Abstract
Structural studies of membrane proteins are still hampered by difficulties of finding appropriate membrane mimicking media that maintain protein structure and function. Phospholipid nanodiscs seem promising to overcome the intrinsic problems of detergent containing environments. While nanodiscs can offer a near native environment, the large particle size complicates their routine use in the structural analysis of membrane proteins by solution NMR. Here, we introduce nanodiscs assembled from shorter ApoA-I protein variants that are of markedly smaller diameter and show that the resulting discs provide critical improvements for the structure determination of membrane proteins by NMR. Using the bacterial outer membrane protein OmpX as an example, we demonstrate that the combination of small nanodisc size, high deuteration levels of protein and lipids and the use of advanced non-uniform NMR sampling methods enable the NMR resonance assignment as well as the high-resolution structure determination of polytopic membrane proteins in a detergent-free, near-native lipid bilayer setting. By applying this method to bacteriorhodopsin we show that our smaller nanodiscs can also be beneficial for the structural characterization of the important class of seven-transmembrane helical proteins. Our set of engineered nanodiscs of subsequently smaller diameters can be used to screen for optimal NMR spectral quality for small to medium sized membrane proteins while still providing a functional environment. In addition to their key improvements for de novo structure determination, due to their smaller size these nanodiscs enable the investigation of interactions between membrane proteins and their (soluble) partner proteins, unbiased by the presence of detergents that might disrupt biological relevant interactions.
Keywords: Dynamics, Nanodiscs, NMR, OmpX, Structure
INTRODUCTION
Compared to the plethora of structural data on soluble proteins much less is known about structures of integral membrane proteins. This is primarily due to difficulties of producing integral membrane proteins in folded and active forms as well as in sufficient yields. A major part of this problem is the selection of an appropriate membrane-mimicking environment supporting both function and stability of a particular membrane protein. Usually detergents are employed for membrane protein preparation and detergent micelles are the most common media for structural investigations1. However, crystal structures of membrane proteins solubilized in detergents often contain bound lipids emphasizing the beneficial effect of a lipid environment for their structure and stability2. Detergents may also lower membrane protein stability, and abolish their biological function3. In addition, detergents can hamper interaction studies between membrane proteins and soluble factors, as they tend to destabilize or even denature soluble interaction partners. The use of phospholipid bilayers can overcome these problems, as they resemble a considerably more native membrane environment. Phospholipid bilayers can be prepared to match any desired lipid composition, required to stabilize the respective membrane protein. Until recently, phospholipid liposomes were the only means to harbor membrane proteins in a detergent-free bilayer environment. Unfortunately, liposomes are not compatible with crystallization and these particles are too big for solution-state NMR as well. Among NMR methods, solid-state NMR has made progress in studying membrane proteins in large phospholipid bilayer systems6 but signal overlap and low sensitivity are in most cases preventing structural work at high-resolution.
The recent introduction of phospholipid nanodiscs as membrane mimetic5,7,8 appears promising for studying membrane proteins in phospholipid bilayers by solution NMR. The formation of nanodiscs is based on the observation that apolipoprotein A-I (ApoA-I) can wrap around small patches of bilayer creating small membrane-like particles of defined size9,10. Different versions of Apo A-I have been engineered for biophysical studies and are called membrane-scaffolding proteins (MSPs)5. Ideally, two copies of MSP wrap around a patch of a lipid bilayer to form a disc-like particle or nanodisc (Fig. 1A). This system has the potential to be a widely used membrane mimetic with the advantage of closely resembling a native-like lipid environment5. To this end, nanodiscs are the only available detergent-free membrane mimetic for solution NMR spectroscopy and are in addition suitable for studying protein-protein interactions in an unbiased lipid bilayer environment. It is therefore highly desirable to be able to structurally characterize membrane proteins in this promising system by NMR. The shortest commonly used version of the MSP constructs produces discs of around 10 nm in diameter, which translates into a molecular weight of 150–200 kDa. These nanodiscs were already successfully used for the incorporation of membrane proteins like VDAC-111 and -212, CD4mut13 and the voltage-sensing domain of the potassium channel KvAP14 for 2D-heteronuclear NMR experiments. However, the use of nanodiscs for multidimensional NMR experiments as required for structure determination remained elusive due to the still large molecular weight of these particles.
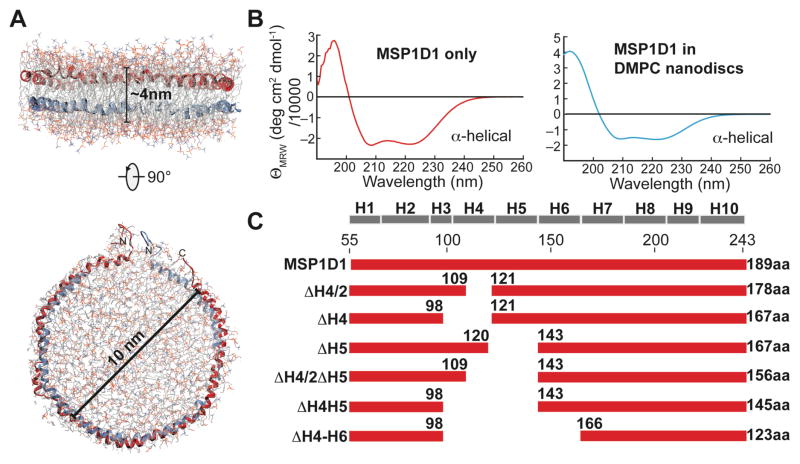
Figure 1.
Construction of truncated membrane scaffold protein (MSP) variants. (A) Proposed architecture of a phospholipid nanodisc where two copies of membrane scaffold protein (MSP) wrap around a patch of phospholipid bilayer, thereby stabilizing its hydrophobic edge. The most commonly used nanodisc has a diameter of 10 nm. Coordinates of the MSP were taken from ref.4. (B) Far-UV CD spectra of MSP alone (left) or in an assembled nanodisc (right) show that MSP adopts α-helical secondary structure in both cases. (C) Deletion constructs of MSP1D15 used in this study. The predicted secondary structure of MSP1D1 is shown on top and the length of each construct is indicated.
Here, we present a set of truncated ApoA-I variants that form smaller nanodiscs suitable for NMR studies of small to medium sized membrane proteins. We were able to reduce the nanodisc diameter by up to 3.0 nm from the 10 nm obtained with wild-type MSP1D15 protein construct, as estimated by size exclusion chromatography (SEC), dynamic light scattering (DLS) and electron microscopy (EM). To assess the NMR spectroscopic properties, we incorporated the bacterial outer membrane β-barrel protein OmpX and the seven transmembrane α-helical protein bacteriorhodopsin into this set of nanodiscs. We evaluated the spectral quality by 2D-[15N;1H]-TROSY and TROSY for rotational correlation times (TRACT) experiments and used circular dichroism (CD) spectroscopy to assess the thermal stability of OmpX in each nanodisc. Furthermore, we show that OmpX incorporated into smaller nanodiscs is accessible to multidimensional NMR experiments, enabling resonance assignment and high-resolution structure determination. The obtained structure of OmpX in phospho-lipid nanodiscs was compared to the structure in DPC detergent micelles determined here, as well as to the previously reported NMR structure in di-hexanoyl-glycero-phosphocholine (DHPC) detergent micelles15 and to the crystal structure in n-octyltetraoxyethylene (C8E4)16. Finally, we characterized the dynamics of the obtained structures in phospholipid nanodiscs and dodecylphosphocholine (DPC) micelles using NMR relaxation experiments and molecular dynamics simulations.
RESULTS
Construction of truncated MSP variants
The most commonly used nanodisc has a diameter of around 10 nm and a molecular weight of 150–200kDa (Fig. 1A). In order to construct a truncated set of MSPs we assumed that the size of a nanodisc particle is solely governed by the length of the membrane scaffold protein (MSP)7. To get a raw estimate on the correlation between MSP length and nanodisc size we analyzed the apolipoprotein A-I crystal structure (pdb code: 1av117).
The protein consists of a single α-helix interrupted by kinks caused by proline residues. The α-helical secondary structure content of this protein in solution was confirmed by CD spectroscopy (Fig. 1B). Taking into account the number of amino acids of MSP1D1 (189aa) and assuming an α-helical pitch per residue of 0.15 nm a theoretical length of 28.5 nm can be derived resulting in a diameter of 9.1 nm. The thickness of the helical MSP contributes approximately 0.5 nm to the diameter on each side of the disc resulting in a total diameter of about 10 nm, which is close to the value of 9.6 nm determined experimentally5. We also modeled the dimensions of the nanodisc formed by MSP1D1 using the empirically determined number of 80 DMPC lipid molecules per bilayer leaflet, which resulted in a diameter of 8 nm for the lipid bilayer alone (Fig. 1C). Adding 1 nm for the diameter of the MSP protein at each side of the nanodisc results in a final diameter of 10 nm, which is in excellent agreement with the experimentally determined size5. It seems generally possible to predict the size of the assembled nanodiscs based on either the length of the MSP protein or the number of lipids per MSP.
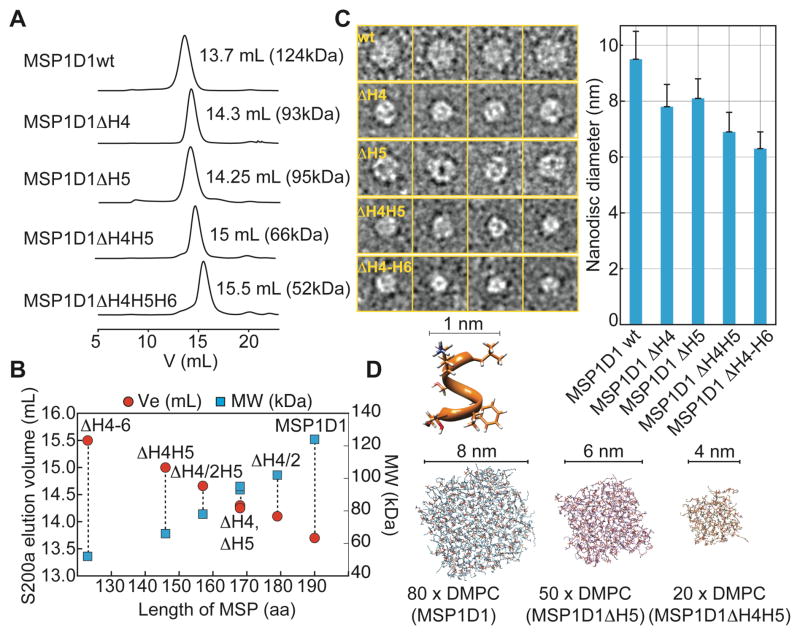
In order to design smaller nanodiscs we focused on the deletion of internal regions of the MSP resulting in the construction of a series of MSPs lacking either (half of) helix 4, helix 5, helix 4 and 5 or helix 4 to 6 (Fig. 1D). Deletion of N-terminal residues of MSP1D1 produced only slightly smaller nanodiscs (Fig. S1), which is in agreement with previous observations5. However, DMPC nanodiscs formed with the internally shortened MSPs showed markedly altered lipid to MSP ratios and elution profiles in analytical size exclusion chromatography experiments (Fig. 2A), yielding nanodiscs of subsequently smaller sizes (Fig. 2B). Using different MSP-to-lipid (DMPC) ratios (Tab. 1) we empirically optimized the number of lipid molecules per nanodisc. The smallest disc (MSP1ΔH4-H6) harbors only 10 DMPC molecules per MSP protein, which corresponds to an area of roughly 500 Å2 per bilayer leaflet. The size of this series of nanodiscs was further estimated by DLS and EM (Tab. 1, Fig. 2C, Fig. S2). In line with the SEC data, a decrease in nanodisc diameter by as much as ~ 3 nm could be detected from the initial MSP1D1 construct using EM. In good agreement with the experimental data, modeling of a bilayer based on the known number of lipids in each disc predicts their size fairly well (Fig. 2D, Tab. 1). We furthermore validated the long-term stability of the smaller discs by recording SEC experiments 50 days after nanodisc assembly. As evident by the gel filtration elution profiles (Fig. S2), the smallest nanodisc presented here (with MSP1D1ΔH4-6) shows a tendency to form larger assemblies of approximately 11 nm diameter over time; a similar behavior was previously observed for Apolipophorin-III lipoprotein particles18. To a lower extent, this feature can also be observed for the MSP1D1ΔH4H5 nanodisc whereas the other discs presented here are stable over the investigated time period (Fig. S2).
Figure 2.
Characterization of the smaller nanodiscs. (A) Size exclusion chromatograms of DMPC nanodiscs formed with various MSP1 deletion variants as indicated. The molecular weight was obtained by calibration of an analytical S200 column. (B) The elution volumes and the calculated molecular weights are linearly dependent on the length of the MSP protein that is in contact with lipids. (C) Representative negative-stain electron micrograms showing single nanodisc particles for the MSP variants as indicated. Right: Average diameter of nanodisc preparations determined by single particle analysis showing a decrease in nanodisc diameter from 9.5 to 6.3 nm with shorter MSP variants. Error bars indicate standard deviations (see also SI Fig. 2). (D) Assembly and equilibration of the experimentally determined number of DMPC lipids for MSP1D1, MSP1D1ΔH5 and MSP1D1ΔH4H5 by molecular dynamics simulations provide a fairly good estimate of the diameter of each nanodisc. To obtain the final diameter, one layer of the helical MSP protein (1 nm) was added to each side of the lipid bilayer.
Table 1.
Size and lipid content of nanodiscs used in this study
| MSP protein | MSP: DMPC ratio | Size exclusion chromatography (Ve, MW, Rh×2)† | Diameter from EM (nm) | Rotational correlation time τc from NMR‡ (calc. MW) | DLS$ Rh×2 (nm), MW | Calc. diameter (nm)# |
|---|---|---|---|---|---|---|
| MSP1D1wt | 1:80 | 13.7 mL, 124kDa, 10.2nm | 9.5±1.1 | 48ns (158kDa) | 9.4±0.9 (126kDa) | 9.5 |
| ΔH4/2 | 1:60 | 14.10 mL, 102kDa, 9.4nm | n.d. | n.d. | n.d. | 8.9 |
| ΔH4 | 1:45 | 14.3 mL, 93kDa, 9.1nm | 7.8±0.8 | 33ns (102kDa) | 8.2±0.9 (91kDa) | 8.2 |
| ΔH5 | 1:50 | 14.25 mL, 95kDa, 9.2nm | 8.2±0.6 | 34ns (107kDa) | 8.4±0.7 (98kDa) | 8.4 |
| ΔH4/2H5 | 1:30 | 14.66 mL, 78 kDa, 8.4nm | n.d. | n.d. | n.d. | 7.9 |
| ΔH4H5 | 1:20 | 15 mL, 66 kDa, 7.8nm | 6.9±0.8 | 27ns (81kDa) | 7.3±0.3 (70kDa) | 7.1 |
| ΔH4-H6 | 1:~10 | 15.5 mL, 52kDa, 6.8nm | 6.3±0.6 (10.9±0.9) | n.d. | 6.4±0.5 (52kDa) | 6.3 |
Rh: hydrodynamics radius
Obtained with [15N,1H]-TRACT experiments at 318K with 2H,15N labeled OmpX
DLS: dynamic light scattering
Calculated using the formula: D = (n(aa)·0.15nm/π) + 1nm, where n(aa) is the number of amino acids in contact with lipids, which is 180 for MSP1D1
Application to the seven transmembrane-helical protein bacteriorhodopsin (bR)
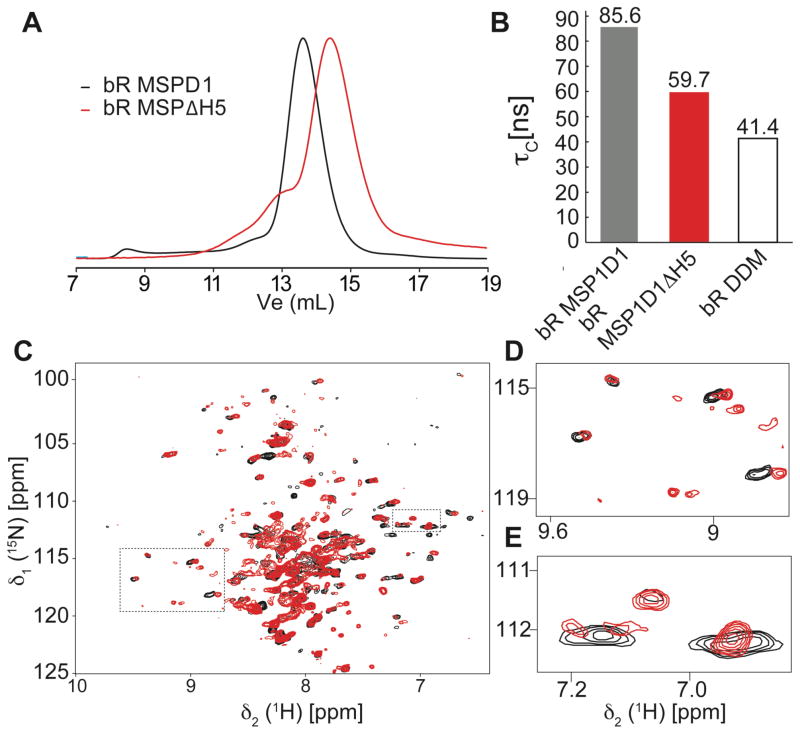
In order to test the smaller discs for the use in NMR spectroscopy we first used the seven-transmembrane helical protein bacteriorhodopsin (bR). bR is a well-characterized membrane protein that can be produced in rather good amounts by E. coli cell-free expression techniques19 (see SI methods). Analytical size exclusion chromatography indicates that bR can be incorporated into our smaller discs (Fig. 3A). The functionality and the presence of an intact tertiary structure of the preparation were further corroborated by its characteristic purple color. The apparent correlation time of 2H and 15N labeled bR in MSP1ΔH5 and MSP1D1wt DMPC nanodiscs and n-dodecyl β-D-maltoside (DDM) micelles was estimated with [15N,1H]-TRACT experiments20 (Fig. 3B). A pronounced reduction (>20 ns) in the apparent correlation time of bR can be seen with the ΔH5 variant as compared with wild-type MSP. However, bR in DDM micelles shows an even smaller correlation time. These results are in line with reduced proton linewidths of the 1H-15N correlation signals in a 2D-TROSY experiment (Fig. 3D–E, Fig. S3). Our data hence suggest that the optimized discs have the potential of significantly improving the spectral quality of 7-transmembrane helical proteins when compared to the nanodisc constructs reported so far, promoting their use for the NMR investigation of proteins with similar topology.
Figure 3.
Analysis of bacteriorhodopsin (bR) in different nanodiscs. (A) Analytical SEC traces of bR assembled into nanodiscs of different sizes. (B) Apparent rotational correlation time (τc) of bR in DMPC MSP1D1 (black) and MSP1D1ΔH5 (red) nanodiscs and in DDM micelles (white). (C) Overlay of 15N-1H-TROSY spectra of bR in MSP1D1 (black) and MSP1D1ΔH5 (red). (D,E) Expansion of boxed regions in (C).
Even though NMR spectral quality of bR is better in detergent micelles, the higher stability in phospholipid nanodiscs may still be beneficial for performing long-term experiments and interaction studies. This may be even more critical for less stable proteins with a comparable membrane topology.
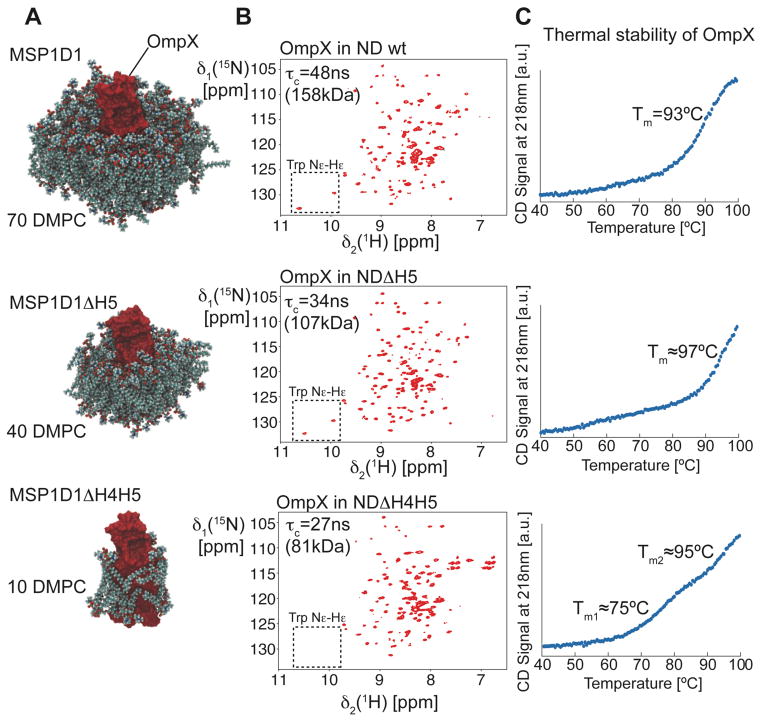
A strategy for screening the optimal nanodisc size for OmpX
We next chose the bacterial outer membrane protein OmpX for incorporation into phospholipid nanodiscs. The smaller size of this membrane protein (148aa) is expected to allow the use of a wide range of nanodisc variants. We compared DMPC/DMPG (3:1) nanodiscs made with MSP1D1wt and the ΔH5 and ΔH4H5 variants by recording 2D-[15N,1H]-TROSY and TRACT experiments. A cartoon of the resulting OmpX nanodiscs is depicted in Fig. 4A, showing OmpX and the determined number of lipids in each preparation. The nanodisc made from MSP1D1ΔH4H5 accommodates approximately 10 lipid molecules in addition to OmpX, thus barely forming a one-layered ring of lipids around the protein. 2D-[15N,1H]-TROSY spectra of each preparation is shown in Fig. 4B. Compared to the wild-type nanodisc, the spectral quality of the OmpX 2D-[15N,1H]-TROSY improves with the MSP1D1ΔH5 nanodisc. Due to the low amount of lipid present in the smallest discs (MSP1D1ΔH4H5), the sidechain resonances of the two tryptophan residues (boxes in Fig. 4B) disappear most likely due to altered dynamics or enhanced relaxation caused by direct contact between OmpX and the MSP protein. The rotational correlation times estimated with TRACT experiments (Fig. S4) indicate smaller particle sizes with shorter MSP variants (τc and MW statements in Fig. 4B). However, CD-detected thermal melting curves show that only MSP1D1wt and ΔH5 represent a stable environment for OmpX whereas the melting transition of the ΔH4H5 nanodisc at around 75°C indicates lower stability. A comparison of proton relaxation rates of lipid resonances in wild-type and ΔH5 nanodiscs suggests similar lipid dynamics in both systems indicating that the restrictions imposed by the scaffold protein on the phospholipids in the bilayer is very similar (Fig. S5). In summary, the MSP1D1ΔH5 nanodisc of an apparent molecular weight of 107 kDa was best suited for further NMR studies of OmpX and was therefore selected for all subsequent experiments. Furthermore, the CD-detected high thermal stability of this nanodisc preparation permits NMR experiments at elevated temperatures.
Figure 4.
Optimization of nanodisc size for the target protein OmpX. (A) Equilibrated box with OmpX and the number of DMPC lipids per bilayer leaflet determined experimentally. For clarity, the surrounding MSP protein is not shown. (B) 2D-[15N,1H]-TROSY experiments with 2H,15N-labeled OmpX in nanodiscs of different sizes, as indicated in the figure. The spectral region of the tryptophan side chain resonances is marked with a box. (C) Circular dichroism (CD)-detected thermal unfolding traces at 218nm of OmpX in different nanodiscs.
NMR resonance assignment and structure determination of OmpX in phospholipid bilayer nanodiscs
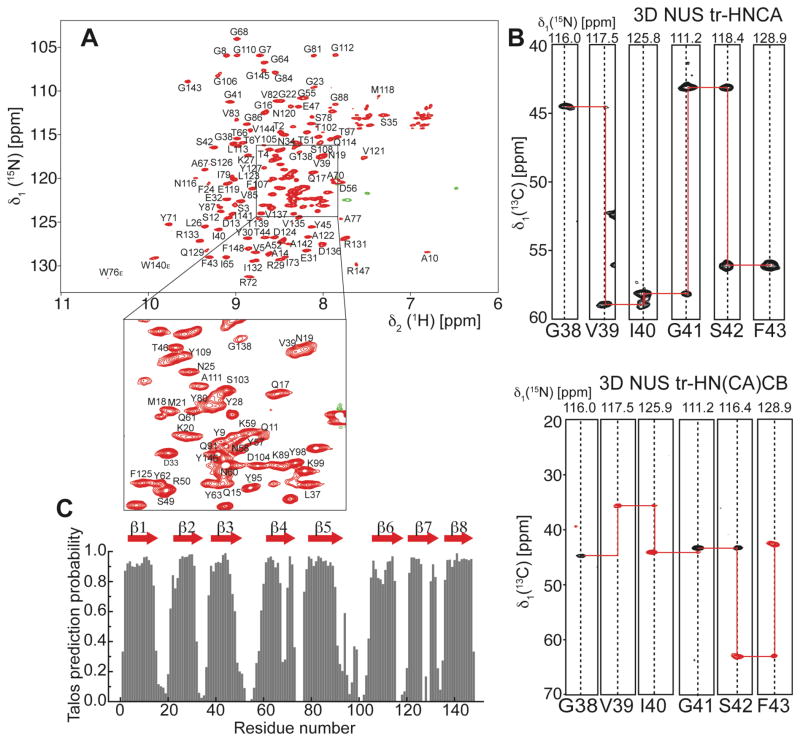
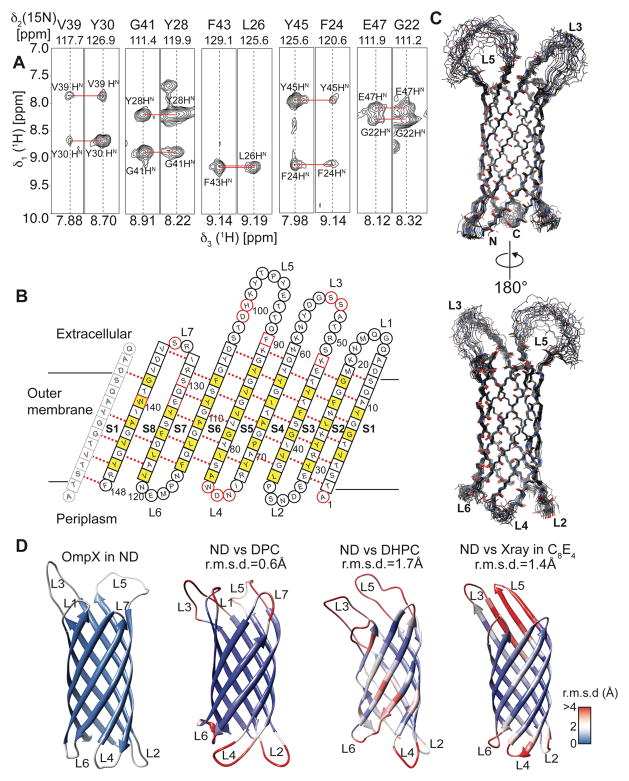
For 3D backbone resonance assignment experiments, we used uniformly 2H, 13C, 15N labeled OmpX in MSP1D1ΔH5 nanodiscs assembled with deuterated DMPC/DMPG (3:1) lipids. A set of TROSY 3D-triple resonance experiments consisting of HNCO, HN(CA)CO, HNCA, HN(CO)CA and HN(CA)CB22 was recorded in a non-uniformly sampled (NUS) manner at 45°C. The sampling schedules for all 3D experiments were generated using the Poisson-Gap method23 and the final spectra were reconstructed by the iterative soft threshold method24. This procedure produced sharp line widths and enabled the detection of most of the expected resonances in each of the NMR experiments. In contrast to the smaller nanodiscs, HNCACB-type experiments were not successful with MSP1D1 nanodiscs due to their large size leading to enhanced transverse relaxation, which prevented the desired coherence transfer. The good quality and high resolution of the 3D NMR experiments (Fig. 5A,B) in smaller nanodiscs facilitated the resonance assignment of 90% of all non-proline residues. Some residues in loop regions and around Trp140 and Ser130 could not be assigned most likely because of unfavorable dynamics and fast amide hydrogen exchange at 45°C. The extracted chemical shift data were used to identify the eight β-strands in the protein (Fig. 5C). Furthermore, we recorded a 15N-edited 3D-TROSY-NOESY experiment and were able to detect long-range inter-β-strand contacts (Fig. 6A) that enabled the structure determination of OmpX in nanodiscs. The degree of assignment and the location of the detected inter-strand NOE contacts are graphically summarized in Fig. 6B. In order to compare the structure of OmpX in a nanodisc and a micellar environment, we additionally performed NMR backbone assignment and NOESY experiments with OmpX in DPC micelles (Fig. S6&S7).
Figure 5.
NMR backbone assignment and secondary structure determination of OmpX in MSP1D1ΔH5 nanodiscs. (A) 2D-[15N,1H]-TROSY spectrum of OmpX in MSP1D1ΔH5 DMPC/DPMG (3:1) nanodiscs at 45 °C and pH6.8. All assigned non-proline backbone amide resonances (90%) are labeled in the spectrum. (B) Example strips taken from the 3D-NUS HNCA and HN(CA)CB experiments show high spectral quality and resolution required for backbone resonance assignment. (C) Secondary structure prediction taken from the program Talos+21 indicates 8 β-strands and long loop regions between strands 3 and 4 and strands 5 and 6.
Figure 6.
Structure determination of OmpX in nanodiscs. (A) Example strips taken from a 3D-15N-edited-[1H,1H]-NOESY experiment showing long-range inter-β-strand contacts used for structure calculation. (B) Topology plot of OmpX. Residues facing towards the lipid bilayer are shown in yellow, unassigned residues in red. Residues in b-sheets are represented by squares whereas residues in loop regions are represented by circles. (C) The best-energy structural ensemble of (20 structures) of OmpX in nanodiscs has an r.m.s.d. of 0.32 Å between residues in β-sheets. (D) Comparison of the OmpX structure in nanodiscs (ND) with the NMR structures of OmpX in the detergents do-decylphosphocholine (DPC) (determined here), di-hexanoyl-glycero-phosphocholine (DHPC)15 and the crystal structure in n-octyltetraoxyethylene (C8E4)16. These structures are color-coded according to the r.m.s.d. values to individual residues of the nanodisc structure, as shown by the legend on the lower right. The r.m.s.d to ordered residues of the NMR structure in nanodiscs is indicated above each structure.
A similar degree of backbone resonances and NOE contacts could be assigned (Tab. 2). Chemical shift data, NOE distance and hydrogen bond restraints were subsequently used to determine the structure of OmpX in both membrane mimics (Tab. 2). The ensemble of the 20 lowest-energy models of OmpX in phospholipid nanodiscs (Fig. 6C) shows an r.m.s.d. of 0.32 Å for ordered secondary structural elements. In both environments the residues in the extracellular loops 3 and 5 show no long-range NOEs, exhibit rapid amide-hydrogen exchange, and in contrast to the crystal structure of OmpX16 do not adopt a rigid β-sheet conformation. The chemical shift values of OmpX in both environments are very similar except for the long loop regions and, to a smaller extent, in some of the β-stands (Fig. S7C). A comparison of the different OmpX structures obtained here and from previous studies15,16 shows that the biggest differences are present within the extracellular loops, (color-coding and r.m.s.d. values in Fig. 6D). The central 8-stranded β-barrel is present in all of the structures, yet there are slight differences in the length and relative orientation of these strands among the displayed structures, highlighting the influence of the different membrane mimics on the protein conformation.
Dynamics of OmpX in phospholipid nanodiscs and DPC micelles
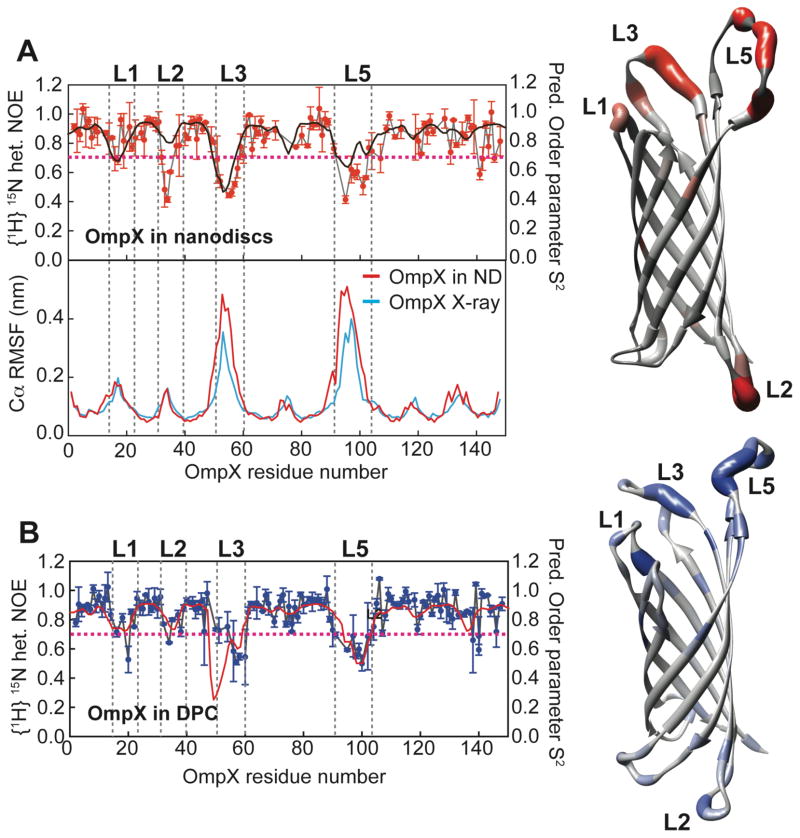
In order to probe the dynamical properties of OmpX in phospholipid bilayers and detergent micelles, we acquired 15N heteronuclear NOE experiments for both systems (Fig. 7A, B). The obtained data show that the dynamics in the ns-ps range of most regions in the protein are not affected by the membrane mimic, in particular in the β-sheet region. These differences are more pronounced for the loop regions, especially loop 2 and loop 3. Loop 2 shows no marked dynamics (high hetNOE) in DPC micelles whereas in the bilayer environment this region appears to be more flexible. The same tendency holds true for the long loop 3. Noteworthy, the generalized order parameter S2 of OmpX in DPC and in nanodiscs calculated from chemical shift data agrees very well with the profile of experimental heteronuclear NOE values (black and red lines in Fig. 7A,B).
Figure 7.
Dynamics of OmpX in nanodiscs and in DPC. (A) Upper left: {1H}-15N-heteronuclear NOE for OmpX in nanodiscs (red symbols) overlaid by the predicted squared order parameter S2 (black line). Right: Heteronuclear NOE values mapped onto the structure of OmpX (from grey to red). Lower left: Molecular dynamics simulations of OmpX in a phospholipid bilayer using the NMR structure obtained here (red line) or the previous X-ray structure (blue line)16 as starting conformations. The root mean square fluctuations (RMSF) of amino acid residues in OmpX are shown. Both simulations were run for 25 ns duration at 45ºC. Of note, even the well-ordered X-ray structure exhibits large fluctuations in the external long loop regions. (B) {1H}-15N-heteronuclear NOE for OmpX in DPC (blue symbols) overlaid by the predicted squared order parameter S2 (red line) based on chemical shift data. Right: Heteronuclear NOE values mapped onto the structure of OmpX in DPC (from grey to blue). Except at loop 2, these values are almost identical to the hetNOE obtained in nanodiscs. The dotted line at a hetNOE value of 0.7 represents the cutoff for coloring of residues in the structure.
These data emphasize that besides structural differences, alterations in the membrane-mimicking environment can affect dynamical properties of OmpX, which might be relevant in terms of its functional features. In order to validate the structural differences between OmpX in the crystal and in solution, we further performed 25 ns molecular dynamics (MD) simulations using the OmpX crystal structure (1qj8.pdb16) and the solution structure in nanodiscs obtained here as starting models, respectively. As can be seen in Fig. 7A, the magnitude of the root mean square fluctuation (RMSF) values of the Cα atoms in both simulations resembles the pattern of the heteronuclear NOE values of OmpX in nanodiscs. This finding corroborates the NMR NOE, chemical shift and dynamics data and indicates, in line with previous results26, that loops 3 and 5 in OmpX do not adopt a well-ordered secondary structure in solution. The extracellular extruding β-strands observed in the crystal structure also exhibited large RMSF values in these simulations emphasizing the flexibility of this region.
DISCUSSION
In this study, we showed that a detailed solution NMR characterization of integral polytopic membrane proteins in nanodiscs is feasible. The herein introduced truncated MSPs that assemble into markedly smaller nanodiscs are an important step toward that goal. In addition, the use of highly deuterated protein and lipids and advanced NMR sampling methods23,24,27 also contribute to high-quality NMR spectra that can be recorded in short experimental time. Deuteration of lipids appears to be beneficial in particular for experiments involving side-chain coherence-transfer steps like the 3D-HNCACB. We show that smaller nanodiscs can be constructed and adapted to the size of the target protein of interest. The smallest nanodisc produced here harbors only around 10 lipids per MSP molecule (20 per nanodisc) and might be suited for single trans-membrane spanning helices and membrane anchored soluble proteins, yet the lower stability of this smallest nanodisc might restrict its use to short-term experiments. However, the very stable ΔH4 and ΔH5 MSP variants are large enough to accommodate membrane proteins up to ~30 kDa in molecular weight while still offering sufficiently improved spectral quality required for structural studies, as shown here with the 148-residue-protein OmpX and the 22 kDa seven-transmembrane α-helical protein bR. The inherent dynamics of the lipids in the smaller ΔH5 nanodisc as compared to the wild-type nanodisc does not seem to be restricted as shown by proton relaxation experiments of the lipid resonances (Fig. S5). For larger membrane proteins or membrane protein complexes, the use of the wild-type MSP might be required solely because of size constraints imposed by the chosen membrane protein.
While so far, high-resolution NMR structures of membrane proteins could be obtained in detergent micelles or bicelles that facilitate structural work due to their small size, the presence of detergents may be unfavorable for stability, alter the interaction properties with partner proteins and/or distort the native protein structure. Here, we report a NMR derived solution structure of a polytopic integral membrane protein in detergent-free phospholipid nanodiscs, which we believe has not been achieved before. The obtained structure of OmpX in nanodiscs shows the same overall fold and topology for the transmembrane part as previously determined NMR15 and X-ray structures16. However, it differs from the crystal structure determined in n-octyltetraoxyethylene and NMR structures determined in micelles. The X-ray structure differs most prominently at the extracellular extensions of the β-sheet. Presumably these extracellular segments are immobilized by crystal contacts and the cryogenic temperature used during data collection. In nanodiscs in solution, these external β-sheet regions do not form a regular structure as shown here by chemical shifts, NOE, NMR dynamics data and MD simulations. This is consistent with previous solution NMR15 and MD26 data. A comparison with this previous NMR solution structure solved in DHPC micelles identifies differences in the arrangement and lengths of the β-strands, which might be caused by the use of different structure calculation software and/or the choice of detergent. To clarify the role of the membrane mimetic on protein structure and dynamics, we pursued backbone resonance assignments and a structure determination of OmpX in a different detergent (DPC vs. DHPC) but using the same buffer as for nanodiscs. We calculated a structural ensemble using the same approach as demonstrated for the nanodisc system (Fig. S6&S7; Tab. 2). The resulting structure in DPC micelles is comprised of a well-defined β-barrel and shows an overall fold that is very similar to the structure determined in DHPC micelles. However, compared to the nanodisc structure on average each strand is up to two residues shorter (Fig. 6D, Fig. S7), which might be due to the slightly denaturing effect of the detergent at the water boundary and differences in hydrophobic coverage. In addition to these structural differences, dynamics in the ns-ps time scale as measured by 15N-heteronuclear NOE experiments reveal differences between OmpX in nanodiscs and in DPC micelles, mainly in the loop regions at the surface of the membrane mimetic. Our data hence indicate that the membrane mimetic can have a considerable impact on the conformational space of the embedded protein.
Table 2.
Structural statistics of OmpX in phospholipid nanodiscs and DPC
| OmpX in nanodiscs | OmpX in DPC | |
|---|---|---|
| Structural information | ||
| HN-HN NOEs (inter-strand contacts) | 58 | 50 |
| Hydrogen bond restraints | 67 | 65 |
| Dihedral angle restraints (TALOS21) | 291 | 275 |
| Backbone rmsd for β-sheets (Å)+ | 0.32±0.16 | 0.30±0.14 |
| Backbone rmsd for all residues (Å) | 1.32±0.31 | 1.72±0.33 |
| Ramachandran map analysis$ | ||
| Most favored regions | 93.4% | 90.2% |
| Additionally allowed regions | 4.1% | 9.0% |
| Generously allowed regions | 1.6% | 0.8% |
| Disallowed regions | 0.8% | 0.0% |
| Deviations from restraints and idealized geometry | ||
| Distance restraints (Å) | 0.039 ±0.001 | 0.044 ±0.0008 |
| Dihedral angle restraints (°) | 0.127 ±0.007 | 0.236±0.01 |
| Bonds (Å) | 0.0021 ±0.00006 | 0.0023 ±0.00008 |
| Angles (°) | 0.61±0.01 | 0.65±0.01 |
| Impropers (°) | 1.52±0.07 | 2.35±0.06 |
Analysis of the 20 lowest-energy structures.
ordered secondary structure elements were used for structural superimposition: 3–14, 20–30, 38–48, 60–71, 78–90, 104–115, 122–132, 135–147; r.m.s.d. values are calculated relative to a non-minimized average structure of each ensemble.
Ramachandran analysis with PROCHECK-NMR25 was performed on the lowest-energy structure.
CONCLUSIONS
We presented here the first example of a membrane protein structure solved in phospholipid nanodiscs. The use of the herein introduced smaller nanodiscs was a critical step in this process and enabled a detailed NMR spectroscopic characterization of OmpX in a phospholipid bilayer system. We expect that the use of a native-like membrane environment might be even more important for marginally stable membrane proteins, which so far eluted structural studies due to limited stability in detergent micelles. Phospholipid nanodiscs may turn out to be an ideal choice for maintaining longer-term stability for structural studies, preserving a particular membrane protein in its functional form and enabling binding studies in a detergent free environment. With the introduction of the smaller MSP variants described here, solution NMR spectroscopy shows great promise to address important functional and structural questions of membrane proteins in their native lipid bilayer environment.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported by NIH grants GM047467 and GM094608 (to GW). FH was supported by EMBO and Human Frontier Science Program long-term fellowships. The molecular electron microscopy facility at Harvard Medical School is maintained with funds from NIH grant PO1 GM62580 (to Stephen C. Harrison). Maintenance and operation of the high-field NMR instruments used were supported by NIH grant P41-EB002026 (to Robert Griffin).
FB reagents (Cambridge, MA) is gratefully acknowledged for providing deuterated lipids (d54-DMPC/d54-DMPG). We want to thank Melissa Chambers and Tom Walz (Harvard Medical School) for help with electron microscopy and Scott Robson and Sven Hyberts (Harvard Medical School) for help with non-uniform sampling NMR setup. The simulations in this paper were run on the Orchestra cluster supported by the Harvard Medical School Research Information Technology Group.
ABBREVIATIONS
- bR
bacteriorhodopsin
- DMPC
Dimyristoylphosphatidylcholine
- DMPG
Dimyristoylphosphatidylglycine
- DPC
dodecylphosphocholine
- DDM
dodecyl-β-D-maltopyranoside
- MSP
membrane scaffold protein
- NMR
nuclear magnetic resonance
- TRACT
TROSY for rotational correlation times
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The NMR backbone assignments and structural coordinates of OmpX in MSP1D1ΔH5 nanodiscs and in DPC micelles were deposited at the BMRB (accession codes 18796 and 18797) and RCSB (accession codes 2m06 and 2m07) databanks.
Experimental details, size exclusion and electron microscopy data, as well as NMR data on OmpX in DPC. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Linke D. Methods Enzymol. 2009;463:603. doi: 10.1016/S0076-6879(09)63034-2. [DOI] [PubMed] [Google Scholar]
- 2.Lee AG. Biochim Biophys Acta. 2003;1612:1. doi: 10.1016/s0005-2736(03)00056-7. [DOI] [PubMed] [Google Scholar]
- 3.Seddon AM, Curnow P, Booth PJ. Biochim Biophys Acta. 2004;1666:105. doi: 10.1016/j.bbamem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Frauenfeld J, Gumbart J, Sluis EO, Funes S, Gartmann M, Beatrix B, Mielke T, Berninghausen O, Becker T, Schulten K, Beckmann R. Nat Struct Mol Biol. 2011;18:614. doi: 10.1038/nsmb.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denisov IG, Grinkova YV, Lazarides AA, Sligar SG. J Am Chem Soc. 2004;126:3477. doi: 10.1021/ja0393574. [DOI] [PubMed] [Google Scholar]
- 6.Judge PJ, Watts A. Curr Opin Chem Biol. 2011;15:690. doi: 10.1016/j.cbpa.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 7.Ritchie TK, Grinkova YV, Bayburt TH, Denisov IG, Zolnerciks JK, Atkins WM, Sligar SG. Methods Enzymol. 2009;464:211. doi: 10.1016/S0076-6879(09)64011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nath A, Atkins WM, Sligar SG. Biochemistry. 2007;46:2059. doi: 10.1021/bi602371n. [DOI] [PubMed] [Google Scholar]
- 9.Krieger M, Brown MS, Faust JR, Goldstein JL. J Biol Chem. 1978;253:4093. [PubMed] [Google Scholar]
- 10.Fang Y, Gursky O, Atkinson D. Biochemistry. 2003;42:13260. doi: 10.1021/bi0354031. [DOI] [PubMed] [Google Scholar]
- 11.Raschle T, Hiller S, Yu TY, Rice AJ, Walz T, Wagner G. J Am Chem Soc. 2009;131:17777. doi: 10.1021/ja907918r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu TY, Raschle T, Hiller S, Wagner G. Biochim Biophys Acta. 2012;1818:1562. doi: 10.1016/j.bbamem.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gluck JM, Wittlich M, Feuerstein S, Hoffmann S, Willbold D, Koenig BW. J Am Chem Soc. 2009;131:12060. doi: 10.1021/ja904897p. [DOI] [PubMed] [Google Scholar]
- 14.Shenkarev ZO, Lyukmanova EN, Paramonov AS, Shingarova LN, Chupin VV, Kirpichnikov MP, Blommers MJ, Arseniev AS. J Am Chem Soc. 2010;132:5628. doi: 10.1021/ja9097498. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez C, Adeishvili K, Wuthrich K. Proc Natl Acad Sci U S A. 2001;98:2358. doi: 10.1073/pnas.051629298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogt J, Schulz GE. Structure. 1999;7:1301. doi: 10.1016/s0969-2126(00)80063-5. [DOI] [PubMed] [Google Scholar]
- 17.Borhani DW, Rogers DP, Engler JA, Brouillette CG. Proc Natl Acad Sci U S A. 1997;94:12291. doi: 10.1073/pnas.94.23.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer NO, Blanchette CD, Segelke BW, Corzett M, Chromy BA, Kuhn EA, Bench G, Hoeprich PD. PLoS One. 2010;5:e11643. doi: 10.1371/journal.pone.0011643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwarz D, Junge F, Durst F, Frolich N, Schneider B, Reckel S, Sobhanifar S, Dotsch V, Bernhard F. Nat Protoc. 2007;2:2945. doi: 10.1038/nprot.2007.426. [DOI] [PubMed] [Google Scholar]
- 20.Lee D, Hilty C, Wider G, Wuthrich K. J Magn Reson. 2006;178:72. doi: 10.1016/j.jmr.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Shen Y, Delaglio F, Cornilescu G, Bax A. J Biomol NMR. 2009;44:213. doi: 10.1007/s10858-009-9333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salzmann M, Pervushin K, Wider G, Senn H, Wuthrich K. Proc Natl Acad Sci U S A. 1998;95:13585. doi: 10.1073/pnas.95.23.13585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyberts SG, Takeuchi K, Wagner G. J Am Chem Soc. 2010;132:2145. doi: 10.1021/ja908004w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyberts SG, Milbradt AG, Wagner AB, Arthanari H, Wagner G. J Biomol NMR. 2012;52:315. doi: 10.1007/s10858-012-9611-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laskowski RA, Rullmannn JA, MacArthur MW, Kaptein R, Thornton JM. J Biomol NMR. 1996;8:477. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- 26.Choutko A, Glattli A, Fernandez C, Hilty C, Wuthrich K, van Gunsteren WF. Eur Biophys J. 2011;40:39. doi: 10.1007/s00249-010-0626-7. [DOI] [PubMed] [Google Scholar]
- 27.Hyberts SG, Arthanari H, Wagner G. Top Curr Chem. 2012;316:125. doi: 10.1007/128_2011_187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.