Abstract
Simple and rapid extraction of human genomic DNA remains a bottle neck for genome analysis and disease diagnosis. Current methods using microfilters require cumbersome, multiple handling steps in part because salt conditions must be controlled for attraction and elution of DNA in porous silica. We report a novel extraction method of human genomic DNA from buccal swab- and saliva samples. DNA is attracted on to a gold-coated microchip by an electric field and capillary action while the captured DNA is eluted by thermal heating at 70 °C. A prototype device was designed to handle 4 microchips, and a compatible protocol was developed. The extracted DNA using microchips was characterized by qPCR for different sample volumes, using different lengths of PCR amplicon, and nuclear and mitochondrial genes. In comparison with a commercial kit, an equivalent yield of DNA extraction was achieved with fewer steps. Room-temperature preservation for one month was demonstrated for captured DNA, facilitating straightforward collection, delivery and handling of genomic DNA in an environment-friendly protocol.
Keywords: DNA extraction, Microtip, Electric field, Human genomic DNA, Human samples
Introduction
There are significant needs for simpler and higher-throughput methods to extract human genomic DNA from body samples. DNA extraction is also crucial for medical, forensic, environmental, or military purposes [1]. Popular sources are saliva- [2, 3] and buccal swab samples [4, 5] because the sample collection is minimally invasive.
For DNA extraction, solid phase extraction (SPE) methods using porous silica are commercially available[5, 6]. Cell lysates are infiltrated into silica micropores by high salt and chaotropic solutions, which bind DNA by electrostatic charge. After washing with alcohol, the DNA is eluted in a low salt solution by electrostatic repulsion. The extraction yield is high but multiple centrifuge steps are required along with the use of toxic reagents. In the process, DNA can be degraded by alkaline solutions [7–9] and flow-induced shear of DNA [10] during centrifugation. For on-chip systems, silica chips [11], silica beads [12] or polymers [13] can be integrated into microfluidic devices. However, the actual use is limited to a small sample volume (e.g. 1μL). In microfluidic devices, electric field-induced methods have shown limited success to concentrate DNA in buffer solutions [14–16]. DNA extraction from human samples using an electric field has yet to be demonstrated.
Preservation of DNA at room temperature is also important to medical, forensic, environmental, and military purposes. In particular, long term storage is a critical issue in genomic analysis [17] and forensic applications [18]. Preservation in aqueous solutions is detrimental to DNA molecules, susceptible to chemical changes [19–22]. Extended storage requires freezing or the use of specialized preservatives [23].
This paper reports a rapid DNA preparation method. DNA is attracted on to a microchip using an AC electric field and capillary action. The captured DNA is eluted in buffer by thermal heating at 70 °C. Two protocols for buccal swab- and saliva samples are presented. Using real-time PCR (qPCR), the yield of DNA extraction is compared with that of a commercial kit.
Materials and Methods
Device Operation
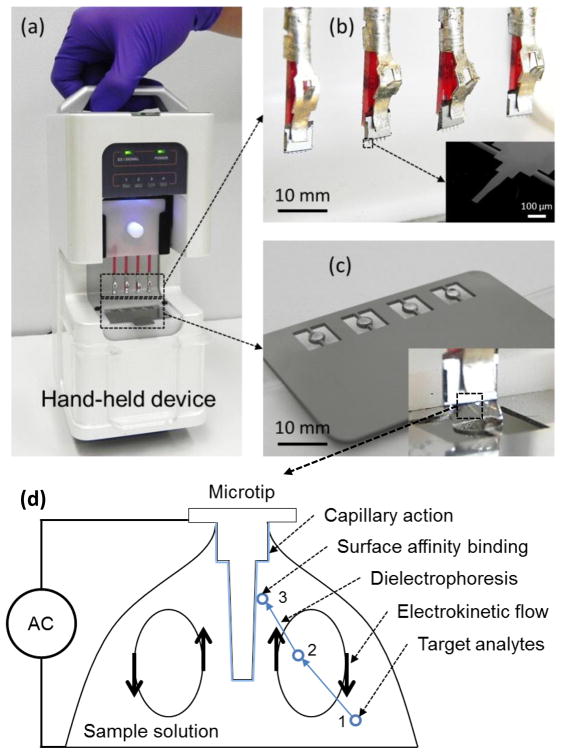
A DNA extraction device was designed to process four DNA samples in one batch [Fig. 1(a)]. Four chips were loaded on a plastic coupon [Fig. 1(b)]. Each individual chip has five microtips. In this paper, ‘microtip’ means one of five microtips in a microchip, ‘microchip’ means a whole chip composed of microtips and a silicon chip, and ‘a microtip device’ means a prototype device in Fig. 1(a). The microtips were made of 1 μm-thick silicon nitride layer supported on a 500 μm-thick silicon layer [24]. The top side of the microtips was coated with a 20 nm-thick gold layer for electrical connection and preservation of DNA. Metallic rings were used to suspend sample solutions by surface tension [Fig. 1 (c)].
Figure 1.
(a) Portable microtip device for DNA extraction (b) magnified view of four chips. Each chip has five microtips. The inset shows an SEM image of a single microtip. (c) An array of rings holds four sample solution drops by surface tension. The inset shows a chip immersed in a solution drop. (See Electronic Supplementay Material, movie for operation) (d) Working principle of a microtip: DNA in a sample is concentrated by electroosmotic flow from location 1 to 2. The concentrated DNA is further attracted to the microtip by dielectrophoresis from location 2 to 3. The attracted DNA is captured on the microtip surface with capillary action when the microtip is withdrawn from the solution. The captured DNA is eluted in buffer by thermal heating at 70 °C.
For device operation, 4 sample solutions of 5 μL were suspended in the metal rings. The chips were immersed into the sample solutions as shown in the inset image of Fig. 1 (c). An AC voltage of 20 Vpp (peak to peak voltage) at 5 MHz was applied between a chip and a ring for 30 seconds. The chips were withdrawn from the sample solutions at a speed of 100 μm/s with continuous application of an AC potential. After complete withdrawal, the chips were dried for two minutes in air. In the evaporation process, DNA could be adhered and preserved on the Au surface of microchips at room temperature. The captured DNA was eluted in PCR tubes by immersing microchips in 30 uL of 1X TE buffer 8.5 pH at 70 °C for 4 minutes.
In the DNA extraction process, 20 Vpp was chosen to avoid electrical breakdown of sample solution on the microtips. In our previous study, λ-DNA spiked in buffer could be concentrated on to microtip surface by dielectrophoresis and electrokinetic flow [25]. An electric field of 10 MHz and a DC bias showed the highest capturing yield measured by a fluorescence microscope. However, such conditions attracted other charged particles, which inhibited q-PCR reactions. In our repeated tests using human samples, an AC field between 100 kHz and 5 MHz showed the highest yield in q-PCR. In the frequency region, 5 MHz was chosen because frequencies below 1 MHz could potentially attract PCR inhibitors.
Experimental methods
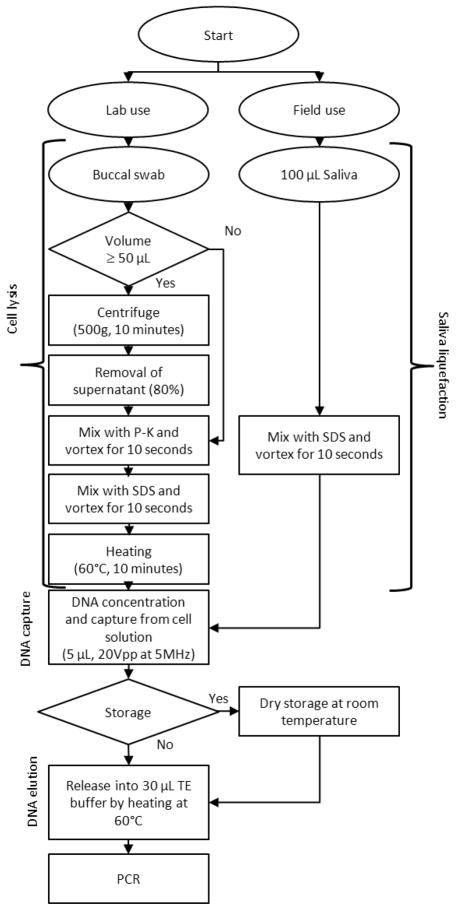
Two kinds of samples, buccal swab and saliva, were collected from de-identified volunteers. Buccal swab samples were evaluated for a laboratory protocol while saliva samples were evaluated for a field-deployable protocol (Fig. 2).
Figure 2.
Protocols for cell lysis, DNA capture, and elution from for buccal swab- and saliva samples.
For buccal swab samples, Whatman (Piscataway, NJ) omni sterile buccal swabs were used. After sample collection, the swab was completely dried. For elution of cells, the swab was immersed in 1 mL of 1X TRIS-EDTA (TE) buffer (pH 7.5, Invitrogen, Carlsbad, CA, USA). After the vortexing of 30 seconds, sample volume of 700 μL was collected from one swab sample.
To evaluate the extraction yield, sample volumes of 5, 10, 50 and 100 μL were pipetted from a 700 μL extract. The sample solutions were lysed by using 600 AU/L proteinase-K (P-K) (Qiagen®, Valencia, CA) and sodium dodecyl sulfate (SDS) (Sigma Aldrich, St. Louis, MO). For the large volumes of 50 and 100 μL, the sample solutions were centrifuged to make the final volumes of 10 and 20 μL, respectively. P-K of 1 μL (600 AU/L) and SDS of 4 μL (0.28 g/mL) were added per cell solution of 20 μL. After mixing of the reagents, the sample solution was heated at 60 °C for 10 minutes to lyse the cells. An aliquot of 5 μL was subjected to processing by the microtip device. The captured DNA was eluted in PCR tubes by immersing chips in 30 μL of 1X TE buffer (pH 8.5) at 70 °C for 4 minutes. For a reproducibility test, 24 samples from different volunteers of 100 μL volume were tested only with the microtip device.
To compare the extraction yield with a commercial kit (Qiagen® QIAmp DNA mini kit), the sample volumes of 5, 10, 50 and 100 μL were also collected from the same extract in the same way as the microtip device. The 50 and 100 μL samples were not centrifuged before the use of the commercial kit. The commercial kit required about 6 centrifugation steps in the extraction process. To evaluate the potential damage of genomic DNA, both 100 and 1500 bp of PCR amplicons were used for the extracted DNA from both the microchips and the commercial kit. For saliva samples, SDS was added to obtain a final concentration of 0.08 g/mL. This was achieved by adding 4 μL of 2 g/mL SDS per 100 μL of saliva followed by vortexing for 10 seconds. The treatment may not have lysed cells but reduced the viscosity of saliva. Using microchips, DNA was captured from 5 μL of the processed samples. The captured DNA was eluted in PCR tubes in the same way as the buccal swab samples. For comparison with the commercial kit, 5 μL of the same saliva samples was used. For the evaluation of reproducibility, 24 saliva samples were tested by the microchips. To test the DNA integrity in one-month preservation, 16 different chips were used to extract DNA from single sample mixture. The chips were stored in a vial at room temperature without dessicants. The DNA on the chips was tested at days of 1, 8, 15, and 30 (n=4 for each day). For the commercial kit, the eluted DNA was also tested likewise in parallel.
To measure the yield of extracted DNA from the microchips and the commercial kit, qPCR was mainly used (See Electronic Supplementary Material for qPCR analysis and gene sequences). UV measurement and gel electrophoresis were also attempted. However, because the concentrations of DNA and protein were smaller than 1μg/mL, the results were not reliable, and hence not reported.
Results
When DNA was extracted from buccal swab samples with volumes of 5, 10, 50 and 100 μL, the average threshold cycles for the microtip device using the 100 bp amplicon were 23.92, 23.85, 21.22 and 21.87, respectively [Fig. 3(a)]. The corresponding threshold cycles of the commercial kit were 25.58, 24.47, 22.10 and 21.49, respectively. For 1500 bp amplicon, the microtip devices yielded threshold cycles of 27.37, 26.37, 23.38 and 22.99 for the sample volumes of 5, 10, 50 and 100 μL while the corresponding cycles of the commercial kit were 28.99, 27.09, 24.95, and 23.69 [Fig. 2(b)]. The performance of the microtip device was equivalent to that of the commercial kit in all volumes.
Figure 3.
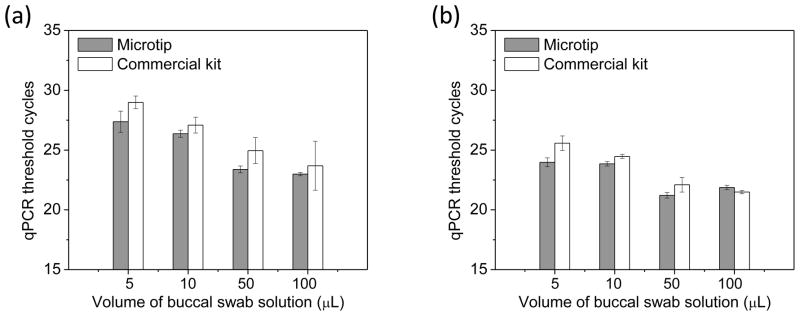
qPCR analysis for buccal swab samples. Sample volumes are 5, 10, 50 and 100 μL for the microtip device and the commercial kit. (a) The length of amplicon is 100 bp (n=4) (b) The length of amplicon is 1500 bp (n=4).
For saliva samples, the average threshold cycle of 100 bp amplicon of nuclear DNA for the microtip device was 25.95 ± 0.21 while that of the commercial kit was 27.06 ± 0.52 [Fig. 4(a)]. The average threshold cycle for 100 bp amplicon of mitochondrial DNA for the microtip device was 23.71 ± 0.65 while that of the commercial kit was 24.55 ± 0.49. For the reproducibility test, DNA was extracted for 24 different saliva samples and tested only with microtip device. The threshold cycle using the microtip device was 25.18 ± 1.50, which was also in the similar range of the average threshold cycle of the automated commercial device, 23.1±0.25 in literature [2]. For a preservation test, both 100 and 1500 bp amplicons were used [Fig. 4 (b) and (c)]. The threshold cycles for 100 bp amplicon showed the better results than those for 1500 bp amplicon by two cycles. The threshold cyles were maintained for one month without significant damage.
Figure 4.
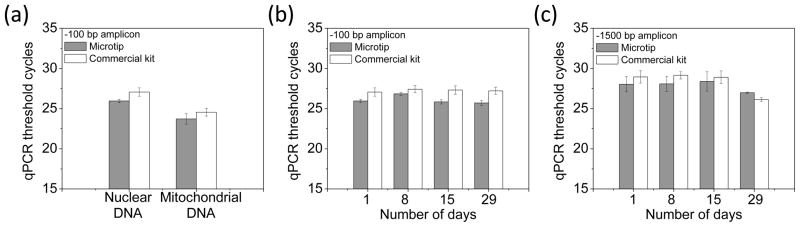
qPCR analysis of human genomic DNA from saliva samples. The microtip device is compared with a commercial kit. (a) qPCR threshold cycles of nuclear DNA (β-actin gene) and mitochondrial DNA (Cytochrome C oxidase gene) (n=4) (b) Preservation test: qPCR threshold cycles of 100 bp amplicon in β-actin gene (n=4) (c) Preservation test: qPCR threshold cycles of 1500 bp amplicon of nuclear DNA (β-globin gene) (n=4).
Discussion
In working principle, the electric field and the capillary effect were the dominant mechanisms for the DNA capture. To investigate the contribution of the capillary effect and the electric field, λ-DNA molecules were used for the comparison. When, λ-DNA molecules were used for the recovery, the difference between in the presence and absence of an electric field was 7 cycles for, λ-DNA molecules in buffer (Electronic Supplementary Material, Fig. S1). When saliva samples were used, only 1~2 cycle difference was observed with larger error bars in the absence of an electric field. The significantly higher yield in buffer could be caused by an electric field because dielectrophoresis was more dominant than capillary action in low-conductivity buffer. For saliva, capillary action became more dominant in complex samples.
In this paper, both buccal swab and saliva samples were chosen in consideration of minimally invasive human samples. Both samples could be collected with minimal pain and treated with the reagents less than 5 μL. Such field-collection capability was also aligned with the portable design of the microtip device. The device was designed to run 60 batches using 8 of AA batteries. However, when the microtip device is applied for more complex human samples, such as blood, the DNA extraction protocol should be modified for more rigorous lysis and purification. For example, after capturing of DNA onto microtip surface, the tip should be washed to remove excessive protein and reagents. Such protocols are being developed to apply the microtip device for various samples.
In terms of an extraction yield of genomic DNA, the microtip device was similar to the commercial kit within an error range. In case of the buccal swab samples, the threshold cycles by qPCR were similar between the microtip device and the commercial kit. When 4 microchips ran for 100 μL of buccal swab samples, the total volume of the extracted DNA in buffer was 120 μL, which was similar to the elution volume of the commercial kit. Thus the total mass of DNA was equivalent for the microtip device and the commercial kit. In the case of the saliva samples, the microtip device advanced the commercial kit by one or two cycles. But the elution volume was one quarter of the commercial kit. In an error range of 5% (i.e. 1.5 cycles in 30 cycles of qPCR analysis), the total mass of DNA was equivalent for both kits.
In terms of the procedure time, the microtip device was much faster and simpler than the commercial kit. The microtip device could extract DNA from saliva within 10 minutes. The commercial kit required about 30 minutes for one sample, including multiple centrifugation processes. The microtip device did not require a centrifugation step for the volumes smaller than 50 μL, which could reduce potential human errors. The centrifuge-free process could be also advantageous for zero-gravity environment, such as space applications.
In combination with the simple process, the microchip did not require any toxic solutions. Moreover, the smallest sample volume for DNA extraction was 1 μL with microtip. In the smaller volume, even the volume of reagent will be negligible. Therefore, the microtip device could facilitate environment-friendly extraction. The commercial kit required 2000 μL of liquid phase reagents including 1400 μL of toxic reagents, which also increased the risk of contamination. The performance of the microtip device is summarized in Table 1 in comparison with the commercial kit.
Table 1.
Comparison between the microtip device and the commercial kit for saliva samples
| For a saliva sample (5 μL) | Microtip device | Commercial kit |
|---|---|---|
| Threshold cycles/eluted volume | 26.0/30 μL* | 27.1/120 μL* |
| Number of steps | 4 | 23 |
| Approximate processing time (single sample) | 10 minutes | 30 minutes |
| Reagent volumes: toxic reagents total reagents | None/4 μL | 1400/2000 μL |
| Number of centrifugations | None | 6 |
| Storage time at room temperature | 1month | 1 month |
| Portability | Yes (60 assays with 8 AA-batteries) | No |
Data excerpted from nuclear DNA in Fig. 4(a).
The microtip-based method could cause less shear of DNA than a porous silica-based method, which is advantageous for long-range PCR. To compare the damage of DNA in the microtip device and the commercial kit, gel electrophoresis and atomic force microscopy (AFM) were used for recovery study of λ-DNA from buffer (Electronic Supplementary Material, Fig. S1). By gel electrophoresis, the band of the microtip device was similar to that of the original λ-DNA solution. In contrast, the λ-DNA by the commercial kit appeared damaged, which was shown as the smeared band. However, in the qPCR analysis, the threshold cycles were the same. In qPCR using buccal swab samples, large error bars were observed for 1500 bp [Fig. 3(b)], indicating the damaged DNA molecules. In our AFM study, DNA of K562 human leukemia cells was extracted by the microtip device and the commercial kit (Electronic Supplementary Material, Fig. S2). A few micrometers-long DNA was easily found for the microtip device. However, the presence of long DNA could not be clearly observed for the commercial kit. Considering the gel electrophoresis, AFM, and qPCR results, the microtip device could damage DNA less than the silica-based microfilters.
One of the major advantages of the microtip device is the capability of long-term preservation of genomic DNA in a dried form at room temperature. The preservation of DNA in liquid is known to damage more DNA than that under dried condition [22]. The storage capability enables field collection of DNA and does not require freezers or refrigerators, which has been a challenge for forensic analysis. One month storage of DNA from saliva samples was demonstrated in Fig. 4(c). In addition, the DNA extracted from human cells could be preserved for 6 months (Electronic Supplementary Material, Fig. S3). The capability of the room-temperature preservation renders the microtip device ideal for field-deployable collection of genomic DNA from saliva samples. Further preservation test for a long term (e.g. year) should be conducted to clearly differentiate the advantages of DNA storage in a dried condition.
For the scalability of the microtip device, the microchips are manufactured as an array by microfabrication steps. Using a 100 mm-diameter Si wafer, 350 chips are manufactured in one wafer. Typically 25~50 wafers are processed in one batch, which can significantly reduce the manufacturing cost and thus the assay cost. For a higher throughput, the microtip device does not require centrifuge steps, which yields the flexibility for a microwell-plate compatible design. For example, a 96 well-plate compatible microtip device can be developed to handle 96 samples simultaneously, which will be future work of the microtip device.
Conclusion
A prototype microtip device was developed and characterized for rapid DNA extraction by using microtips. A combination of an electric field and capillary action were used for attraction of DNA while thermal heating was used for the elution. The extraction yield in terms of qPCR was equivalent to that offered by a commercial kit. The environment-friendly steps with less reagent could complete the DNA extraction less than 10 minutes from small-volume saliva samples. The simple process can also significantly reduce the assay cost and potential human errors. The long-term storage capability can facilitate easy collection of DNA for developing a database of human genomic DNA from saliva. The microtip device can potentially benefit human genome projects, disease diagnosis, and forensic analysis by reducing the initial barrier of high-throughput sample preparation.
Supplementary Material
Figure 5.
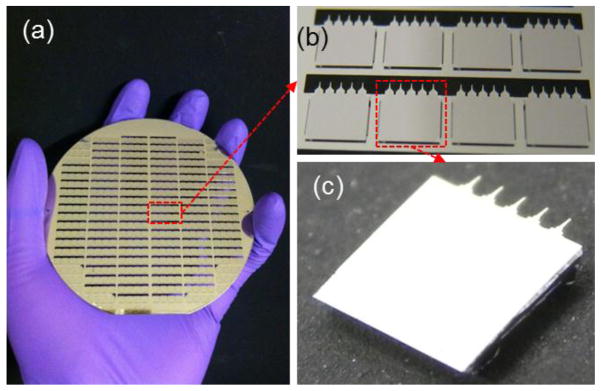
Scalability of microchips (a) 350 microchips in a 100mm-diameter wafer (b) Exploded view of microchips (c) Microchip composed of 5 microtips.
Acknowledgments
We would like to acknowledge Ms. Sijie Sun, Department of Bioengineering at University of Washington for help with gel electrophoresis. We would like to acknowledge Dr. Xia You and Dr. John Stamatoyannopoulos at Department of Genome Sciences at University of Washington for providing K562 cells. We acknowledge the support of NSF STTR II award (0956876), NSF Career Award (ECCS-0846454) and NIH SBIR (NIH/NIGMS 1R43GM099347).
Contributor Information
D. Kalyanasundaram, Department of Mechanical Engineering, University of Washington, Seattle, WA 98195, USA
J.-H. Kim, Department of Mechanical Engineering, University of Washington, Seattle, WA 98195, USA
W.-H. Yeo, Department of Mechanical Engineering, University of Washington, Seattle, WA 98195, USA
K. Oh, NanoFacture, Inc., P.O. Box 52651, Bellevue, WA 98015, USA
K.-H. Lee, NanoFacture, Inc., P.O. Box 52651, Bellevue, WA 98015, USA
M.-H. Kim, KNR systems, Inc., Yongin-si, Gyeonggi-do, 449-881, Republic of Korea
S.-M. Ryew, KNR systems, Inc., Yongin-si, Gyeonggi-do, 449-881, Republic of Korea
S.-G. Ahn, Department of Industrial Design, University of Washington, Seattle, WA 98195, USA
D. Gao, Department of Mechanical Engineering, University of Washington, Seattle, WA 98195, USA
G. A. Cangelosi, Washington Department of Environmental and Occupational Health Sciences, University of Washington, Seattle, WA 98195, USA
J.-H. Chung, Department of Mechanical Engineering, University of Washington, Seattle, WA 98195, USA
References
- 1.Gyorgy C. Present and future of rapid and/or high-throughput methods for nucleic acid testing. Clin Chim Acta. 2006;363:6–31. doi: 10.1016/j.cccn.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Keijzer H, Endenburg SC, Smits MG, Koopmann M. Automated genomic DNA extraction from saliva using the QIAxtractor. Clin Chem Lab Med. 2010;48:641–3. doi: 10.1515/CCLM.2010.139. [DOI] [PubMed] [Google Scholar]
- 3.Koni AC, Scott RA, Wang G, Bailey MES, Peplies J, Bammann K, Pitsiladis YP. DNA yield and quality of saliva samples and suitability for large-scale epidemiological studies in children. Int J Obes. 2011;35:S113–S8. doi: 10.1038/ijo.2011.43. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Closas M, Egan KM, Abruzzo J, Newcomb PA, Titus-Ernstoff L, Franklin T, Bender PK, Beck JC, Le Marchand L, Lum A, Alavanja M, Hayes RB, Rutter J, Buetow K, Brinton LA, Rothman N. Collection of genomic DNA from adults in epidemiological studies by buccal cytobrush and mouthwash. Cancer Epidemiol Biomarkers Prev. 2001;10(6):687–696. [PubMed] [Google Scholar]
- 5.Hanselle T, Otte M, Schnibbe T, Smythe E, Krieg-Schneider F. Isolation of genomic DNA from buccal swabs for forensic analysis, using fully automated silica-membrane purification technology. Legal Med. 2003;5:S145–S9. doi: 10.1016/s1344-6223(02)00099-8. [DOI] [PubMed] [Google Scholar]
- 6.Carter MJ, Milton ID. An inexpensive and simple method for DNA purifications on silica particles. Nucleic Acids Res. 1993;21(4):1044. doi: 10.1093/nar/21.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner JG, Petry TW, Roth RA. Characterization of monocrotaline pyrrole-induced dna cross-linking in pulmonary-artery endothelium. Am J Physiology. 1993;264:L517–L522. doi: 10.1152/ajplung.1993.264.5.L517. [DOI] [PubMed] [Google Scholar]
- 8.Fornace AJ, Dobson PP, Kinsella TJ. Analysis of the effect of DNA alkylation on alkaline elution. Carcinogenesis. 1986;7(6):927–932. doi: 10.1093/carcin/7.6.927. [DOI] [PubMed] [Google Scholar]
- 9.Ageno M, Dore E, Frontali C. The Alkaline Denaturation of DNA. Biophys J. 1969;9(11):1281–311. doi: 10.1016/S0006-3495(69)86452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price CW, Leslie DC, Landers JP. Nucleic acid extraction techniques and application to the microchip. Lab Chip. 2009;9:2484–2494. doi: 10.1039/b907652m. [DOI] [PubMed] [Google Scholar]
- 11.Christel LA, Petersen K, McMillan W, Northrup MA. Rapid, automated nucleic acid probe assays using silicon microstructures for nucleic acid concentration. J Biomech Eng-T ASME. 1999;121:22–27. doi: 10.1115/1.2798037. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe KA, Breadmore MC, Ferrance JP, Power ME, Conroy JF, Norris PM, Landers JP. Toward a microchip-based solid-phase extraction method for isolation of nucleic acids. Electrophoresis. 2002;23:727–33. doi: 10.1002/1522-2683(200203)23:5<727::AID-ELPS727>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 13.Witek MA, Llopis SD, Wheatley A, McCarley RL, Soper SA. Purification and preconcentration of genomic DNA from whole cell lysates using photoactivated polycarbonate (PPC) microfluidic chips. Nucleic Acids Res. 2006;34(10):e74. doi: 10.1093/nar/gkl146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pethig R. Review Article-Dielectrophoresis: Status of the theory, technology, and applications. Biomicrofluidics. 2010;4(2):022811. doi: 10.1063/1.3456626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lapizco-Encinas BH, Rito-Palomares M. Dielectrophoresis for the manipulation of nanobioparticles. Electrophoresis. 2007;28:4521–38. doi: 10.1002/elps.200700303. [DOI] [PubMed] [Google Scholar]
- 16.Bakewell DJ, Morgan H. Dielectrophoresis of DNA: Time- and frequency-dependent collections on microelectrodes. IEEE T Nanobiosci. 2006;5(1):1–8. [PubMed] [Google Scholar]
- 17.Bonnet J, Colotte M, Coudy D, Couallier V, Portier J, Morin B, Tuffet S. Chain and conformation stability of solid-state DNA: implications for room temperature storage. Nucleic Acids Res. 2010;38(5):1531–1546. doi: 10.1093/nar/gkp1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frippiat C, Zorbo S, Leonard D, Marcotte A, Chaput M, Aelbrecht C, Noel F. Evaluation of novel forensic DNA storage methodologies. Forensic Sci Int: Gen. 2011;5(5):386–392. doi: 10.1016/j.fsigen.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Lindahl T, Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972;11:3610–8. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- 20.Lindahl T, Karlstrom O. Heat-induced depyrimidination of deoxyribonucleic acid in neutral solution. Biochemistry. 1973;12(25):5151–4. doi: 10.1021/bi00749a020. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro R, Klein RS. The Deamination of Cytidine and Cytosine by Acidic Buffer Solutions. Mutagenic Implications Biochemistry. 1966;5:2358–2362. doi: 10.1021/bi00871a026. [DOI] [PubMed] [Google Scholar]
- 22.Anchordoquy TJ, Molina MC. Preservation of DNA. Cell Preserv Technol. 2007;5:180–188. [Google Scholar]
- 23.Lee SB, Clabaugh KC, Silva B, Odigie KO, Coble MD, Loreille O, Scheible M, Fourney RM, Stevens J, Carmody GR, Parsons TJ, Pozder A, Eisenberg AJ, Budowle B, Ahmad T, Miller RW, Crouse CA. Assessing a novel room temperature DNA storage medium for forensic biological samples. Forensic Sci Int: Gen. 2012;6:31–40. doi: 10.1016/j.fsigen.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Kim J-H, Yeo W-H, Shu Z, Soelberg SD, Inoue S, Kalyanasundaram D, Ludwig J, Furlong CE, Riley JJ, Weigel K, Cangelosi GA, Oh K, Lee K-H, Gao D, Chung J-H. Immunosensor towards Low-Cost, Rapid Diagnosis of Tuberculosis. Lab Chip. 2012;12(8):1437–1440. doi: 10.1039/c2lc21131a. [DOI] [PubMed] [Google Scholar]
- 25.Kalyanasundaram D, Inoue S, Kim J-H, Lee H-B, Kawabata Z, Yeo W-H, Cangelosi G, Oh K, Gao D, Lee K-H, Chung J-H. Electric field-induced concentration and capture of DNA onto microtips. Microfluid Nanofluid. 2012;13(2):217–225. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.