Abstract
Testicular torsion is one of the few emergencies in pediatric urology which requires an accurate and timely diagnosis in order to avoid testis loss. It is not an uncommon event affecting a young male population. In fact, testicular torsion is more common than testicular tumors for this same age group, yet testicular torsion has not been given the public attention it deserves as a male health risk. In this review we highlight the new information published over the past four years regarding testicular torsion. We will discuss a variety of topics associated with torsion including: medical legal issues, etiology and genetics, imaging diagnostics, innovative surgical techniques, management controversies, fertility, and new drug therapies.
Keywords: testicular torsion, orchiectomy, orchiopexy, fertility
Introduction
Unilateral scrotal pain of sudden onset is commonly due to acute testicular torsion, wherein the testicle suddenly spins in the scrotum twisting the blood vessels to the testicle and halting testicular blood flow. For every 100,000 males less than 25 years of age, about 4.5 males will have testicular torsion per year. [1] Time is of the essence for this urological emergency, since pain lasting more than 4 to 8 hours is highly associated with testicular death if no intervention occurs.[1] Unfortunately, at surgical exploration, one third of testes will be considered dead and orchiectomy is performed.[1–3] For salvaged testes, many may have damage with diminished testicular size after healing is complete, with possible contralateral testis injury as well. The possible impact on fertility has always been a concern for these patients. In addition, many practitioners and parents are surprised to learn that testicular torsion is more common than testicular tumors for this same age group, yet testicular torsion has not been given the public attention it deserves as a male health risk. We herein summarize important facts on testicular torsion with emphasis on new information that has come forward over the last few years including: medical legal issues, etiology and genetics, imaging diagnostics, innovative surgical techniques, management controversies, fertility, and new drug therapies.
Medical Legal Pitfalls
Many factors make torsion an active area of litigation, including the urgency needed in its diagnosis and treatment, the diagnostic uncertainties and errors, delays in presentation, a relatively common rate of adverse outcome (testicular loss) and the psychological impact related to the loss of a testicle. These facts have induced many adult urologists to shy away from dealing with this condition, and perhaps they may have good reason to do so. From 1985–2000, a retrospective review was performed of all 2283 closed pediatric claims originating in the emergency department and urgent care center in the Physician Insurers Association of America (P IAA) database.[4] This database contains data from 20 major malpractice firms insuring 25% of US physicians. Testicular torsion was the third most common diagnosis of a claim involving children ages 12–17 years.
It is important to note that this is not an issue in the US alone where lawsuits are known to be frequent. Similar litigation issues have been reported even in countries like England and Canada. In England from 2005–2010, of 195 closed pediatric cases involving financial compensation to the claimant, the 12th most common incident leading to successful litigation against the National Health Service (NHS) involving children was delayed diagnosis of testicular torsion.[5, 6] According to the Canadian Medical Protective Association, 55% of the closed legal actions involving testicular torsion between 2001 and 2005 were settled as expert support could not be obtained. This is in contrast to their usual rate of <30% settled and 66% dismissed.[7]
To avoid missing the diagnosis of a testicular torsion, some may prefer to use an aggressive approach of exploring every single acute scrotum. A retrospective series by Molokwu et al using this approach, diagnosed torsion in only 51% of the cases explored.[8] While this approach will correctly identify all cases of torsion, it will lead to a high rate of unnecessary surgery which could also lead to litigation. It is the believe of the authors that while litigation issues may be inevitable, as with other conditions, urologist should apply an evidence based approach to the diagnose and treatment of testicular torsion.
Testicular Torsion Genetics and Etiology
An anatomical predisposition wherein a lack of normal fixation of the testis and epididymis to the scrotum, also known as ‘bell clapper deformity,’ continues to be the most commonly described etiology for intravaginal testicular torsion. While this is a common finding in patients who undergo scrotal exploration for torsion, it is not observed in all torsion cases. Furthermore, it is found during autopsies in up to 12% of cases who presumably never experienced torsion, a much higher rate than the incidence of testicular torsion.[9] Despite these autopsy findings, the ‘bell clapper deformity’ continues to be the only anatomical risk factor for the intravaginal torsion at this time. Nevertheless, no explanation currently exists concerning how the ‘bell clapper deformity’ arises, or whether this is a congenital anomaly which occurs from anomalous embryonic development of the scrotum, spermatic cord and testis. A long mesorchium and cryptorchidism are anatomical variants that have been implicated with testicular torsion.[10] Moreover, no new insight has come concerning the precipitating factors which trigger the acute torsion event other than trauma and exercise (in particular bicycle riding), among others.[11] A hypothesis that occurrence of testicular torsion was correlated with colder weather months has recently been refuted by a temporal review of testicular torsion cases from a US national pediatric database[12] but was supported by work from Scotland[8].
To date, there are 20 published reports of familial testicular torsion, which has raised the possibility of a genetic basis for the disease.[3, 13–22] A prospective series of 70 boys with torsion identified familial testicular torsion in 11.4% and one case with a three generational family.[3] Although previously there had been no candidate genes for human testicular torsion, recently the INSL3 hormone and its receptor, RXLF2, have been investigated as candidate genes. The impetus for this was the observation that Insl3 knockout mice, which uniformly manifest intraabdominal bilateral cryptorchidism with accompanying heat-induced testicular atrophy in adulthood, additionally have spontaneous testicular torsion. The risk for active torsion was greatest in the ‘adolescent’ mice and loss of the testis (not just testicular atrophy) was observed the most in the older adult mice. Thus, the altered anatomy predisposed the mice to the torsion event, which was witnessed most commonly peripubertally. [23, 24] When comparing this mouse model to the human condition, similarities include that torsion is primarily a peripubertal event while differences include that human testicular torsion occurs in the scrotum and not intraabdominally, as in the mice. It is well established that in humans and mice, INSL3 is produced by testicular Leydig cells. By studying the Insl3 mutant mice, it has become clear that INSL3 acts early on the embryonic gubernaculum to masculinize and enlarge it.[23] This allows for the later action, mediated by testosterone, which may induce transinguinal testicular descent with gubernacular regression and potentially scrotal fixation. This particular function makes this hormone-receptor signaling cascade a strong candidate for having at least a partial role in the etiology of human torsion, as it has been associated in human cryptorchidism.
With this in mind, our group tested genomic DNA samples from 39 males (11 neonatal: 21 pre- or peri-pubertal; 7 pubertal) with surgically confirmed testicular torsion for mutations in INSL3 and RXFP2 [25]. Bilateral testicular torsion was present in 2/11 (18%) neonatal and 2/28 (7%) older cases. A positive family history of torsion was noted in 29% of neonatal and 33% of older cases. We did not detect any functionally significant mutations in INSL3 or RXFP2, leading one to conclude that mutations in these genes are not a common de novo or inherited cause for testicular torsion. However, this ligand-receptor signaling system may still be important in human testicular torsion at other yet to be determined levels of regulation.
New Concepts in Imaging and Testing
Many cases of testicular torsion do not need imaging to confirm the need for surgical intervention, such as when a thorough history and physical exam are pathognomonic. However, color Doppler sonography (CDS) remains the first-line radiological test to evaluate a patient with an equivocal acute scrotum in order to rule out testicular torsion.[26] Recently, CDS has been used to predict the chance for testicular salvage. In cases with loss of testicular arterial blood flow by Doppler, the additional finding of parenchymal heterogeneity of the testicular echotexture was reported to be 100% predictive of testicular loss at exploration.[27] Thus, if this finding is present, orchiectomy with contralateral fixation may be undertaken in a less emergent manner since the torsed testis is unsalvageable. The urgent versus elective nature could be debated, however, given the concern of contralateral testicular damage from torsion, the longer duration of pain that the patient might incur, and whether rescue from testicular compartment syndrome is feasible (see below).
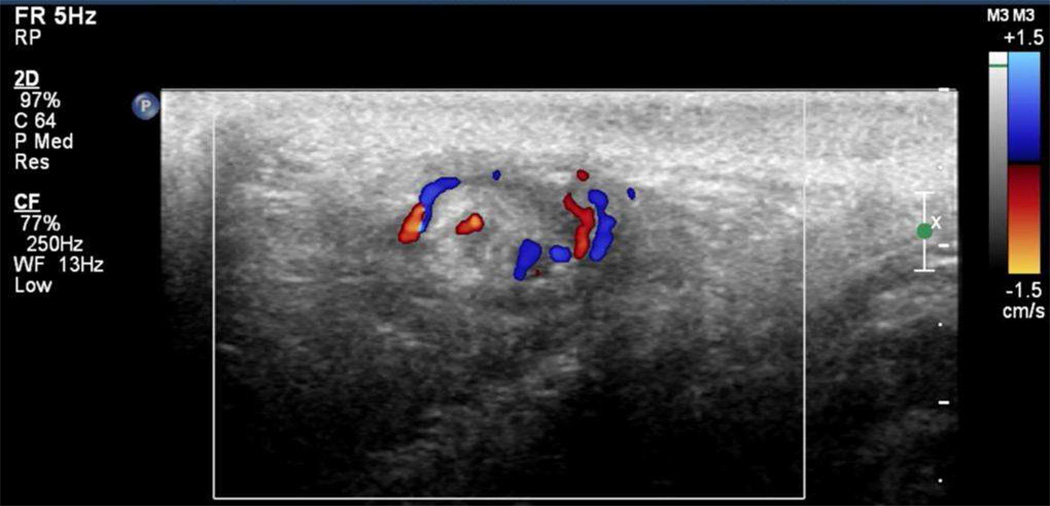
Unfortunately, CDS is not always accurate and false negative results have been reported.[28–30] In order to address the sensitivity of CDS for testicular torsion, Kalfa et al described the advantage of high-resolution ultrasonography (HRUS) for the direct visualization of twisting of the spermatic cord. This sign of torsion found on HRUS is seen as an inhomogeneous mass at the inguinal or paratesticular position and was initially described as the spiral twist of the cord or the “Whirlpool Sign”. [28, 31] (Figure 1) The authors in this 919 patient multicenter report (mean age 9 years; range 1 day to 18 years) found CDS of the scrotum to have a sensitivity of 76%, while HRUS of the spermatic cord for linear or twist configuration reached 96% and 99% sensitivity and specificity, respectively.[30] These results reinforce the need to include a thorough spermatic cord evaluation to the routine scrotal CDS in order to improve diagnostic accuracy. Since this is a relatively new observation, it may be up to adult and pediatric urologists to specifically request that the ultrasonography technician perform a thorough examination of the spermatic cord even up to the level of the internal ring and not just the testes.
FIGURE 1.
Whirlpool sign seen by Doppler ultrasound indicating the presence of the supercoiled spermatic cord involved in testicular torsion. (Photo from S. Boopathy Vijayaraghavan, MD)
Despite being a reliable test to diagnose torsion, CDS has a few drawbacks. It requires a skilled technician and has to be read by a radiologist, both of these steps which could lead to increase in the time to make the diagnosis. When it comes to saving a torsed testis, time is of the essence. Thus, if one could design the perfect diagnostic test for torsion, it should have the accuracy and reliability of CDS but also have the ability to be a point of care type of test that could be done by the ER physician with immediate positive or negative results. In many ways similar to pulse oximetry, near-infrared spectroscopy (NIRS) is a technology that uses infrared light to obtain continuous, noninvasive transcutaneous monitoring of deep tissue oxygen saturation (%StO2). Pediatric applications of NIRS are well-documented for other body organs, including 4 randomized trials utilizing NIRS for monitoring cerebral oxygenation in patients with congenital heart disease [32]. Unlike pulse oximetry, the transcutaneous NIRS probe provides a venous weighted tissue %StO2 (approximately 3:1 ratio of venous to arterial blood) and thus is ideal in non-pulsatile or low-flow conditions [33], as would be expected in testicular torsion. NIRS has been studied in sheep, rabbit, and rat models of testicular torsion, demonstrating prompt and consistent identification of significantly lower %StO2 values in torsed versus nontorsed testes [34–36]. Recently, our adult human pilot study applying a transscrotal NIRS probe found significantly lower %StO2 values in the affected testis compared to the contralateral, unaffected testis in 11 patients (mean age 18.27 years) with surgically confirmed torsion. Moreover, in the 5 patients without torsion, the %StO2 in the affected testis was either higher or no more than 10 points lower than the contralateral unaffected testis in all cases [37]. This preliminary data demonstrates feasibility and accuracy of NIRS in testicular torsion in adult humans and an NIH-funded trial in children is underway (NIH R21 DK092654). While additional data is required to definitively validate these findings, we believe that NIRS may have the potential to become the point of care imaging test modality in the diagnosis of testicular torsion. Regardless, no study is flawless and, when it comes to testicular torsion, the physician should always rely on the history and a thorough physical exam to make the diagnosis and perform surgical intervention if the diagnosis is equivocal.
Novel Surgical Techniques
Most urologists are very familiar with the surgical technique to correct a torsed testis, as well as the need to perform orchiopexy of the contralateral testis. In 2008, the Philadelphia group proposed a concept that implies further testicular injury may occur following detorsion secondary to ‘testicular compartment syndrome’.[38] This hypothesis postulates that after the reperfusion following the surgical correction of a torsed testis, especially with prolonged ischemia time, increased blood flow to the affected testis will lead to edema. Given the testis is surrounded by its relatively inelastic tunica albuginea, the edema in this confined space would increase ‘testicular compartment’ pressure with decreased perfusion to the testis and further ischemic injury, even after the spermatic cord was untwisted.
To counteract such events, a recent report has proposed that during surgical detorsion an incision should be made over the tunica albuginea, in similar fashion of a fasciotomy, with placement of a tunica vaginalis patch. This should allow for edema to ensue without increasing the compartmental pressure. Despite the small number of patient in the report (n=3) and the ischemia time of at least 6 hours in all patients, they were able to salvage the testis in all patients with preservation of size as well as symmetrical growth on 1 year follow up.[38] Moritoki et al, corroborate these finding in an animal model. They showed in rats that smaller reductions in the intratesticular pressure following an episode of detorsion were more likely to result in disturbances of spermatogenesis.[39] These results are quite promising and should encourage a multi-institutional study to evaluate the efficacy of this surgical technique.
For testicular torsion cases resulting in orchiectomy, another novel surgical approach has been described by Bush and colleagues wherein testicular prosthesis placement is performed simultaneously with the torsion orchiectomy with excellent early results.[40] Designed for postpubertal males that have already achieved full testicular growth, this strategy gives cost advantages of a single surgical procedure and likely aids body self-esteem. It is performed through a midline scrotal incision used for the scrotal exploration of the testicular torsion and so far no prosthesis infections have occurred.
Management Controversies
Management of perinatal testicular torsion continues to be a topic of significant controversy among pediatric urologists and surgeons dealing with this pathology.[41] In perinatal torsion, the spermatic cord, testis and tunica vaginalis twist together as a unit (extravaginal torsion) as opposed to the more common intravaginal torsion where only the spermatic cord and testis rotate. Perinatal torsion has been commonly subdivided into prenatal (event occurring prior birth) and postnatal (event occurring from birth to one month of life) torsion.
The main controversy lies in the timing of surgical management of prenatal torsion. While some believe early intervention to be necessary, others argue that a more expectant approach may be used. The main argument for performing early intervention is based on the risk of asynchronous contralateral torsion. Perinatal torsion can be bilateral in up to 22% of the cases, [42] with bilateral torsion occurring synchronous in 67% and asynchronous in 33%.[43] Proponents of delayed intervention base their argument on beliefs including 1) there is increased risk of anesthesia associated with the first month of life, especially in the setting of a hospital without pediatric anesthesiologists[44] and 2) the perinatal torsed testis in invariably unsalvageable.[45]
Concerning pediatric anesthetic risks, a 2007 Mayo Clinic study reviewing 92,881 pediatric anesthetics between 1988–2005 determined that the incidence of anesthesia-related cardiac arrest in children < 18 years of age was very low (0.3 per 10,000) for patients undergoing noncardiac surgery and classified as American Society of Anesthesiologists physical status (ASA PS) I or II.[46] Concerning the specific subgroup of neonates ages 0–30 days, 4 cardiac arrests occurred in 1,014 anesthetics (39.4 cardiac arrests per 10,000 anesthetics; 95% CI 10.8–100.7) however all 4 cases were ASA PS IV or V and all 4 died.[46] Thus, ASA PS is a strong determinant in neonatal anesthesia. Given most neonates with torsion do not have co-morbidities (low ASA PS), the anesthetic concern may be overinflated for our patients if pediatric facilities and fellowship-trained pediatric anesthesiologists are present. If such resources are not available, we would recommend immediate transfer to such a facility.
Concerning the viability of prenatal torsion, Roth et al recently reported on six cases of bilateral prenatal torsion in which 3 patients with unilateral prenatal torsion were incidentally found to have contralateral torsion at the time of surgical exploration. [47] Unilateral testicular salvage was successful in all three of these cases, as well as one other case of recognized asynchronous torsion. Moreover, one patient who was followed with serum markers of testicular function had documented salvaged endocrine function after orchiopexy, the first reported case in the literature in a bilateral torsion case. In light of their experience with incidentally diagnosed asynchronous prenatal torsion, those authors have adopted the approach of emergent exploration with contralateral orchiopexy in healthy newborns with prenatal torsion. We concur with this philosophy.
Management of synchronous bilateral prenatal torsion appears to be less controversial, and most would advocate immediate exploration and orchiopexy rather than orchiectomy. The logic behind maintaining the ischemic testes is that in animal models, Leydig cells tolerate severe ischemia better than the other testicular cell populations, and thus the possibility of endogenous testosterone production may persist. [48–50]
Postnatal infant torsion should be managed with emergent exploration in similar fashion of that an older age patient with torsion. This statement is based in the fact that in this condition there is a real chance for testicular conservation. [42, 45] Regardless, even with the use of an immediate surgical approach for perinatal testicular torsion (prenatal and postnatal), the results for testicular salvage without severe atrophy are disappointing. [42, 45, 47]
Impact on Fertility
The true effect that an episode of torsion may have on fertility is yet not fully understood. Since torsion is most commonly a peripubertal event, there is a paucity of long term follow-up in adults who had torsion when younger. Only one study from 1994 has attempted this, with 1–12 year follow-up data on only 25 of 64 patients. They noted that in all cases of surgical detorsion in which torsion lasted more than 24 hours and viability of the testis was questionable, subsequent atrophy was the rule.[51] A piece of indirect evidence that implies that torsion has a low impact on fertility is that testicular torsion is the main diagnosis in only 0.5% of the patients evaluated for infertility.[52] Nevertheless, recent published data has shed some light in the possible long term effects of testicular torsion. Two articles that have evaluated fersemen analyses following testicular torsion found that all semen parameters were abnormal when compared to normal standard values, including sperm count, motility and morphology. One of the reports also looked at men with other causes of monorchia (including trauma, tumor and cryptorchidism) and found no difference in parameters compared with the torsion group.[53, 54] Thus, it seems that if a decrease in fertility does exist, it is likely due to the loss of germ cell tissue.[53]
In 2007, Arap et al investigated fertility parameters in patients who had testicular torsion at median age 15 years and underwent either orchiopexy (n=9) or orchiectomy (n=15), with median follow-up of 10 and 6 years, respectively. Results were compared to healthy controls (n=20). Their findings, surprisingly, showed no significant difference between subjects and the control group in relation to sperm count and motility. Sperm morphology was abnormal for all patients and controls according to the parameter used, but patients subjected to orchiopexy had the worst scores followed by orchiectomy and controls, respectively. Levels of anti-sperm antibody were abnormal in patients with torsion but not statistically different from normal controls.[55] Additionally, Romeo et al evaluated late hormonal function in patients with torsion who underwent detorsion and orchiopexy (n=12) or orchiectomy (n=8).[56] Serum follicle-stimulating hormone, luteinizing hormone, and testosterone were within normal reference ranges for all torsion patients. However, inhibin B levels were significantly reduced in both the orchiopexy and orchiectomy groups when compared to age-matched controls, although not significantly different between the torsion groups.
These results suggest that despite the relatively high incidence of testicular torsion in the young male population at 1 in 4000,[1] this disease is not a major contributor to the male infertility population. Additional long-term studies are needed to assess the outcomes of fertility parameters in this patient population to determine infertility frequency.
Drugs to Modulate Ischemic Germ Cell Damage
Recent publications have focused attention to the role of ischemia reperfusion injury in long-term testicular injury. It is believed that the recruitment of neutrophils and subsequent creation of reactive oxygen species (ROS) lead to DNA damage and apoptosis of germ cells, thus playing a major role in this type of injury following detorsion. Thus, it has been hypothesized that ROS scavengers may protect the germ cell population against this type of injury. This concept has led to reports on a number of different substances that may potentially reduce the ischemia-reperfusion injury following the surgical correction of a testicular torsion event. The theory is that these drugs can lead to improvement of the testicular function (particularly germ cell function) following an episode of torsion when the testis is salvaged. Although not tested in human testicular torsion, drugs such as vardenafil [57], sildenafil [58], rosuvastatin [59] and poly(adenosine diphosphate-ribose) polymerase (PARP) inhibitors [60, 61], and supplements such as coenzyme Q10 [62], lycopene [63] and ginkgo biloba [64] have been studied in rat models of testicular torsion.
Two reports have shown the efficacy of Phosphodiesterase type 5 (PDE5) inhibitors in reducing the amount of injury cause by ischemia/reperfusion injury.[57, 58] In separate studies, vardenafil and sildenafil were used in rat models undergoing torsion-detorsion procedures, with medication given intraperitoneally 30 minutes prior to detorsion. At necroscopy, tissue levels of malondialdehyde and testicular nitric oxide synthase expression were significantly lower and total testicular antioxidant levels higher in rats given medication as compared to those who simply underwent torsion/detorsion. PARP is an enzyme responsible for DNA repair and is activated during ischemia/reperfusion injury, leading to the consumption of large amounts of energy by the cell in order to repair DNA. The overexpression of PARP has been linked with progression to apoptosis. PARP inhibitors such as nicotinamide have been studied in a comparable manner and shown to demonstrate similar results of PDE5 inhibitors.[60, 61]
Ginkgo biloba, a compound with known antioxidative effects, was given to rats for one month prior to the torsion–detorsion event. After one hour of torsion and 2 hours post-detorsion, testes were retrieved for histopathologic evaluation and compared to animals subjected to a sham operation. Testes in the animals treated with ginkgo biloba showed a significant decrease in the number of apoptotic cells and nitric oxide synthase expression when compared to the untreated group.[64] Two additional studies were similarly performed administering lycopene and coenzyme Q10, both supplements that are also believed to have analogous antioxidative effects. Results were similar in regards to protection against ischemia/reperfusion injury to data reported with ginkgo biloba.[62, 63]
Rosuvastatin, a synthetic statin used for the treatment of hyperlipidemia, has been demonstrated to have anti-inflammatory effect. It has been shown to reduce ischemia/reperfusion injury in brain, intestine and heart tissue. This medication was given in a rat testicular torsion model via intraperitoneal injection prior to detorsion. In contrast to previous studies, the parameter measured was blood flow to the testis as measured by laser Doppler flowmeter before and during torsion as well as after detorsion. The rosuvastatin group had return of blood flow following detorsion equal to the control group (sham operation) and significantly better than the torsion group.[59] Interpretation of these results indicates that rosuvastatin may preserve or salvage tissue perfusion in an experimental testicular torsion animal model.
In addition to the above mention substances there have been studies with similar results using a variety of other compounds such as: Dexmedetomidine; Melatonin; L-Carnitine; Cyclosporine-A; FK-506; Erythropoietin; N-Acetylcysteine; and Molsidomine.[65–71] All of these compounds demonstrated a propensity towards decreasing the damage created by the ischemia-reperfusion injury when compared to placebo in murine models. These are interesting findings which will hopefully lead to the development of medications that can be given pre- or intra-operatively in males with testicular torsion in order to decrease the injury to the testis following detorsion.
Conclusions
Testicular torsion is an important and frequent condition affecting the young male population, carrying with it a significant risk for testicular loss and uncertain impact on fertility. While many areas of controversy remain, areas of future research in testicular torsion should include investigations concerning the etiology, rapid diagnosis, surgical techniques, and drug therapies for minimizing testicular injury and maximizing testicular salvage.
Acknowledgments
Supported in part by NIH R01 HD 048838 (PI: Baker, L.A.) and NIH R21 DK092654-01A1 (PI: Baker, LA)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
Ethical Approval: Not required
REFERENCES
- 1.Mansbach JM, Forbes P, Peters C. Testicular torsion and risk factors for orchiectomy. Arch Pediatr Adolesc Med. 2005;159(12):1167–1171. doi: 10.1001/archpedi.159.12.1167. [DOI] [PubMed] [Google Scholar]
- 2.Cost NG, et al. Pediatric testicular torsion: demographics of national orchiopexy versus orchiectomy rates. J Urol. 2011;185(6 Suppl):2459–2463. doi: 10.1016/j.juro.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Cubillos J, et al. Familial testicular torsion. J Urol. 2011;185(6 Suppl):2469–2472. doi: 10.1016/j.juro.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Selbst SM, Friedman MJ, Singh SB. Epidemiology and etiology of malpractice lawsuits involving children in US emergency departments and urgent care centers. Pediatr Emerg Care. 2005;21(3):165–169. [PubMed] [Google Scholar]
- 5.Raine JE. An analysis of successful litigation claims in children in England. Arch Dis Child. 2011;96(9):838–840. doi: 10.1136/adc.2011.212555. [DOI] [PubMed] [Google Scholar]
- 6.Osman NI, Collins GN. Urological litigation in the UK National Health Service (NHS): an analysis of 14 years of successful claims. BJU Int. 2011;108(2):162–165. doi: 10.1111/j.1464-410X.2011.10130.x. [DOI] [PubMed] [Google Scholar]
- 7.Scrotal pain may point to testicular torsion. 2008 Available from: < http://www.cmpa-acpm.ca/cmpapd04/docs/resource_files/infoletters/2006/com_il0630_2-e.cfm>, 2008.
- 8.Molokwu CN, Somani BK, Goodman CM. Outcomes of scrotal exploration for acute scrotal pain suspicious of testicular torsion: a consecutive case series of 173 patients. BJU Int. 107(6):990–993. doi: 10.1111/j.1464-410X.2010.09557.x. [DOI] [PubMed] [Google Scholar]
- 9.Caesar RE, Kaplan GW. Incidence of the bell-clapper deformity in an autopsy series. Urology. 1994;44(1):114–116. doi: 10.1016/s0090-4295(94)80020-0. [DOI] [PubMed] [Google Scholar]
- 10.Favorito LA, Cavalcante AG, Costa WS. Anatomic aspects of epididymis and tunica vaginalis in patients with testicular torsion. Int Braz J Urol. 2004;30(5):420–424. doi: 10.1590/s1677-55382004000500014. [DOI] [PubMed] [Google Scholar]
- 11.Cuckow PM, Frank JD. Torsion of the testis. BJU Int. 2000;86(3):349–353. doi: 10.1046/j.1464-410x.2000.00106.x. [DOI] [PubMed] [Google Scholar]
- 12.Casey JT, et al. Cold weather causing testicular torsion is an urban legend; Moderated poster at AUA Annual Meeting; 2011. [Google Scholar]
- 13.Gorbonos A, Cheng EY. Perinatal testicular torsion in siblings. J Pediatr Urol. 2007;3(6):514–515. doi: 10.1016/j.jpurol.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Collins K, Broecker BH. Familial torsion of the spermatic cord. J Urol. 1989;141(1):128–129. doi: 10.1016/s0022-5347(17)40618-5. [DOI] [PubMed] [Google Scholar]
- 15.Okeke LI, Ikuerowo OS. Familial torsion of the testis. Int Urol Nephrol. 2006;38(3–4):641–642. doi: 10.1007/s11255-006-0049-7. [DOI] [PubMed] [Google Scholar]
- 16.Castilla EE, et al. Neonatal testicular torsion in two brothers. J Med Genet. 1975;12(1):112–113. doi: 10.1136/jmg.12.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunningham RF. Familial occurrence of testicular torsion. JAMA. 1960;174:1330–1331. doi: 10.1001/jama.1960.63030100018026b. [DOI] [PubMed] [Google Scholar]
- 18.Picard J. 4 testicular torsions in twin brothers. J Urol Nephrol (Paris) 1975;81(6):460–461. [PubMed] [Google Scholar]
- 19.Lisk CH, Wilding RP. Torsion of the testicle in homozygous twins. Br J Urol. 1984;56(5):544–545. doi: 10.1111/j.1464-410x.1984.tb06276.x. [DOI] [PubMed] [Google Scholar]
- 20.Stewart JO, Maiti AK. Familial torsion of the testicle. Br J Urol. 1985;57(2):190–191. doi: 10.1111/j.1464-410x.1985.tb06420.x. [DOI] [PubMed] [Google Scholar]
- 21.Sinisi AA, et al. Late gonadal function and autoimmunization in familial testicular torsion. Arch Androl. 1993;30(3):147–152. doi: 10.3109/01485019308987748. [DOI] [PubMed] [Google Scholar]
- 22.Shteynshlyuger A, Freyle J. Familial testicular torsion in three consecutive generations of first-degree relatives. J Ped Urol. 2011;7:86–91. doi: 10.1016/j.jpurol.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Nef S, Parada LF. Cryptorchidism in mice mutant for Insl3. Nat Genet. 1999;22(3):295–299. doi: 10.1038/10364. [DOI] [PubMed] [Google Scholar]
- 24.Sozubir S, et al. Loss of Insl3: a potential predisposing factor for testicular torsion. J Urol. 2010;183(6):2373–2379. doi: 10.1016/j.juro.2010.02.2390. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, et al. Screening For A Genetic Basis For Testicular Torsion: The Insulin-3 (Insl3) And Lgr8 Genes. J Urol. 2008;179(4):147. (Abstract #413). [Google Scholar]
- 26.Baker LA, et al. An analysis of clinical outcomes using color doppler testicular ultrasound for testicular torsion. Pediatrics. 2000;105(3 Pt 1):604–607. doi: 10.1542/peds.105.3.604. [DOI] [PubMed] [Google Scholar]
- 27.Kaye JD, et al. Parenchymal echo texture predicts testicular salvage after torsion: potential impact on the need for emergent exploration. J Urol. 2008;180(4 Suppl):1733–1736. doi: 10.1016/j.juro.2008.03.104. [DOI] [PubMed] [Google Scholar]
- 28.Kalfa N, et al. Ultrasonography of the spermatic cord in children with testicular torsion: impact on the surgical strategy. J Urol. 2004;172(4 Pt 2):1692–1695. doi: 10.1097/01.ju.0000138477.85710.69. discussion 1695. [DOI] [PubMed] [Google Scholar]
- 29.Frauscher F, Klauser A, Radmayr C. Ultrasonographic assessment of the scrotum. Lancet. 2001;357(9257):721–722. doi: 10.1016/S0140-6736(05)71481-1. [DOI] [PubMed] [Google Scholar]
- 30.Kalfa N, et al. Multicenter assessment of ultrasound of the spermatic cord in children with acute scrotum. J Urol. 2007;177(1):297–301. doi: 10.1016/j.juro.2006.08.128. discussion 301. [DOI] [PubMed] [Google Scholar]
- 31.Vijayaraghavan SB. Sonographic differential diagnosis of acute scrotum: real-time whirlpool sign, a key sign of torsion. J Ultrasound Med. 2006;25(5):563–574. doi: 10.7863/jum.2006.25.5.563. [DOI] [PubMed] [Google Scholar]
- 32.Hirsch JC, et al. Near-infrared spectroscopy: what we know and what we need to know--a systematic review of the congenital heart disease literature. J Thorac Cardiovasc Surg. 2009;137(1):154–159. doi: 10.1016/j.jtcvs.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Mittnacht AJ. Near infrared spectroscopy in children at high risk of low perfusion. Curr Opin Anaesthesiol. 2010;23(3):342–347. doi: 10.1097/ACO.0b013e3283393936. [DOI] [PubMed] [Google Scholar]
- 34.Capraro GA, et al. Feasibility of using near-infrared spectroscopy to diagnose testicular torsion: an experimental study in sheep. Ann Emerg Med. 2007;49(4):520–525. doi: 10.1016/j.annemergmed.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 35.Hallacoglu B, et al. Noninvasive assessment of testicular torsion in rabbits using frequency-domain near-infrared spectroscopy: prospects for pediatric urology. Journal of Biomedical Optics. 2009;14(5) doi: 10.1117/1.3253318. 054027. [DOI] [PubMed] [Google Scholar]
- 36.Aydogdu O, et al. Near infrared spectroscopy to diagnose experimental testicular torsion: comparison with Doppler ultrasound and immunohistochemical correlation of tissue oxygenation and viability. J Urol. 2012;187(2):744–750. doi: 10.1016/j.juro.2011.09.145. [DOI] [PubMed] [Google Scholar]
- 37.Burgu B, et al. Feasibility of transscrotal near-infrared spectroscopy (NIRS) in the evaluation of acute scrotum: a pilot human study; Moderated poster at AUA Annual Meeting; 2011. [Google Scholar]
- 38.Kutikov A, et al. Testicular compartment syndrome: a new approach to conceptualizing and managing testicular torsion. Urology. 2008;72(4):786–789. doi: 10.1016/j.urology.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 39.Moritoki Y, et al. Intratesticular pressure after testicular torsion as a predictor of subsequent spermatogenesis: a rat model. BJU Int. 2011 doi: 10.1111/j.1464-410X.2011.10279.x. [DOI] [PubMed] [Google Scholar]
- 40.Bagrodia A, Preuss D, Bush N. American Urological Association National Convention. Washington, D.C.: 2011. Combined Orchiectomy and Prosthetic Exchange: Surgical Technique. [Google Scholar]
- 41.Guerra LA, et al. Management of neonatal testicular torsion: Which way to turn? Can Urol Assoc J. 2008;2(4):376–379. doi: 10.5489/cuaj.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yerkes EB, et al. Management of perinatal torsion: today, tomorrow or never? J Urol. 2005;174(4 Pt 2):1579–1582. doi: 10.1097/01.ju.0000179542.05953.11. discussion 1582-3. [DOI] [PubMed] [Google Scholar]
- 43.Baglaj M, Carachi R. Neonatal bilateral testicular torsion: a plea for emergency exploration. J Urol. 2007;177(6):2296–2299. doi: 10.1016/j.juro.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Cohen MM, Cameron CB, Duncan PG. Pediatric anesthesia morbidity and mortality in the perioperative period. Anesth Analg. 1990;70(2):160–167. doi: 10.1213/00000539-199002000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Kaye JD, et al. Neonatal torsion: a 14-year experience and proposed algorithm for management. J Urol. 2008;179(6):2377–2383. doi: 10.1016/j.juro.2008.01.148. [DOI] [PubMed] [Google Scholar]
- 46.Flick RP, et al. Perioperative cardiac arrests in children between 1988 and 2005 at a tertiary referral center: a study of 92,881 patients. Anesthesiology. 2007;106(2):226–237. doi: 10.1097/00000542-200702000-00009. quiz 413-4. [DOI] [PubMed] [Google Scholar]
- 47.Roth CC, Mingin GC, Ortenberg J. Salvage of bilateral asynchronous perinatal testicular torsion. J Urol. 2011;185(6 Suppl):2464–2468. doi: 10.1016/j.juro.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 48.Baker LA, Turner TT. Leydig cell function after experimental testicular torsion despite loss of spermatogenesis. J Androl. 1995;16(1):12–17. [PubMed] [Google Scholar]
- 49.Turner TT, Bang HJ, Lysiak JJ. Experimental testicular torsion: reperfusion blood flow and subsequent testicular venous plasma testosterone concentrations. Urology. 2005;65(2):390–394. doi: 10.1016/j.urology.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 50.Callewaert PR, Van Kerrebroeck P. New insights into perinatal testicular torsion. Eur J Pediatr. 2009 doi: 10.1007/s00431-009-1096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tryfonas G, et al. Late postoperative results in males treated for testicular torsion during childhood. J Pediatr Surg. 1994;29(4):553–556. doi: 10.1016/0022-3468(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 52.Sigman M, Jarow JP. Male Infertility. In: Alan M, Wein J PhD, editors. Campbell-Walsh Urology. Philadelphia: Saunders Elsievier; 2007. pp. 609–657. [Google Scholar]
- 53.Ferreira U, et al. Comparative study of the fertility potential of men with only one testis. Scand J Urol Nephrol. 1991;25(4):255–259. doi: 10.3109/00365599109024555. [DOI] [PubMed] [Google Scholar]
- 54.Schutte B, Becker H, Vydra G. Exocrine and endocrine testicular function following unilateral torsion--a retrospective clinical study of 36 patients. Urologe A. 1986;25(3):142–146. [PubMed] [Google Scholar]
- 55.Arap MA, et al. Late hormonal levels, semen parameters, and presence of antisperm antibodies in patients treated for testicular torsion. J Androl. 2007;28(4):528–532. doi: 10.2164/jandrol.106.002097. [DOI] [PubMed] [Google Scholar]
- 56.Romeo C, et al. Late hormonal function after testicular torsion. J Pediatr Surg. 2010;45(2):411–413. doi: 10.1016/j.jpedsurg.2009.10.086. [DOI] [PubMed] [Google Scholar]
- 57.Erol B, et al. Vardenafil reduces testicular damage following ischemia/reperfusion injury in rats. Kaohsiung J Med Sci. 2009;25(7):374–380. doi: 10.1016/S1607-551X(09)70530-3. [DOI] [PubMed] [Google Scholar]
- 58.Beheshtian A, et al. Protective effects of sildenafil administration on testicular torsion/detorsion damage in rats. World J Urol. 2008;26(2):197–202. doi: 10.1007/s00345-008-0243-6. [DOI] [PubMed] [Google Scholar]
- 59.Karakaya E, et al. Rosuvastatin protects tissue perfusion in the experimental testicular torsion model. Int Urol Nephrol. 2009 doi: 10.1007/s11255-009-9633-y. [DOI] [PubMed] [Google Scholar]
- 60.Bozlu M, et al. The effect of poly (adenosine diphosphate-ribose) polymerase inhibitors on biochemical changes in testicular ischemia-reperfusion injury. J Urol. 2003;169(5):1870–1873. doi: 10.1097/01.ju.0000049228.37887.4d. [DOI] [PubMed] [Google Scholar]
- 61.Bozlu M, et al. Inhibition of poly(adenosine diphosphate-ribose) polymerase decreases long-term histologic damage in testicular ischemia-reperfusion injury. Urology. 2004;63(4):791–795. doi: 10.1016/j.urology.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 62.Erol B, et al. Coenzyme Q(10) treatment reduces lipid peroxidation, inducible and endothelial nitric oxide synthases, and germ cell-specific apoptosis in a rat model of testicular ischemia/reperfusion injury. Fertil Steril. 2009 doi: 10.1016/j.fertnstert.2009.07.981. [DOI] [PubMed] [Google Scholar]
- 63.Hekimoglu A, et al. Lycopene, an antioxidant carotenoid, attenuates testicular injury caused by ischemia/reperfusion in rats. Tohoku J Exp Med. 2009;218(2):141–147. doi: 10.1620/tjem.218.141. [DOI] [PubMed] [Google Scholar]
- 64.Akgul T, et al. Ginkgo biloba (EGB 761) affects apoptosis and nitric-oxide synthases in testicular torsion: an experimental study. Int Urol Nephrol. 2009;41(3):531–536. doi: 10.1007/s11255-008-9511-z. [DOI] [PubMed] [Google Scholar]
- 65.Hanci V, et al. Effect of dexmedetomidine on testicular torsion/detorsion damage in rats. Urol Int. 2010;84(1):105–111. doi: 10.1159/000273476. [DOI] [PubMed] [Google Scholar]
- 66.Kanter M. Protective effects of melatonin on testicular torsion/detorsion-induced ischemia-reperfusion injury in rats. Exp Mol Pathol. 2010;89(3):314–320. doi: 10.1016/j.yexmp.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 67.Guan Y, et al. Protective effects of L-carnitine upon testicular ischemia-reperfusion damage in rats. Zhonghua Yi Xue Za Zhi. 2009;89(26):1858–1861. [PubMed] [Google Scholar]
- 68.Nezami BG, et al. Protective effects of immunophilin ligands on testicular torsion/detorsion damage in rats. Int Urol Nephrol. 2009;41(1):93–99. doi: 10.1007/s11255-008-9453-5. [DOI] [PubMed] [Google Scholar]
- 69.Ergur BU, et al. Protective effect of erythropoietin pretreatment in testicular ischemia-reperfusion injury in rats. J Pediatr Surg. 2008;43(4):722–728. doi: 10.1016/j.jpedsurg.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 70.Payabvash S, et al. Salutary effects of N-acetylcysteine on apoptotic damage in a rat model of testicular torsion. Urol Int. 2007;79(3):248–254. doi: 10.1159/000107958. [DOI] [PubMed] [Google Scholar]
- 71.Dokucu AI, et al. The effects of molsidomine on hypoxia inducible factor alpha and Sonic hedgehog in testicular ischemia/reperfusion injury in rats. Int Urol Nephrol. 2009;41(1):101–108. doi: 10.1007/s11255-008-9460-6. [DOI] [PubMed] [Google Scholar]