Abstract
The dermal extracellular matrix (ECM) provides strength and resiliency to skin. The ECM consists mostly of type I collagen fibrils, which are produced by fibroblasts. Binding of fibroblasts to collagen fibrils generates mechanical forces, which regulate cellular morphology and function. With aging, collagen fragmentation reduces fibroblast-ECM binding and mechanical forces, resulting in fibroblast shrinkage and reduced function including collagen production. Here, we report that these age-related alterations are largely reversed by enhancing structural support of the ECM. Injection of dermal filler, cross-linked hyaluronic acid, into the skin of persons over seventy years-old stimulates fibroblasts to produce type I collagen. This stimulation is associated with localized increased of mechanical forces, indicated by fibroblast elongation/spreading, and mediated by up-regulation of type II TGF-β receptor and connective tissue growth factor. Interestingly, enhanced mechanical support of the ECM also stimulates fibroblast proliferation, expands vasculature, and increases epidermal thickness. Consistent with our observations in human skin, injection of filler into dermal equivalent cultures causes elongation of fibroblasts, coupled with type I collagen synthesis, which is dependent on the TGF-β signaling pathway. Thus, fibroblasts in aged human skin retain their capacity for functional activation, which is restored by enhancing structural support of the ECM.
INTRODUCTION
The dermal extracellular matrix (ECM) plays vital roles in structural support, immunity, circulation, and sensory perception (Fisher et al., 2008; Quan et al., 2009; Uitto and Bernstein, 1998). Dermal ECM supports the epidermis and consists mostly of type I collagen fibrils, which are synthesized by fibroblasts. As the most abundant structural protein in the dermis, type I collagen provides strength and resiliency to skin (Fisher et al., 2008).
A wealth of evidence indicates that interactions between adherent cells, such as fibroblasts, and the ECM are critical for cellular function (Dupont et al., 2011; Eckes et al., 2006; Fisher et al., 2008; Grinnell, 2003; Ingber, 1997; Kessler et al., 2001; Lambert et al., 1992; Ruoslahti, 1997; Silver et al., 2003). In healthy young skin, dermal fibroblasts attach to collagen fibrils through transmembrane integrin receptors. Engagement of integrins with the ECM triggers formation of focal adhesion complexes, which couple the ECM to the intracellular actin cytoskeleton (Delon and Brown, 2007; Eckes et al., 2006; Lambert Ch et al., 1998; Olson and Nordheim, 2010). The actin cytoskeletal machinery generates mechanical forces that determine cell shape, which in turn greatly influences fibroblast function (Fisher et al., 2008; Grinnell, 2003; Ingber, 2006; Olson and Nordheim, 2010; Silver et al., 2003).
With aging, dermal collagen fibrils undergo enzyme-catalyzed cleavage (Fisher et al., 2002; Fisher et al., 2008; Fligiel et al., 2003). This degenerative process compromises the mechanical microenvironment of the dermis and impairs fibroblast attachment to the ECM, resulting in reduced mechanical forces (Varani et al., 2006). Consequently, fibroblasts in aged skin display a collapsed cytoplasm and rounded shape, which contrasts with the spread shape of fibroblasts in young skin. Importantly, fibroblasts with a collapsed morphology down-regulate production of type I collagen and up-regulate production of collagen-degrading matrix metalloproteinases (MMPs) (Fisher et al., 2008; Fligiel et al., 2003; Varani et al., 2006; Varani et al., 2001).
The TGF-β signaling pathway is influenced by mechanical force and pivotal to dermal fibroblast function (Eckes et al., 2006; Fisher et al., 2009; Rittie and Fisher, 2002; Varga and Jimenez, 1995). TGF-β is a multifunctional cytokine that acts through a receptor complex composed of type I, II, and III TGF-β receptors (TβR) (Massague and Gomis, 2006). TGF-β induces connective tissue growth factor (CTGF/CCN2), which in concert with TGF-β regulates fibroblast function, including synthesis of type I procollagen and other ECM proteins (Duncan et al., 1999; Oliver et al., 2010; Quan et al., 2002a; Quan et al., 2010; Rittie and Fisher, 2002). In fibroblasts in aged skin, reduced TGF-β-mediated signaling and CTGF/CCN2 expression contribute to decreased collagen production (Quan et al., 2006; Quan et al., 2010).
Clinically, impaired fibroblast function, coupled with reduced collagen synthesis, translates into atrophy, wrinkling, and fragility of aged skin (Fisher et al., 2008; Lapiere, 1990). We hypothesized that fibroblast function in naturally aged skin could be stimulated by enhancing structural support of the ECM with an injectable space-filling material, cross-linked hyaluronic acid (Wang et al., 2007). Hyaluronic acid (HA), which is a component of the extracellular matrix in all tissues, is a glycosaminoglycan disaccharide composed of repeating units of d-glucuronic acid and N-acetyl-d-glucosamine. Hyaluronic acid-based dermal fillers are composed of disacharide chains ranging in molecular weight from 500,000–6,000,000 that are cross-linked with butanediol diglycidyl ether (Kablik et al., 2009). We report that injection of this filler induces fibroblast spreading, in turn stimulating type I collagen production. Our data, therefore, demonstrate that fibroblasts in aged skin retain their capacity for functional activation, highlighting the importance of the ECM microenvironment in regulating fibroblast behavior. Thus, aging of connective tissue in skin, and perhaps other organs, is largely attributable to alterations in the extracellular microenvironment, in addition to inherent cellular changes.
RESULTS
Expression of type I procollagen in aged human skin is restored by enhancing structural support of the dermal ECM
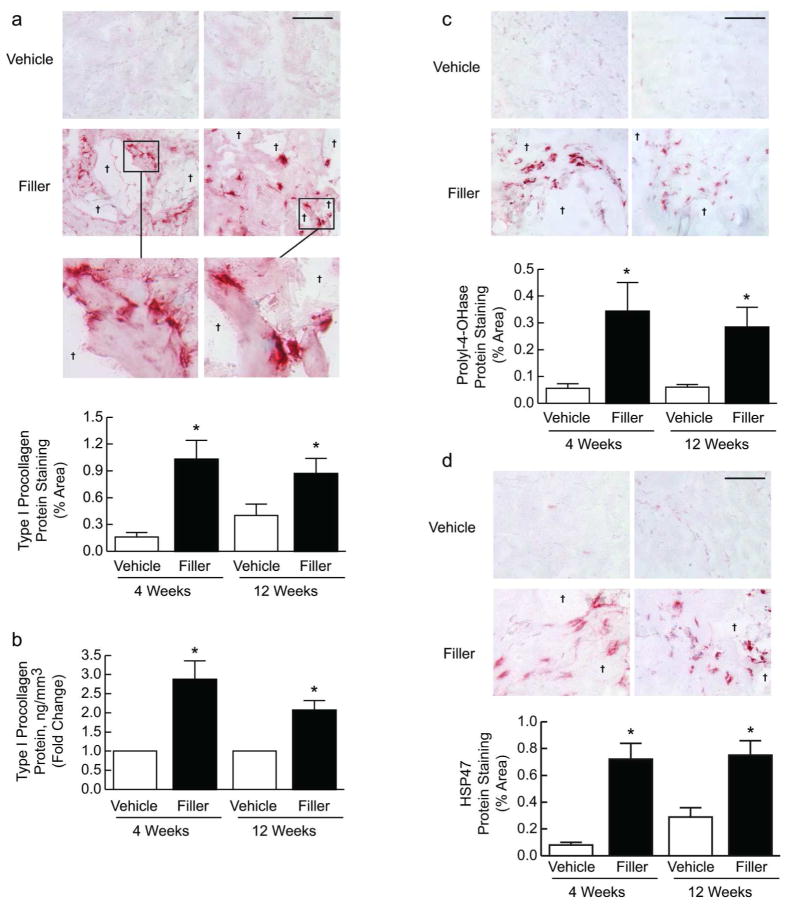
We injected vehicle (saline) or filler into buttock skin of aged individuals (81.4±1.0 years-old) and obtained biopsies at 1, 2, 4 and 12 weeks later. We initially evaluated whether injection caused an inflammatory response. Neither clinical nor histological inflammation (determined by immunostaining for immune/inflammatory monocytes, macrophages, neutrophils) was observed in any subject during the course of the study (data not shown). This lack of inflammation following injection of cross-linked hyaluronic acid has been previously reported (Flynn et al., 2011). As reduced fibroblast function and ECM synthesis are prominent features of aged skin, we first analyzed the localization of type I procollagen protein expression by immunohistochemistry. Type I procollagen is the precursor of mature type I collagen fibrils. Compared with vehicle injection, filler injection induced intense immunostaining within the ECM and dermal fibroblasts (Fig. 1A). Staining was particularly strong adjacent to pockets of injected filler, which were present primarily in the mid to lower dermis. Interestingly, positively stained fibroblasts tended to align around pockets of injected filler and exhibited an enlarged, elongated morphology, indicating increased mechanical force and structural support within the dermal ECM (Fig. 1A, inset). Elongated fibroblasts were mostly embedded within ECM fibers surrounding pockets of injected filler, but not directly contacting the filler material. Overall, the amount of staining was increased 6-fold at 4 weeks post-filler injection and remained elevated at least 12 weeks (Fig. 1A). Quantitation by ELISA confirmed type I procollagen protein induction (Fig. 1B).
Figure 1. Type I procollagen expression in aged human skin is restored by enhancing structural support of the dermal microenvironment.
Aged human skin was biopsied 4 and 12 weeks after injection of vehicle or filler. (A) Immunostaining of type I procollagen protein at 4 weeks (left panels) and 12 weeks (right panels) (n=21). Insets display elongated morphology of immunostained fibroblasts adjacent to pools of filler (†). Graphs display staining quantification. (B) The level of type I procollagen protein was determined by ELISA (n=22). Immunostaining localization of (C) prolyl-4-hydroxylase (n=22) and (D) heat shock protein 47 (HSP47, n=10) to fibroblasts adjacent to pools of filler (†) at 4 weeks (left panels) and 12 weeks (right panels), with quantification of staining. All images, bars=100μm; means+SEM. *p<0.05.
Additionally, we performed immunostaining for two proteins induced in fibroblasts actively producing type I procollagen. Prolyl-4-hydroxylase catalyzes the formation of hydroxyproline, which is required for stable assembly of the triple helical region of type I collagen, and heat shock protein 47 (HSP47) is an intracellular molecular chaperone required for shuttling type I procollagen through the endoplasmic reticulum during synthesis. Staining patterns for prolyl-4-hydroxylase (Fig. 1C) and HSP47 (Fig. 1D) were similar to that of type I procollagen, with elongated/spread fibroblasts surrounding filler displaying increased and intense staining at 4 and 12 weeks, compared with vehicle-treated skin (Fig. 1C,D).
To complement our immunostaining results, we measured gene expression of type I procollagen, prolyl-4-hydroxylase, HSP47, and type III procollagen, the precursor of type III collagen, which associates with type I collagen fibrils. Expression of these genes was significantly induced at 4 weeks post-filler injection, and with the exception of HSP47, their expression remained elevated at least 12 weeks (Supplemental Fig. 1). These data indicate that enhanced structural support of the dermal ECM induces fibroblast elongation and procollagen synthesis in aged human skin.
Enhanced structural support of the dermal ECM up-regulates type I procollagen expression and the TGF-β pathway specifically in elongated fibroblasts in aged human skin
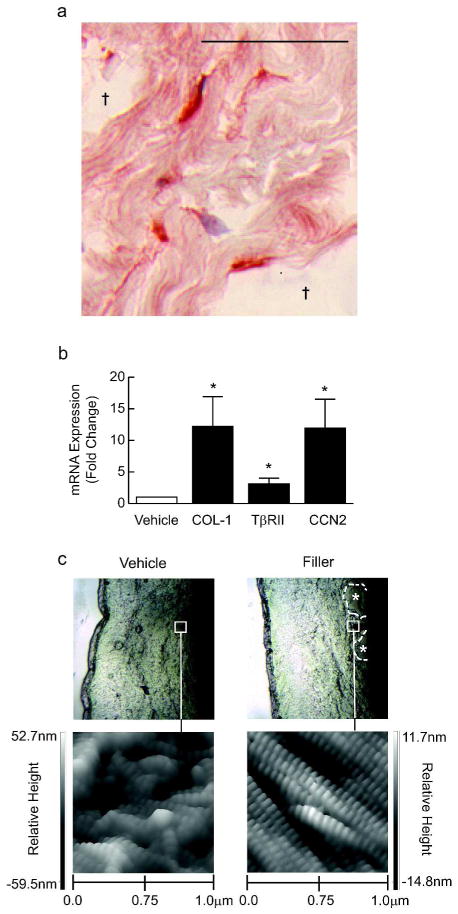
Since procollagen-producing fibroblasts appeared elongated and aligned around pockets of deposited filler (Fig. 2A), we next used laser capture microscopy (LCM) to specifically isolate these cells and analyze their gene expression. Consistent with our immunostaining results, elongated fibroblasts surrounding injected filler demonstrated a 12-fold induction of type I procollagen gene expression, compared with an equivalent number of fibroblasts from the middle and deep dermis of vehicle-injected skin (Fig. 2B).
Figure 2. Enhanced structural support up-regulates TGF-β pathway and collagen deposition in aged human skin fibroblasts.
Skin was obtained 4 weeks after injection of vehicle or filler. (A) Image of pools of injected filler (†), with adjacent elongated fibroblasts immunostained for type I procollagen (bar=50μm). (B) Fibroblasts from vehicle- and filler-injected skin were isolated by laser capture microdissection, and analyzed for type I procollagen (COL-1, n=9), type II TGF-β receptor (TβRII, n=9) and connective tissue growth factor (CCN2, n=7). Means+SEM, *p<0.05. (C) Nanoscale structure of collagen fibrils imaged by atomic force microscopy (n=3). Upper panels display probe location in mid dermis. Collagen fibrils with characteristic banded pattern appear intact, tightly packed, and spatially organized in filler-injected skin, but fragmented and disorganized in vehicle-injected skin.
Additionally, we measured TβRII and CTGF/CCN2 gene expression in LCM-captured fibroblasts. Elongated fibroblasts adjacent to filler exhibited a 3-fold and 10-fold induction of TβRII and CTGF/CCN2, respectively, compared with cells from vehicle-injected skin (Fig. 2B). These data indicate that enhanced structural support of the dermal ECM up-regulates the TGF-β pathway through induction of TβRII and CTGF/CCN2 in elongated fibroblasts in aged human skin.
Deposition of mature collagen is increased by enhancing structural support of the dermal ECM in aged human skin
Having found that enhanced mechanical support of the ECM promotes type I procollagen synthesis, we next considered whether newly made procollagen is processed to form stable collagen fibrils. To address this question, we used atomic force microscopy (AFM) to assess the nanoscale structure of collagen fibrils. In vehicle-injected skin, collagen fibrils in the mid and deep dermis appeared disorganized and fragmented (Fig. 2C, left panel). However, in areas adjacent to injected filler, we observed highly organized, dense bundles of collagen fibrils, with characteristic banded structure (D-spacing) representing the staggered alignment of individual collagen molecules within fibrils (Fig. 2C, right panel). These highly-organized bundles extended from pockets of injected filler as far away as approximately 500 μm. More distantly, collagen fibrils appeared similar to those in vehicle-injected skin (Supplemental Fig. 2). Additionally, we performed a metabolic labeling assay to measure the rate of production of insoluble collagen fibrils. Skin samples obtained 4 weeks after vehicle or filler injection were incubated with [14C]-proline, and insoluble collagen was extracted after 48 hours. The level of radioactivity was 90% greater in filler- versus vehicle-injected skin (p<0.05, data not shown). These findings indicate that enhanced structural support of the dermal ECM stimulates synthesis of procollagen, which is processed into mature collagen in aged human skin.
Enhanced structural support of the dermal ECM is associated with increased epidermal proliferation and thickening in aged human skin
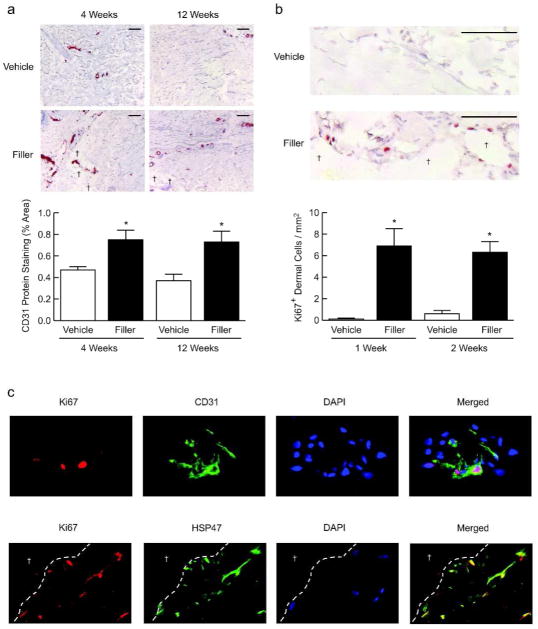
Aged human skin is characterized by a thinned epidermis, caused in part by decreased proliferation of basal keratinocytes (Zouboulis and Makrantonaki, 2011). Interestingly, we noticed that epidermal thickness appeared greater following injection of filler, compared with vehicle. Indeed, quantitative morphometric analyses revealed that epidermal thickness was increased 19% and 14% at 4 and 12 weeks, respectively, after filler injection (Fig. 3A). Additionally, keratinocyte proliferation, assessed by Ki67 immunostaining, was significantly increased within 1–2 weeks after filler injection (Fig. 3B). Thus, enhanced structural support of the dermal ECM is associated with increased keratinocyte proliferation and epidermal thickening.
Figure 3. Increased epidermal thickening and keratinocyte proliferation is associated with enhanced structural support of the dermal microenvironment in aged human skin.
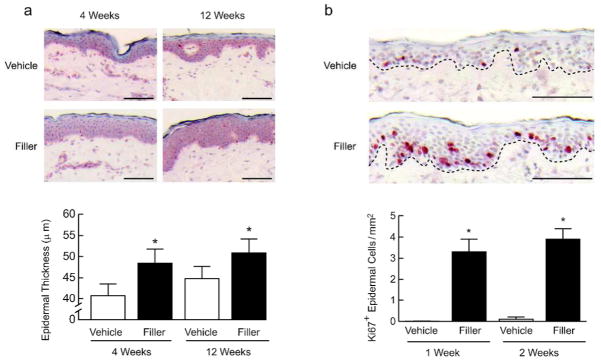
Aged human skin was injected with vehicle or filler. Skin samples were obtained between 1 and 12 weeks later. (A) Epidermal thickness of skin samples was measured by computerized image analysis (n=21, bars=100μm). (B) Immunostaining of the proliferation marker Ki67, with positive cell staining in the epidermis quantified by image analysis (n=6, bars=100μm), and representative images at 1 week post-injection shown. Bar graphs display means+SEM. *p<0.05.
Enhanced structural support of the dermal ECM is associated with proliferation of endothelial cells and fibroblasts in aged human skin
In addition to epidermal changes, we noticed increased prominence of blood vessels in the mid to deep dermis in filler-injected skin. Indeed, immunostaining for the endothelial cell marker CD31 revealed increased staining by 60% and 97% at 4 and 12 weeks, respectively, after filler injection, compared with vehicle injection (Fig. 4A).
Figure 4. Proliferation of endothelial cells and fibroblasts is associated with enhanced structural support of the dermal microenvironment in aged human skin.
Aged human skin was injected with vehicle or filler, and biopsied between 1 and 12 weeks later. Immunostaining of (A) Endothelial cell marker CD31 (n=10, bar=100μm) and (B) Ki67 (n=6, bar=100μm) near pockets of injected filler (†), with quantification of staining. (C) Double-label immunofluorescence staining of the dermis near injected filler, with Ki67 (red) plus CD31 (green, top panels) or HSP47 (green, bottom panels) (n=6). Nuclei are stained with DAPI (blue). Dashed lines separate dermal ECM from pools containing injected filler (†). All representative images are from 2 weeks post-filler injection. Bar graphs, means+SEM. *p<0.05.
Next, having observed increased proliferation of epidermal cells and increased prominence of endothelial cells, we examined proliferation of dermal cells. As early as 1–2 weeks after filler injection, dermal cell proliferation, assessed by Ki67 immunostaining, was readily evident in filler-injected skin, particularly in areas adjacent to the filler material (Fig. 4B). Dermal cell proliferation was rarely detected in vehicle-injected skin.
Positively stained cells in filler-injected skin appeared to include a fraction of endothelial cells and fibroblasts. Therefore, we performed double-label immunofluorescence staining to confirm the identity of proliferating cells. We found Ki67/CD31-positive endothelial cells in vessel structures near pockets of injected filler (Fig. 4C, upper row). Similarly, Ki67/HSP47-positive fibroblasts were localized to areas adjacent to injected filler (Fig. 4C, bottom row). Together, these data indicate enhanced structural support of the dermal ECM is associated with proliferation of endothelial cells and fibroblasts in aged human skin.
Enhanced structural support in three-dimensional collagen lattices induces fibroblast elongation and up-regulates collagen production via the TGF-β signaling pathway
To further elucidate mechanisms by which enhanced structural support stimulates fibroblasts, we employed dermal equivalent cultures, composed of human dermal fibroblasts embedded in three-dimensional (3-D) collagen lattices. After focal injection into collagen lattices, filler material remained confined to pockets at injection sites, where it caused localized expansion of lattices (Fig. 5A). Thus, the space filling property of injected filler in collagen lattices appeared similar to that observed in human skin. Injection of vehicle had no observable effect on lattices.
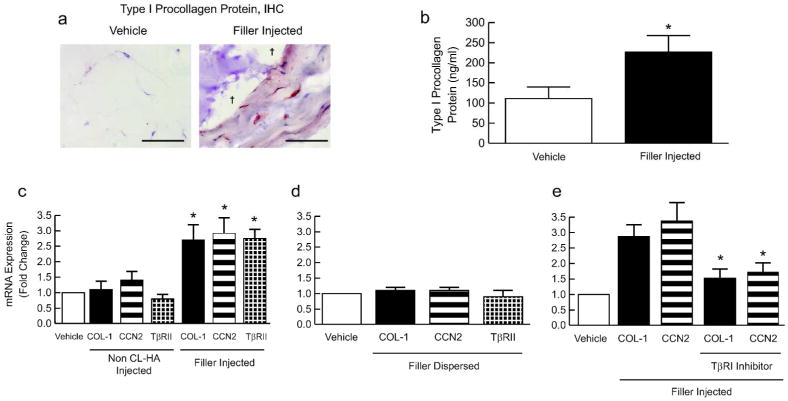
Figure 5. Enhanced structural support of dermal equivalent cultures induces fibroblast elongation and up-regulates TGF-β pathway and collagen production.
Dermal equivalent cultures were analyzed 2 days after treatment. (A) Immunostaining of type I procollagen within elongated fibroblasts adjacent to pools of injected filler (†, purple) (n=3, bar=100μm). (B) Type I procollagen protein secreted into culture media was quantified by ELISA (n=3). Type I procollagen (COL-1), CCN2, and type II TGF-β receptor (TβRII) gene expression following : (C) vehicle, non cross-linked hyaluronic acid (CL-HA), or filler injection into preformed lattices (n=4, *p≤0.05 vs. vehicle), (D) vehicle or filler dispersed in collagen solution prior to lattice formation (n=4), (E) addition of type I TGF-β receptor (TβRI) kinase inhibitor prior to injection of vehicle or filler (n=4,*p<0.05 vs. filler injection without inhibitor).
Consistent with our findings in human skin, we observed intense immunostaining of type I procollagen within elongated fibroblasts adjacent to pockets of injected filler (Fig. 5A). Additionally, protein levels of secreted type I procollagen were increased approximately 2-fold in filler-injected cultures, compared with vehicle injection (Fig. 5B). Filler injection also increased the gene expression of HSP47 and prolyl-4-hydroxylase (Supplemental Fig. 3).
As mentioned previously, expression of type I procollagen is dependent on the TGF-β/CCN2 axis (Quan et al., 2006; Quan et al., 2010). Similar to our data in human skin, we found that type I procollagen, TβRII, and CTGF/CCN2 gene expression were significantly induced following filler injection into dermal equivalent cultures (Fig. 5C). As noted above, expansion of collagen lattices occurs after filler injection. To examine the role of this expansion, non cross-linked hyaluronic acid, which readily diffuses within the lattices and does not cause expansion, was injected. Similar to vehicle injection, injection of non cross-linked hyaluronic acid had no effect on type I procollagen, TβRII, and CTGF/CCN2 gene expression (Fig. 5C).
To further examine the role of lattice expansion in inducing procollagen production, filler material was dispersed into collagen solution prior to lattice formation. Under these conditions, fibroblast morphology appeared similar to that in untreated lattices or lattices injected with vehicle or non-cross-linked hyaluronic acid (data not shown). Furthermore, dispersal of filler, as opposed to injection into preformed lattices, failed to induce type I procollagen, TβRII, or CTGF/CCN2 (Fig. 5D). Thus, lattice deformation was required for up-regulation of fibroblast function.
Finally, we investigated the role of the TGF-β pathway in procollagen induction following filler injection. Addition of TβRI kinase inhibitor to collagen lattices prior to filler injection prevented up-regulation of type I procollagen and CTGF/CCN2 (Fig. 5E), indicating that collagen up-regulation following filler injection is dependent on the TGF-β signaling pathway.
DISCUSSION
We have proposed that accumulation of fragmented collagen during natural skin aging negatively impacts fibroblast function (Fisher et al., 2002; Fisher et al., 2008; Varani et al., 2006; Varani et al., 2004; Varani et al., 2001). Collagen fragmentation alters the physical properties of the dermal microenvironment and reduces ECM binding by fibroblasts, which in turn lessens mechanical force. Under these conditions, fibroblasts down-regulate collagen production and up-regulate MMPs (Fisher et al., 2009; Fisher et al., 2008; Varani et al., 2006). This cellular response promotes further loss and fragmentation of collagen, thereby promoting self-perpetuating progression of the aged phenotype in human skin. Inherent to our model is the concept that quality of the ECM, rather than chronologic age of dermal fibroblasts, is a key determinant of age-dependent decline of fibroblast function.
In this study, we used a space-filling material, cross-linked hyaluronic acid, as a tool to test the hypothesis that enhanced structural support could stimulate fibroblast function in aged skin. We observed that the filler, when injected focally into skin, distributes in the dermis as large pools, filling space and pushing against the surrounding ECM. Adjacent to these pockets of filler, fibroblasts display an elongated morphology, indicating increased mechanical force and structural support within the dermal ECM. Importantly, fibroblast elongation is associated with up-regulation of the TGF-β signaling pathway, and its downstream targets CTGF/CCN2 and type I procollagen. Thus, we found that structural properties of the dermal ECM play a significant role in modulating fibroblast function during human skin aging. Furthermore, we conclude that impaired fibroblast function in aged human skin is not solely due to irreversible cellular alterations, but instead dynamically responsive and, in part, reversible via manipulation of the ECM microenvironment (Varani et al., 2002).
We considered the possibility that fibroblast stimulation may occur by direct binding of filler to cellular receptors. Addition of exogenous, monomeric hyaluronic acid (HA) to cultured fibroblasts has been reported to trigger TGF-β signaling and collagen production (David-Raoudi et al., 2008; Mast et al., 1993). Some of these responses are mediated by binding of HA to CD44, a cell surface glycoprotein (David-Raoudi et al., 2008). However, other studies have not reproduced these observations (Croce et al., 2001; Huang et al., 2009). Here, we observed that collagen-producing, elongated fibroblasts were embedded within the ECM adjacent to injected filler and did not appear to directly contact the filler. Moreover, uniform dispersion of filler in dermal equivalent cultures, unlike focal injection, failed to induce fibroblast elongation or procollagen synthesis. These data make it unlikely that injected filler acts through direct interactions with fibroblast receptors.
Our findings, in fact, suggest that collagen production following filler injection occurs by enhanced structural support within the dermal ECM. Supporting this interpretation is a wealth of evidence, derived from model systems indicating that morphology and function of adherent cells are linked by mechanical properties of the ECM (Dupont et al., 2011; Eckes et al., 2006; Fisher et al., 2008; Grinnell, 2003; Ingber, 1997; Kessler et al., 2001; Lambert et al., 1992; Ruoslahti, 1997; Silver et al., 2003; Varani et al., 2006; Varani et al., 2002). For instance, when cultured with fragmented collagen fibrils, fibroblasts display a collapsed appearance, indicative of low mechanical force. These cells lack direct attachment to the ECM and adopt a catabolic phenotype, with decreased collagen synthesis and up-regulation of MMP-1 (Eckes et al., 2006; Kessler et al., 2001; Varani et al., 2006; Varani et al., 2002; Varani et al., 2004). This situation reflects aged human skin (Fisher et al., 2009; Fisher et al., 2008; Fligiel et al., 2003). In contrast, immobilized 3-D matrices composed of intact collagen fibrils provide a stable framework for fibroblast adherence via integrins (Barczyk et al., 2010). In this setting, fibroblasts display an elongated/spread morphology, coupled with procollagen synthesis. This scenario reflects healthy young human skin (Fisher et al., 2008; Fligiel et al., 2003; Quan et al., 2010; Silver et al., 2003; Varani et al., 2006).
Interestingly, fibroblasts cultured in mechanically stiff ECM also up-regulate production of TGF-β and its effector CTGF/CCN2 (Eckes et al., 2006; Garrett et al., 2004; Kessler et al., 2001; Skutek et al., 2001). TGF-β-mediated signaling, in turn, modulates fibroblast responses to mechanical force by stimulating integrin expression and reorganization of the actin cytoskeleton, suggesting that TGF-β is a “mechanoregulatory” growth factor (Brown et al., 2002; Grinnell and Ho, 2002). Additionally, expression of TβRII is reduced in dermal fibroblasts in aged human skin, thus decreasing cellular responsiveness to TGF-β (Quan et al., 2006).
Given these observations, we propose that filler injection into aged skin stiffens the ECM, which induces fibroblast elongation and activation. The result is up-regulation of the TGF-β pathway leading to synthesis and deposition of collagen. Since mature collagen has an estimated half-life of 15 years (Verzijl et al., 2000), it is likely that newly formed collagen fibrils facilitate additional elongation/spreading of fibroblasts and, hence, further activation of TGF-β signaling.
We found that filler injection stimulates localized proliferation of fibroblasts, many of which are synthetically active. Fibroblast proliferation can be driven by numerous mechanisms, including increased mechanical force (Eckes et al., 2006; Varani et al., 2002). It has been reported that substrate stiffness controls proliferation in a variety of cell types through integrin-dependent signaling to FAK, Rac, and cyclin D1 (Klein et al., 2009). Similar mechanisms may be operative in human skin in response to enhanced structural support by injection of cross-linked hyaluronic acid. Increased fibroblast number would be expected to contribute to collagen production following filler injection.
Along with decreased fibroblast function and proliferation, reduced vasculature and epidermal thinning contribute to fragility and impaired wound healing in aged skin (Chung and Eun, 2007; Holt et al., 1992; Zouboulis and Makrantonaki, 2011). Here, we observed that enhanced structural support of the dermal ECM is associated with proliferation of endothelial cells and keratinocytes. Based on previous studies, proliferation of these cell types might result from increased mechanical force (Chen et al., 1997; Reichelt, 2007), or production of diffusible mediators, such as vascular endothelial growth factor (VEGF), TGF-β, CTGF/CCN2, and/or cysteine-rich angiogenic inducer 61 (Chen et al., 2001; Chen and Du, 2007; Kessler et al., 2001; Shirakata, 2010). Additional studies may clarify which of these mechanisms are involved in stimulating endothelial and keratinocyte proliferation following enhancement of structural support.
Together, our findings extend current knowledge of mechanisms of skin aging beyond intrinsic cellular processes to include the dermal ECM microenvironment. Our data indicate that collagen production in aged skin can be substantially restored. Restoration of this synthetic capacity is intimately linked with structural integrity/support of the dermal ECM, which dynamically interacts with fibroblasts and modulates their function and proliferation. Our data also suggest that proliferation and function of other cell types, including endothelial cells and keratinocytes, can be enhanced in aged skin. These findings provide a rationale for maintaining and/or enhancing the structural integrity of dermal ECM, which in turn may improve the health, function, and wound healing capacity of aged human skin.
METHODS
Procurement of skin specimens
This study was approved by our Institutional Review Board, and conducted according to the Declaration of Helsinki principles. Healthy volunteers (74–95 years-old, 18 females, 10 males) provided written informed consent and underwent 2 injections of filler (Restylane®, cross-linked hyaluronic acid, Medicis, Scottsdale, AZ) and 2 injections of vehicle (0.9% NaCl in sterile water) into buttock skin. Injections were each 0.5ml and spaced 2–4 cm apart. Later, sites were located reliably, and punch biopsies (4 mm) were obtained under local anesthesia (lidocaine) at 4 and 12 weeks (n=22), or 1 and 2 weeks (n=6). Specimens were stored in OCT at −80°C, or immediately placed into cell culture medium for collagen-labeling studies.
Type I procollagen ELISA
Using whole cell extracts from cryosections (1000μm), type I procollagen levels were measured, according to manufacturer protocol (Takara Bio, Japan), as previously described (Orringer et al., 2011).
Immunohistochemical/immunofluorescence staining
Staining was performed as described previously (Fisher et al., 2009; Quan et al., 2002b; Quan et al., 2006; Wang et al., 2007) using antibodies against HSP47 (Stressgen Biotechnologies, Victoria, Canada), prolyl-4-hydroxylase (Acris, Hiddenhausen, Germany), type I procollagen (Takara Bio; used in human skin studies), type I procollagen (obtained as described previously (Kang et al., 2005); used in organ culture studies), Ki67 (BioGenex, San Ramon, CA) and CD31 (BD Pharmingen, San Diego, CA). Sections were analyzed with Image-Pro Plus v4.1 (Media Cybernetics, Silver Spring, MD). Matching subtype, non-immune antibodies were used as controls to determine non-specific signal. In all cases, immune antibodies were used at concentrations and fixation conditions that yielded no observable non-specific staining.
Measurement of gene expression
After total RNA extraction (RNeasy Micro kit, Qiagen, Valencia, CA), real-time PCR was performed, as previously described (Quan et al., 2002a, 2004).
Laser capture microdissection
As previously described (Quan et al., 2002a, 2004), approximately 200 fibroblasts from each cryosection (14μm) were collected in lysis buffer (RNeasy Micro kit, Qiagen), followed by RNA extraction and RT-PCR, as described above.
Collagen synthesis in skin organ culture
For each subject, 2 punch biopsies (2mm) of vehicle-injected skin and 2 punch biopsies (2mm) of filler-injected skin were cultured in labeling medium (DMEM/Ham-F12 1:1, v/v, 1% FBS, 50μg/ml L-ascorbic acid, 2 μCi/ml [14C]-proline, and 46 μg/ml L-proline). After incubation at 37°C under 5% CO2 for 2 days, samples were rinsed 3 times in PBS, frozen in liquid nitrogen, powdered, and weighed. Soluble proteins were extracted under rotation for 24 hours at 4°C in 10mM Tris, pH 7.5, 0.15M NaCl, 5mM EDTA, and protease inhibitors (Complete Mini, Roche, Indianapolis, IN), followed by centrifugation at 16,000g at 4°C for 30 minutes. Mature, extractable collagens were released from the resulting pellet by adding 1mg/ml pepsin (Sigma-Aldrich, St. Louis, MO) in 0.5M acetic acid at 4°C for 16 hours, repeated 5 times. Remaining insoluble, cross-linked material was used for radioactivity counting. Counts per minute were normalized to mg of tissue.
Atomic force microscopy
Cryosections (10μm) were mounted on microscope cover glass (1.2mm diameter, Fisher Scientific, Pittsburgh, PA), allowed to air dry at least 24 hours, and examined using a Dimension Icon AFM system (Bruker AXS, Santa Barbara, CA) in tapping mode, with a silicon-etched cantilever (NSC15/AIBS, MikroMasch, San Jose, CA) with a full tip cone angle ~40° and tip radius of curvature ~10 nm. Images were acquired at a scan rate of 1.0Hz at 512×512 pixel resolution, with integral and proportional gain settings of 0.4 and 0.6, respectively. Image quality was optimized by dynamically lowering the scan rate and setpoint, and increasing the gains and drive amplitude. Images were analyzed using NanoScope Analysis software v1.20 (BrukerAXS).
Dermal equivalent cultures
Collagen lattices were prepared using early passage (<10 passages) primary adult dermal fibroblasts (2×105), obtained as previously described (Fisher et al., 1991), mixed with type I collagen from calf skin (6mg/ml, Elastin Products Company, Owensville, MO) and medium (pH7.2, DMEM, 44mM NaHCO3, 4mM L-glutamine, 9mM folic acid, and 1N NaOH). After formation of lattices (0.5ml/well) (Fisher et al., 2009), 10–15 μl of filler, non cross-linked hyaluronic acid (0.2mg.ml, Sigma Chemical Co., St Louis), or vehicle (PBS) was injected into lattice centers and incubated for 48 hours at 37°C under 5% CO2. Viable cells were recovered, as previously described (Fisher et al., 2009). For certain wells, filler was dispersed/mixed throughout the medium before lattice formation. For TβRI kinase inhibition, lattices were pretreated overnight with SB431542 (10μM, Sigma-Aldrich) or an equivalent volume of vehicle (DMSO), prior to filler injection.
Statistics
Data are presented as means±SEM. When appropriate, logarithmic transformation of data was performed to achieve normality. For data with a small sample size (n<9), normality was assumed. Comparisons between treatment groups were assessed at each time point using the paired t-test. An overall α-level of 0.05 was used to determine statistical significance, and all tests were two-sided. Data were analyzed using SAS v9.2 (SAS Institute, Cary, NC).
Supplementary Material
Acknowledgments
We thank Stephanie Cooke and Ting Li for technical support; Heather Chubb for statistical analyses; Laura VanGoor and Diane Fiolek for assistance with graphical material; and Suzan Rehbine, LPN, for tissue procurement.
Funding/Support
Medicis, Inc., donated filler for research purposes, but had no involvement in the design or conduct of this study or in the collection, analysis, and interpretation of the data. Medicis had no role in the preparation or review of this manuscript, and has not seen the manuscript. This study was supported by AG019364(G.F.), AG031452(G.F.), AG025186(G.F.), and 5T32 AR007197(F.W.) from the National Institutes of Health.
Footnotes
The authors declare no conflicts of interest.
References
- Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–80. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battegay EJ, Raines EW, Seifert RA, Bowen-Pope DF, Ross R. TGF-beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell. 1990;63:515–24. doi: 10.1016/0092-8674(90)90448-n. [DOI] [PubMed] [Google Scholar]
- Brown RA, Sethi KK, Gwanmesia I, Raemdonck D, Eastwood M, Mudera V. Enhanced fibroblast contraction of 3D collagen lattices and integrin expression by TGF-beta1 and -beta3: mechanoregulatory growth factors? Exp Cell Res. 2002;274:310–22. doi: 10.1006/excr.2002.5471. [DOI] [PubMed] [Google Scholar]
- Chen CC, Mo FE, Lau LF. The angiogenic factor Cyr61 activates a genetic program for wound healing in human skin fibroblasts. J Biol Chem. 2001;276:47329–37. doi: 10.1074/jbc.M107666200. [DOI] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–8. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- Chen Y, Du XY. Functional properties and intracellular signaling of CCN1/Cyr61. J Cell Biochem. 2007;100:1337–45. doi: 10.1002/jcb.21194. [DOI] [PubMed] [Google Scholar]
- Chung JH, Eun HC. Angiogenesis in skin aging and photoaging. J Dermatol. 2007;34:593–600. doi: 10.1111/j.1346-8138.2007.00341.x. [DOI] [PubMed] [Google Scholar]
- Croce MA, Dyne K, Boraldi F, Quaglino D, Jr, Cetta G, Tiozzo R, et al. Hyaluronan affects protein and collagen synthesis by in vitro human skin fibroblasts. Tissue Cell. 2001;33:326–31. doi: 10.1054/tice.2001.0180. [DOI] [PubMed] [Google Scholar]
- David-Raoudi M, Tranchepain F, Deschrevel B, Vincent JC, Bogdanowicz P, Boumediene K, et al. Differential effects of hyaluronan and its fragments on fibroblasts: relation to wound healing. Wound Repair Regen. 2008;16:274–87. doi: 10.1111/j.1524-475X.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- Delon I, Brown NH. Integrins and the actin cytoskeleton. Curr Opin Cell Biol. 2007;19:43–50. doi: 10.1016/j.ceb.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Duncan MR, Frazier KS, Abramson S, Williams S, Klapper H, Huang X, et al. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down-regulation by cAMP. FASEB J. 1999;13:1774–86. [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–83. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Eckes B, Zweers MC, Zhang ZG, Hallinger R, Mauch C, Aumailley M, et al. Mechanical tension and integrin alpha 2 beta 1 regulate fibroblast functions. J Investig Dermatol Symp Proc. 2006;11:66–72. doi: 10.1038/sj.jidsymp.5650003. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Henderson PA, Voorhees JJ, Baldassare JJ. Epidermal growth factor-induced hydrolysis of phosphatidylcholine by phospholipase D and phospholipase C in human dermal fibroblasts. J Cell Physiol. 1991;146:309–17. doi: 10.1002/jcp.1041460216. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–70. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Quan T, Purohit T, Shao Y, Cho MK, He T, et al. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am J Pathol. 2009;174:101–14. doi: 10.2353/ajpath.2009.080599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol. 2008;144:666–72. doi: 10.1001/archderm.144.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fligiel SE, Varani J, Datta SC, Kang S, Fisher GJ, Voorhees JJ. Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase-1 in vitro. J Invest Dermatol. 2003;120:842–8. doi: 10.1046/j.1523-1747.2003.12148.x. [DOI] [PubMed] [Google Scholar]
- Flynn TC, Sarazin D, Bezzola A, Terrani C, Micheels P. Comparative histology of intradermal implantation of mono and biphasic hyaluronic acid fillers. Dermatol Surg. 2011;37:637–43. doi: 10.1111/j.1524-4725.2010.01852.x. [DOI] [PubMed] [Google Scholar]
- Frazier K, Williams S, Kothapalli D, Klapper H, Grotendorst GR. Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J Invest Dermatol. 1996;107:404–11. doi: 10.1111/1523-1747.ep12363389. [DOI] [PubMed] [Google Scholar]
- Garrett Q, Khaw PT, Blalock TD, Schultz GS, Grotendorst GR, Daniels JT. Involvement of CTGF in TGF-beta1-stimulation of myofibroblast differentiation and collagen matrix contraction in the presence of mechanical stress. Invest Ophthalmol Vis Sci. 2004;45:1109–16. doi: 10.1167/iovs.03-0660. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 2003;13:264–9. doi: 10.1016/s0962-8924(03)00057-6. [DOI] [PubMed] [Google Scholar]
- Grinnell F, Ho CH. Transforming growth factor beta stimulates fibroblast-collagen matrix contraction by different mechanisms in mechanically loaded and unloaded matrices. Exp Cell Res. 2002;273:248–55. doi: 10.1006/excr.2001.5445. [DOI] [PubMed] [Google Scholar]
- Grotendorst GR. Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev. 1997;8:171–9. doi: 10.1016/s1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]
- Holt DR, Kirk SJ, Regan MC, Hurson M, Lindblad WJ, Barbul A. Effect of age on wound healing in healthy human beings. Surgery. 1992;112:293–7. [PubMed] [Google Scholar]
- Huang L, Gu H, Burd A. A reappraisal of the biological effects of hyaluronan on human dermal fibroblast. J Biomed Mater Res A. 2009;90:1177–85. doi: 10.1002/jbm.a.32173. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–99. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–27. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- Kablik J, Monheit GD, Yu L, Chang G, Gershkovich J. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009;35(Suppl 1):302–12. doi: 10.1111/j.1524-4725.2008.01046.x. [DOI] [PubMed] [Google Scholar]
- Kang S, Cho S, Chung JH, Hammerberg C, Fisher GJ, Voorhees JJ. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691–9. doi: 10.1016/s0002-9440(10)62479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler D, Dethlefsen S, Haase I, Plomann M, Hirche F, Krieg T, et al. Fibroblasts in mechanically stressed collagen lattices assume a “synthetic” phenotype. J Biol Chem. 2001;276:36575–85. doi: 10.1074/jbc.M101602200. [DOI] [PubMed] [Google Scholar]
- Klein EA, Yin L, Kothapalli D, Castagnino P, Byfield FJ, Xu T, et al. Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Curr Biol. 2009;19:1511–8. doi: 10.1016/j.cub.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert CA, Soudant EP, Nusgens BV, Lapiere CM. Pretranslational regulation of extracellular matrix macromolecules and collagenase expression in fibroblasts by mechanical forces. Lab Invest. 1992;66:444–51. [PubMed] [Google Scholar]
- Lambert Ch A, Nusgens BV, Lapiere Ch M. Mechano-sensing and mechano-reaction of soft connective tissue cells. Adv Space Res. 1998;21:1081–91. doi: 10.1016/s0273-1177(98)00031-3. [DOI] [PubMed] [Google Scholar]
- Lapiere CM. The ageing dermis: the main cause for the appearance of ‘old’ skin. Br J Dermatol. 1990;122(Suppl 35):5–11. doi: 10.1111/j.1365-2133.1990.tb16119.x. [DOI] [PubMed] [Google Scholar]
- Massague J, Gomis RR. The logic of TGFbeta signaling. FEBS Lett. 2006;580:2811–20. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Mast BA, Diegelmann RF, Krummel TM, Cohen IK. Hyaluronic acid modulates proliferation, collagen and protein synthesis of cultured fetal fibroblasts. Matrix. 1993;13:441–6. doi: 10.1016/s0934-8832(11)80110-1. [DOI] [PubMed] [Google Scholar]
- Oliver N, Sternlicht M, Gerritsen K, Goldschmeding R. Could aging human skin use a connective tissue growth factor boost to increase collagen content? J Invest Dermatol. 2010;130:338–41. doi: 10.1038/jid.2009.331. [DOI] [PubMed] [Google Scholar]
- Olson EN, Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol. 2010;11:353–65. doi: 10.1038/nrm2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orringer JS, Rittie L, Hamilton T, Karimipour DJ, Voorhees JJ, Fisher GJ. Intraepidermal erbium:YAG laser resurfacing: impact on the dermal matrix. J Am Acad Dermatol. 2011;64:119–28. doi: 10.1016/j.jaad.2010.02.058. [DOI] [PubMed] [Google Scholar]
- Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Connective tissue growth factor: expression in human skin in vivo and inhibition by ultraviolet irradiation. J Invest Dermatol. 2002a;118:402–8. doi: 10.1046/j.0022-202x.2001.01678.x. [DOI] [PubMed] [Google Scholar]
- Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Ultraviolet irradiation alters transforming growth factor beta/smad pathway in human skin in vivo. J Invest Dermatol. 2002b;119:499–506. doi: 10.1046/j.1523-1747.2002.01834.x. [DOI] [PubMed] [Google Scholar]
- Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-beta type II receptor/Smad signaling. Am J Pathol. 2004;165:741–51. doi: 10.1016/s0002-9440(10)63337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, He T, Shao Y, Lin L, Kang S, Voorhees JJ, et al. Elevated cysteine-rich 61 mediates aberrant collagen homeostasis in chronologically aged and photoaged human skin. Am J Pathol. 2006;169:482–90. doi: 10.2353/ajpath.2006.060128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ, Fisher GJ. Matrix-degrading metalloproteinases in photoaging. J Investig Dermatol Symp Proc. 2009;14:20–4. doi: 10.1038/jidsymp.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, Shao Y, He T, Voorhees JJ, Fisher GJ. Reduced expression of connective tissue growth factor (CTGF/CCN2) mediates collagen loss in chronologically aged human skin. J Invest Dermatol. 2010;130:415–24. doi: 10.1038/jid.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt J. Mechanotransduction of keratinocytes in culture and in the epidermis. Eur J Cell Biol. 2007;86:807–16. doi: 10.1016/j.ejcb.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Rittie L, Fisher GJ. UV-light-induced signal cascades and skin aging. Ageing Res Rev. 2002;1:705–20. doi: 10.1016/s1568-1637(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Stretching is good for a cell. Science. 1997;276:1345–6. doi: 10.1126/science.276.5317.1345. [DOI] [PubMed] [Google Scholar]
- Shirakata Y. Regulation of epidermal keratinocytes by growth factors. J Dermatol Sci. 2010;59:73–80. doi: 10.1016/j.jdermsci.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Silver FH, Siperko LM, Seehra GP. Mechanobiology of force transduction in dermal tissue. Skin Res Technol. 2003;9:3–23. doi: 10.1034/j.1600-0846.2003.00358.x. [DOI] [PubMed] [Google Scholar]
- Skutek M, van Griensven M, Zeichen J, Brauer N, Bosch U. Cyclic mechanical stretching modulates secretion pattern of growth factors in human tendon fibroblasts. Eur J Appl Physiol. 2001;86:48–52. doi: 10.1007/s004210100502. [DOI] [PubMed] [Google Scholar]
- Uitto J, Bernstein EF. Molecular mechanisms of cutaneous aging: connective tissue alterations in the dermis. J Investig Dermatol Symp Proc. 1998;3:41–4. [PubMed] [Google Scholar]
- Varani J, Dame MK, Rittie L, Fligiel SE, Kang S, Fisher GJ, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168:1861–8. doi: 10.2353/ajpath.2006.051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J, Perone P, Fligiel SE, Fisher GJ, Voorhees JJ. Inhibition of type I procollagen production in photodamage: correlation between presence of high molecular weight collagen fragments and reduced procollagen synthesis. J Invest Dermatol. 2002;119:122–9. doi: 10.1046/j.1523-1747.2002.01810.x. [DOI] [PubMed] [Google Scholar]
- Varani J, Schuger L, Dame MK, Leonard C, Fligiel SE, Kang S, et al. Reduced fibroblast interaction with intact collagen as a mechanism for depressed collagen synthesis in photodamaged skin. J Invest Dermatol. 2004;122:1471–9. doi: 10.1111/j.0022-202X.2004.22614.x. [DOI] [PubMed] [Google Scholar]
- Varani J, Spearman D, Perone P, Fligiel SE, Datta SC, Wang ZQ, et al. Inhibition of type I procollagen synthesis by damaged collagen in photoaged skin and by collagenase-degraded collagen in vitro. Am J Pathol. 2001;158:931–42. doi: 10.1016/S0002-9440(10)64040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga J, Jimenez SA. Modulation of collagen gene expression: its relation to fibrosis in systemic sclerosis and other disorders. Ann Intern Med. 1995;122:60–2. doi: 10.7326/0003-4819-122-1-199501010-00010. [DOI] [PubMed] [Google Scholar]
- Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275:39027–31. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- Wang F, Garza LA, Kang S, Varani J, Orringer JS, Fisher GJ, et al. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007;143:155–63. doi: 10.1001/archderm.143.2.155. [DOI] [PubMed] [Google Scholar]
- Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011;29:3–14. doi: 10.1016/j.clindermatol.2010.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.