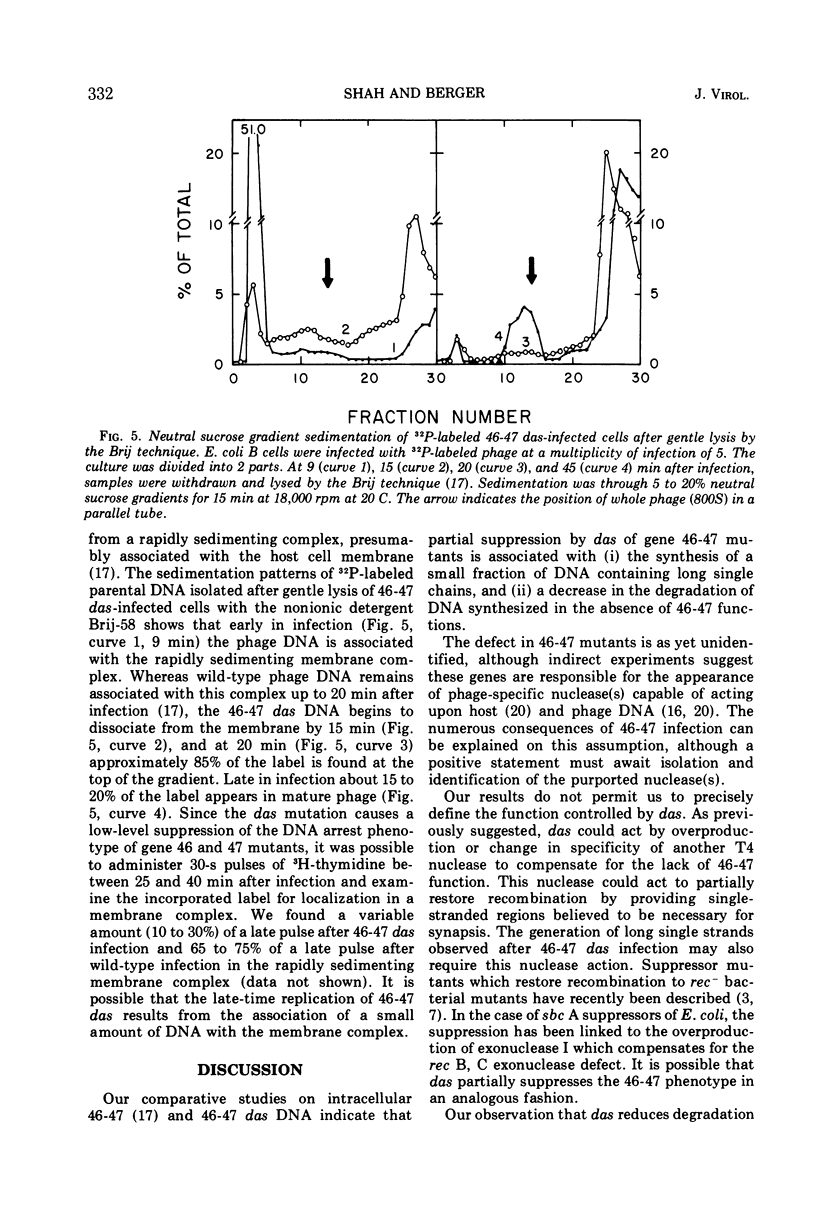
Mutants in genes 46 and 47 of bacteriophage T4 exhibit early cessation of DNA synthesis, inability to form a normal rapidly sedimenting DNA intermediate (200S), reduced genetic recombination, and reduced viable phage production. A gene-specific suppressor mutation called das partially restores many of the pleiotropic effects of gene 46-47 mutants (13). Our results indicate that this partial suppression by das is associated with (i) the synthesis of a small fraction of DNA containing long single chains not detectable in 46-47 infection and (ii) a decrease in an “early” function which participates in the degradation of DNA synthesized in the absence of 46-47 functions. However, das does not restore the formation of a normal rapidly sedimenting (200S) DNA intermediate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S., Lerman L. S. Kinetics and intermediates in the intracellular synthesis of bacteriophage T4 deoxyribonucleic acid. J Mol Biol. 1970 Jun 14;50(2):235–261. doi: 10.1016/0022-2836(70)90190-7. [DOI] [PubMed] [Google Scholar]
- Barbour S. D., Nagaishi H., Templin A., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. II. Rec+ revertants caused by indirect suppression of rec- mutations. Proc Natl Acad Sci U S A. 1970 Sep;67(1):128–135. doi: 10.1073/pnas.67.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H., Warren A. J., Fry K. E. Variations in genetic recombination due to amber mutations in T4D bacteriophage. J Virol. 1969 Feb;3(2):171–175. doi: 10.1128/jvi.3.2.171-175.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein H. Repair and recombination in phage T4. I. Genes affecting recombination. Cold Spring Harb Symp Quant Biol. 1968;33:325–331. doi: 10.1101/sqb.1968.033.01.037. [DOI] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: requirements for late messenger synthesis. J Mol Biol. 1968 Apr 28;33(2):339–362. doi: 10.1016/0022-2836(68)90193-9. [DOI] [PubMed] [Google Scholar]
- Clark A. J. Toward a metabolic interpretation of genetic recombination of E. coli and its phages. Annu Rev Microbiol. 1971;25:437–464. doi: 10.1146/annurev.mi.25.100171.002253. [DOI] [PubMed] [Google Scholar]
- Earhart C. F., Tremblay G. Y., Daniels M. J., Schaechter M. DNA replication studied by a new method for the isolation of cell membrane-DNA complexes. Cold Spring Harb Symp Quant Biol. 1968;33:707–710. doi: 10.1101/sqb.1968.033.01.079. [DOI] [PubMed] [Google Scholar]
- Frankel F. R. Evidence for long DNA strands in the replicating pool after T4 infection. Proc Natl Acad Sci U S A. 1968 Jan;59(1):131–138. doi: 10.1073/pnas.59.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel F. R. Studies on the nature of replicating DNA in T4-infected Escherichia coli. J Mol Biol. 1966 Jun;18(1):127–143. doi: 10.1016/s0022-2836(66)80081-5. [DOI] [PubMed] [Google Scholar]
- Frankel F. R. Studies on the nature of the replicating DNA in Escherichia coli infected with certain amber mutants of phage T4. J Mol Biol. 1966 Jun;18(1):144–155. doi: 10.1016/s0022-2836(66)80082-7. [DOI] [PubMed] [Google Scholar]
- Frankel F. R. The absence of mature phage DNA molecules from the replicating pool of T-even-infected Escherichia coli. J Mol Biol. 1966 Jun;18(1):109–126. doi: 10.1016/s0022-2836(66)80080-3. [DOI] [PubMed] [Google Scholar]
- Hercules K., Wiberg J. S. Specific suppression of mutations in genes 46 and 47 by das, a new class of mutations in bacteriophage T4D. J Virol. 1971 Nov;8(5):603–612. doi: 10.1128/jvi.8.5.603-612.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda J., Mathews E., Jansen B. Role of genes 46 and 47 in bacteriophage T4 reproduction. I. In vivo deoxyribonucleic acid replication. J Virol. 1971 Oct;8(4):372–387. doi: 10.1128/jvi.8.4.372-387.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W., Lin T. H. Early intracellular events in the replication of T4 phage DNA. I. Complex formation of replicative DNA. Proc Natl Acad Sci U S A. 1965 Jul;54(1):273–278. doi: 10.1073/pnas.54.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutter E. M., Wiberg J. S. Degradation of cytosin-containing bacterial and bacteriophage DNA after infection of Escherichia coli B with bacteriophage T4D wild type and with mutants defective in genes 46, 47 and 56. J Mol Biol. 1968 Dec;38(3):395–411. doi: 10.1016/0022-2836(68)90394-x. [DOI] [PubMed] [Google Scholar]
- Shah D. B., Berger H. Replication of gene 46-47 amber mutants of bacteriophage T4D. J Mol Biol. 1971 Apr 14;57(1):17–34. doi: 10.1016/0022-2836(71)90117-3. [DOI] [PubMed] [Google Scholar]
- Shalitin C., Naot Y. Role of gene 46 in bacteriophage T4 deoxyribonucleic acid synthesis. J Virol. 1971 Aug;8(2):142–153. doi: 10.1128/jvi.8.2.142-153.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg J. S. Mutants of bacteriophage T4 unable to cause breakdown of host DNA. Proc Natl Acad Sci U S A. 1966 Mar;55(3):614–621. doi: 10.1073/pnas.55.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]


