Abstract
Purpose
Although epilepsy and migraine are known to co-occur within individuals, the contribution of a shared genetic susceptibility to this comorbidity remains unclear. We investigated the hypothesis of shared genetic effects on migraine and epilepsy in the Epilepsy Phenome/Genome Project (EPGP) cohort.
Methods
We studied prevalence of a history of migraine in 730 EPGP participants aged ≥12 years with non-acquired focal epilepsy (NAFE) or generalized epilepsy (GE) from 501 families containing ≥2 individuals with epilepsy of unknown cause. Information on migraine without aura (MO) and migraine with aura (MA) was collected using an instrument validated for individuals ≥12 years. Since many individuals have both MO and MA, we considered two non-overlapping groups of individuals with migraine: those who met criteria for MA in any of their headaches (MA), and those who did not (“MO-only”). EPGP participants were interviewed about the history of seizure disorders in additional non-enrolled family members. We evaluated associations of migraine prevalence in enrolled subjects with family history of seizure disorders in additional non-enrolled relatives, using generalized estimating equations to control for the non-independence of observations within families.
Key Findings
Prevalence of a history of MA (but not MO-only) was significantly increased in enrolled participants with ≥2 additional affected first degree relatives.
Significance
These findings support the hypothesis of a shared genetic susceptibility to epilepsy and MA.
Keywords: Epilepsy, Seizure, Migraine, Genetic, Comorbidity
INTRODUCTION
The comorbidity of migraine and epilepsy is well established. The prevalence of epilepsy in migraineurs substantially exceeds that of the general population (Andermann & Lugaresi, 1987; Tellez-Zenteno et al., 2005), and the incidence of migraine headaches in individuals with epilepsy is nearly twice that of individuals without epilepsy (Ottman & Lipton, 1994). Although both epilepsy and migraine are individually influenced by genetic factors, the contribution of a shared genetic susceptibility to their comorbidity remains unclear (Ottman & Lipton, 1996).
Several lines of evidence support shared genetic effects on migraine and epilepsy. First, migraine and epilepsy have been linked to the same chromosomal regions within individual families. Linkage of migraine, epilepsy, or the combined phenotype has been reported for loci on 9q21-q22, 12q24.2-24.3, and 14q12-q23 (Deprez et al., 2007; Polvi et al., 2012). Second, epilepsy and the rare migraine variant, familial hemiplegic migraine (FHM), have overlapping genetic contributions. Mutations in CACNA1A and ATP1A2 were identified in both FHM and epilepsy (Deprez et al., 2008; Dichgans et al., 2005; Imbrici et al., 2004) and mutations within the SCN1A gene may cause FHM (Dichgans et al., 2005), genetic epilepsy with febrile seizures plus (GEFS+), or Dravet’s syndrome (Claes et al., 2001; Escayg et al., 2000). Third, migraine was found to be nearly twice as common in probands with rolandic epilepsy and their siblings without epilepsy than in controls without epilepsy and their siblings without epilepsy (Clarke et al., 2009a). Taken together, these studies support the existence of shared genetic influences on migraine and epilepsy; however, they are all restricted to single families and/or specific epilepsy or migraine syndromes.
Results from the Epilepsy Family Study of Columbia University (EFSCU) did not support a shared genetic susceptibility to migraine and epilepsy (Ottman & Lipton, 1996). In EFSCU, migraine prevalence was not increased in individuals with a family history of epilepsy in first-degree relatives, compared to those without a family history of epilepsy. Similarly, the risk of epilepsy was not increased in the first-degree relatives of individuals with both epilepsy and migraine, compared to the relatives of those with epilepsy but without migraine. These results corroborated those of another study that failed to find an increased prevalence of migraine in relatives of probands with epilepsy compared with relatives of controls (Andermann & Andermann, 1987). Thus the extent to which a shared genetic susceptibility for epilepsy and migraine underlies the co-morbidity of these two disorders is unclear.
We examined the occurrence of migraine in families with generalized epilepsy (GE) or non-acquired focal epilepsy (NAFE) from the Epilepsy Phenome/Genome Project. In EPGP, participants were evaluated for a history of migraine and were asked to list additional relatives with seizures beyond those family members already enrolled. We were therefore able to assess the relationship between the number of additional relatives with seizure disorders in the family and the prevalence of a history of migraine in EPGP participants, taking into account the closeness of relationship of these additional affected relatives to enrolled participants.
METHODS
The Epilepsy Phenome/Genome Project (EPGP)
EPGP is an ongoing study whose goal is to recruit, perform detailed phenotyping on, and collect DNA from 3,750 participants with epilepsy. EPGP includes 27 clinical centers in the United States, Canada, Argentina, Australia, and New Zealand. Participants are ascertained through screening of patients at local centers, referrals, and a national recruitment campaign. At each clinical site, detailed information is collected on epilepsy phenotype, family history, electrophysiologic characteristics, neuroimaging findings, demographic variables, and response to anticonvulsant medications.
One arm of EPGP focuses on the enrollment of affected sibling pairs and parent-child pairs with epilepsy of unknown cause. Participants in this arm of the study are required to have well-characterized GE or NAFE; families need not necessarily be concordant for epilepsy type to be eligible. The analysis presented here is restricted to these NAFE and GE families
Each site’s IRB has approved the use of human subjects for this study and all participants provided informed consent.
Participants
Eligibility requirements for GE/NAFE participants included a lifetime history of two or more unprovoked seizures or one seizure with epileptiform electroencephalogram (EEG) activity. In the GE/NAFE arm of the study, participation (enrollment) of at least an affected sibling pair or parent-child pair with epilepsy was required; less commonly, more than two relatives were enrolled (e.g. three siblings, or a sibling pair plus a parent). Individuals with only febrile or other acute symptomatic seizures were excluded, as were those with a history of acquired central nervous system injury before epilepsy onset. Individuals with autistic disorder, pervasive developmental disorder, or severe developmental delay prior to the onset of seizures and medication use were also excluded. Potential participants were considered ineligible if they had an identified pathogenic mutation in a previously identified epilepsy gene.
To be classified as NAFE, neuroimaging was required to be normal or demonstrate mesial temporal sclerosis (MTS) or focal cortical dysplasia (FCD). Individuals with MTS or FCD were not excluded because these lesions are not clearly a result of exogenous injury. Patients with NAFE were also required to have focal EEG abnormalities or unambiguous clinical semiology consistent with focal seizures. Participants with benign rolandic epilepsy diagnosed by clinical presentation were not required to have neuroimaging.
To be classified as GE, participants had to have generalized onset seizures, normal neuroimaging if done (though not required), and an EEG showing generalized epileptiform activity with a normal posterior dominant rhythm for age.
Data Collection
Phenotypic information was collected using telephone or in-person interviews (Nesbitt et al.) and medical record abstraction. Participants with epilepsy were administered a detailed semi-structured diagnostic interview designed to ascertain seizure types, semiology, seizure frequency, age at onset, history of status epilepticus, epilepsy syndrome, anticonvulsant response, and additional medical conditions including migraine. The interview was modified from a previously validated instrument (Ottman et al., 1990; Ottman et al., 1993b). Basic laboratory data (e.g., serum AED levels), high-resolution MRI scans, EEGs, and results of video-EEG telemetry were abstracted from the medical record. A blood sample was drawn from each participant and sent to the National Institute for Neurological Disorders and Stroke (NINDS) Human Genetics DNA and Cell Line Repository at the Coriell Institute for Medical Research for DNA extraction. Representative EEG and MRI and medical records were reviewed by EPGP’s Electrophysiology and Imaging Cores, respectively. A final diagnosis was completed by the local site principal investigator based on all the collected information, and served as the source of the NAFE and GE diagnoses for our analyses. The diagnoses of a subset of cases were reviewed independently by two members of EPGP’s Data Review Core to ensure data quality and consistency. We restricted analysis to individuals who had completed final diagnoses at the time of the analysis (April, 2012), and who had either GE or NAFE, but not both.
Migraine in EPGP participants was assessed using a standardized and validated interview, as recommended in the NINDS Common Data Elements Project (Lipton et al., 2001). Because this interview is validated for individuals aged 12 or older, we restricted our analyses to individuals in this age range. Individuals were diagnosed with migraine with aura (MA) or migraine without aura (MO) based on the criteria of the International Classification of Headache Disorders (2004). Since many individuals have both MO and MA, we considered two independent groups of individuals with migraine: those who met criteria for MA in any of their headaches (denoted MA) and those who did not (denoted “MO-only”).
Data on history of seizure disorders in other family members were collected through a single interview question that asked enrolled participants to report any additional relatives affected with epilepsy or a seizure disorder. For each family, we identified the total number of relatives reported to be affected beyond the enrolled participants (denoted “additional” affected relatives below), and also the number who were first-degree relatives of enrolled participants (i.e., parents, siblings, or offspring of enrolled participants).
Statistical Analysis
We computed odds ratios (ORs) and 95% confidence intervals (CIs) for migraine in EPGP participants, using the history of seizure disorders in additional relatives as the predictor. For this analysis we used generalized estimating equation (GEE) models to control for the non-independence of participants within the same family. To control for potential confounding by age, sex, participant type (e.g. proband, parent, or sibling), and epilepsy type (GE or NAFE), we added these variables to the model and computed adjusted ORs and CIs. These analyses were repeated using any migraine, MO-only, and MA as the outcome, and using as the predictor either the total number of relatives reported to have had seizure disorders (0,1, or ≥2), or the number of affected first degree relatives. We also examined the effect of stratifying by epilepsy type (GE or NAFE) in enrolled participants.
RESULTS
The analysis included 730 EPGP participants (probands, siblings, or parents) aged 12 or older with either GE or NAFE (but not both), completed epilepsy diagnoses, and complete information on migraine history. These participants were from 501 families (Table 1), averaging 1.5 (range 1–4) EPGP participants and 0.4 (range 0–4) additional relatives reported to have had seizure disorders per family. (Although all EPGP families contain ≥2 affected individuals, in many cases only one EPGP participant per family met the inclusion criteria for the current analysis.) The distribution of ILAE syndromes in the included participants is shown in Table 2.
Table 1.
Distribution of EPGP families by number of enrolled and non-enrolled affected individuals
| Number of additional (non-enrolled) family members with seizure disorders |
Number of enrolled family members with GE or NAFE |
Total | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| 0 | 199 | 164 | 6 | 1 | 370 |
| 1 | 56 | 37 | 1 | 0 | 94 |
| 2 | 20 | 6 | 0 | 0 | 26 |
| 3 | 5 | 3 | 0 | 0 | 8 |
| 4 | 1 | 2 | 0 | 0 | 3 |
| Total | 281 | 212 | 7 | 1 | 501 |
Table 2.
Distribution of ILAE syndromes in participants included in analysis.
| ILAE SYNDROME | N | % |
|---|---|---|
| 1000 LRE-NOS | 23 | 3.2 |
| 1100 Idiopathic LRE-NOS | 47 | 6.4 |
| 1110 BECTS | 12 | 1.6 |
| 1120 Childhood epilepsy with occipital paroxysms | 1 | 0.1 |
| 1200 cLRE-NOS | 90 | 12.3 |
| 1210 cTLE-NOS | 68 | 9.3 |
| 1211 cTLE-Amygdalohippocampal | 8 | 1.1 |
| 1212 cTLE-Lateral | 4 | 0.5 |
| 1220 cryp Parietal | 3 | 0.4 |
| 1230 cryp Occipital | 7 | 1.0 |
| 1240 cryp Frontal-NOS | 9 | 1.2 |
| 1300 symp LRE-NOS | 4 | 0.5 |
| 1310 symp TLE-NOS | 6 | 0.8 |
| 1311 symp TLE-Amygdalohippocampal | 7 | 1.0 |
| 1312 symp TLE-Lateral | 1 | 0.1 |
| 1340 symp Frontal-NOS | 5 | 0.7 |
| 2000 GE-NOS | 6 | 0.8 |
| 2100 IGE-NOS | 170 | 23.3 |
| 2140 CAE | 62 | 8.5 |
| 2145 CAE-JAE indistinguishable (onset age 9–11) | 27 | 3.7 |
| 2150 JAE (onset 12 or older) | 21 | 2.9 |
| 2160 JME (onset 10 or older) | 102 | 14.0 |
| 2170 Epilepsy with GTCs on awakening | 2 | 0.3 |
| 2180 IGE with sz precip by spec modes of activation | 6 | 0.8 |
| 2190 Other IGEs not defined above | 13 | 1.8 |
| 2191 Late-onset IGE, NOS | 5 | 0.7 |
| 2192 CAE-JME indistinguishable | 3 | 0.4 |
| 2193 JAE-JME indistinguishable | 8 | 1.1 |
| 2200 Gen cryp or symp epil-NOS | 2 | 0.3 |
| 2240 Epilepsy with myoclonic-astatic seizures | 2 | 0.3 |
| 2250 Epilepsy with myoclonic absences | 2 | 0.3 |
| 2290 Other cryp-symp epilepsy | 2 | 0.3 |
| 2300 Symp generalized epilepsy NOS | 1 | 0.1 |
| 4120 Isolated unprovoked seizure* TOTAL |
1 730 |
0.1 |
NOS= Not otherwise specified; LRE=Localiization related epilepsy; BECTS=Benign epilepsy with centrotemporal spikes; cTLE=cryptogenic TLE; cryp=cryptogenic; symp=symptomatic; GE=generalized epilepsy; IGE=idiopathic generalized epilepsy; CAE=Childhood absence epilepsy; JAE=Juvenile absence epilepsy; JME=Juvenile myoclonic epilepsy; GTC=Generalized tonic-clonic seizure.
Individuals with isolated unprovoked seizures were enrolled in EPGP if they had epileptiform EEG abnormalities. The single individual with an isolated unprovoked seizure had a generalized epileptiform EEG abnormality and was therefore coded as GE in our analyses.
We examined the factors associated with prevalence of a history of migraine in EPGP participants (Tables 3, 4, 5). Prevalence of a history of any migraine in enrolled participants (probands, siblings, or parents) was significantly greater in women (32%) than in men (15%; Table 3), and in older vs. younger individuals. Migraine prevalence in probands was also higher in women (30%) than in men (17%). Prevalence was higher in parents than probands or siblings, but this difference disappeared after adjustment, suggesting that it was attributable to older age and a greater proportion of female vs. male parents compared with probands and siblings (72% of parents were female, compared with 55% of probands and 63% of siblings). Prevalence did not differ significantly by epilepsy type (GE vs. NAFE). These trends were similar for MO-only (Table 4) and MA (Table 5).
Table 3.
Any Migraine in EPGP Participants
| No. participants | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| Total | With Any Migraine (%) |
OR* | (95% CI) | OR** | (95% CI) | |
| All participants | 730 | 184 (25.2) | -- | -- | -- | -- |
| Female | 445 | 140 (31.5) | 2.5 | (1.72–3.66) | 2.4 | (1.62–3.52) |
| Male | 285 | 44 (14.5) | 1.0 | (referent) | 1.0 | (referent) |
| Age (years) | ||||||
| 12–24 | 340 | 56 (16.5) | 1.0 | (referent) | 1.0 | (referent) |
| 25–39 | 189 | 66 (34.9) | 2.7 | (1.79–4.13) | 2.3 | (1.50–3.67) |
| ≥40 | 201 | 62 (30.8) | 2.3 | (1.48–3.46) | 1.6 | (1.02–2.82) |
| Probands | 371 | 87 (23.5) | 1.0 | (referent) | 1.0 | (referent) |
| Siblings | 231 | 52 (22.5) | 1.0 | (0.65–1.38) | 0.9 | (0.62–1.35) |
| Parents | 128 | 45 (35.2) | 1.8 | (1.16–2.70) | 1.2 | (0.70–1.95) |
| Generalized epilepsy | 435 | 103 (23.7) | 1.0 | (referent) | 1.0 | (referent) |
| Focal epilepsy | 295 | 81 (27.5) | 1.2 | (0.86–1.73) | 1.2 | (0.83–1.73) |
| No. additional first-degree relatives with seizure disorders | ||||||
| None | 549 | 131 (23.9) | 1.0 | (referent) | 1.0 | (referent) |
| 1 | 133 | 33 (24.8) | 1.1 | (0.66–1.69) | 1.0 | (0.59–1.58) |
| ≥2 | 48 | 20 (41.7) | 2.3 | (1.19–4.38) | 2.0 | (0.99–3.85) |
Bivariate odds ratio and 95% confidence interval, computed by generalized estimating equations.
Multivariate odds ratio and 95% confidence interval, computed by generalized estimating equations, with all variables in table included in the model.
Table 4.
Migraine without Aura Only (MO-only) in EPGP Participants
| No. participants | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| Total | With MO- only (%) |
OR* | (95% CI) | OR** | (95% CI) | |
| All participants | 730 | 102 (14.0) | -- | -- | -- | -- |
| Female | 445 | 74 (16.6) | 1.8 | (1.15–2.93) | 1.8 | (1.11–2.83) |
| Male | 285 | 28 (9.8) | 1.0 | (referent) | 1.0 | (referent) |
| Age (years) | ||||||
| 12–24 | 340 | 34 (10.0) | 1.0 | (referent) | 1.0 | (referent) |
| 25–39 | 189 | 39 (20.6) | 2.3 | (1.43–3.84) | 2.1 | (1.23–3.46) |
| ≥40 | 201 | 29 (14.4) | 1.5 | (0.87–2.65) | 1.2 | (0.62–2.35) |
| Probands | 371 | 53 (14.3) | 1.0 | (referent) | 1.0 | (referent) |
| Siblings | 231 | 25 (10.8) | 0.7 | (0.44–1.20) | 0.7 | (0.43–1.19) |
| Parents | 128 | 24 (18.8) | 1.4 | (0.83–2.31) | 1.2 | (0.63–2.14) |
| Generalized epilepsy | 435 | 59 (13.6) | 1.0 | (referent) | 1.0 | (referent) |
| Focal epilepsy | 295 | 43 (14.6) | 1.1 | (0.70–1.68) | 1.1 | (0.70–1.69) |
| No. additional first-degree relatives with seizure disorders | ||||||
| None | 549 | 76 (13.8) | 1.0 | (referent) | 1.0 | (referent) |
| 1 | 133 | 18 (13.5) | 1.0 | (0.54–1.76) | 0.9 | (0.49–1.63) |
| ≥2 | 48 | 8 (16.7) | 1.3 | (0.56–2.76) | 1.1 | (0.50–2.38) |
Bivariate odds ratio and 95% confidence interval, computed by generalized estimating equations.
Multivariate odds ratio and 95% confidence interval, computed by generalized estimating equations, with all variables in table included in the model.
Table 5.
Migraine with Aura (MA) in EPGP Participants
| No. participants | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| Total | With MA (%) |
OR* | (95% CI) | OR** | (95% CI) | |
| All participants | 730 | 82 (11.2) | -- | -- | -- | -- |
| Female | 445 | 66 (14.8) | 2.9 | (1.67–5.13) | 2.7 | (1.51–4.76) |
| Male | 285 | 16 (5.6) | 1.0 | (referent) | 1.0 | (referent) |
| Age (years) | ||||||
| 12–24 | 340 | 22 (6.5) | 1.0 | (referent) | 1.0 | (referent) |
| 25–39 | 189 | 27 (14.3) | 2.4 | (1.31–4.43) | 2.1 | (1.07–3.98) |
| ≥40 | 201 | 33 (16.4) | 2.8 | (1.63–4.93) | 2.2 | (1.15–4.22) |
| Probands | 371 | 34 (9.2) | 1.0 | (referent) | 1.0 | (referent) |
| Siblings | 231 | 27 (11.7) | 1.3 | (0.83–2.08) | 1.2 | (0.74–2.11) |
| Parents | 128 | 21 (16.4) | 1.9 | (1.10–3.43) | 1.1 | (0.57–2.14) |
| Generalized epilepsy | 435 | 44 (10.1) | 1.0 | (referent) | 1.0 | (referent) |
| Focal epilepsy | 295 | 38 (12.9) | 1.3 | (0.83–2.08) | 1.3 | (0.77–2.04) |
| No. additional first-degree relatives with seizure disorders | ||||||
| None | 549 | 55 (10.0) | 1.0 | (referent) | 1.0 | (referent) |
| 1 | 133 | 15 (11.3) | 1.1 | (0.60–2.16) | 1.1 | (0.55–2.09) |
| ≥2 | 48 | 12 (25.0) | 3.0 | (1.42–6.32) | 2.5 | (1.13–5.46) |
Bivariate odds ratio and 95% confidence interval, computed by generalized estimating equations.
Multivariate odds ratio and 95% confidence interval, computed by generalized estimating equations with all variables in table included in the model.
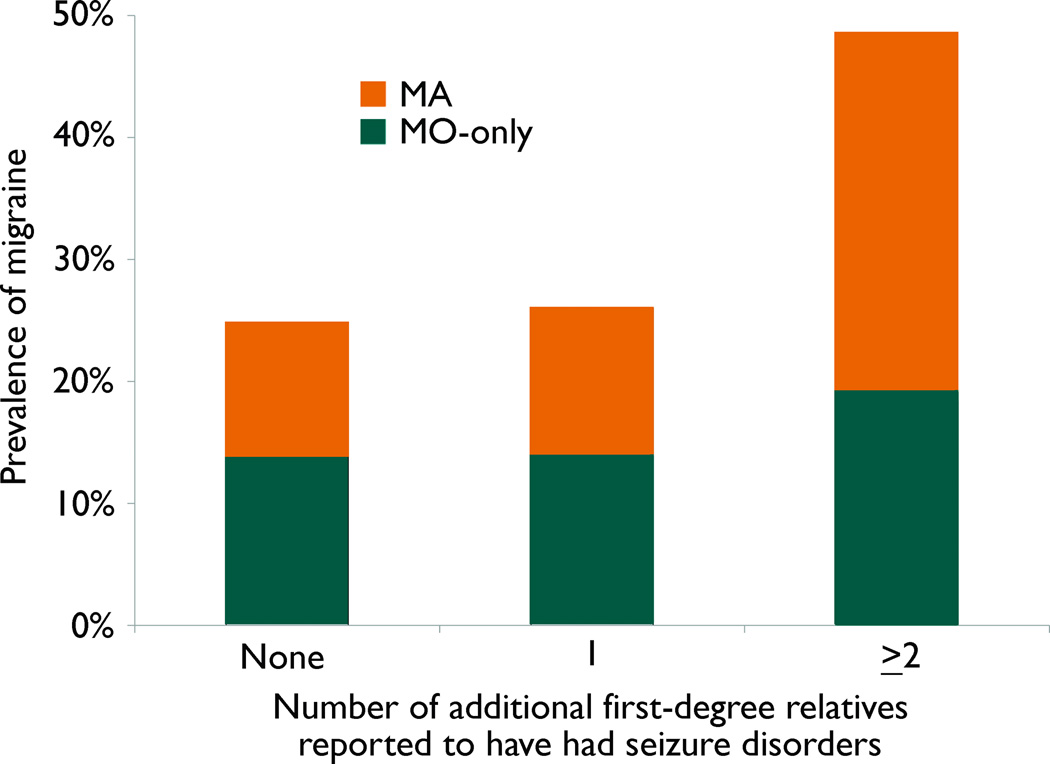
The prevalence of any migraine and MA in enrolled participants (i.e., siblings, probands, or parents) increased when 2 or more additional first-degree relatives were reported to have seizure disorders in the family (Tables 3 and 5), but the prevalence of MO-only did not (Table 4). The association of MA prevalence with number of additional affected relatives was restricted to first degree relatives with seizure disorders; when MA prevalence was examined in relation to the total number of relatives with seizure disorders, the OR was much reduced and was not significant (adjusted OR for ≥2 vs. 0 affected relative of any type=1.4, 95% CI 0.77–2.53). The effect of the number of affected relatives with epilepsy on MA prevalence did not differ by epilepsy type (GE vs. NAFE), though small numbers in each group widened confidence intervals for the ORs (not shown in tables: adjusted OR for GE= 2.8 (0.96–8.05); NAFE=2.2 (0.72–6.52)).
DISCUSSION
To our knowledge, this is the first demonstration of a shared genetic susceptibility to migraine and epilepsy in a large cohort of participants with non-acquired generalized and focal epilepsy. The association of migraine only with first degree relatives rather than any affected relatives, and with two or more first degree relatives (and not one additional affected relative) also provides support for a shared genetic etiology for migraine and epilepsy, because migraine is more prevalent in families in which a stronger genetic effect on epilepsy appears to be operating. A history of seizure disorders in additional (non-enrolled) relatives was associated with MA, but not MO-only, in enrolled relatives. This effect persisted in both GE and NAFE. These results suggest that the genetic influences we observed are specific to MA and epilepsy, but act across epilepsy types (GE and NAFE).
Because the EPGP questionnaire elicits lifetime headache history, our results are roughly similar to cumulative incidence; thus to compare the rates of migraine we observed in EPGP families with rates in the general population, we examined previous estimates of population cumulative incidence up to the median age of our EPGP participants (23 years in males, 29 years in females) (Stewart et al., 2008). Previous estimates of population cumulative incidence to these ages are approximately 10% in males (vs. 17% in our study) and 25% in females (vs. 30% in our study) (Stewart et al., 2008). The slightly elevated rates of migraine in this familial epilepsy sample are consistent with a shared genetic susceptibility to migraine and epilepsy.
Our data provide evidence for a shared genetic susceptibility to epilepsy and MA but not MO-only. This finding is consistent with previously published reports of within-individual (as opposed to within-family) comorbidity of migraine and epilepsy. In a rural Norwegian population-based study, the prevalence of active epilepsy was increased among individuals with MA but not among individuals with any migraine, compared with controls (Brodtkorb et al., 2008). In Iceland, prevalence of MA, but not MO, was higher among children with a first unprovoked seizure when compared to age-matched controls (Ludvigsson et al., 2006). These previous studies differ from ours in their focus on comorbidity within individuals rather than in families; hence our findings provide the first evidence for a shared genetic cause linking MA and commonly occurring epilepsies.
Evidence supporting a shared genetic susceptibility for epilepsy and migraine is not universally restricted to MA, however. In the study of rolandic epilepsy referred to above, no proband or sibling was diagnosed with MA (Clarke et al., 2009a). In a Finnish family with significant evidence of linkage for a combined migraine and epilepsy phenotype to chromosome 12q24.2–24.3, MO was the most frequent migraine phenotype reported (Polvi et al., 2012).
The prevalence of migraine in our subjects did not differ significantly by epilepsy type (GE vs. NAFE). This is consistent with results from the EFSCU cohort (Ottman & Lipton, 1994), and the Icelandic children’s study (Ludvigsson et al., 2006).
Migraine is comorbid with specific pediatric partial epilepsy syndromes, including rolandic epilepsy (Clarke et al., 2009b) and idiopathic childhood epilepsy of Gastaut, a focal occipital epilepsy in which partial seizures are frequently followed by migraine headaches (Panayiotopoulos et al., 2008). The comorbidity of migraine and partial epilepsy is not unique to these specific syndromes; migraine headaches occur with similar frequency in age-matched cohorts of rolandic and cryptogenic/symptomatic partial epilepsies (Wirrell & Hamiwka, 2006).
Genes implicated in migraine also play a role in both generalized and focal epilepsies. A point mutation within the CACNA1A gene was found in a single family with absence (generalized) epilepsy and ataxia (Imbrici et al., 2004). In two families, ATP1A2 mutations have been found to be associated with mostly partial epilepsies, sometimes with secondary generalization (Deprez et al., 2008). Mutations within the SCN1A gene raise risk for generalized epilepsy, specifically GEFS+ and the Dravet syndromes (Claes et al., 2001; Escayg et al., 2000).
We considered the possibility that a few large multiplex families might be driving the results we observed, possibly families with FHM. Among 37 families with two or more additional (non-enrolled) first-degree relatives reported to have seizure disorders in our analysis, the number of additional relatives reported to be affected was two in 21 families, three in 11 families, and four in the remaining five families. There was no evidence that the effect arose primarily from a small number of multiplex families. The presence of FHM was not systematically ascertained, but syndromes with a known genetic cause were excluded.
Our study design has several potential limitations. EPGP is not a population-based study; most enrolled subjects were identified from a tertiary-care referral population of individuals with familial epilepsy. However, specialized populations such as the EPGP cohort, enriched for genetic causes because of the requirements of multiple affected individuals per family and non-acquired etiologies for epilepsy, are well-suited for the identification of the genetic underpinnings of comorbidity. Furthermore, the EPGP requirement of detailed medical records, diagnostic interviews, imaging, and EEG data, allows the examination of a great deal of phenotypic detail which would not be possible in a population-based study.
A second potential limitation is that no controls were collected in the GE/NAFE arm of the EPGP study. For this reason, we could not assess migraine prevalence in relatives of unaffected individuals, which would be of interest in a typical familial aggregation design. However this is not a familial aggregation study; rather, it is an assessment of the predictors of migraine occurrence among individuals with familial epilepsy. In our analysis, the number of individuals with additional relatives reported to have epilepsy in the family served as a proxy for the strength of a genetic influence on epilepsy in the family, and the prevalence of migraine in families with a strong family history (2 or more additional affected relatives) was compared with the prevalence of migraine in families with no affected additional relatives.
We did not systematically collect information on family size in EPGP, and therefore were unable to compute the proportion of all family members affected with epilepsy. This should not have biased our results--although family size is likely to be associated with the number of additional relatives with epilepsy (larger families more likely to contain additional affected relatives), it is unlikely to be related to the history of migraine in EPGP participants.
Another potential limitation is that we did not perform direct interview of non-enrolled family members reported to have seizure disorders; information was collected indirectly, in the interview with the enrolled family members. Family history information collected in this way has been shown to have reasonably high sensitivity and specificity for identifying first-degree relatives with epilepsy; if anything, underreporting is more likely than artificially inflated rates of seizure disorders, particularly for affected parents (Ottman et al., 2011). For our analysis, the most important issue with regard to family history reporting is bias -- whether sensitivity of the family history data is likely to differ according to migraine history of the EPGP participants – which we believe is unlikely.
We were not able to assess history of migraine in the non-enrolled family members, because family history information for migraine (unlike that for seizure disorders) has poor sensitivity and specificity (Ottman et al., 1993a). We therefore could not examine the effect of the number of individuals with known epilepsy in the family on the history of migraine in non-enrolled relatives. In future studies expanding on the EPGP population and infrastructure, we hope to be able to enroll these additional reported family members, and obtain direct information from them about their history of seizures and migraine, as well as other neuropsychiatric comorbidities that have been shown to co-occur with epilepsy.
Our results differ from those in EFSCU, although a similar analytic strategy was used in that study (i.e., assessment of association between occurrence of migraine and family history of epilepsy, among individuals with epilepsy). One major difference between EFSCU and EPGP is that the EPGP sample is entirely familial epilepsy, whereas the EFSCU sample was ascertained independently of family history, so that the majority of families contained only one affected individual. Given our finding that migraine in EPGP participants was associated with a family history of epilepsy only when 2 or more additional affected relatives were reported (i.e., beyond the enrolled pair), an effect might not have been observed in EFSCU because of the very small proportion of families with so many affected individuals.
Our results provide a foundation for the exploration of the genetics of neuropsychiatric comorbidity in epilepsy. Neuropsychiatric comorbidity presents a tremendous problem for people with epilepsy and their caregivers, affecting quality of life, treatment success, and mortality (Baca et al., 2011; Christensen et al., 2007; Hitiris et al., 2007; Kanner, 2007; Kanner, 2009). In fact, comorbidities have been shown to have a greater impact on quality of life in people with epilepsy than the seizures themselves (Gilliam et al., 2003). Comorbidity in epilepsy is an NIH benchmark (Area III) and is emphasized in the Institute of Medicine report on epilepsy because of the widespread recognition of the importance of research in this area (www.iom/edu). Elucidation of the genetic contributions to neuropsychiatric comorbidity in epilepsy can help clarify the relationships among these diseases, identify neurobiological underpinnings of comorbidity, and suggest novel targets for therapy and prevention (Johnson & Shorvon, 2011; Mula, 2012). Future innovative phenotype definition in epilepsy will incorporate more than single disorders alone, and begin to focus on constellations of disorders with shared pathophysiology. This may improve recognition and treatment of comorbid disorders, and help re-conceptualize disease boundaries (Jensen, 2011).
Figure 1.
Lifetime Prevalence of Migraine in Enrolled Participants, by History of Seizure Disorders in First-Degree Relatives
MO-only = Migraine without aura only
MA = Migraine with aura
ACKNOWLEDGEMENTS
Supported by NINDS U01 NS 053998
We would like to acknowledge the recruitment contributions of the EPGP Community Referral Network (CRN). The CRN consists of healthcare professionals not paid by the EPGP grant who refer eligible families to EPGP. A list of individual contributors can be found at www.epgp.org.
In addition, we would like to acknowledge the efforts of the clinical coordinators, the site principal investigators, neurologists, and support staff at our EPGP clinical centers who have contributed significant effort into recruitment, data acquisition and storage, and extensive phenotyping. Finally, we extend our sincere appreciation to the participants with epilepsy and their families who have contributed to this research effort.
Appendix 1: The EPGP Investigators (Excluding authors listed above)
Bassel Abou-Khalil, MD; Data Review Core, Local PI; Vanderbilt University Medical Center
Brian Alldredge, PharmD; AED Core; University of California, San Francisco
Jocelyn Bautista, MD; Local PI; Cleveland Clinic
Sam Berkovic, MD; Local PI; The University of Melbourne
Alex Boro, MD; EEG Core; Albert Einstein College of Medicine
Gregory Cascino, MD; MRI Core, Local PI; Mayo Clinic College of Medicine Rochester, Minnesota
Damian Consalvo, MD, PhD; Local PI; Hospital General de Agudos José Maria Ramos Mejía
Patricia Crumrine, MD; Local PI; Children's Hospital of Pittsburgh of UPMC
Orrin Devinsky, MD; Phenotyping Core, Local PI; New York University School of Medicine
Dennis Dlugos, MD, MCSE; EEG Core, Phenotyping Core, Local PI; The Children’s Hospital of Philadelphia
Michael Epstein, PhD; Data Analysis Core; Emory University School of Medicine
Robyn Fahlstrom, MPH; EPGP Statistician; University of California San Francisco School of Medicine
Miguel Fiol, MD; Referral Center PI; University of Minnesota Medical Center
Nathan Fountain, MD; Data Review Core, Local PI; University of Virginia Health System
Jacqueline French, MD; AED Core; New York University School of Medicine
Daniel Friedman, MD; Local Co-PI; New York University School of Medicine
Eric Geller, MD; Local Co-PI; St. Barnabas Health Care System
Tracy Glauser, MD; AED Core, Local PI; Cincinnati Children's Hospital Medical Center
Simon Glynn, MD; Local PI; University of Michigan
Sheryl Haut, MD, MS; Local PI; Albert Einstein College of Medicine
Jean Hayward, MD; Referral Center PI; Kaiser Permanente: Oakland Medical Center
Sandra Helmers, MD; Local PI; Emory University School of Medicine
Andres Kanner, MD; AED Core; Rush University Medical Center
Heidi Kirsch, MD, MS; Local PI; University of California, San Francisco
Robert Knowlton, MD; MRI Core, Local PI; University of Alabama at Birmingham School of Medicine
Eric Kossoff, MD; Local Co-PI; The Johns Hopkins University School of Medicine
Rachel Kuperman, MD; Local Referral Center PI; Children’s Hospital & Research Center Oakland
Ruben Kuzniecky, MD; Study PI; New York University School of Medicine
Daniel Lowenstein, MD; Study PI; University of California, San Francisco
Shannon McGuire, MD; Local PI; Louisiana State University Health Sciences Center
Paul Motika, MD; Local Co-PI; Rush University Medical Center
Edward Novotny, MD; Local PI; Seattle Children's Hospital
Ruth Ottman, PhD; Phenotyping Core, Data Analysis Core, Local PI; Columbia University
Juliann Paolicchi, MD; Local PI; Vanderbilt University Medical Center
Jack Parent, MD; Local Co-PI; University of Michigan
Kristen Park, MD; Local PI; The Children's Hospital Denver
Annapurna Poduri, MD; Data Review Core, Local PI; Children's Hospital Boston
Neil Risch PhD; Data Analysis Core; University of California, San Francisco
Lynette Sadleir, MBChB, MD; Local PI; Wellington School of Medicine and Health Sciences, University of Otago
Ingrid Scheffer, MBBS, PhD; Data Review Core, Local PI; The University of Melbourne
Renee Shellhaas, MD; EEG Core; University of Michigan
Elliot Sherr, MD, PhD; Phenotyping Core; University of California, San Francisco
Jerry Shih, MD; Data Review Core, Local PI; Mayo Clinic College of Medicine Jacksonville, Florida
Shlomo Shinnar, MD, PhD; Phenotyping Core; Albert Einstein College of Medicine
Rani Singh, MD; Local Co-PI; University of Michigan
Joseph Sirven, MD; Local PI; Mayo Clinic College of Medicine Scottsdale, Arizona
Michael Smith, MD; Local PI; Rush University Medical Center
Joe Sullivan, MD; EEG Core; University of California, San Francisco
Liu Lin Thio, MD, PhD; Local PI; Washington University in St. Louis
Anu Venkatasubramanian, MD; Local Co-PI; The Children’s Hospital of Philadelphia
Eileen Vining, MD; Local PI; The Johns Hopkins University School of Medicine
Gretchen Von Allmen, MD; Local PI; University of Texas Health Science Center at Houston
Judith Weisenberg, MD; Local PI; Washington University in St. Louis
Peter Widdess-Walsh, MD; Local PI and Data Review Core; St. Barnabas Health Care System
Administrative and Informatics Core Members contributing to the manuscript (Contributors):
Catharine Freyer, Project Director
Kristen Schardein, RN, MS, Recruitment Director
Sabrina Cristofaro, RN, BSN, Phenotyping Director
Gerry Nesbitt, EPGP CIO
Footnotes
Melodie R. Winawer MD MS has no conflicts of interest to disclose.
Robert D. Connors received $1000 from the Committee for Interns and Residents (CIR) to attend a conference in 2011.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- The International Classification of Headache Disorders. Cephalalgia. (2nd edition) 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- Andermann E, Andermann F. Migraine-epilepsy relationships: epidemiological and genetic aspects. In: Andermann F, Lugaresi E, editors. Migraine and epilepsy. Boston: Butterworths; 1987. pp. 281–291. [Google Scholar]
- Andermann F, Lugaresi E. Migraine and epilepsy. Boston: Butterworths; 1987. [Google Scholar]
- Baca CB, Vickrey BG, Caplan R, Vassar SD, Berg AT. Psychiatric and medical comorbidity and quality of life outcomes in childhood-onset epilepsy. Pediatrics. 2011;128:e1532–e1543. doi: 10.1542/peds.2011-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodtkorb E, Bakken IJ, Sjaastad O. Comorbidity of migraine and epilepsy in a Norwegian community. Eur J Neurol. 2008;15:1421–1423. doi: 10.1111/j.1468-1331.2008.02353.x. [DOI] [PubMed] [Google Scholar]
- Christensen J, Vestergaard M, Mortensen PB, Sidenius P, Agerbo E. Epilepsy and risk of suicide: a population-based case-control study. Lancet Neurol. 2007;6:693–698. doi: 10.1016/S1474-4422(07)70175-8. [DOI] [PubMed] [Google Scholar]
- Claes L, Del-Favero J, Ceulemans B, Lagae L, Van Broeckhoven C, De Jonghe P. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet. 2001;68:1327–1332. doi: 10.1086/320609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke T, Baskurt Z, Strug L, Pal D. Evidence of shared genetic risk factors for migraine and rolandic epilepsy. Epilepsia. 2009a;50:2428–2433. doi: 10.1111/j.1528-1167.2009.02240.x. [DOI] [PubMed] [Google Scholar]
- Clarke T, Baskurt Z, Strug LJ, Pal DK. Evidence of shared genetic risk factors for migraine and rolandic epilepsy. Epilepsia. 2009b;50:2428–2433. doi: 10.1111/j.1528-1167.2009.02240.x. [DOI] [PubMed] [Google Scholar]
- Deprez L, Peeters K, Van Paesschen W, Claeys KG, Claes LR, Suls A, Audenaert D, Van Dyck T, Goossens D, Del-Favero J, De Jonghe P. Familial occipitotemporal lobe epilepsy and migraine with visual aura: linkage to chromosome 9q. Neurology. 2007;68:1995–2002. doi: 10.1212/01.wnl.0000262764.78511.17. [DOI] [PubMed] [Google Scholar]
- Deprez L, Weckhuysen S, Peeters K, Deconinck T, Claeys KG, Claes LR, Suls A, Van Dyck T, Palmini A, Matthijs G, Van Paesschen W, De Jonghe P. Epilepsy as part of the phenotype associated with ATP1A2 mutations. Epilepsia. 2008;49:500–508. doi: 10.1111/j.1528-1167.2007.01415.x. [DOI] [PubMed] [Google Scholar]
- Dichgans M, Freilinger T, Eckstein G, Babini E, Lorenz-Depiereux B, Biskup S, Ferrari MD, Herzog J, van den Maagdenberg AM, Pusch M, Strom TM. Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. Lancet. 2005;366:371–377. doi: 10.1016/S0140-6736(05)66786-4. [DOI] [PubMed] [Google Scholar]
- Escayg A, MacDonald BT, Meisler MH, Baulac S, Huberfeld G, An-Gourfinkel I, Brice A, LeGuern E, Moulard B, Chaigne D, Buresi C, Malafosse A. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nat Genet. 2000;24:343–345. doi: 10.1038/74159. [DOI] [PubMed] [Google Scholar]
- Gilliam F, Hecimovic H, Sheline Y. Psychiatric comorbidity, health, and function in epilepsy. Epilepsy Behav. 2003;4(Suppl 4):S26–S30. doi: 10.1016/j.yebeh.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Hitiris N, Mohanraj R, Norrie J, Sills GJ, Brodie MJ. Predictors of pharmacoresistant epilepsy. Epilepsy Res. 2007;75:192–196. doi: 10.1016/j.eplepsyres.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Imbrici P, Jaffe SL, Eunson LH, Davies NP, Herd C, Robertson R, Kullmann DM, Hanna MG. Dysfunction of the brain calcium channel CaV2.1 in absence epilepsy and episodic ataxia. Brain. 2004;127:2682–2692. doi: 10.1093/brain/awh301. [DOI] [PubMed] [Google Scholar]
- Jensen FE. Epilepsy as a spectrum disorder: Implications from novel clinical and basic neuroscience. Epilepsia. 2011;52(Suppl 1):1–6. doi: 10.1111/j.1528-1167.2010.02904.x. [DOI] [PubMed] [Google Scholar]
- Johnson MR, Shorvon SD. Heredity in epilepsy: neurodevelopment, comorbidity, and the neurological trait. Epilepsy Behav. 2011;22:421–427. doi: 10.1016/j.yebeh.2011.07.031. [DOI] [PubMed] [Google Scholar]
- Kanner AM. Epilepsy and Mood Disorders. Epilepsia. 2007;48:20–22. doi: 10.1111/j.1528-1167.2007.01395.x. [DOI] [PubMed] [Google Scholar]
- Kanner AM. Psychiatric issues in epilepsy: the complex relation of mood, anxiety disorders, and epilepsy. Epilepsy Behav. 2009;15:83–87. doi: 10.1016/j.yebeh.2009.02.034. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646–657. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- Ludvigsson P, Hesdorffer D, Olafsson E, Kjartansson O, Hauser WA. Migraine with aura is a risk factor for unprovoked seizures in children. Ann Neurol. 2006;59:210–213. doi: 10.1002/ana.20745. [DOI] [PubMed] [Google Scholar]
- Mula M. Epilepsy: Bidirectional link between epilepsy and psychiatric disorders. Nat Rev Neurol. 2012;8:252–253. doi: 10.1038/nrneurol.2012.69. [DOI] [PubMed] [Google Scholar]
- Nesbitt G, McKenna K, Mays V, Carpenter A, Miller K, Williams M The EPGP Investigators. The Epilepsy Phenome/Genome Project (EPGP) informatics platform. Int J Med Inform. doi: 10.1016/j.ijmedinf.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottman R, Barker-Cummings C, Leibson CL, Vasoli VM, Hauser WA, Buchhalter JR. Accuracy of family history information on epilepsy and other seizure disorders. Neurology. 2011;76:390–396. doi: 10.1212/WNL.0b013e3182088286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottman R, Hauser WA, Stallone L. Semistructured interview for seizure classification: agreement with physicians' diagnoses. Epilepsia. 1990;31:110–115. doi: 10.1111/j.1528-1157.1990.tb05368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottman R, Hong S, Lipton RB. Validity of family history data on severe headache and migraine. Neurology. 1993a;43:1954–1960. doi: 10.1212/wnl.43.10.1954. [DOI] [PubMed] [Google Scholar]
- Ottman R, Lee JH, Hauser WA, Hong S, Hesdorffer D, Schupf N, Pedley TA, Scheuer ML. Reliability of seizure classification using a semistructured interview. Neurology. 1993b;43:2526–2530. doi: 10.1212/wnl.43.12.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottman R, Lipton RB. Comorbidity of migraine and epilepsy. Neurology. 1994;44:2105–2110. doi: 10.1212/wnl.44.11.2105. [DOI] [PubMed] [Google Scholar]
- Ottman R, Lipton RB. Is the comorbidity of epilepsy and migraine due to a shared genetic susceptibility? Neurology. 1996;47:918–924. doi: 10.1212/wnl.47.4.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayiotopoulos CP, Michael M, Sanders S, Valeta T, Koutroumanidis M. Benign childhood focal epilepsies: assessment of established and newly recognized syndromes. Brain. 2008;131:2264–2286. doi: 10.1093/brain/awn162. [DOI] [PubMed] [Google Scholar]
- Polvi A, Siren A, Kallela M, Rantala H, Artto V, Sobel EM, Palotie A, Lehesjoki AE, Wessman M. Shared loci for migraine and epilepsy on chromosomes 14q12-q23 and 12q24.2-q24.3. Neurology. 2012;78:202–209. doi: 10.1212/WNL.0b013e31823fcd87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart WF, Wood C, Reed ML, Roy J, Lipton RB. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28:1170–1178. doi: 10.1111/j.1468-2982.2008.01666.x. [DOI] [PubMed] [Google Scholar]
- Tellez-Zenteno JF, Matijevic S, Wiebe S. Somatic comorbidity of epilepsy in the general population in Canada. Epilepsia. 2005;46:1955–1962. doi: 10.1111/j.1528-1167.2005.00344.x. [DOI] [PubMed] [Google Scholar]
- Wirrell EC, Hamiwka LD. Do children with benign rolandic epilepsy have a higher prevalence of migraine than those with other partial epilepsies or nonepilepsy controls? Epilepsia. 2006;47:1674–1681. doi: 10.1111/j.1528-1167.2006.00639.x. [DOI] [PubMed] [Google Scholar]