Abstract
Introduction
Transplant recipients are at risk of post-transplant lymphoproliferative disease (PTLD). Methods: Thirty-six pediatric transplant recipients were evaluated (18 hematopoietic stem cell and 18 liver recipients; 12 had PTLD). We studied 207 longitudinal plasma samples from these recipients for three markers of B-cell activation or clonality: immunoglobulin free light chains (FLCs), soluble CD30 (sCD30), and monoclonal immunoglobulins (M-proteins).
Results
Kappa FLCs, lambda FLCs, and sCD30 were elevated in 20.8%, 28.0%, and 94.2% of plasma specimens, respectively. FLC and sCD30 levels increased significantly 1.18–1.82 fold per log10 Epstein Barr virus (EBV) load in peripheral blood. Five PTLD cases manifested elevated FLCs with an abnormal kappa/lambda ratio, suggesting monoclonal FLC production. M-proteins were present in 91% of PTLD cases, vs. 50–67% of other recipients with high or low EBV loads (p=0.13). Concordance of FLCs, M-proteins, and PTLD tumor light chain restriction was imperfect. For example, one PTLD case with an IgG lambda M-protein had a tumor that was kappa restricted, and another case with an M-protein had a T-cell PTLD. In an additional case, an IgM kappa M-protein and excess kappa FLCs were both detected in plasma at PTLD diagnosis; while the tumor was not restricted at diagnosis, kappa restriction was present 5 years later when the PTLD relapsed.
Discussion
Plasma markers of B-cell dysfunction are frequent following transplantation and associated with poor EBV control. These abnormal markers may be produced by oligoclonal B-cell populations or PTLD tumor cells, and could potentially help identify recipients at high risk of PTLD.
Keywords: post transplant lymphoproliferative disease, Epstein-Barr virus (EBV), B cell, immune monitoring, immunoglobulins, cytokines
Introduction
Transplantation provides life-saving therapy for patients with severe disease. Transplant outcomes have improved markedly over time, but substantial morbidity still results from immunosuppressive therapy administered to prevent graft rejection (for solid organ transplants) or graft vs. host disease (for HSCT).
Post-transplant lymphoproliferative disorder (PTLD), an important complication of transplantation, comprises a heterogeneous spectrum of conditions ranging from benign lymphoid hyperplasia to malignant neoplasms of lymphocytes (mostly non-Hodgkin lymphoma [NHL], but also including rare cases of multiple myeloma) (1;2). Epstein Barr virus (EBV) is present in most PTLD tumors and plays a crucial role in lymphocyte transformation (1–3).
Transplant-related immunosuppression mainly involves depressed T-cell function, but disordered B-cell function is also present, probably as a consequence of T-cell dysregulation. Hypogammaglobulinemia is frequent and predisposes to infection in both solid organ and HSCT recipients (4;5). Circulating monoclonal immunoglobulin proteins (termed “M-proteins”) are also frequent among both solid organ and HSCT recipients (6–12). For immunocompetent individuals, detection of an M-protein as an isolated finding (i.e., monoclonal gammopathy of undetermined significance [MGUS]) indicates the presence of an abnormal clone of plasma cells and is associated with development of multiple myeloma (13). Although most PTLD tumors are derived from B-cells and might therefore be capable of producing immunoglobulins (1), the clinical relevance of detection of M-proteins in transplant recipients as a risk marker for PTLD is uncertain (6–11;14;15).
Of interest, we recently showed that, in the setting of solid organ transplantation, elevated levels of kappa and lambda immunoglobulin free light chains (FLCs) in peripheral blood were associated with an increased risk of subsequently developing PTLD (16). FLCs are produced and released by B-cells along with intact immunoglobulins (17). CD30 is a cell surface receptor expressed on activated lymphocytes, and elevated circulating levels of this protein (i.e., soluble CD30 [sCD30]) have also been associated with PTLD in solid organ recipients (18;19).
In the present study, we evaluated markers of monoclonal and polyclonal B-cell activation in longitudinal peripheral blood samples from pediatric liver and HSCT recipients. In particular, we focused on the relationships of these markers with EBV replication and the development of PTLD.
Results
The study included 12 low-EBV recipients, 12 high-EBV recipients, and 12 PTLD cases with a total of 207 plasma samples (Table 1). Eighteen subjects were HSCT recipients (donors were haploidentical relatives in 3 transplants and unrelated in 14 transplants, while one transplant was syngeneic) and the remaining 18 were deceased-donor liver recipients. The median age at transplantation was 3 years (range 1–19 years).
Table 1.
Characteristics of transplant recipients evaluated for plasma markers of B-cell activation and clonality
| All recipients (N=36) | Low-EBV recipients (N=12) | High-EBV recipients (N=12) | PTLD cases (N=12) | |
|---|---|---|---|---|
| HSCT, n (%) | 18 (50) | 8 (67) | 4 (33) | 6 (50) |
| Male, n (%) | 21 (58) | 11 (92) | 4 (33) | 6 (50) |
| Age at transplant, median (range) | 3 (1–19) | 6 (1–19) | 3 (1–9) | 2 (1–10) |
| Total samples | 207 | 60 | 63 | 84 |
| Number of samples per subject, median (range) | 5 (1–10) | 4 (3–8) | 5 (1–8) | 7 (1–10) |
| Number of samples relative to time since transplant, (%) | ||||
| 0–0.5 yr | 81 (39) | 33 (55) | 19 (30) | 29 (35) |
| 0.6–1 yr | 43 (21) | 13 (22) | 18 (29) | 12 (14) |
| 1.1+ yrs | 83 (40) | 14 (23) | 26 (41) | 43 (51) |
| Number of samples relative to PTLD diagnosis, (%) | ||||
| Before diagnosis | -- | -- | -- | 18 (21) |
| At diagnosis | -- | -- | -- | 11 (13) |
| After diagnosis | -- | -- | -- | 55 (55) |
| EBV detectable in sample, n (%)* | 136 (66) | 10 (17) | 56 (90) | 70 (84) |
| EBV viral load among detectable samples, median (range)* | 3760 (20–490,000) | 97 (36–569) | 3970 (44–246,000) | 4190 (20–490,000) |
Abbreviations: EBV Epstein Barr virus, PTLD post-transplant lymphoproliferative disorder, HSCT hematopoietic stem cell transplant
EBV viral load data were missing for 2 samples.
Among HSCT recipients, 2 were EBV seronegative at the time of transplantation, 15 were seropositive, and 1 had unknown serostatus; all donors were EBV seropositive. Among liver recipients, 10 were EBV seronegative, 6 were seropositive, and 2 had unknown serostatus; all donors had unknown serostatus. As expected, following transplantation EBV was less frequently detected in plasma samples from low-EBV recipients, and when detected, EBV loads were lower than in high-EBV recipients or PTLD cases (Table 1).
Plasma free light chains and sCD30
The median plasma level (interquartile range) was 1.09 mg/dL (0.63–1.74) for kappa FLC, 1.61 mg/dL (0.94–2.82) for lambda FLC, and 124 ng/ml (80.1–184) for sCD30. Levels were above normal for 20.8%, 28.0%, and 94.2% of kappa, lambda, and sCD30 measurements, respectively. Kappa and lambda FLC levels were correlated with each other (R=0.77), and both were correlated with sCD30 (R=0.40–0.47).
In univariate analyses (not shown), levels of all three biomarkers levels were higher in liver than HSCT recipients, and increased with EBV load and time since transplantation. In Table 2, we show results from multivariable models that adjust for the category of recipient and treatment directed at PTLD or high EBV load. The most consistent finding was a significant increase in each biomarker with higher EBV load (i.e., 1.18–1.82 fold increases for each log10 increase in EBV load). For each biomarker, levels appeared to increase after the first 6 months following transplantation, although a significant trend was present only for sCD30. sCD30 levels decreased with the age of the recipient, and kappa levels were almost 4-fold higher in liver than HSCT recipients (Table 2).
Table 2.
Associations with plasma levels of free light chains and sCD30 among transplant recipients
| Kappa fold change (95%CI)* | Lambda fold change (95%CI)* | sCD30 fold change (95%CI)* | |
|---|---|---|---|
| Category of recipient | |||
| Low-EBV | Reference | Reference | Reference |
| High-EBV | 0.30 (0.10–0.92) | 0.22 (0.06–0.75) | 0.97 (0.69–1.35) |
| PTLD | 0.44 (0.16–1.25) | 0.37 (0.11–1.18) | 1.11 (0.79–1.55) |
| EBV viral load, per 1og10 | 1.52 (1.15–2.00) | 1.82 (1.29–2.58) | 1.18 (1.10–1.26) |
| Liver, vs. HSCT | 3.81 (1.44–10.1) | 1.40 (0.45–4.35) | 1.00 (0.77–1.31) |
| Age at transplant, years | |||
| 1 | 0.42 (0.16–1.12) | 1.13 (0.31–4.11) | 1.52 (1.08–2.16) † |
| 2–5 | 0.59 (0.21–1.64) | 1.43 (0.58–3.49) | 1.44 (0.98–2.11) † |
| 6+ | Reference | Reference | Reference |
| Time since transplant, years | |||
| 0.0–0.5 | Reference | Reference | Reference |
| 0.6–1.0 | 2.62 (1.34–5.13) † | 1.96 (1.03–3.74) | 1.14 (0.92–1.40) † |
| 1.1+ | 2.13 (1.01–4.50) † | 1.49 (0.65–3.41) | 1.34 (1.03–1.75) † |
| Prior treatment of PTLD or high EBV load | 0.87 (0.50–1.50) | 0.97 (0.60–1.58) | 0.92 (0.72–1.19) |
Abbreviations: EBV Epstein Barr virus, PTLD post-transplant lymphoproliferative disorder, HSCT hematopoietic stem cell transplant
For each biomarker, results are from a multivariable model that included each of the indicated variables. Fold changes indicate the factor by which the marker is multiplied across the indicated category.
Test of trend across categories p<0.05.
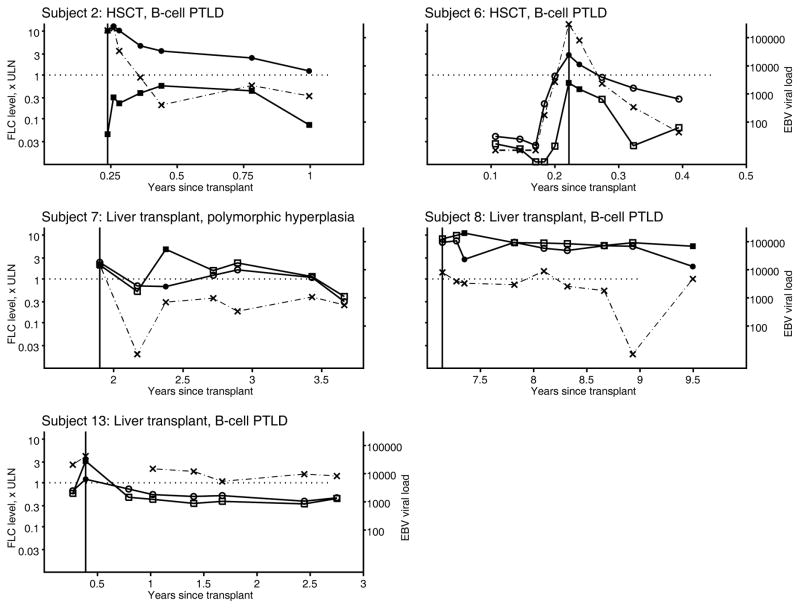
Overall, 53 samples (26%) showed a polyclonal FLC elevation, 13 (6%) showed a monoclonal elevation, 10 (5%) showed an abnormal FLC ratio without elevation, and 131 (63%) manifested a normal FLC pattern (Table 3). Polyclonal FLC elevations were more common with increasing time following transplantation (p=0.02) and in samples that demonstrated higher sCD30 levels (p=0.02). Among PTLD cases, only 6 (50%) had one or more plasma samples available from before PTLD diagnosis, but 11 (92%) had a specimen at PTLD diagnosis. All samples with a monoclonal elevation were in PTLD cases (13 samples in 5 subjects, the earliest of which was at PTLD diagnosis). As shown in Figure 1, three of these PTLD cases (subjects 2, 6, 13) had monoclonal FLC elevations at PTLD diagnosis, while the other two cases (subjects 7 and 8) had monoclonal FLC elevations later. FLC levels tended to parallel EBV load results (Figure 1).
Table 3.
Characteristics associated with free light chain patterns in plasma specimens from transplant recipients
| Polyclonal FLC elevation, n (%) | Monoclonal FLC elevation, n (%) | No FLC elevation with abnormal ratio, n (%) | Normal FLCs, n (%) | P-value | |
|---|---|---|---|---|---|
| Category of subject | 0.85 | ||||
| Low-EBV | 16 (27) | 0 (0) | 3 (5) | 41 (68) | |
| High-EBV | 16 (25) | 0 (0) | 2 (3) | 45 (71) | |
| PTLD | 21 (25) | 13 (15) | 5 (6) | 45 (54) | |
| EBV load, copies/ug | 0.33 | ||||
| <1000 | 19 (18) | 3 (3) | 7 (7) | 76 (72) | |
| 1000+ | 34 (34) | 10 (10) | 3 (3) | 53 (53) | |
| Transplant type | 0.33 | ||||
| Liver | 39 (36) | 4 (4) | 0 (0) | 65 (60) | |
| HSCT | 14 (14) | 9 (9) | 10 (10) | 66 (67) | |
| Sex | 0.92 | ||||
| Female | 22 (26) | 2 (2) | 2 (2) | 58 (69) | |
| Male | 31 (25) | 11 (9) | 8 (7) | 73 (59) | |
| Age at transplant, years | 0.61 | ||||
| 1 | 28 (37) | 10 (13) | 0 (0) | 37 (49) | |
| 2–5 | 17 (25) | 3 (4) | 7 (10) | 41 (60) | |
| 6+ | 8 (13) | 0 (0) | 3 (5) | 53 (83) | |
| Time since transplant, years | 0.02 | ||||
| 0.0–0.5 | 6 (7) | 8 (10) | 10 (12) | 57 (70) | |
| 0.6–1.0 | 15 (35) | 2 (5) | 0 (0) | 26 (60) | |
| 1.1+ | 32 (39) | 3 (4) | 0 (0) | 48 (58) | |
| Timing among PTLD cases | 0.21 | ||||
| Before PTLD | 1 (6) | 0 (0) | 2 (11) | 15 (83) | |
| At PTLD | 4 (36) | 3 (27) | 0 (0) | 4 (36) | |
| After PTLD | 16 (29) | 10 (18) | 3 (5) | 26 (47) | |
| sCD30, ng/ml | 0.02 | ||||
| <120 | 12 (13) | 4 (4) | 4 (4) | 76 (79) | |
| 120+ | 41 (37) | 9 (8) | 6 (5) | 55 (50) |
Results are shown for N=207 plasma specimens in N=36 transplant recipients.
Abbreviations: FLCs free light chains, EBV Epstein Barr virus, PTLD post-transplant lymphoproliferative disorder, HSCT hematopoietic stem cell transplant, sCD30 soluble CD30
Figure 1.
Longitudinal measurements of plasma free light chain and EBV loads in five PTLD cases. Results are shown for five transplant recipients who developed PTLD and who had at least one plasma sample that manifested a monoclonal pattern of free light chain elevation. A monoclonal pattern is present when at least one free light chain is above the upper limit of normal and the kappa:lambda ratio is abnormal. Results are shown for kappa (squares) and lambda (circles), according to multiples of the upper limit of normal (left axis). Sold symbols represent observations for which a monoclonal FLC pattern was present, and open symbols when a monoclonal FLC pattern was absent. The EBV load is shown as a dashed line with X symbols, using a logarithmic scale (right axis). The PTLD diagnosis date is shown with a vertical line. The panels also include information about the transplant (liver vs. hematopoietic stem cell transplant) and type of PTLD. Note that the time scale differs across panels
For subject 13, PTLD tumor tissue at initial diagnosis did not show light chain restriction, in contrast with the FLC pattern in plasma at the time of PTLD diagnosis, which was consistent with a monoclonal excess of kappa. However, 5 years after initial diagnosis, this subject again developed PTLD, and at that time the tumor was kappa restricted; no plasma was available at the time of recurrence. Two additional PTLD cases with monoclonal FLC patterns (subjects 7 and 8) also had PTLD tumors that did not show light chain restriction. Tumor tissue was not obtained for the remaining two PTLD cases (subjects 2 and 6), so there were no data on light chain restriction in tumor cells.
M-proteins detected by protein electrophoresis and immunofixation
Twenty-four of 35 evaluated recipients (69%) had detectable M-proteins, of which 11 were seen with both protein electrophoresis and immunofixation and 13 were seen only with immunofixation. M-proteins appeared more frequently in PTLD cases (n=10 cases, 91%) than high-EBV recipients (n=6, 50%) or low-EBV recipients (n=8, 67%), but this difference was not significant (p=0.13).
Among the 24 samples with M-proteins, 12 (50%) had FLC measurements consistent with polyclonal elevations, 2 (8%) had monoclonal FLC elevations that corresponded to the light chain detected in their immunofixation, 1 (4%) had no elevation but a skewed FLC ratio that matched the immunofixation result, and 8 (33%) had normal FLC levels. The remaining sample with an M-protein was from subject 6, who developed PTLD: the immunofixation revealed an IgM kappa M-protein, but the FLCs manifested monoclonal elevation of lambda FLCs.
Of the 10 PTLD cases with M-proteins, 3 lacked tumor tissue or staining to assess for light chain restriction, and 4 tumors were noted to lack light chain restriction. Of the remaining PTLD cases, one with an IgG lambda M-protein had a tumor that was kappa restricted. Another case had an IgG kappa M-protein, but the tumor was a T-cell PTLD. The final PTLD case with an M-protein was subject 13, for whom an IgM kappa M-protein was detected at PTLD diagnosis, in agreement with the plasma FLC result. As noted above, the tumor in this case did not show light chain restriction at diagnosis, but kappa restriction was present 5 years later with a relapse.
Discussion
In the present study of pediatric liver and HSCT recipients, we evaluated multiple markers of B-cell dysfunction, including elevated plasma levels of FLCs and sCD30, and the presence of circulating M-proteins. While the predominant immune deficit among transplant recipients is related to iatrogenic T-cell suppression, disordered B-cell function is also common (4–12;16;18–20). Furthermore, since most PTLDs are derived from B-cells, markers of B-cell abnormalities may help identify recipients at high risk of this adverse outcome.
Overall, FLC abnormalities were very common among the recipients we evaluated, with abnormally elevated levels detected in 20–28% of plasma specimens. The most common abnormality was a polyclonal FLC excess in which kappa and lambda FLCs were proportionately increased, so that the kappa/lambda FLC ratio remained within the normal range. Additionally, levels of sCD30 were increased in the vast majority (94%) of samples. These findings are consistent with frequent polyclonal B-cell dysfunction and activation among solid organ and HSCT recipients.
Causes of B-cell dysfunction in recipients include profound T-cell deficits from immunosuppression, as well as chronic immune stimulation from the organ allograft or graft vs. host disease (20–22). Furthermore, in the present study three biomarkers (kappa and lambda FLCs, sCD30) were strongly increased in association with higher EBV loads. Elevated EBV loads likely reflect, in part, increased proliferation of EBV-infected B-cells, and our results support that this process leads to detectable increases in circulating B-cell activation markers.
Our detection of M-proteins in more than half of recipients who did not have PTLD is consistent with prior studies of HSCT (prevalence of 18–52%) and solid organ transplant (28–50%) (6–12). In the U.S. general population, for comparison, one survey found M-proteins in approximately 3% of adults (largely non-Hispanic whites) over age 50 (23). Perhaps surprisingly, we did not find concordance between the light chain component of M-proteins and circulating FLCs. The most notable discrepancy was for subject 6, a PTLD case who had an IgM kappa M-protein but an excess of lambda FLCs. A similar lack of agreement between M-proteins and FLCs has been described in a minority of multiple myeloma patients (24).
In the setting of transplantation, the detection of M-proteins may also largely be a manifestation of generalized B-cell dysfunction, reflecting the presence of oligoclonal B-cell populations that secrete immunogloublins at varying levels, rather than a true monoclonal population of tumor cells (as seen in multiple myeloma). Among transplant recipients, both plasma cells and EBV-transformed lymphocytes are likely sources of circulating immunoglobulins (25). Among the transplant recipients in the present study, most M-proteins were present at low concentration, as more than half were only seen with immunofixation. In addition, detection of M-proteins is often transient among transplant recipients and people with human immunodeficiency virus (an immunosuppressed state with T-cell deficits analogous to transplantation) (7–9;26;27). Babel and colleagues previously noted a positive association between the detection of M-proteins and EBV load among kidney recipients (28). We did not find a similar difference in M-proteins between low-EBV and high-EBV recipients, although our sample size was limited. Among HSCT recipients, the presence of an M-protein is more common when chronic graft vs. host disease is present (6), further highlighting the importance of immune dysregulation.
Several observations suggest that these abnormalities in circulating immunoglobulin proteins are more frequent in PTLD cases than other transplant recipients. All but one PTLD case (91%) had a detectable M-protein, and all of the plasma samples that showed monoclonal FLC elevations were from PTLD cases. Nonetheless, we did not find consistent evidence that PTLD tumor cells were actually the source of the abnormal immunoglobulins. Of the 7 PTLD cases who had M-proteins and who also had evaluated tumors, none had M-proteins that matched the light chain restriction in the tumor. Indeed, one PTLD case with a circulating M-protein had a T-cell PTLD. Moreover, monoclonal FLC elevations were not limited to recipients whose tumors showed monoclonal light chain restriction. Thus, in addition to lack of agreement between M-proteins and plasma FLCs, a striking finding was that these circulating monoclonal immunoglobulin proteins (intact M-proteins and FLCs) did not match the proteins produced by tumor cells. Tsai et al. similarly reported a substantial lack of agreement between circulating M-proteins among solid organ recipients and the light chain restriction of PTLD tumor cells (14).
Given that PTLD tumors are sometimes polyclonal (1;29), one possibility is that some of these discrepancies between tumors and circulating immunoglobulins occurred because the biopsied tumor tissue did not include cells from the B-cell clone producing the circulating proteins. One PTLD case (subject 13) is instructive in this regard. In this recipient, a plasma sample at PTLD diagnosis revealed both an IgM kappa M-protein and a monoclonal excess of kappa FLCs. At the time of PTLD diagnosis, when the M-protein and FLC excess were detected, this recipient’s tumor did not show light chain restriction, but 5 years later, a relapse of PTLD was kappa-restricted. The kappa restriction of the tumor at relapse may be a coincidence, but it is also possible that a kappa-restricted B-cell clone, which later gave rise to the relapse, was already present at the time of the initial PTLD diagnosis.
Although our findings regarding PTLD are inconclusive, results of previous studies support associations of circulating FLCs and sCD30 with PTLD. In our prior case-control study of solid organ recipients (16), PTLD cases were more likely to have elevated FLC levels than controls. Haque et al. previously demonstrated higher plasma levels of sCD30 in PTLD cases than in thoracic organ recipients without PTLD (18). Among HIV-infected people, circulating FLC and sCD30 levels are predictive of the development of NHL (27;30–32), and levels of sCD30 are also predictive of NHL in the general population (19).
Strengths of our study were the inclusion of both solid organ and HSCT recipients, recipients with and without PTLD, and recipients with differing EBV loads. Also, we evaluated multiple samples from transplant recipients, including samples from before PTLD diagnosis, which allowed longitudinal assessment of these markers. A limitation was that the study subjects were selected from participants in previous clinical studies, so they may therefore not be representative of other transplant recipients. It is unknown how associations with these markers might differ for other groups of recipients (e.g., adults, recipients of other solid organs). A further limitation was the small sample size, which precluded detailed analyses of subsets of transplant recipients (e.g., separately for liver and HSCT recipients). We did not evaluate associations of these B-cell markers with some relevant clinical conditions (e.g., use of immunosuppressive medications, rejection, graft-vs.-host disease), and assessing these relationships would be of interest for further research.
Given the high risk of PTLD among transplant recipients, there is a need for clinical assays to identify those with incipient PTLD. The detection of an elevated EBV load is associated with the presence of PTLD, but previous studies have demonstrated that this finding is somewhat non-specific (22;33;34). It would be of substantial interest if markers of B-cell dysfunction could help identify a subset of recipients at greatest risk of PTLD, especially well in advance of when PTLD becomes clinically apparent. Of interest, our prior study demonstrated that FLC elevations can predict the subsequent development of PTLD over a period of about half a year (16). In the present study we did not observe any PTLD cases with prior monoclonal FLC elevations (the earliest was at the time of PTLD diagnosis), although some cases did not have specimens prior to diagnosis that could be evaluated. Additional work is needed to further assess the utility of these B-cell markers for identifying transplant recipient at high risk of developing PTLD in a prospective setting.
In conclusion, we observed a high prevalence of plasma markers indicating abnormal B-cell activation in both solid organ and HSCT recipients. The frequent detection of monoclonal patterns of FLC elevation and M-proteins was associated with elevated EBV load and PTLD. Nonetheless, the markers were not entirely consistent with each other, and we could not demonstrate evidence that the FLCs and M-proteins were produced by PTLD tumor cells. In addition to pursuing possible clinical applications of these markers, future studies should elucidate mechanisms of B-cell dysfunction in transplant recipients.
Materials and Methods
Subject selection
Study subjects were children (age 0–19 years at transplantation) drawn from clinical studies of PTLD surveillance or therapy, whose protocols and results have been previously reported (35–39). They were included in the present investigation if sufficient stored plasma specimens were available. Approval was obtained from the Baylor institutional review board to study longitudinal plasma samples from subjects in these prior studies.
Subjects fell into three categories based on clinical data and previous EBV load testing: recipients who developed PTLD (i.e., PTLD cases), recipients with high EBV loads who never developed PTLD (high-EBV recipients), and recipients whose EBV load remained low (low-EBV recipients). High EBV loads were defined as more than 1000 copies/ug of peripheral blood DNA.
Laboratory methods and tumor evaluation
Heparinized plasma samples were tested for FLCs on a PLUS Special Protein Analyzer Plus platform using Freelite reagents (The Binding Site, Birmingham, United Kingdom) (40). For interpretation, we describe FLC levels as multiples of the upper limit of normal (ULN: 1.94 mg/dL for kappa, 2.63 mg/dL for lambda). Based on the kappa/lambda FLC ratio (normal range: 0.26–1.65), we also describe FLC elevations in four categories: polyclonal elevation (elevated kappa and/or lambda with normal ratio), monoclonal elevation (elevated kappa and/or lambda, with an abnormal ratio), no elevation with an abnormal ratio, and normal (41). sCD30 levels were measured using an enzyme linked immunoassay (eBioscience, San Diego, California). The upper limit of normal for sCD30 levels in heparanized plasma is 43.3 ng/ml.
For assessment of M-proteins in PTLD cases, we chose one plasma sample close to PTLD diagnosis; results are unavailable for one case. For high-EBV recipients, we selected the sample with highest EBV load, while for low-EBV recipients, we chose a sample in the first 6–12 months after transplant (if possible). Protein electrophoresis and immunofixation were performed using a SPIFE 3000 analyzer (Helena Laboratories, Beaumont, TX).
EBV load was measured from peripheral blood mononuclear cells (PBMCs) as described previously (42), by a real time polymerase chain reaction assay using primers specific for the EBER-1 region of the EBV genome. Results are expressed as copies/ug peripheral blood DNA, with a detection limit of 10 copies/ug. Transplant recipients were diagnosed with PTLD according to standard criteria (1). Light chain restriction in tumor cells was measured by immunohistochemistry.
Statistical analyses
We compared levels of kappa and lambda FLCs and sCD30 across categories defined by recipient characteristics, EBV load, and time since transplant. These analyses used generalized linear models that accounted for repeated measurements on subjects using an independent working correlation matrix (GENMOD procedure, SAS 9.1; Cary, NC). In a multivariable model, we included characteristics associated in univariate analyses with at least one of the three biomarkers, as well as the category of the recipient (PTLD, high-EBV, low-EBV) and whether there was prior treatment for PTLD or high EBV load (defined as cytoreductive surgery, rituximab, chemotherapy, or EBV-directed cytotoxic T lymphocytes) (22). FLC and sCD30 levels were log10-transformed to better approximate normality, so that model coefficients correspond to relative differences (i.e., fold changes) across the categories. Residual plots from these models indicated that the assumptions of normality were met in the log10-transformed data. In analyses that assessed log10-transformed EBV load as a predictor, EBV-negative samples were assigned a value of 5 copies/ug (i.e., half the lower limit of detection).
We also evaluated whether the frequencies of FLCs defined in the four-way classification (polyclonal elevation, monoclonal elevation, no elevation with an abnormal ratio, normal) differed across recipient categories using a Fisher exact test; given the dependence of the repeated samples from the same recipients, we obtained permutation-based p-values for this test.
Acknowledgments
This study was supported by the Intramural Research Program of the National Cancer Institute. It was also supported by grant P50CA126752 from the National Institutes of Health and a Specialized Center of Research Program of the Leukemia and Lymphoma Society.
List of abbreviations
- EBV
Epstein Barr virus
- FLC
free light chain
- M-protein
monoclonal immunoglobulin protein
- HSCT
hematopoietic stem cell transplantation
- NHL
non-Hodgkin lymphoma
- PTLD
post-transplant lymphoproliferative disorder
- sCD30
soluble CD30
Footnotes
The authors of this manuscript have no conflicts of interest to disclose.
Author contributions
Eric A. Engels: oversight of project, research design, statistical analyses, wrote manuscript.
Barbara Savoldo: research design, subject recruitment, reviewed and commented on manuscript.
Ruth M. Pfeiffer: statistical analysis, reviewed and commented on manuscript.
Rene Costello: laboratory assays, reviewed and commented on manuscript.
Adriana Zingone: laboratory assays, reviewed and commented on manuscript.
Helen E. Heslop: research design, subject recruitment, reviewed and commented on manuscript.
Ola Landgren: research design, laboratory assays, reviewed and commented on manuscript.
Reference List
- 1.Harris NL, Swerdlow SH, Frizzera G, Knowles DM. Post-transplant lymphoproliferative disorders. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001. pp. 264–69. [Google Scholar]
- 2.Andreone P, Gramenzi A, Lorenzini S, Biselli M, Cursaro C, Pileri S, et al. Posttransplantation lymphoproliferative disorders. Arch Intern Med. 2003;163:1997–2004. doi: 10.1001/archinte.163.17.1997. [DOI] [PubMed] [Google Scholar]
- 3.Curtis RE, Travis LB, Rowlings PA, Socie G, Kingma DW, Banks PM, et al. Risk of lymphoproliferative disorders after bone marrow transplantation: a multi-institutional study. Blood. 1999;94:2208–16. [PubMed] [Google Scholar]
- 4.Raanani P, Gafter-Gvili A, Paul M, Ben Bassat I, Leibovici L, Shpilberg O. Immunoglobulin prophylaxis in hematopoietic stem cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2009;27:770–781. doi: 10.1200/JCO.2008.16.8450. [DOI] [PubMed] [Google Scholar]
- 5.Mawhorter S, Yamani MH. Hypogammaglobulinemia and infection risk in solid organ transplant recipients. Curr Opin Organ Transplant. 2008;13:581–85. doi: 10.1097/MOT.0b013e3283186bbc. [DOI] [PubMed] [Google Scholar]
- 6.Mitus AJ, Stein R, Rappeport JM, Antin JH, Weinstein HJ, Alper CA, et al. Monoclonal and oligoclonal gammopathy after bone marrow transplantation. Blood. 1989;74:2764–68. [PubMed] [Google Scholar]
- 7.Nagashima T, Muroi K, Kawano-Yamamoto C, Komatsu N, Ozawa K. Paraproteinemia after hematopoietic stem cell transplantation. Leuk Lymphoma. 2004;45:135–37. doi: 10.1080/1042819031000139729. [DOI] [PubMed] [Google Scholar]
- 8.Lim ZY, Ingram W, Brand R, Akthari M, Milojkovic D, Ho AY, et al. Clonal gammopathies following alemtuzumab-based reduced intensity conditioning haematopoietic stem cell transplantation: association with chronic graft-versus-host disease and improved overall survival. Bone Marrow Transplant. 2007;40:747–52. doi: 10.1038/sj.bmt.1705805. [DOI] [PubMed] [Google Scholar]
- 9.Hammarstrom L, Smith CI. Frequent occurrence of monoclonal gammopathies with an imbalanced light-chain ratio following bone marrow transplantation. Transplantation. 1987;43:447–49. doi: 10.1097/00007890-198703000-00028. [DOI] [PubMed] [Google Scholar]
- 10.Badley AD, Portela DF, Patel R, Kyle RA, Habermann TM, Strickler JG, et al. Development of monoclonal gammopathy precedes the development of Epstein-Barr virus-induced posttransplant lymphoproliferative disorder. Liver Transpl Surg. 1996;2:375–82. doi: 10.1002/lt.500020508. [DOI] [PubMed] [Google Scholar]
- 11.Peest D, Schaper B, Nashan B, Wonigeit K, Raude E, Pichlmayr R, et al. High incidence of monoclonal immunoglobulins in patients after liver or heart transplantation. Transplantation. 1988;46:389–93. doi: 10.1097/00007890-198809000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Myara I, Quenum G, Storogenko M, Tenenhaus D, Guillemain R, Moatti N. Monoclonal and oligoclonal gammopathies in heart-transplant recipients. Clin Chem. 1991;37:1334–37. [PubMed] [Google Scholar]
- 13.Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–69. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 14.Tsai DE, Aqui NA, Tomaszewski JE, Olthoff KM, Ahya VN, Kotloff RM, et al. Serum protein electrophoresis abnormalities in adult solid organ transplant patients with post-transplant lymphoproliferative disorder. Clin Transplant. 2005;19:644–52. doi: 10.1111/j.1399-0012.2005.00388.x. [DOI] [PubMed] [Google Scholar]
- 15.Rosselet A, Vu DH, Meylan P, Chaubert AS, Schapira M, Pascual M, et al. Associations of serum EBV DNA and gammopathy with post-transplant lymphoproliferative disease. Clin Transplant. 2009;23:74–82. doi: 10.1111/j.1399-0012.2008.00904.x. [DOI] [PubMed] [Google Scholar]
- 16.Engels EA, Preiksaitis J, Zingone A, Landgren O. Circulating antibody free light chains and risk of posttransplant lymphoproliferative disorder. Am J Transplant. 2012;12:1268–74. doi: 10.1111/j.1600-6143.2011.03954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradwell AR, Carr-Smith HD, Mead GP, Tang LX, Showell PJ, Drayson MT, et al. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem. 2001;47:673–80. [PubMed] [Google Scholar]
- 18.Haque T, Chaggar T, Schafers J, Atkinson C, McAulay KA, Crawford DH. Soluble CD30: a serum marker for Epstein-Barr virus-associated lymphoproliferative diseases. J Med Virol. 2011;83:311–16. doi: 10.1002/jmv.21953. [DOI] [PubMed] [Google Scholar]
- 19.Purdue MP, Lan Q, Martinez-Maza O, Oken MM, Hocking W, Huang WY, et al. A prospective study of serum soluble CD30 concentration and risk of non-Hodgkin lymphoma. Blood. 2009;114:2730–2732. doi: 10.1182/blood-2009-04-217521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Claas FH. Clinical relevance of circulating donor-specific HLA antibodies. Curr Opin Organ Transplant. 2010;15:462–66. doi: 10.1097/MOT.0b013e32833b9c38. [DOI] [PubMed] [Google Scholar]
- 21.Lim ZY, Ingram W, Brand R, Akthari M, Milojkovic D, Ho AY, et al. Clonal gammopathies following alemtuzumab-based reduced intensity conditioning haematopoietic stem cell transplantation: association with chronic graft-versus-host disease and improved overall survival. Bone Marrow Transplant. 2007;40:747–52. doi: 10.1038/sj.bmt.1705805. [DOI] [PubMed] [Google Scholar]
- 22.Heslop HE. How I treat EBV lymphoproliferation. Blood. 2009;114:4002–8. doi: 10.1182/blood-2009-07-143545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354:1362–69. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 24.Singhal S, Vickrey E, Krishnamurthy J, Singh V, Allen S, Mehta J. The relationship between the serum free light chain assay and serum immunofixation electrophoresis, and the definition of concordant and discordant free light chain ratios. Blood. 2009;114:38–39. doi: 10.1182/blood-2009-02-205807. [DOI] [PubMed] [Google Scholar]
- 25.Wroblewski JM, Copple A, Batson LP, Landers CD, Yannelli JR. Cell surface phenotyping and cytokine production of Epstein-Barr Virus (EBV)-transformed lymphoblastoid cell lines (LCLs) J Immunol Methods. 2002;264:19–28. doi: 10.1016/s0022-1759(01)00565-8. [DOI] [PubMed] [Google Scholar]
- 26.Ahdieh L, Gange SJ, Greenblatt r, Minkoff H, Anastos K, Young M, et al. Selection by indication of potent antiretroviral therapy use in a large cohort of women infected with human immunodeficiency virus. Am J Epidemiol. 2000;152:923–33. doi: 10.1093/aje/152.10.923. [DOI] [PubMed] [Google Scholar]
- 27.Landgren O, Goedert JJ, Rabkin CS, Wilson WH, Dunleavy K, Kyle RA, et al. Circulating serum free light chains as predictive markers of AIDS-related lymphoma. J Clin Oncol. 2010;28:773–79. doi: 10.1200/JCO.2009.25.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babel N, Schwarzmann F, Pruss A, Volk HD, Reinke P. Monoclonal gammopathy of undetermined significance (MGUS) is associated with an increased frequency of Epstein-Barr Virus (EBV) latently infected B lymphocytes in long-term renal transplant patients. Transplant Proc. 2004;36:2679–82. doi: 10.1016/j.transproceed.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 29.Chadburn A, Cesarman E, Liu YF, Addonizio L, Hsu D, Michler RE, et al. Molecular genetic analysis demonstrates that multiple posttransplantation lymphoproliferative disorders occurring in one anatomic site in a single patient represent distinct primary lymphoid neoplasms. Cancer. 1995;75:2747–56. doi: 10.1002/1097-0142(19950601)75:11<2747::aid-cncr2820751119>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 30.Breen EC, Fatahi S, Epeldegui M, Boscardin WJ, Detels R, Martinez-Maza O. Elevated serum soluble CD30 precedes the development of AIDS-associated non-Hodgkin’s B cell lymphoma. Tumour Biol. 2006;27:187–94. doi: 10.1159/000093022. [DOI] [PubMed] [Google Scholar]
- 31.Pizzolo G, Vinante F, Morosato L, Nadali G, Chilosi M, Gandini G, et al. High serum level of the soluble form of CD30 molecule in the early phase of HIV-1 infection as an independent predictor of progression to AIDS. AIDS. 1994;8:741–45. doi: 10.1097/00002030-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Vendrame E, Martinez-Maza O. Assessment of pre-diagnosis biomarkers of immune activation and inflammation: insights on the etiology of lymphoma. J Proteome Res. 2011;10:113–19. doi: 10.1021/pr100729z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen U, Preiksaitis J. Epstein-barr virus and posttransplant lymphoproliferative disorder in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl 4):S87–96. S87–S96. doi: 10.1111/j.1600-6143.2009.02898.x. [DOI] [PubMed] [Google Scholar]
- 34.Lee TC, Savoldo B, Barshes NR, Rooney CM, Heslop HE, Gee AP, et al. Use of cytokine polymorphisms and Epstein-Barr virus viral load to predict development of post-transplant lymphoproliferative disorder in paediatric liver transplant recipients. Clin Transplant. 2006;20:389–93. doi: 10.1111/j.1399-0012.2006.00498.x. [DOI] [PubMed] [Google Scholar]
- 35.Savoldo B, Rooney CM, Quiros-Tejeira RE, Caldwell Y, Wagner HJ, Lee T, et al. Cellular immunity to Epstein-Barr virus in liver transplant recipients treated with rituximab for post-transplant lymphoproliferative disease. Am J Transplant. 2005;5:566–72. doi: 10.1111/j.1600-6143.2004.00693.x. [DOI] [PubMed] [Google Scholar]
- 36.Savoldo B, Goss JA, Hammer MM, Zhang L, Lopez T, Gee AP, et al. Treatment of solid organ transplant recipients with autologous Epstein Barr virus-specific cytotoxic T lymphocytes (CTLs) Blood. 2006;108:2942–49. doi: 10.1182/blood-2006-05-021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–35. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leen AM, Christin A, Myers GD, Liu H, Cruz CR, Hanley PJ, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114:4283–92. doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12:1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 40.Drayson M, Tang LX, Drew R, Mead GP, Carr-Smith H, Bradwell AR. Serum free light-chain measurements for identifying and monitoring patients with nonsecretory multiple myeloma. Blood. 2001;97:2900–2902. doi: 10.1182/blood.v97.9.2900. [DOI] [PubMed] [Google Scholar]
- 41.Maurer MJ, Cerhan JR, Katzmann JA, Link BK, Allmer C, Zent CS, et al. Monoclonal and polyclonal serum free light chains and clinical outcome in chronic lymphocytic leukemia. Blood. 2011;118:2821–26. doi: 10.1182/blood-2011-04-349134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savoldo B, Goss J, Liu Z, Huls MH, Doster S, Gee AP, et al. Generation of autologous Epstein-Barr virus-specific cytotoxic T cells for adoptive immunotherapy in solid organ transplant recipients. Transplantation. 2001;72:1078–86. doi: 10.1097/00007890-200109270-00017. [DOI] [PubMed] [Google Scholar]