Abstract
Objective
This study tested two related hypotheses: 1) that brain blood flow is reduced during the postmenopausal hot flash; and, 2) the magnitude of this reduction in brain blood flow is greater during hot flashes where blood pressure is reduced.
Methods
Eleven healthy, normotensive, postmenopausal women rested in a temperature-controlled laboratory (~25°C) for approximately 120 minutes while waiting for a hot flash to occur. The onset of a hot flash was objectively identified by an abrupt increase in sternal sweat rate (capacitance hygrometry). Middle cerebral artery blood velocity (MCAv, transcranial Doppler) and mean arterial pressure (Finometer®) were measured continuously. Each hot flash was divided into 8 equal segments and the segment with the largest reduction in MCAv and mean arterial pressure identified for each hot flash.
Results
Twenty-five hot flashes occurred during the experimental sessions (lasting 6.2 ± 2.8 min, 3 ± 1 hot flashes per participant). Seventy-six percent of hot flashes were accompanied by a clear reduction (greater than 5%) in brain blood flow. For all hot flashes, the average maximum decrease in MCAv was 12 ± 9% (7 ± 6 cm.s−1). This value did not correlate with corresponding changes in mean arterial pressure (R=0.36).
Conclusion
These findings demonstrate that hot flashes are often accompanied by clear reductions in brain blood flow that do not correspond with acute reductions in mean arterial blood pressure.
Keywords: Menopause, hot flash, brain blood flow
Introduction
Frequent hot flash episodes are a primary symptom of the female menopause (1), affecting ~70% of women and persisting for typically 1–5 years following the onset of menopause (2–4). Notably, the rate and severity of hot flashes becomes even higher (90–100%) in surgically induced postmenopausal women and oncological female patients (3, 5). Hot flashes are defined as sudden sensations of heat that can also be accompanied by sensations of cold, perspiration, anxiety, frustration, depression, and nausea (1, 6, 7). Despite the prevalence and impact of hot flashes on symptomatic menopausal women, physiological responses during the hot flash are relatively unknown.
During a hot flash there is a significant increase in cutaneous vascular conductance and skin blood flow, which elevates peripheral blood flow (8, 9). Similarly, under hyperthermic conditions, humans dissipate heat by increasing skin blood flow and sweating. These heat loss mechanisms redistribute blood from central regions to the periphery, increasing systemic vascular conductance and reducing central blood volume (10, 11). Hot flashes can acutely reduce blood pressure in many symptomatic women (9) indicating that, as with heat stressed, hot flashes may increase systemic vascular conductance and reduce central blood volume. During heat stress, reductions in central blood volume and end-tidal carbon dioxide contribute to accompanying reductions in brain blood flow (12, 13). Notably, the magnitude of these heat-related responses corresponds to the degree of heat stress (14). It is not known whether the smaller and perhaps more acute increase in skin blood flow and sweat rate accompanying a hot flash similarly affects brain perfusion. To that end, the first objective of this study was to test the hypothesis that brain blood flow is reduced during the postmenopausal hot flash.
Previously, our laboratory has shown that hot flashes are accompanied by acute reductions in blood pressure in many symptomatic women (9). Brain blood flow is not independent of changes in blood pressure (15), and thus it is possible that the acute reduction in blood pressure during hot flashes is a primary mechanism responsible for the hypothesized reductions in brain blood flow. Therefore, the second purpose of this study was to test the hypothesis that the magnitude of the reduction in brain blood flow during a hot flash, should this occur, will be greater if that hot flash is accompanied by reductions in blood pressure.
Methods
Participants
Eleven healthy postmenopausal women (two surgically and nine naturally menopausal) participated in this study (age: 53 ± 3 years; mass: 62 ± 6 kg; body mass index: 24 ± 2 kg/m2). All women were amenorrheic for at least 1 year and had a minimum of four hot flash episodes per day, as indicated from a 7 day journal. Participants were healthy, with no history of cardiovascular, neurological, or metabolic diseases, and were not taking hormone therapy or other therapies designed to reduce hot flash frequency/intensity. Written informed consent was obtained before participation in this study, which was approved by the University of Texas Southwestern Medical Center, Texas Health Presbyterian Hospital Dallas and complies with the Declaration of Helsinki. Prior to each experimental visit, participants abstained from exercise and alcohol for 24 h and from caffeine for 12 h prior to testing.
Instrumentation
Beat-to-beat arterial blood pressure was measured using finger cuff photoplethysmography (Finometer Pro, FMS, Amsterdam, the Netherlands and NexFin HD, BMEYE B.V, Amsterdam, Netherlands). Blood velocity in the right middle cerebral artery (MCAv) was measured using a 2 MHz pulsed Doppler ultrasound system (Multiflow, DWL Elektronische Systeme, Singen, Germany). The Doppler probe was maintained in position, at a fixed angle, using a commercial headpiece. Cerebrovascular conductance was calculated from the ratio of MCAv to mean arterial pressure (MAP). Heart rate was collected from an electrocardiogram signal (Agilent, Munich, Germany) interfaced with a cardiotachometer (1000 Hz sampling rate, CWE, Ardmore, PA, USA). Chest skin blood flow and sweat rate were measured simultaneous via an integrated laser-Doppler probe (model DP7a; Moor Instruments, Wilmington, DE), secured in an acrylic sweat rate capsule (0.78-cm2 area), respectively. Sweat rate was measured using the capacitance hygrometry ventilated-capsule method (Vaisala, Woburn, WA), with compressed nitrogen delivered at a rate of 300 mL.min−1. Core body temperature was measured using a telemetry temperature pill swallowed ~1 h before the onset of data collection (HQ Inc., Palmetto, FL, USA). Whole-body mean skin temperature (Tsk) was measured from the electrical average of six thermocouples (16) fixed to the skin with porous adhesive tape. Perceived severity of the hot flash was rated on a 10 point scale (17).
Experimental protocol
Experiments were performed in a temperature-controlled laboratory (~25°C). Participants dressed in water perfused, tube-lined trousers (Med-Eng, Ottawa, ON, Canada) that covered their lower limbs. In some participants, warm water (40–44 °C) was perfused through these trousers, as hot flashes are more frequent during peripheral warming (2, 18). After instrumentation, participants rested for approximately 120 minutes while waiting for a hot flash to occur. Data were obtained continuously throughout this ~120 min period. Participants pressed a trigger interfaced with the data acquisition system at the perceived onset and end of each hot flash.
Data analysis
Data were acquired continuously at 50 Hz throughout the experiment (Biopac, Santa Barbara, CA, USA). Hot flash onset was objectively identified by a rapid and large increase in sternal sweat rate (rate greater than 0.001mg/cm−2·second−1) that dissipated upon the cessation of the flash, as previously described (9, 18, 19). Each hot flash was divided into 8 equal segments to normalize varying hot flash durations between and within participants (Figure 1). For each participant, a non-hot flash period (equivalent to each participant’s average hot flash duration, ~6 min) was selected and divided into 8 equal segments to identify normal variations in MCAv and MAP.
Figure 1.
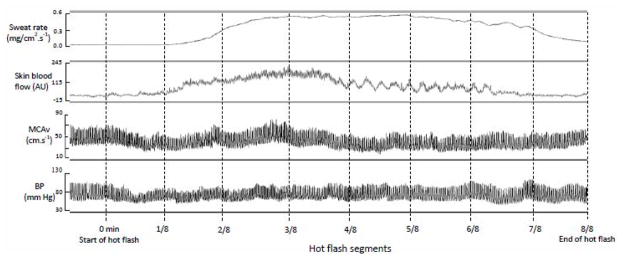
Representative changes in sternal sweat rate and skin blood flow, middle cerebral artery blood velocity (MCAv) and blood pressure (BP), during a hot flash segmented in 1/8 segments.
To address hypothesis 1, the segment of each hot flash with the largest drop in MCAv was identified and compared to 1 min of baseline (immediately prior to the onset of the hot flash) and 1 min of recovery (2 min post hot flash) values. A repeated measures, one-way ANOVA was used to assess changes in MCAv and MAP during all hot flashes across these three periods. Pearson correlations were used to determine the relationship between the change in MCAv relative to the following: perceived ratings of hot flash severity, skin blood flow, sweat rate and MAP. R and P values are reported.
To address hypothesis 2, each hot flash was categorized as a “Responder” or a “Non-Responder”, as previously performed (9). Briefly, a Responder was identified when MAP decreased ≥5 mm Hg during any hot flash segment. The segment with the maximum drop in MAP was identified for each hot flash. Baseline (immediately prior hot flash), as well as recovery (2 min post hot flash) values for each hot flash were averaged over 1 minute. Differences in thermoregulatory and hemodynamic responses between the Responder and Non-responder hot flashes before, during, and after the hot flash periods were evaluated using a two-way mixed model analysis of variance with main effects of time (repeated variable) and group (non-repeated variable). Differences in hot flashes duration and perceived severity between Responder and Non-responder hot flashes were assessed using t tests. Values are reported as means ± SEM. P values ≤ 0.05 were considered statistically significant. A post-hoc power analysis (α = 0.05, Power ≈ 0.8) indicated that 13 hot flashes gave adequate power to the ANOVA statistical test to evaluate differences in MCAv during the hot flash.
Results
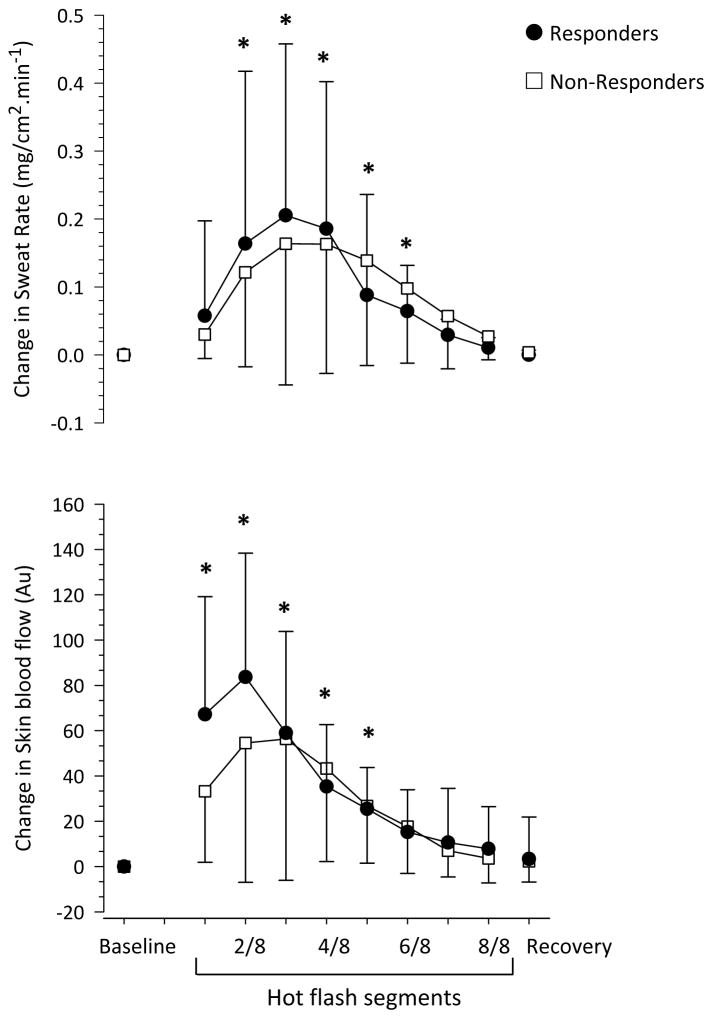
Twenty-five hot flashes occurred during the experimental sessions (lasting 6.2 ± 2.8 min, averaging 3 ± 1 hot flashes per participant). Consistent with the employed objective definition of hot flashes, sternal sweat rate increased at the onset of each hot flash, peaking at the 3/8 segment of the hot flash, and then returning to baseline values at recovery (P <0.01, Figure 2). Skin blood flow was elevated at the onset of the hot flash, peaking at the 1/8 segment (P <0.01).
Figure 2.
Change in sternal sweat rate and skin blood flow during 25 hot flashes, in 11 postmenopausal women, segregated as Responders (n=16 hot flashes, ●; defined as ≥5 mm Hg reduction in mean arterial pressure during the hot flash) and Non-responders (n= 9 hot flashes, □; defined ≤5 mm Hg reduction in mean arterial pressure during the hot flash). *P <0.05 versus baseline for both groups.
Hypothesis 1: Brain blood flow during the hot flash
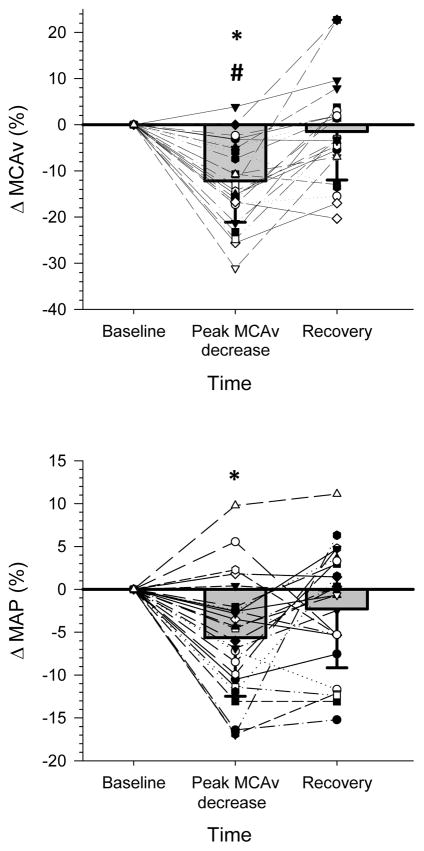
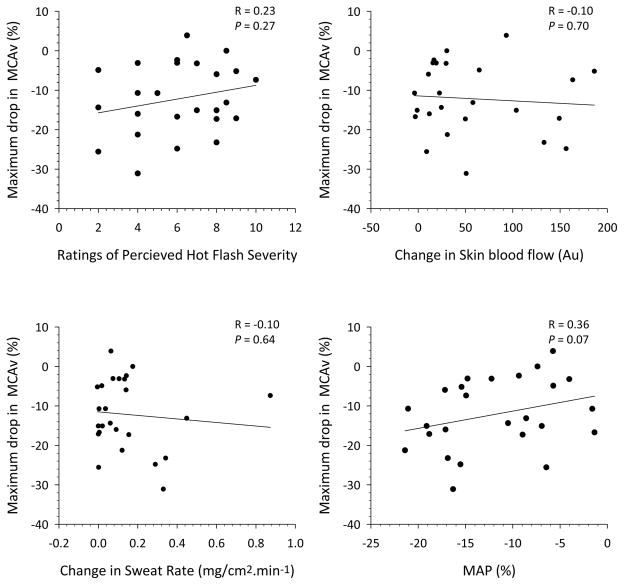
For all hot flashes, the peak decrease in MCAv averaged 7 ± 6 cm.s−1 (12 ± 9%) (P <0.01, Figure 3) and this was accompanied by an average decrease in MAP of 5 ± 6 mm Hg (6 ± 7%; P <0.01). Seventy-six percent of the hot flashes were accompanied with a decrease in MCAv of ≥5%, 60% showed a decrease in MCAv of ≥10%, and 44% showed a decrease in MCAv of ≥15%. Across all hot flashes, during the segment with the largest decrease in MCAv, there was no relationship between the magnitude of the reduction in MCAv relative to assessed responses (i.e., MAP, skin blood flow, sweat rate, or rating of hot flash severity; see Figure 4). During non-hot flash periods, there were no significant changes in MCAv (3 ± 1%; 2 ± 1 cm.s−1) or MAP (3 ± 1%; 3 ± 1 mm Hg).
Figure 3.
Relative change (i.e., % from pre-hot flash baseline) in middle cerebral artery blood velocity (MCAv, upper panel) and mean arterial pressure (MAP, lower panel) during all 25 hot flashes in 11 postmenopausal women at the segment with the peak decrease in MCAv. *P <0.05 versus pre hot flash baseline, # P <0.05, versus post hot flash recovery.
Figure 4.
Relationships between the maximal change in MCAv during 25 hot flashes in 11 postmenopausal women with 1) ratings of hot flash severity, 2) change in skin blood flow, 3) change in sweat rate and 4) mean arterial pressure (MAP) during hot flashes. Hemodynamic and sweating variables were obtained during the same segment with the maximal change in MCAv, while rating of hot flash severity was for the entire hot flash. Regardless of the comparative variable, there was no relationship between that variable and the reduction in brain perfusion as indicated by changes in MCAv.
Hypothesis 2: Responders verses Non-responders
Sixteen out of 25 hot flashes (64%) were classified as Responders, defined by a ≥5 mm Hg decrease in MAP, with the peak decrease in MAP during these hot flashes averaging 12 ± 4 mm Hg (13 ± 3%; P <0.01). Hot flash duration (6.0 ± 3.4 min vs. 6.3 ± 4.2 min; P >0.05) and perceived severity (6 ± 2 vs. 5 ± 2; P >0.05) were not different between Responder and Non-responder flashes, respectively. The magnitude of the elevation in sweat rate and skin blood flow were also not different between these groups of hot flashes (P >0.05, Figure 2, Table 1).
Table 1.
Cardiovascular, cerebrovascular and thermoregulatory responses to hot flashes, categorized as Responders (n=16) and Non-responders (n= 9).
| Responders | Non-responders | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Baseline | Peak MAP decrease | Recovery | Baseline | Peak MAP decrease | Recovery | |
| MAP (mm Hg) | 90 ± 13 | 78 ± 12 *# | 85 ± 13 * | 91 ± 17 | 90 ± 19 | 92 ± 17 |
| SBP (mm Hg) | 120 ± 20 | 100 ± 19 *# | 115 ± 19* | 120 ± 27 | 118 ± 31 | 123 ± 27 |
| DBP (mm Hg) | 68 ± 9 | 57 ± 7 *# | 66 ± 10 | 68 ± 13 | 64 ± 7 * | 71 ± 12 |
| MCAv (cm.s−1) | 54 ± 20 | 47 ± 17 * | 51 ± 19 | 58 ± 17 | 54 ± 15 | 54 ± 14 |
| CBVC (cm.s−1.mm Hg−1) | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.3 | 0.7 ± 0.3 | 0.6 ± 0.2 | 0.6 ± 0.2 |
| HR (bpm) | 70 ± 7 | 74 ± 10 *# | 70 ± 6 | 75 ± 8 | 78 ± 11 # | 73 ± 6 |
| Δ Skin blood flow (AU) | 0 ± 0 | 41 ± 65*# | −8 ± 43 | 0 ± 0 | 47 ± 62*# | 0 ± 13 |
| Δ Sweat rate (mg/cm2.min−1) | 0 ± 0 | 0.22 ± 0.24*# | 0.00 ± 0.01 | 0 ± 0 | 0.18 ± 0.20*# | 0.00 ± 0.01 |
| Tcore (° C) | 37.2 ± 0.3 | 37.3 ± 0.3 | 37.2 ± 0.3 | 37.3 ± 0.1 | 37.4 ± 0.1 | 37.4 ± 0.2 |
| Tsk (° C) | 35.3 ± 0.9 | 35.4 ± 0.8 | 35.3 ± 0.7 | 36.0 ± 0.4 | 36.7 ± 1.8‡ | 36.7 ± 2.2‡ |
A Responder was identified upon a reduction in mean arterial pressure (MAP) of ≥5 mm Hg in any segment of the hot flash. SBP, systolic blood pressure; DPB, diastolic blood pressure, MCAv, middle cerebral artery blood velocity; CBVC, cerebrovascular conductance; HR, heart rate; Δ, delta change; Tcore; core body temperature; Tsk, mean skin temperature.
P <0.05, versus pre hot flash baseline;
P <0.05, versus post hot flash recovery;
P <0.05, Non-responders different from Responders.
In the Responder group, the peak decrease in MAP occurred in concert with a 6 ± 6 cm.s−1 (10 ± 8%; P =0.04) decrease in MCAv. At recovery, MAP remained 4 ± 6 mm Hg (4 ± 7%) lower (P <0.01) than baseline values, while MCAv returned (P >0.05) to near baseline values in the Responder group. In the Non-responder group, there was no significant change in MCAv during these hot flashes (P >0.05). Heart rate in both the Responder and Non-Responder group increased ~4 bpm (P <0.05) during the hot flash, while there were no changes in calculated cerebrovascular conductance or Tcore (P >0.05, Table 1).
Discussion
These findings demonstrate that approximately 76% of hot flashes are accompanied by a clear reduction (greater than 5%) in brain blood flow. Furthermore, a hot flash can be accompanied by a significant acute reduction in MAP in symptomatic postmenopausal women. However, hot flash-related reductions in brain blood flow do not consistently correlate to acute reductions in MAP.
Hypothesis 1: Brain blood flow during the hot flash
Seventy-six percent of the evaluated hot flashes were accompanied by at least a 5% reduction in brain blood flow; 60% were accompanied by at least a 10% reduction; and 44% were accompanied by at least a 15% reduction in brain blood flow. When an individual transitions from the supine to the upright position, there is an ~15% reduction in brain blood flow (20). Accordingly, in nearly half of the evaluated hot flashes, the reduction in brain blood flow was equivalent to that observed with standing. These data indicate that the hot flash, like standing, can present a significant physiological challenge to brain blood flow, and that feelings of faintness often accompanying hot flashes (6) may be associated with acute reductions in brain blood flow. Estrogen has neuroprotective properties (21) influencing brain blood flow (22), cerebral reactivity (23) and cerebral microvascular vasomotor tone (24). Subsequently, it is possible that reduced estrogen levels in postmenopausal women contribute to the large reductions in brain brood flow observed during some hot flashes. It is recognized that the minimum change in MCAv (i.e., a 5% decrease) could result from normal biological fluctuations in brain blood flow. In the current study, however, MCAv had a maximum decrease of 3 ± 1% during a non-hot flash period. Thus, a ≥5% reduction in MCAv during a hot flash exceeded normal, brain blood flow oscillations in these symptomatic postmenopausal women. Furthermore, over 60% of the hot flashes were accompanied by greater than a 10% reduction in brain perfusion.
Peak hot flash-related changes in brain blood flow varied from +4% to −31% (see Figure 3). These changes were unrelated to hot flash severity; as quantified by sweat rate, skin blood flow and hot flash severity perception. Furthermore, when all hot flashes were evaluated, MAP changes during hot flashes were not correlated with changes in brain blood flow (R=0.36). This is, perhaps, unexpected as blood pressure can influence brain blood flow (15, 25). Other major modulators of brain blood flow are cerebral metabolism, cardiac output, sympathetic activity and the arterial content of carbon dioxide (25). Therefore, it is possible that one or a combination of these major modulators underlie hot flash-related reductions in brain perfusion; though, the magnitude of any such modulating effect appears unrelated to hot flash severity. For example, ventilation increases during the hot flash (26) and subsequently, the arterial content of carbon dioxide may be reduced, which would contribute to reductions in brain perfusion. In the current study an attempt was made to sample the partial pressure of end-tidal carbon dioxide from a nasal cannula. However, many participants markedly changed their breathing pattern during a hot flash and as a consequence, breathed deeply through the mouth and not the nose, thereby invalidating the end-tidal carbon dioxide measures. As a result, end tidal carbon dioxide values are not reported.
Hypothesis 2: Responders verses Non-responders
As observed in the present and prior findings (9), the hot flash can cause significant decreases in blood pressure in many symptomatic women. The second objective was designed to more closely evaluate a possible relationship between hot flash-related reductions in blood pressure and reductions in brain blood flow. This objective was accomplished by comparing MCAv responses between hot flashes where MAP decreased ≥5 mm Hg (responders) to hot flashes where MAP did not (non-responders). Sixteen out of the 25 hot flashes showed a clear decrease in MAP. For three participants all hot flashes were classified as Responders, for two participants all hot flashes were classified as Non-Responders, and five participants had hot flashes that were classified as both Responders and Non-responders. Thus, it appears that blood pressure responses to hot flashes can vary between and within symptomatic women.
In the Responder group, MAP decreased 13 ± 3% during hot flashes and this coincided with a small but significant decrease (i.e., 10 ± 8%) in brain perfusion (see Table 1). In the Non-responder group, the maximal change in MAP (−2 ± 3%) coincided with a 6 ± 14% decrease in brain blood flow. However, this decrease in MCAv was not significant due to the large variability (± 14%) in these hot flashes. These finding indicate that blood pressure alone was not the only modulator influencing brain perfusion during a hot flash. Indeed, some of the largest reductions in MCAv occurred in the Non-Responder group, where MAP decreased less than 5 mm Hg. Interestingly, MAP did not completely return to pre-hot flash values during the recovery period in the Responder group of hot flashes. Decreases in total vascular resistance most likely cause MAP reductions during the hot flash (9). It is possible that a sustained decrease in total vascular resistance in the Responder group slowed the return of MAP to pre-hot flash values. Although, the return of skin blood flow (and presumably cutaneous vascular conductance) to pre-hot flash values does not support this presupposition.
Limitations
Mild peripheral warming (legs only) was used to induce a hot flash in some participants. This technique increases lower limb skin temperature without inducing sternal or forearm sweating, and has successfully been used by our (9, 27) and other laboratories (2, 18) to increase the incidence of hot flashes. In our experience, ~60% of the participants who do not experience a hot flash within the first ~2 hours of assessment will experience at least one hot flash during this mild peripheral warming. In the current study, ~40% of women did not need this heating stimulus to trigger a hot flash. Importantly, data from the current study shows that cardiovascular responses (i.e., heart rate and MAP) were not different (P >0.05) between spontaneous and warm-induced flashes. The interpretation of these data is limited to the characteristics of the evaluated participants. It may be that hot flash-related changes in MCAv and MAP are affected by variables not considered in the present investigation such as age, co-morbidities, race, etc.
Conclusions
In summary, the postmenopausal hot flash can cause pronounced transient reductions in brain perfusion, even while supine, in most symptomatic women. Subsequently, the hot flash can present a significant physiological challenge to brain blood flow, with transient reductions in brain blood flow possibly contributing to feelings of faintness or nausea associated with hot flashes. However, though a hot flash can be accompanied by an acute reduction in MAP, other mechanisms or modulators likely contribute to hot flash-induced reductions in brain blood flow, as index by MCAv.
Acknowledgments
This study was supported by the National Institutes of Health grant AG030189. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors have no financial or other conflicts of interests to disclose.
References
- 1.Kronenberg F. Menopausal Hot Flashes: A Review of Physiology and Biosociocultural Perspective on Methods of Assessment. J Nutr. 2010;140(7):1380S–1385. doi: 10.3945/jn.109.120840. [DOI] [PubMed] [Google Scholar]
- 2.Kronenberg F. Hot Flashes: Epidemiology and Physiologya. Annals of the New York Academy of Sciences. 1990;592(1):52–86. doi: 10.1111/j.1749-6632.1990.tb30316.x. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann GA. Vasomotor flushes in menopausal women. American Journal of Obstetrics and Gynecology. 1999;180(3, Supplement 1):S312–S316. doi: 10.1016/s0002-9378(99)70725-8. [DOI] [PubMed] [Google Scholar]
- 4.Shanafelt TD, Barton DL, Adjei AA, Loprinzi CL. Pathophysiology and Treatment of Hot Flashes. Mayo Clinic Proceedings. 2002;77(11):1207–1218. doi: 10.4065/77.11.1207. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter JS, Johnson DH, Wagner LJ, Andrykowski MA. Hot Flashes and Related Outcomes in Breast Cancer Survivors and Matched Comparison Women. Oncology Nursing Forum. 2002;29(3):E16–E25. doi: 10.1188/02.ONF.E16-E25. [DOI] [PubMed] [Google Scholar]
- 6.Dormire SL. What We Know About Managing Menopausal Hot Flashes: Navigating Without a Compass. Journal of Obstetric, Gynecologic, & Neonatal Nursing. 2003;32(4):455–464. doi: 10.1177/0884217503255069. [DOI] [PubMed] [Google Scholar]
- 7.Greendale GA, Lee NP, Arriola ER. The menopause. Lancet. 1999;353(9152):571–580. doi: 10.1016/S0140-6736(98)05352-5. [DOI] [PubMed] [Google Scholar]
- 8.Ginsburg J, Swinhoe J, O’Reilly B. Cardiovascular responses during the menopausal hot flush. British Journal of Obstetrics and Gynaecology. 1981;88(9):925–930. doi: 10.1111/j.1471-0528.1981.tb02230.x. [DOI] [PubMed] [Google Scholar]
- 9.Low DA, Davis SL, Keller DM, Shibasaki M, Crandall CG. Cutaneous and hemodynamic responses during hot flashes in symptomatic postmenopausal women. Menopause. 2008;15(2):290–295. doi: 10.1097/gme.0b013e3180ca7cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crandall CG, Wilson TE, Marving J, et al. Effects of passive heating on central blood volume and ventricular dimensions in humans. The Journal of Physiology. 2008;586(1):293–301. doi: 10.1113/jphysiol.2007.143057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowell LB, Brengelmann GL, Murray JA. Cardiovascular Responses to Sustained High Skin Temperature in Resting Man. Journal of Applied Physiology. 1969;27(5):673. doi: 10.1152/jappl.1969.27.5.673. [DOI] [PubMed] [Google Scholar]
- 12.Nelson MD, Haykowsky MJ, Stickland MK, et al. Reductions in cerebral blood flow during passive heat stress in humans: partitioning the mechanisms. The Journal of Physiology. 2011;589(16):4053–4064. doi: 10.1113/jphysiol.2011.212118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brothers RM, Jonathan EW, Kimberly AH, Craig GC. The effects of reduced end-tidal carbon dioxide tension on cerebral blood flow during heat stress. The Journal of Physiology. 2009;587(15):3921–3927. doi: 10.1113/jphysiol.2009.172023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan J-L, Cotter JD, Lucas RAI, Thomas K, Wilson L, Ainslie PN. Human cardiorespiratory and cerebrovascular function during severe passive hyperthermia: effects of mild hypohydration. J Appl Physiol. 2008;105(2):433–445. doi: 10.1152/japplphysiol.00010.2008. [DOI] [PubMed] [Google Scholar]
- 15.Lucas SJE, Tzeng YC, Galvin SD, Thomas KN, Ogoh S, Ainslie PN. Influence of Changes in Blood Pressure on Cerebral Perfusion and Oxygenation. Hypertension. 2010;55(3):698–705. doi: 10.1161/HYPERTENSIONAHA.109.146290. [DOI] [PubMed] [Google Scholar]
- 16.Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol. 1989;66:1586–1592. doi: 10.1152/jappl.1989.66.4.1586. [DOI] [PubMed] [Google Scholar]
- 17.Kronenberg F, Cote LJ, Linkie DM, Dyrenfurth I, Downey JA. Menopausal hot flashes: Thermoregulatory, cardiovascular, and circulating catecholamine and LH changes. Maturitas. 1984;6(1):31–43. doi: 10.1016/0378-5122(84)90063-x. [DOI] [PubMed] [Google Scholar]
- 18.Freedman RR. Laboratory and Ambulatory Monitoring of Menopausal Hot Flashes. Psychophysiology. 1989;26(5):573–579. doi: 10.1111/j.1469-8986.1989.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 19.Freedman RR. Biochemical, metabolic, and vascular mechanisms in menopausal hot flashes. Fertility and Sterility. 1998;70(2):332–337. doi: 10.1016/s0015-0282(98)00137-x. [DOI] [PubMed] [Google Scholar]
- 20.Pott F, van Lieshout JJ, Ide K, Madsen P, Secher NH. Middle cerebral artery blood velocity during a Valsalva maneuver in the standing position. Journal of Applied Physiology. 2000;88(5):1545–1550. doi: 10.1152/jappl.2000.88.5.1545. [DOI] [PubMed] [Google Scholar]
- 21.Roof RL, Hall ED. Gender Differences in Acute CNS Trauma and Stroke: Neuroprotective Effects of Estrogen and Progesterone. Journal of Neurotrauma. 2000;17(5):367–388. doi: 10.1089/neu.2000.17.367. [DOI] [PubMed] [Google Scholar]
- 22.Ohkura T, Teshima Y, Isse K, et al. Estrogen Increases Cerebral and Cerebellar Blood Flows in Postmenopausal Women. Menopause. 1995;2(1):13–18. [Google Scholar]
- 23.Matteis M, Troisi E, Monaldo BC, Caltagirone C, Silvestrini M. Age and Sex Differences in Cerebral Hemodynamics : A Transcranial Doppler Study. Stroke. 1998;29(5):963–967. doi: 10.1161/01.str.29.5.963. [DOI] [PubMed] [Google Scholar]
- 24.Belfort MA, Saade GR, Snabes M, et al. Hormonal status affects the reactivity of the cerebral vasculature. American Journal of Obstetrics and Gynecology. 1995;172(4 Part 1):1273–1278. doi: 10.1016/0002-9378(95)91492-7. [DOI] [PubMed] [Google Scholar]
- 25.Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol. 2009;296(5):R1473–1495. doi: 10.1152/ajpregu.91008.2008. [DOI] [PubMed] [Google Scholar]
- 26.Woodward S, Greville HW, Freedman RR. Ventilatory Response during Menopausal Hot Flashes. Menopause. 1995;2(2):81–88. [Google Scholar]
- 27.Hubing K, Wingo J, Brothers R, Del Coso J, Low D, Crandall C. Nitric oxide synthase inhibition attenuates cutaneous vasodilation during postmenopausal hot flash episodes. Menopause. 2010;17(5):978–82. doi: 10.1097/gme.0b013e3181d674d6. [DOI] [PMC free article] [PubMed] [Google Scholar]