Abstract
We previously demonstrated that sperm heads from amphibians (Xenopus and Rana) and zebrafish (Danio) could form giant lampbrush chromosomes when injected into the nucleus of amphibian oocytes. However, similar experiments with mammalian sperm heads were unsuccessful. Here we describe a slightly modified procedure and demonstrate that human sperm heads can form giant lampbrush chromosomes when injected into the oocyte nucleus of the frog Xenopus laevis or the newt Notophthalmus viridescens. Human and other mammalian chromosomes do not form recognizable lampbrush chromosomes in their own oocytes or in any somatic cells. These experiments thus demonstrate that the lampbrush condition is an inducible state and that the amphibian oocyte nucleus contains all factors required to remodel the inactive chromatin of a mammalian sperm into a transcriptionally active state. They also demonstrate that absence of lampbrush chromosomes from human oocytes must relate to specific features of mammalian oogenesis, not to permanent genetic or epigenetic changes in the chromatin.
Keywords: chromatin, oocyte, reprogramming, RNA polymerase II, sperm, transcription, Xenopus
Introduction
Lampbrush chromosomes (LBCs) are giant meiotic chromosomes first described more than 100 years ago from the oocyte nucleus or germinal vesicle (GV) of the axolotl (Flemming 1882) and a shark (Rückert 1892). LBCs occur in many species, including vertebrates, invertebrates, and even the single-celled alga Acetabularia, but they are best characterized from amphibian and avian oocytes (Callan and Lloyd 1960; Morgan 2002; Gall et al. 2004; Gaginskaya et al. 2009). Each homologue has an axis of chromomeres corresponding to transcriptionally inactive chromatin, from which transcriptionally active pairs of loops extend laterally. A coating of nascent ribonucleoprotein (RNP) fibrils makes these lateral loops visible by conventional light microscopy. Because of their enormous size, LBCs provide an ideal system in which to study transcription, RNA processing and other general features of chromosome organization. The overall organization and significance of LBCs were summarized in the classic monograph by Mick Callan (Callan 1986) (see also www.projects.exeter.ac.uk/lampbrush).
In a previous study, we demonstrated that demembranated sperm heads from the frogs Xenopus and Rana, and the zebrafish Danio can form LBCs when injected into the GV of Xenopus laevis (Gall and Murphy 1998). Although they are unreplicated single chromatids, sperm LBCs are similar to endogenous lampbrush bivalents in morphology and immunofluorescent staining properties. The induction of sperm LBCs in amphibian GVs provides a useful system for identifying cis- and trans-acting factors required for converting condensed chromatin into a transcriptionally active form. In our earlier experiments we failed to induce LBCs from mammalian sperm heads. It was unclear whether the failure was due to technical issues or to more fundamental differences between amphibian and mammalian chromatin. Because mammalian chromosomes do not form recognizable LBCs during meiosis or in any somatic cells, one could postulate that mammalian chromatin is unable to assume the LBC condition. Here we describe new experiments under slightly different conditions in which human sperm heads give rise to transcriptionally active LBCs when injected into the GV of the frog X. laevis or the newt Notophthalmus viridescens. These experiments demonstrate that the amphibian GV contains all factors required to reprogram inactive mammalian chromatin into a transcriptionally active state. Thus the absence of LBCs from mammalian oocytes must relate to specific aspects of mammalian oogenesis and not to permanent genetic or epigenetic features of mammalian chromatin.
Materials and Methods
Oocytes and LBC spreads
Adult frogs X. laevis were purchased from Xenopus 1 (Dexter, MI) and adult newts N. viridescens from the Sullivan Company (Nashville, TN). Oocytes were held at 16–18°C or 24°C in a small Petri dish of OR2 saline (Wallace et al. 1973). Chromosome spreads were prepared from individual GVs as described previously (Gall and Wu 2010).
Oocyte injections
Sperm heads of Xenopus laevis were prepared from testes as described earlier (Newmeyer and Wilson 1991). A sample of normal human sperm was obtained from the Johns Hopkins University Medical School. The research was deemed by the Johns Hopkins Institutional Review Board to qualify for exemption under category (4) of 45 CFR 46.101(b). The sperm tails were removed by sonication and the heads were concentrated by centrifugation. The technique for injection of human sperm heads into the GV was basically as described previously for experiments with Xenopus, Rana, and Danio (Gall and Murphy 1998) with four relatively minor changes. Although the effect of any one of these changes is difficult to assess, the new protocol now gives a higher survival rate and allows us to hold oocytes for longer periods before observing the GV contents. A major difference between Xenopus and human sperm heads is the longer time needed for the human sperm heads to generate recognizable LBCs.
First, in our previous study we injected sperm heads that had been demembranated with lysolecithin, as originally described by Gurdon (Gurdon 1976). In the experiments described here, we found that intact sperm heads started swelling and eventually resolved into individual LBCs at a pace comparable to that of demembranated sperm heads. For this reason, we omitted the lysolecithin step.
Second, we did not defolliculate the oocytes with collagenase before injection. Defolliculated oocytes are softer and easier to penetrate with the injection needle, but are more fragile and prone to contamination. By using sharp needles with smaller tips, we managed to inject oocytes successfully without defolliculation.
Third, we did not centrifuge the oocytes before injection. The major purpose of centrifugation is to bring the GV to the surface, where its position can be detected as a depigmented area. With practice, one can inject sperm heads into the GV even when it lies deeper within the oocyte.
Finally, in earlier experiments we found that the number of sperm heads actually injected decreased dramatically during the course of injecting multiple oocytes. We reasoned that this might be due to adherence of sperm heads to the inside wall of the needle. To prevent such sticking, we added 2–5% polyvinylpyrrolidone (PVP) to the sperm suspension. Subsequently, we found that the number of sperm actually injected remained relatively constant during the course of an experiment. PVP has been used routinely in intracytoplasmic sperm injections involving mammalian sperm and oocytes.
After improving the injection and oocyte handling protocol, we were able to keep injected oocytes alive for several days. The longer incubation time is critical because human sperm heads expand more slowly than Xenopus sperm heads. In our previous experiments most of the injected oocytes were beginning to degenerate before the human sperm heads had expanded.
Immunofluorescence staining and microscopy
GV spreads were stained with antibodies as described previously (Gall and Murphy 1998) with the following modifications. Samples were blocked with 10% horse serum for 30 min before incubation in primary antibody for 4–12 h at room temperature. Primary antibodies used in this study include mouse mAb H14 against Pol II (Bregman et al. 1995); mouse mAb Y12 against the Sm “epitope” (symmetric dimethylarginine) (Lerner et al. 1981; Brahms et al. 2000); and mouse mAb H1 against Xenopus coilin (Tuma et al. 1993). Secondary antibodies were Alexa 488- or Alexa 594-conjugated goat anti-mouse IgG or IgM (Molecular Probes, Eugene, OR). They were used together with 0.01 μg/ml 4′,6 diamidino-2-phenylindole (DAPI) for 4–12 h at room temperature. Slides were mounted in 50% glycerol. Specimens were examined with a Zeiss 63X 1.25 N.A. planapo lens on the Zeiss Axioplan fluorescence microscope. Images were captured with a Micromax charge-coupled device camera (Princeton Instruments, Trenton, NJ) using the IPLab (3.5.5) image acquisition and analysis program (Scanalytics, Fairfax, VA).
Results
Injection of human sperm heads into the Xenopus GV
In earlier experiments we injected sperm heads from Xenopus, Rana, and Danio into Xenopus GVs and saw the formation of morphologically typical LBCs from the originally highly condensed chromatin. The entire process usually required about 24 h for completion. Similar experiments with mouse and human sperm heads were unsuccessful for unknown reasons. To test whether the failure of human sperm heads to form LBCs was due to technical issues or to more fundamental incompatibility between mammalian and amphibian species, we performed new injections but modified the conditions of the experiment. Specifically, we extended the time period over which the observations were made from about 24 hr to several days. Oocytes injected with human sperm heads were incubated in OR2 medium at 16–18°C. Similar to Xenopus sperm, the human sperm heads swelled within 3–6 h after injection. At this time Xenopus sperm heads begin to stain with mAb H14 against the phosphorylated C-terminal domain of pol II, indicative of pol II uptake from the nucleoplasm. However, the human sperm heads did not stain at this time, but instead began to vacuolate. Over the next 15–20 h, the swollen human sperm heads took on a more open configuration, often with prominent nuclear bodies on the surface. At this stage, we could detect staining with mAb H14 (Fig. 1a–c). Thus, it seems that a major difference between the Xenopus and human sperm heads is the time needed for physical expansion and uptake of pol II. Eventually human sperm heads resolved into loose clusters of fuzzy threads about 40–48 h after injection (Figure 1d–f). At still later stages these loose clusters fell apart to form individual DAPI-positive chromosomes with intense pol II staining (Fig. 1g–i). In favourable cases the number of individual chromosomes approximated the human haploid number of 23 (Fig. 1h). Although these chromosomes did not display obvious loops, their fuzzy appearance and staining with an antibody against pol II strongly suggested that they were transcriptionally active.
Fig. 1.
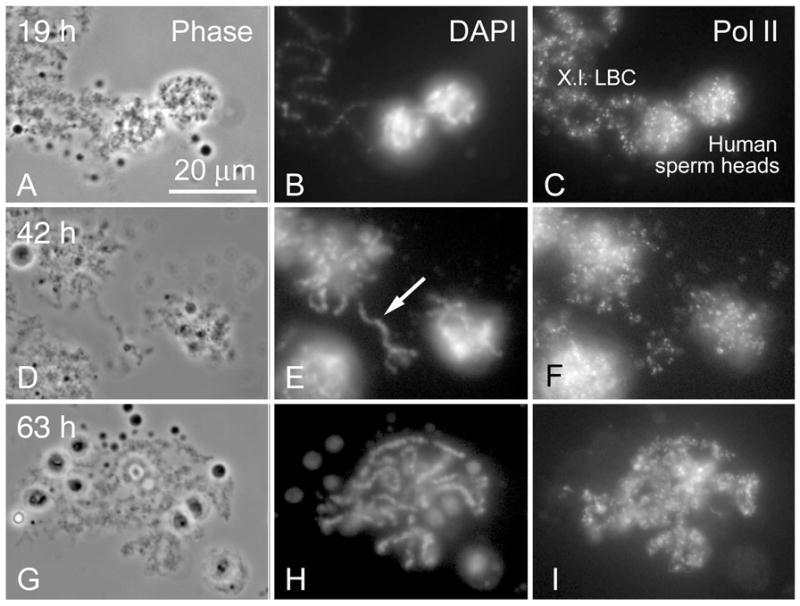
Expansion of human sperm heads injected into the X. laevis GV. a–c Two sperm heads 19 h after injection have swollen and begun to take up pol II. To the left of the sperm heads is part of one endogenous Xenopus LBC. The DAPI stain is much brighter in the human sperm heads, because each contains a complete haploid chromosome set (3.5 pg DNA). The entire endogenous X. laevis LBC set contains roughly 4X as much DNA but spread over 18 extended bivalents. d–f Three sperm heads 42 h after injection are slightly more swollen. An occasional chromosome (arrow) can be found separate from the clusters. g–i A single sperm head 63 h after injection has now resolved into a group of separate chromosomes, presumably the haploid number (23) of univalent chromosomes. Although the chromosomes are covered with pol II, individual transcription loops are not readily visible.
Temperature accelerates the time for formation of LBCs
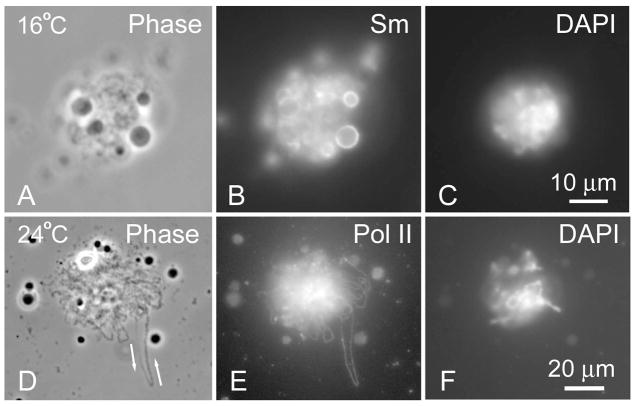
Because the formation of LBCs from human sperm heads was slow, we reasoned that we could speed up the process by increasing the temperature at which the oocytes were incubated after injection. We injected human sperm heads into Xenopus GVs, and then divided the injected oocytes into two groups for incubation at two different temperatures, 16°C–18°C and 24°C. At 44 hr after injection, sperm heads in oocytes held at 16°C–18°C were still condensed (Fig. 2a–c). In contrast, in the group incubated at 24°C, long transcription loops extended from the sperm clusters, even though the clusters as a whole were still compact and had not resolved into individual chromosomes (Fig. 2d–f). These loops occasionally exhibited three stereotypical features of LBC loops: first, the RNP matrix had a thin-to-thick morphology indicative of the direction of transcription (arrows, Fig. 2d); second, the pol II staining appeared as a line of uniform thickness from one end of the loop to the other (Fig. 2e); and finally the loop was DAPI-negative because its DNA axis is so highly extended (Fig. 2f). The thickness of the pol II line is about 0.4 μm and presumably represents the diffraction-limited image of polymerase molecules closely spaced along the DNA axis of the loop (Miller and Hamkalo 1972)
Fig. 2.
Effect of temperature on expansion of sperm heads. a–c A single sperm head 44 h after injection showed minimal expansion when the recipient oocyte was held at 16°C. Note the associated nuclear bodies with a relatively unstained core and a surrounding shell that stains strongly with mAb Y12 against the “Sm” epitope (symmetric dimethylarginine). d–f A single sperm head 42 h after injection was more expanded when the recipient oocyte was held at 24°C. One very long transcription loop extends out from the central cluster. The phase contrast image (a) shows the characteristic “thin-to-thick” loop matrix of ribonucleoprotein, which indicates the direction of transcription (arrows). The pol II antibody (b) shows uniform staining along the entire loop, presumably due to close packing of pol II molecules on the DNA axis. The axis itself is not detectable by DAPI staining (c) because of its extreme attenuation.
Injection of human sperm heads into the newt GV
The most characteristic feature of LBCs is the presence of lateral loops, which consist of either single transcription units (one “thin-to thick” region) or multiple transcription units (two or more such regions)(Gall et al. 1983). Although it was clear from injection of human sperm heads into Xenopus oocytes at 24°C that transcriptionally active LBC loops could be formed, most of the loops were quite short, manifested primarily as a general fuzziness along the chromosome axis. In our earlier experiments with Xenopus sperm heads we noted that the loops on induced LBCs were remarkably large when a heterologous injection was made into the GV of the newt Notophthalmus. Therefore, we carried out a similar experiment by injecting human sperm heads into newt oocytes. The results were dramatic. Not only did the induced human LBCs form more quickly in the newt nuclei, but they were larger and their loops were especially prominent (Fig. 3).
Fig. 3.
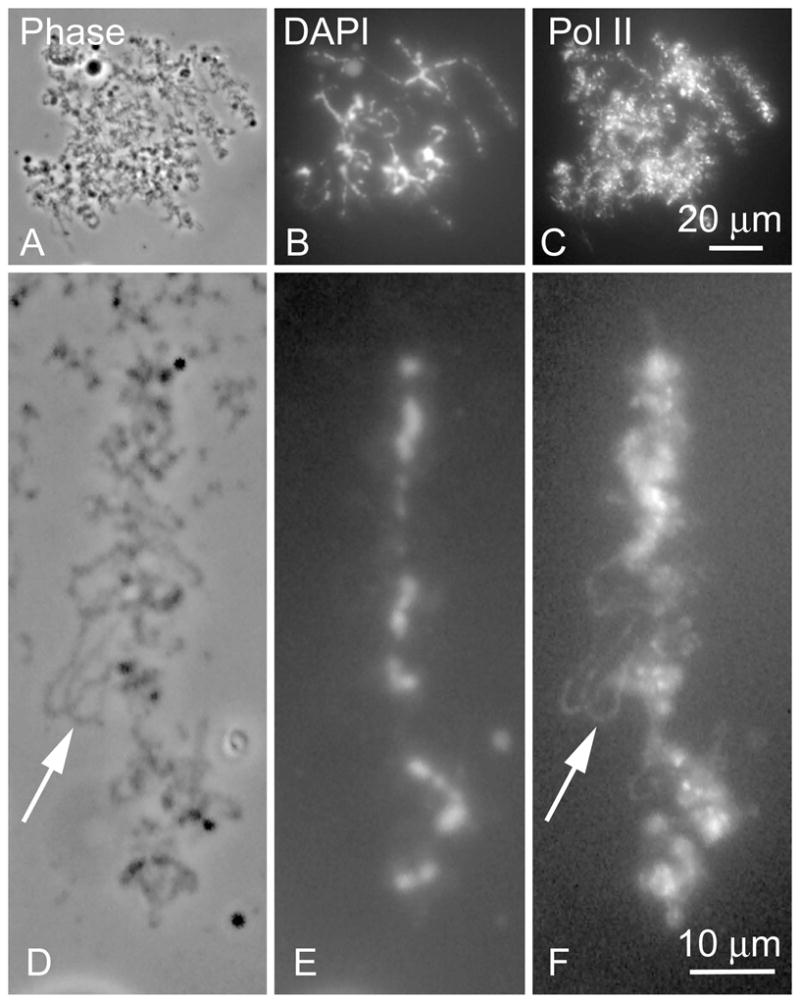
Formation of human LBCs from sperm heads injected into the GV of the newt Notophthalmus. a–c A single sperm head 25 h after injection has resolved into a loose cluster of individual chromatids that show active transcription (pol II stain in c). d–f A single human chromatid with an overall length of about 60 μm. Note the long transcription loops (arrow) visible by phase contrast (d) and by staining with an antibody against pol II (f). DAPI staining reveals condensed chromatin along the chromosome axis (e).
Association of nuclear bodies with human LBCs
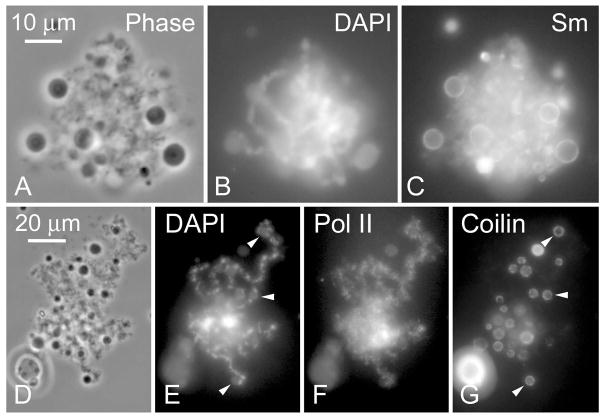
At an early stage in their expansion human sperm heads become associated with one or more spherical, phase-dark bodies up to 6–7 μm in diameter (Fig. 2a and 4a). An antibody against the protein coilin (mAb H1) showed preferential staining of a thin rim on the periphery of these bodies (Fig. 4g), as did an antibody against symmetric dimethylarginine (mAb Y12) (Figs. 2b and 4c). In favourable cases where the human LBCs were individually recognizable, one could see that the spherical bodies were attached directly to the DAPI-positive axis of the chromosomes (Fig. 4e and g, arrowheads). Both the staining pattern and the attachment to the LBCs is highly reminiscent of the newly described “pearls” of X. laevis and X. tropicalis (Nizami and Gall, this volume). Pearls have a coilin-positive rim surrounding a core that contains U85 scaRNA and U3 snoRNA. Pearls are attached to chromosomal loci that stain with antibodies against pol III and they disappear when oocytes are treated with inhibiters of pol III activity. Further study will be needed to confirm that the bodies associated with human LBCs are the same as pearls.
Fig. 4.
Association of nuclear bodies with human LBCs. a–c At an early stage of expansion the human sperm heads are often associated with spherical nuclear bodies (a) whose periphery stains strongly with mAb Y12 against the “Sm” epitope (symmetric dimethylarginine) (c). d–g When the sperm head is more expanded and individual chromosomes are distinguishable, one can see that the nuclear bodies are attached to the DNA axis of the chromosomes (arrowheads in e and g). An antibody against the protein coilin preferentially stains the cortex of the bodies (g).
It is also significant that the bodies associated with the human LBCs stain with anti-coilin mAb H1. This antibody is highly species-specific for Xenopus, not staining coilin in either newt or human cells. Thus it is clear that the coilin in the nuclear bodies associated with the human LBCs is Xenopus coilin. In our earlier injection experiments we showed that the loops on Xenopus LBCs derived by injection of Xenopus sperm heads into newt GVs stained strongly with an antibody against a newt-specific protein. Both examples demonstrate that endogenous proteins are used for assembly of the induced LBCs and associated bodies.
Discussion
The major finding of this study is that condensed human chromatin from sperm heads is able to form typical LBCs when placed in the environment of the amphibian oocyte nucleus. Although a formal definition of a LBC is difficult to make, for purposes of the discussion here we mean a giant chromosome with transcriptionally active lateral loops visible by conventional light microscopy. Our earlier experiments involved a detailed analysis of the LBCs formed when X. laevis sperm heads were injected into the GV of the same species. We showed that the induced LBCs were identical to endogenous LBCs in all essential respects, with the exception that they consisted of single, unpaired chromatids rather than meiotic bivalents. We also showed that sperm heads of the frog Rana pipiens and the zebrafish Danio rerio transformed into recognizable univalent LBCs when injected into the GV of X. laevis. However, experiments with cricket (Acheta domesticus), mouse (Mus musculus), and human sperm heads were unsuccessful, leaving unanswered the question whether the source of the inactive chromatin is important. Our original experiments involved three species that normally have LBCs in their oocyte nuclei, whereas human chromosomes do not go through a LBC stage during oocyte development (or at any other time). Our experiments demonstrate that the absence of LBCs from mammalian oocytes must be due to specific features of mammalian oogenesis and not to permanent genetic or epigenetic features of mammalian chromatin.
Now that we have obtained positive results with human sperm heads, we feel confident in predicting that chromatin from essentially any sperm or germ line source can be converted to the LBC state, so long as it can be made accessible to factors in the GV. Whether chromatin from fully differentiated somatic cells can be similarly converted to the LBC state remains to be demonstrated.
The importance of factors in the GV, as opposed to the source of the chromatin, is underscored by comparing injections into frog and newt GVs. In our earlier experiments we showed that induced Xenopus LBCs were larger and had more prominent lateral loops when formed in the newt GV (Notophthalmus) than in the endogenous frog GV (Xenopus). The same is true in our current experiments with human LBCs. A comparison of the LBCs in Figs. 1 and 4 with those in Fig. 3 shows a dramatic difference. It is well known that LBCs of the newt are much larger than those of Xenopus, and it has frequently been assumed that this difference is related to the large difference in total genomic DNA of the two species (3 pg versus 35 pg in the haploid genome). Our injection experiments raise the interesting possibility that at least some of the differences in LBC size are related to specific factors in the GV, not simply to differences in genomic DNA content.
At present we have very little information concerning the endogenous factors in the GV that are involved. We know that pol II goes onto the chromatin at an early stage, but we assume that the chromatin itself must be modified before transcription can begin. The GV and its giant chromosomes provide a uniquely favourable system in which to study both the changes that occur during reprogramming of the chromatin and the factors in the nucleoplasm that are responsible for the reprogramming.
Acknowledgments
The ideas presented here owe much to discussions with Svetlana Deryusheva, Garry Morgan, Zehra Nizami, and Jun Wei Pek. This work was supported by research grant R01 GM33397 from the National Institute of General Medical Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. JGG is American Cancer Society Professor of Developmental Genetics.
Abbreviations
- DAPI
4′,6-diamidino-2-phenylindole
- GV
germinal vesicle
- LBC
lampbrush chromosome
- Pol II
RNA polymerase 2
- Pol III
RNA polymerase 3
- RNP
ribonucleoprotein
- PVP
polyvinylpyrrolidone
- snRNP
small nuclear ribonucleoprotein
References
- Brahms H, Raymackers J, Union A, de Keyser F, Meheus L, Lührmann R. The C-terminal RG dipeptide repeats of the spliceosomal Sm proteins D1 and D3 contain symmetrical dimethylarginines, which form a major B-cell epitope for anti-Sm autoantibodies. J Biol Chem. 2000;275:17122–17129. doi: 10.1074/jbc.M000300200. [DOI] [PubMed] [Google Scholar]
- Bregman DB, Du L, van der Zee S, Warren SL. Transcription-dependent redistribution of the large subunit of RNA polymerase II to discrete nuclear domains. J Cell Biol. 1995;129:287–298. doi: 10.1083/jcb.129.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan HG. Lampbrush Chromosomes. Springer-Verlag; Berlin: 1986. [Google Scholar]
- Callan HG, Lloyd L. Lampbrush chromosomes of crested newts Triturus cristatus (Laurenti) Philos Trans R Soc Lond B Biol Sci. 1960;243:135–219. [Google Scholar]
- Flemming W. Zellsubstanz, Kern und Zelltheilung. F. C. W. Vogel; Leipzig: 1882. [Google Scholar]
- Gaginskaya E, Kulikova T, Krasikova A. Avian lampbrush chromosomes: a powerful tool for exploration of genome expression. Cytogen Gen Res. 2009;124:251–267. doi: 10.1159/000218130. [DOI] [PubMed] [Google Scholar]
- Gall JG, Diaz MO, Stephenson EC, Mahon KA. The transcription unit of lampbrush chromosomes. In: Subtelny S, Kafatos F, editors. Gene Structure and Regulation in Development. Alan R. Liss; New York: 1983. pp. 137–146. [Google Scholar]
- Gall JG, Murphy C. Assembly of lampbrush chromosomes from sperm chromatin. Mol Biol Cell. 1998;9:733–747. doi: 10.1091/mbc.9.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall JG, Wu Z. Examining the contents of isolated Xenopus germinal vesicles. Methods. 2010;51:45–51. doi: 10.1016/j.ymeth.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall JG, Wu Z, Murphy C, Gao H. Structure in the amphibian germinal vesicle. Exp Cell Res. 2004;296:28–34. doi: 10.1016/j.yexcr.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Gurdon JB. Injected nuclei in frog oocytes: fate, enlargement, and chromatin dispersal. J Embryol Exp Morphol. 1976;36:523–540. [PubMed] [Google Scholar]
- Lerner EA, Lerner MR, Janeway CA, Steitz JA. Monoclonal antibodies to nucleic acid-containing cellular constituents: Probes for molecular biology and autoimmune disease. Proc Natl Acad Sci USA. 1981;78:2737–2741. doi: 10.1073/pnas.78.5.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller OL, Jr, Hamkalo BA. Visualization of RNA synthesis on chromosomes. Int Rev Cytol. 1972;33:1–25. doi: 10.1016/s0074-7696(08)61446-1. [DOI] [PubMed] [Google Scholar]
- Morgan GT. Lampbrush chromosomes and associated bodies: new insights into principles of nuclear structure and function. Chromosome Res. 2002;10:177–200. doi: 10.1023/a:1015227020652. [DOI] [PubMed] [Google Scholar]
- Newmeyer DD, Wilson KL. Egg extracts for nuclear import and nuclear assembly reactions. In: Kay BK, Peng HB, editors. Xenopus laevis: Practical Uses in Cell and Molecular Biology. Academic Press; San Diego, CA: 1991. pp. 607–634. [DOI] [PubMed] [Google Scholar]
- Rückert J. Zur Entwickelungsgeschichte des Ovarialeies bei Selachiern. Anat Anz. 1892;7:107–158. [Google Scholar]
- Tuma RS, Stolk JA, Roth MB. Identification and characterization of a sphere organelle protein. J Cell Biol. 1993;122:767–773. doi: 10.1083/jcb.122.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RA, Jared DW, Dumont JN, Sega MW. Protein incorporation by isolated amphibian oocytes: III. Optimum incubation conditions. J Exp Zool. 1973;184:321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]