Abstract
Wnt/β-catenin signaling plays an important role in hepatic homeostasis especially in liver development, regeneration and cancer, and loss of β-catenin signaling is often associated with increased apoptosis. To elucidate how β-catenin may be regulating hepatocyte survival, we investigated the susceptibility of β-catenin conditional knockout mice (KO) and littermate controls (WT) to Fas and TNF-α, two common pathways of hepatocyte apoptosis. While comparable detrimental effects from Fas activation were observed in WT and KO, a paradoxical survival benefit was observed in KO mice challenged with D-galactosamine (GalN)/lipopolysaccharide (LPS). KO showed significantly lower morbidity and liver injury due to early, robust and protracted activation of NF-κB in absence of β-catenin. Enhanced NF-κB activation in KO was contributed by increased basal inflammation and TLR4 expression and lack of p65-β-catenin complex in hepatocytes. β-Catenin-p65 complex in WT livers underwent temporal dissociation allowing for NF-κB activation to regulate hepatocyte survival following TNF-α-induced hepatic injury. Decrease of total β-Catenin protein but not its inactivation induced, while β-catenin stabilization either chemically or due to mutations repressed, p65 activity in hepatomas in a dose-dependent manner.
Conclusion
β-Catenin-p65 complex in hepatocytes undergoes dynamic changes during TNFα-induced hepatic injury and plays a critical role in NF-κB activation and cell survival. Modulation of β-catenin levels is a unique mode of regulating NF-κB activity and thus may present novel opportunities in devising therapeutics in specific hepatic injuries.
Keywords: Apoptosis, p65, Wnt, liver injury, TNF-α, cell death
Hepatic inflammation due to a number of etiologies, such as viruses, alcohol, and others, is a common cause of hepatic injury and cell death. Liver is particularly susceptibility to apoptosis due to a rich expression of death receptors. Of the six identified death receptors, Fas and TNF-α are considered to have major pathologic significance in liver (1, 2). Activation of the Fas-mediated apoptotic pathway has been linked to liver diseases such as hepatic inflammation, viral hepatitis, alcoholic hepatitis, non-alcoholic steatohepatitis, cholestasis, and Wilson’s disease, while TNF-α mediated activation of apoptosis has been implicated in alcoholic hepatitis, ischemia/reperfusion, and fulminant hepatic failure (1, 2).
Fas-mediated cell death, experimentally induced by intravenous injection of Jo-2 antibody, causes activation of Fas receptor and initiation of a downstream cascade that induces massive hepatocyte apoptosis. Treatment with lipopolysaccharide (LPS) also causes hepatotoxicity and lethality, which is primarily mediated by release of TNF-α from macrophages (3). However, binding of TNF-α to its receptor or binding of LPS to toll-like receptor 4 (TLR-4) also activates nuclear factor kappa B (NF-κB) (4, 5), which directly antagonizes the pro-apoptotic effects of TNF-α and prevents cell death (6). Thus, liver injury through TNF-α pathway requires hepatocyte sensitization accomplished by pretreatment with D-galactosamine (GalN) that depletes UTP and inhibits de novo RNA synthesis (7). NF-κB regulates expression of anti- apoptotic genes such as IAPs, c-FLIP, TRAFs, and Bcl family members, among others (8).
Wnt/β-catenin pathway is an important player in liver biology with roles in development, regeneration, and tumorigenesis (Reviewed in (9)). However, little is known about its role in hepatocyte survival, although evidence exists that β-catenin ablation renders hepatocytes susceptible to apoptosis in development, regeneration and more recently in hepatic ischemia-reperfusion injury (10). We utilized β-catenin conditional knockout (KO) mice and their wild-type (WT) littermates to test susceptibility to Fas and TNF-α. While Fas activation had comparable effects in WT and KO, a paradoxical survival advantage was observed in KO after GalN/LPS treatment. We demonstrate that the β-catenin-p65 complex in hepatocytes underwent dynamic changes to regulate NF-κB activation, and decrease in β-catenin protein levels, both in vivo & in vitro, led to robust and protracted p65 nuclear translocation and activation. Conversely, β-catenin stabilization suppressed NF-κB activity. Thus, we provide evidence that β-catenin-NF-κB interactions may be altered in hepatic pathologies and that modulation of the complex may be uniquely exploited therapeutically for certain forms of liver injury.
EXPERIMENTAL PROCEDURES
Animals
Conditional β-catenin knockout mice (C57BL/6) were generated as described previously (11). Ctnnb1loxp/loxp; Alb-Cre+/− are referred to as knockout mice (KO) and Ctnnb1loxp/loxp; Alb-Cre−/− or Ctnnb1loxp/Wt; Alb-Cre−/− are referred to as wild-type mice (WT). All studies were approved by the University of Pittsburgh’s Institutional Animal Care and Use Committee and were in accordance with National Institutes of Health guidelines.
For complete methods, please see Online Supplement.
RESULTS
β-Catenin KO and WT mice exhibit comparable susceptibility to Fas-mediated apoptosis
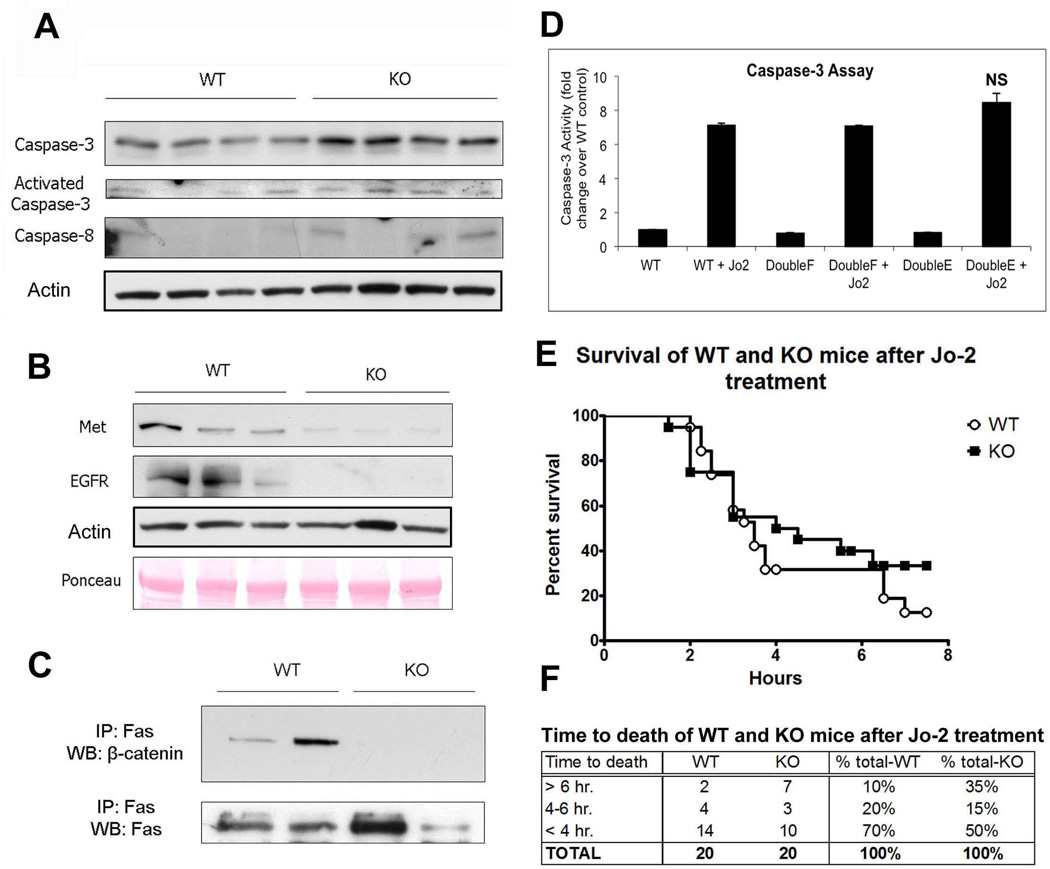
In agreement with our previous study (11), resting KO livers display a small increase in apoptosis, as demonstrated by an increase in caspase-3, active caspase-3, and caspase-8 proteins (Figure 1A). To determine if Fas may be mediating basal cell death in absence of β-catenin, we examined changes in expression of two key receptor tyrosine kinases, epidermal growth factor receptor (EGFR) and the hepatocyte growth factor (HGF) receptor Met, as these signaling pathways are known to prevent Fas-induced liver injury and are also known β-catenin targets (11–14). We found dramatic reduction in Met and EGFR protein in KOs (Figure 1B). Additionally, expression of HGF mRNA is upregulated 9.27-fold in the KOs at baseline (Table 2, Online Supplement).
Figure 1. β-catenin KO animals show alterations in components of the Fas pathway, which does not result in increased susceptibility to Fas-mediated apoptosis.
(A) Caspase protein expression and activity is higher in KOs than in WTs at baseline; actin represents loading control.
(B) Expression of Met and EGFR is decreased in KOs compared to WTs at baseline, as assessed by WB.
(C) IP shows that Fas and β-catenin associate strongly in WT livers at baseline and that this association is absent in KO livers.
(D) Active caspase-3 activity is not significantly changed in Hepa 1–6 cells transfected with either WT, constitutively active, or inactive β-catenin followed by Jo-2 treatment, as measured by fluorometric assay.
(E) Kaplan-Meier survival analysis of WT and KO mice (n=20) after i.v. injection with Jo-2 shows no significant differences in overall survival between the two groups.
(F) Grouping WT and KO animals treated with Jo-2 by time of death shows that KO animals are slightly more resistant to Fas-mediated apoptosis than WT animals.
As previously shown, β-catenin is known to complex with Met (15), which in turn is known to complex with Fas (13) in hepatocytes. We also observe β-catenin-Fas complex by immunoprecipitation studies in WT livers but not in KO (Figure 1C). It has been shown that β-catenin phosphorylation by HGF/Met at tyrosine (Y) 654 & 670 dissociates it from Met (16). To determine if mutation of β-catenin tyrosine residues destabilizes the Met/Fas/β-catenin interactions altering susceptibility of hepatoma cells to Fas-mediated apoptosis, we transfected Hepa 1–6 cells with WT, phospho-mimetic Y654/670E (glutamic acid) or phospho-null Y654/670F (phenylalanine) β-catenin followed by treatment with Jo-2 antibody. Determination of caspase-3 activity by fluorometric assay measuring cleavage of the caspase-3 peptide substrate DEVD-AFC 12 hours after Jo-2 treatment showed insignificant differences in apoptosis between three conditions, suggesting that gain or loss of β-catenin from Met/Fas complex does not alter susceptibility to Fas-ligand (Figure 1D).
Next, we challenged WT and KO mice with Jo-2. Insignificant differences in survival between WT and KO in response to Jo-2 were evident (Figure 1E and F).
KO mice show increased resistance to TNF-α induced apoptosis
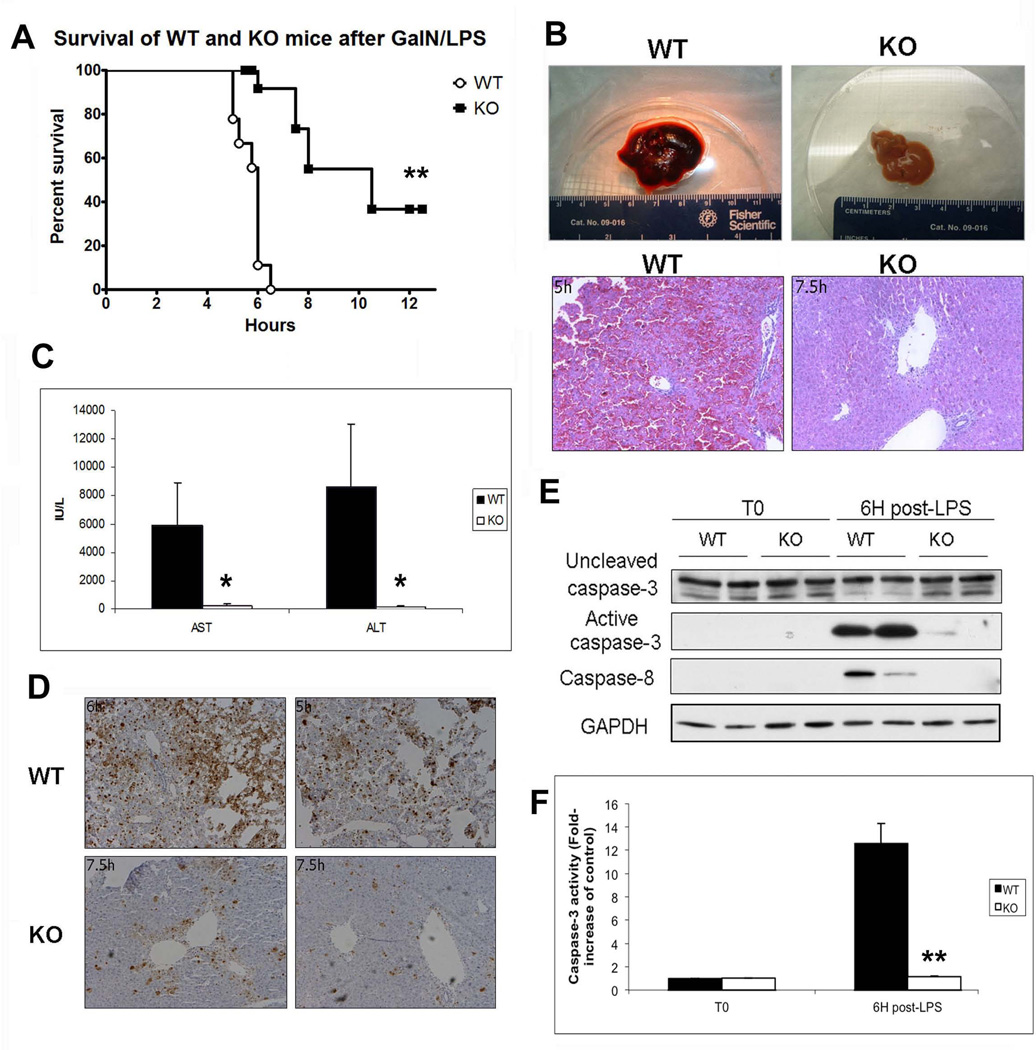
Next, we challenged KO and WT mice with GalN followed 30 minutes later by LPS to activate TNF-α-mediated liver injury (17, 18). As expected, 9/9 WT mice became lethargic and moribund approximately 6 hours (6H) after GalN/LPS administration, while surprisingly, most KO mice (14/15) survived past 6H, with some being uncompromised and healthy as late as 12H post-treatment (Table 1, Online Supplement). Thus, although stimulation of the TNF-α pathway caused predictable morbidity in WT mice, KO mice showed a significant decrease in morbidity and mortality (Figure 2A). The KO mice were also refractory to GalN pretreatment followed by intravenous injection of TNF-α, the major mediator of LPS-induced hepatotoxicity (data not shown) (19).
Figure 2. β-catenin KO mice are protected from injury induced via the TNF-α pathway.
(A) Kaplan-Meier survival analysis of WT and KO mice after treatment with GalN/LPS shows that KOs survive significantly longer than their WT counterparts (n≥9; **P<0.01).
(B) Gross liver specimens from WT and KO mice injected with GalN/LPS demonstrate that WT livers become engorged with blood 6H post-injection, while the KO livers harvested at the same time appear normal. Corresponding histology is characteristic of massive inflammation and apoptotic cell death in WT livers and near normal histology in KO livers as shown by H&E.
(C) Serum AST and ALT levels are significantly higher in WT mice than in KO mice 6H after GalN/LPS treatment (n=3; *P<0.05).
(D) The number of apoptotic hepatocytes 6H after GalN/LPS injection (TUNEL IHC) is dramatically lower in KO mice as compared to WT controls.
(E) Cleaved caspase-3 and caspase-8 is increased in WTs compared to KOs after 6H of GalN/LPS treatment, as shown in WB. GAPDH represents loading control.
(F) The level of active caspase-3 activity is increased in WTs after 6H of GalN/LPS compared to KOs as measured by fluorometric assay (n=3; **P<0.01).
Histological and biochemical analysis of WT and KO livers after GalN/LPS treatment verifies that KO mice are protected from cell death
Livers from WT mice injected with GalN/LPS were harvested when they showed signs of morbidity and KO livers were harvested at comparable and later time points despite lack of any morbidity (Table 1, Online Supplement). Grossly, WT livers were congested and enlarged while KO livers, typically small and pale, remain relatively unaffected by treatment (Figure 2B). Histologically, WT livers showed intense inflammation, massive cell death, and red blood cell sequestration in sinusoidal spaces, with only a few cells spared periportally (Figure 2B). KO livers showed mostly healthy hepatocytes and intact liver with only occasional sinusoidal dilation (Figure 2B). Serum biochemistry revealed a 40-fold increase in serum ALT and a 20-fold increase in serum AST in WT as compared to KO (Figure 2C). Assaying the livers of both genotypes for apoptosis revealed that GalN/LPS-treated KO livers had dramatically fewer hepatocytes with terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL)-positive nuclei as compared to WT (Figure 2D). Finally, WT and KO livers at 6H were assessed for the presence of activated caspases by Western blot (WB) (Figure 2E) and fluorometric measurement of caspase-3 activity (Figure 2F), which showed WT to have significantly greater apoptosis as compared to KO.
KO show mild and self-limited injury, whereas WT display progressive injury to GalN/LPS
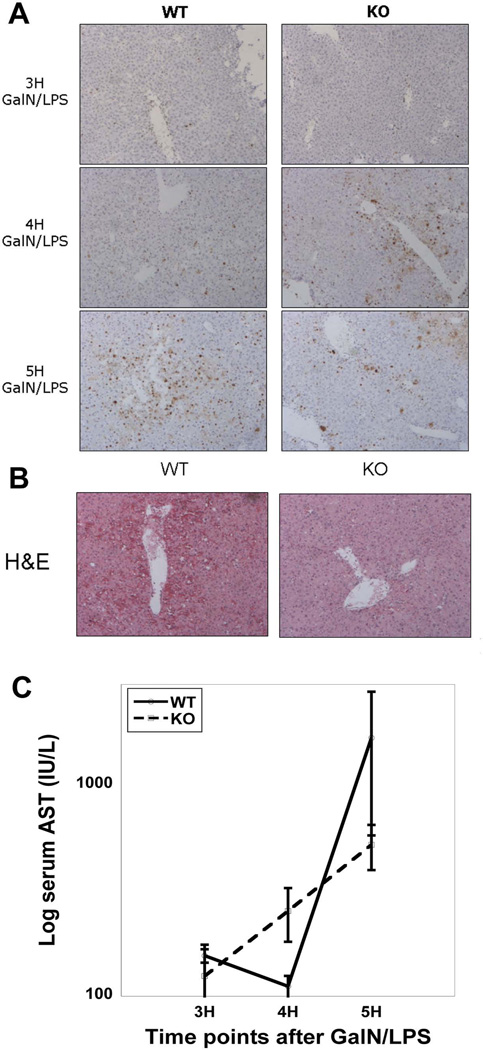
To characterize the initiation and progression of liver injury in WT and KO after GalN/LPS, livers and plasma were obtained at 3H, 4H, and 5H after treatment. TUNEL assay shows few apoptotic cells in either WTs or KOs at 3H while at 4H KO animals display more TUNEL-positive nuclei than WT. However, at 5H TUNEL-positivity in KO has not progressed and may have even improved, whereas extensive apoptosis is evident in WT (Figure 3A), which is also confirmed by H&E (Figure 3B). Consistent with TUNEL, serum AST is low and comparable at 3H, greater in KO at 4H and markedly higher in WT at 5H (Figure 3C). These observations suggest comparable initiation of liver damage in KO and WT after GalN/LPS, and although the damage progresses in WT, it is self-limited in KO.
Figure 3. Progressive damage to WT livers after GalN/LPS, while damage in KO livers is arrested after an early onset.
(A) KO livers have more TUNEL-positive cells compared to WT at 4H post-GalN/LPS. At 5H there is a rapid increase in the number of apoptotic cells in the WT compared to the KOs, which surpasses KO, while KO show no progression in the numbers of TUNEL-positive cells.
(B) WTs have significantly more damage than KOs as early as 5H after GalN/LPS, as shown by H&E staining.
(C) Serum AST levels are increased in KOs at 4H, while at 5H the AST in WTs has surpassed that seen in the KOs.
NF-κB and its downstream targets are upregulated in KO livers 6H post-GalN/LPS
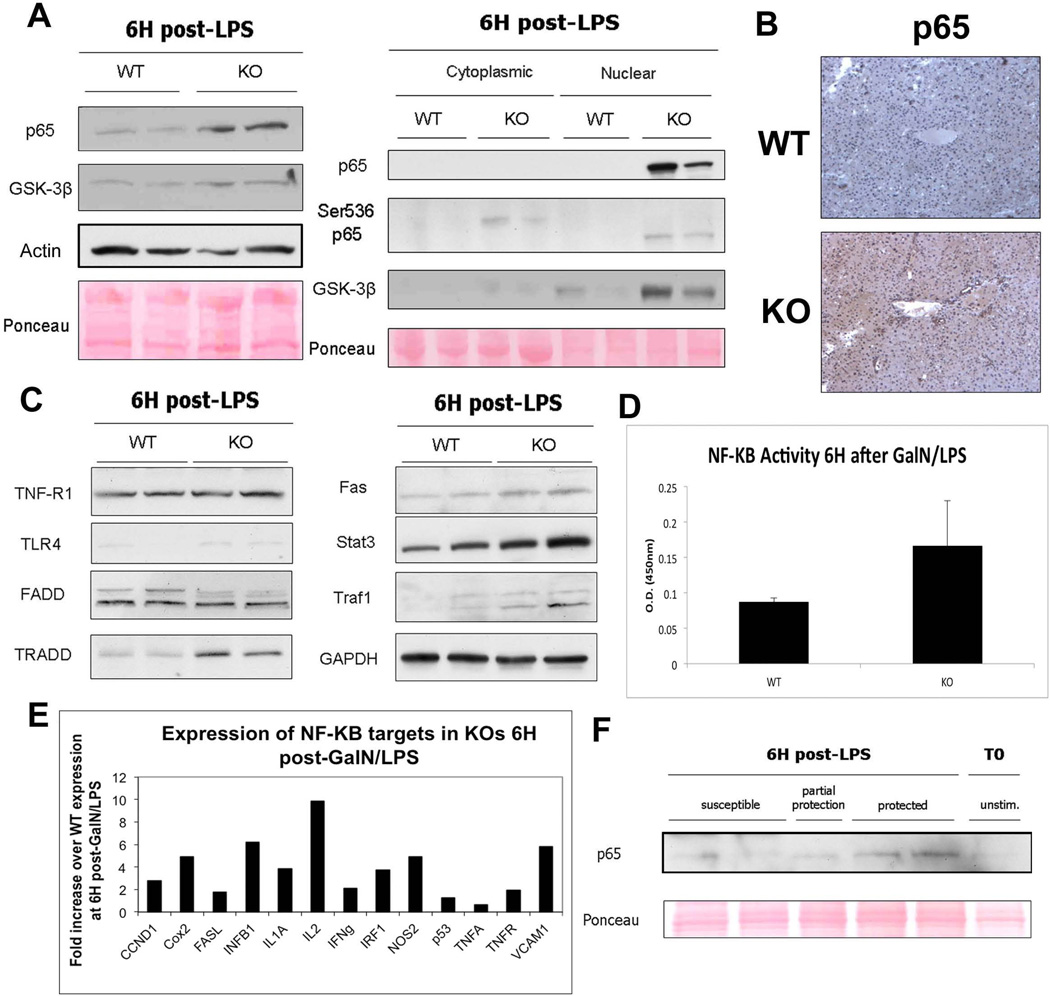
To determine the mechanism of protection in KOs, we examined the expression of NF-κB, a known anti-apoptotic mediator of TNF-α injury. Indeed, 6H after GalN/LPS treatment, there is a clear increase in total p65 levels in KOs, as analyzed by WB (Figure 4A). Similarly, we detect the presence of total- and transcriptionally active Ser-536-phospho-p65 protein in hepatocyte nuclei of KO but not WT livers (20). An increase in GSK-3β, a known NF-κB activator (21), is also observed in KO at 6H suggesting a possible mechanism of p65 phosphorylation (Figure 4A). Extensive cytoplasmic and nuclear p65 in KO is verified by IHC at 5H (Figure 4B). NF-κB activation in KO at 6H after GalN/LPS administration was further substantiated by the increase of NF-κB downstream targets Traf-1 and Fas, as well as Stat3, which is a downstream effector of the NF-κB target gene IL-6 (Figure 4C). In fact, many components of LPS-induced NF-κB protective machinery, including TLR-4 and tumor necrosis factor receptor type 1-associated DEATH domain protein (TRADD) are increased in KOs, while TNF-R1 and Fas-associated death domain protein (FADD), are unchanged (Figure 4C). Measurement of NF-κB transcriptional activity by colorimetric assay confirms an increase in KO livers (Figure 4D). Additional targets including inflammatory mediators and cytokines were analyzed by cDNA array for NF-κB-regulated target genes and also found to be upregulated (Figure 4E).
Figure 4. Cytoprotective proteins and expression of downstream NF-κB targets are higher in KO livers than in WT livers 6H post-GalN/LPS.
(A) WB show that both p65 and GSK-3β are increased in whole-liver lysates from KOs as compared to WTs. Actin and ponceau represent loading controls. Both p65 and GSK-3β are localized mainly to the nuclei of the KO livers, as demonstrated by WB of cell fractionation extracts. Additionally, p65 is present in its active, phosphorylated form in both the cytoplasm and nucleus of KO livers.
(B) IHC for p65 shows a marked increase in p65 expression in the KOs as compared to WTs 5H after GalN/LPS administration.
(C) WB of TNF-α/LPS pathway members and NF-κB target genes shows that expression of targets and some effectors is higher in KOs compared to WTs after GalN/LPS.
(D) Transcriptional activity of NF-κB is increased in KOs as compared to WTs 6H after GalN/LPS, as measured by colorimetric assay.
(E) cDNA analysis of selected NF-κB targets after treatment with GalN/LPS shows that KOs have a several-fold increase in the expression of many NF-κB-induced genes compared to WTs.
(F) Nuclear extracts from KO animals displaying differential susceptibility to GalN/LPS at 7.5H after treatment show that p65 is higher in KO animals that are protected from apoptosis as compared to those that are susceptible.
To further demonstrate that NF-κB is playing a protective role in the KO animals after LPS injury, we examined p65 nuclear expression in KO animals that displayed a range of susceptibility to GalN/LPS. As shown in Supplemental Table 1, around 7.5H after GalN/LPS, 6/15 KO animals were partially susceptible to LPS-induced apoptosis, whereas 9/15 showed prolonged survival. The KO that showed protection showed higher nuclear p65 than those showing injury, indicating a direct correlation between p65 nuclear expression and protection from apoptosis (Figure 4F). Thus protracted NF-κB activation following GalN/LPS injury is the mechanism of protection in β-catenin KO mice.
NF-κB is not activated basally in KO animals
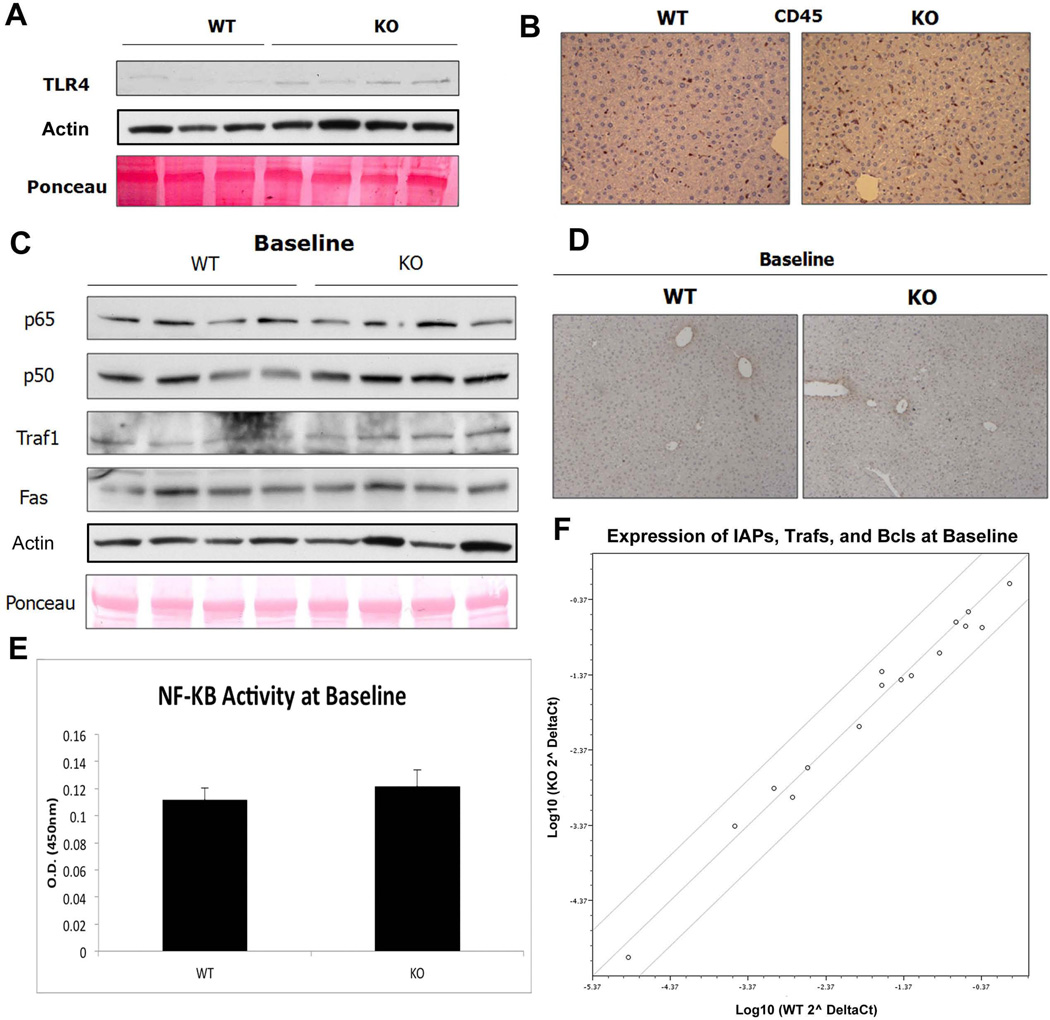
The above observations led us to question the status of NF-κB in resting KO livers. Querying microarray analysis published previously (11) revealed an upregulation of several TNF-α dependent genes in the KO livers at baseline (Supplemental Table 2). Expression of LPS receptor TLR-4, whose activation induces NF-κB signaling, is also increased in KO at baseline (Figure 5A). IHC for CD45, a cell-surface marker of leukocytes, revealed greater numbers of inflammatory cells including macrophages in unstimulated KO livers (Figure 5B).
Figure 5. There is no basal activation of NF-κB in KO livers at baseline.
(A) WB shows a basal increase in TLR-4 in KOs. Actin and Ponceau show comparable loading.
(B) KO livers contain higher numbers of CD45-positive inflammatory cells including macrophages as shown by IHC.
(C) RIPA extracts from unstimulated WT and KO livers show that total p50/p65 is unchanged, as are downstream targets Traf-1 and Fas. Ponceau and actin represent loading controls.
(D) IHC for p65 confirms the absence of active p65 signaling in both KO and WT livers at baseline.
(E) There is no difference in transcriptional activity of NF-κB between KOs and WTs at baseline, as measured by colorimetric assay.
(F) Analysis of NF-κB anti-apoptotic targets by cDNA array shows no difference in expression between WTs and KOs at baseline. Scatter plot compares expression of various known anti-apoptotic genes between WT and KO and is graphed as fold change from WT.
We next wanted to address if protection in KOs was due to a basal increase in NF-κB activity. We examined whole-cell extracts from resting WT and KO livers for the expression of NF-κB subunits and downstream targets. As shown in Figure 5C, there is no difference in total p65 or NF-κB activation between WT and KO livers at baseline. IHC also demonstrates absence of activated p65 in both livers (Figure 5D). Measurement of NF-κB transcriptional activity further confirms these observations (Figure 5E). Finally, expression of anti-apoptotic NF-κB targets, such as IAP and Traf, are equivalent in WT and KO livers as measured by cDNA array (Figure 5F). Therefore, despite an increase in inflammation and TLR-4, there appears to be no frank NF-κB activation at baseline in the KO livers.
p65 associates with β-catenin in WT livers but not in KOs and may be part of an innate response to injury
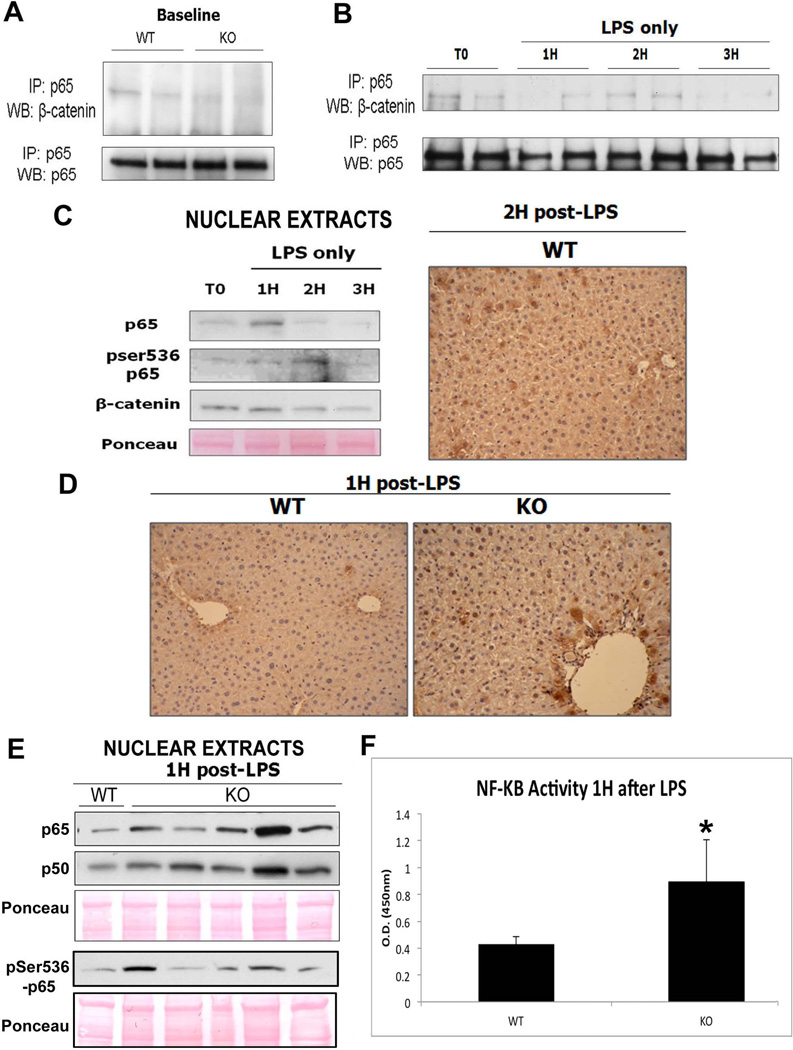
In addition to its well-known interaction with the IκB complex, p65 has also been shown to physically associate with β-catenin in the context of colon, breast, and liver cancer (22, 23). To determine if p65-β-catenin complex is present under normal physiologic conditions in hepatocytes, we immunoprecipitated protein lysates from WT and KO livers with p65 and probed the blots for β-catenin. Figure 6A shows coprecipitation of β-catenin and p65 in both WT and KO livers. However, the association in KO livers is dramatically reduced in KO suggesting the presence of NF-κB-β-catenin complex in hepatocytes and non-parenchymal cells.
Figure 6. The decreased association of p65 and β-catenin in KOs contributes to protection from injury after LPS treatment.
(A) IP shows that p65 and β-catenin associate strongly in WT livers at baseline but less so in KO livers.
(B) Association of p65/β-catenin decreases after administration of LPS in the WT especially at 1H and 3H, as assessed by IP.
(C) Representative WB of WT liver nuclear lysates shows p65 translocation to the nucleus at 1H while its peak activation (ser536-phospho-p65) occurs at 2H after LPS administration. Ponceau represents loading control. The right panel shows representative nuclear expression of ser536-phosho-p65 in around 50% of hepatocytes in WT at 2H after LPS by IHC.
(D) NF-κB is active in the nucleus 1H after LPS treatment in the KO but not in WT, as shown by IHC for ser536-phospho-p65.
(E) Representative WB shows increased p65 and p50 along with ser536-phospho-p65 in the KO livers over the WT at 1H after LPS.
(F) Transcriptional activity of NF-κB is increased in KOs as compared to WTs 1H after LPS treatment alone, as measured by colorimetric assay (*P<0.05).
Lack of β-catenin in hepatocytes primes the liver for NF-κB activation
Next to investigate if p65-β-catenin complex may be undergoing changes and thus modulating NF-κB activation, we harvested WT livers at baseline and at 1H, 2H, and 3H after LPS only treatment. Disruption of β-catenin and p65 association was observed as early as 1 hour after LPS (Figure 6B) along with concomitant p65 nuclear translocation (Figure 6C). While p65 phosphorylation begins to increase simultaneously, it peaks at 2H after LPS treatment, as shown by the appearance of ser536-phospho-p65 in the nuclei (Figure 6C). IHC confirmed the presence of active p65 in around 50% of hepatocytes (Figure 6C) and is consistent with previous reports (24, 25).
We hypothesized that lack of β-catenin in hepatocytes may be lowering the threshold of p65 activation after apoptotic stimuli. To test this, we treated both KO and WT with LPS to compare kinetics of p65 nuclear translocation and activation. While some animal-to-animal variation was evident, KO livers showed a greater increase in nuclear p65 at 1H after LPS treatment as compared to WT (Figure 6E). Additionally, at 1H after LPS, KO but not WT livers showed active ser536-phosho-p65 by both IHC and WB (Figure 6D and E). These results were also confirmed by calorimetric measurement of NF-κB transcriptional activity after 1 hour of LPS, in which KO shows a significant increase over WT (Figure 6F). Thus, loss of β-catenin lowers the threshold to prime the KO livers for early and robust p65 nuclear translocation and activation in response to TNF-α.
Modulation of β-catenin expression alters NF-κB activity in hepatoma cells
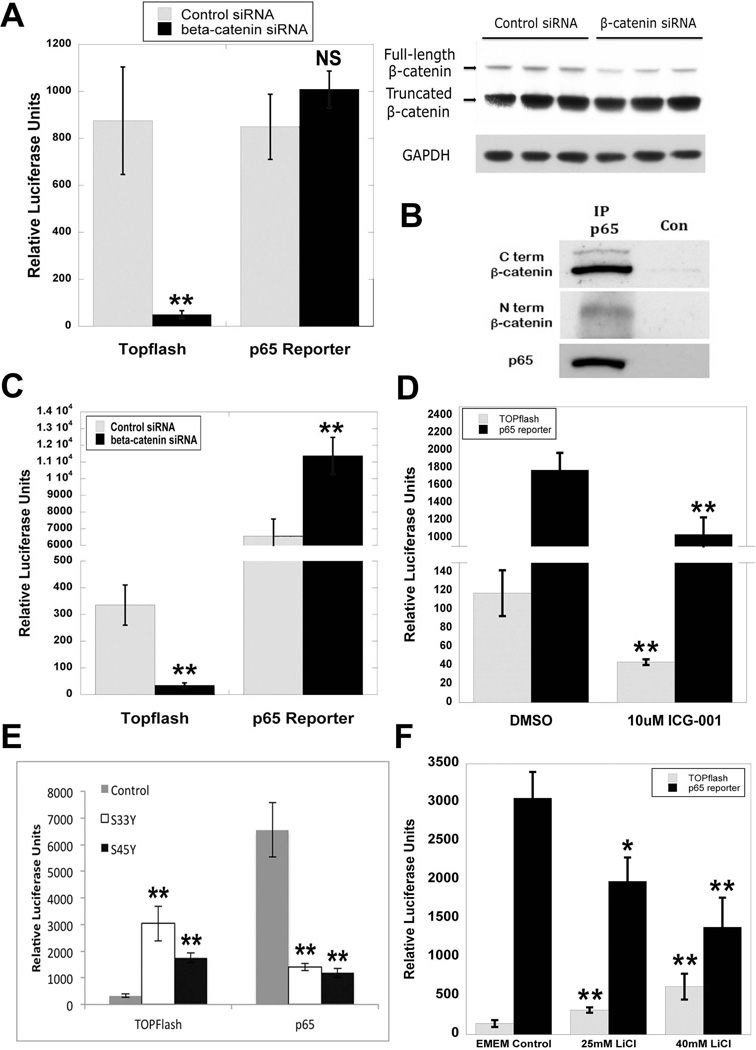
To directly address how β-catenin-p65 interactions may influence NF-κB activity, we first transfected HepG2 cells, which harbor a monoallelic exon-3-deleted constitutively active β-catenin (26), with control or β-catenin siRNA concomitantly with either TOPflash (a luciferase reporter that measures β-catenin/Tcf-dependent transcriptional activation) or p65 luciferase reporter plasmid. Although RNA inhibition caused a reduction in full-length β-catenin at 48H, as shown by WB and TOPflash reporter assay, there was no significant change in p65 activity (Figure 7A). While this was unexpected, further analysis of p65-β-catenin association in HepG2 cells by p65 IP revealed an association between p65 and the predominant truncated as well as the full-length form of β-catenin (Figure 7B), suggesting that despite knockdown of the wild-type form, the presence of the truncated form was sufficient to bind and disallow p65 activation. However, when Hep3B cells that contain full-length, non-mutated β-catenin were transfected with siRNA and reporter plasmids, β-catenin was effectively suppressed, leading to a significant decrease in TOPflash reporter activity and an increase in p65 reporter activity (Figure 7C). We next treated Hep3B cells with ICG-001, which inhibits β-catenin-CREB binding protein (CBP) association and downregulates β-catenin-TCF4 transcriptional activity without reducing total β-catenin levels (27). While ICG-001 expectedly decreased TOPflash reporter activity, it unexpectedly reduced p65 reporter activity, which may be due to an increase in non-CBP-bound pool of β-catenin (Figure 7D). These findings suggest that β-catenin modulation of NF-κB signaling is regulatable through manipulation of β-catenin expression; however, only agents that suppress total β-catenin levels may be useful to induce p65 activation.
Figure 7. Modulation of β-catenin signaling in human hepatoma cell lines regulates p65 activity.
(A) HepG2 cells transfected with either control or β-catenin siRNA showed no significant differences in p65 reporter plasmid activity, despite a significant decrease in TOPflash activity (**P<0.01). WB demonstrates a decrease in full-length but not truncated β-catenin at 48 hours after siRNA treatment in HepG2 cells. GAPDH represents loading control.
(B) IP shows that p65 associates with both the full-length and truncated form of β-catenin in HepG2 cells.
(C) NF-κB activity as measured by p65 luciferase reporter is increased in Hep3B cells transfected with β-catenin siRNA (**P<0.01). Decrease in TOPflash reporter activity confirms knockdown of β-catenin (**P<0.01).
(D) p65 luciferase reporter activity is decreased in Hep3B cells transfected with β-catenin activity inhibitor ICG-001 (**P<0.01). Inhibition of β-catenin signaling is verified by a decrease in TOPflash reporter activity (**P<0.01).
(E) Transfection of Hep3B cells with mutant S33Y and S45Y β-catenin increases TOPflash activity but causes a decrease in p65 luciferase reporter activity as compared to control (**P<0.01).
(F) Treatment of Hep3B cells with escalating doses of LiCl increases TOPflash activity but causes a decrease in p65 luciferase reporter activity as compared to control (*P<0.05; **P<0.01).
Increased β-catenin levels decrease p65 activity and expression in hepatoma cells and HCC patients
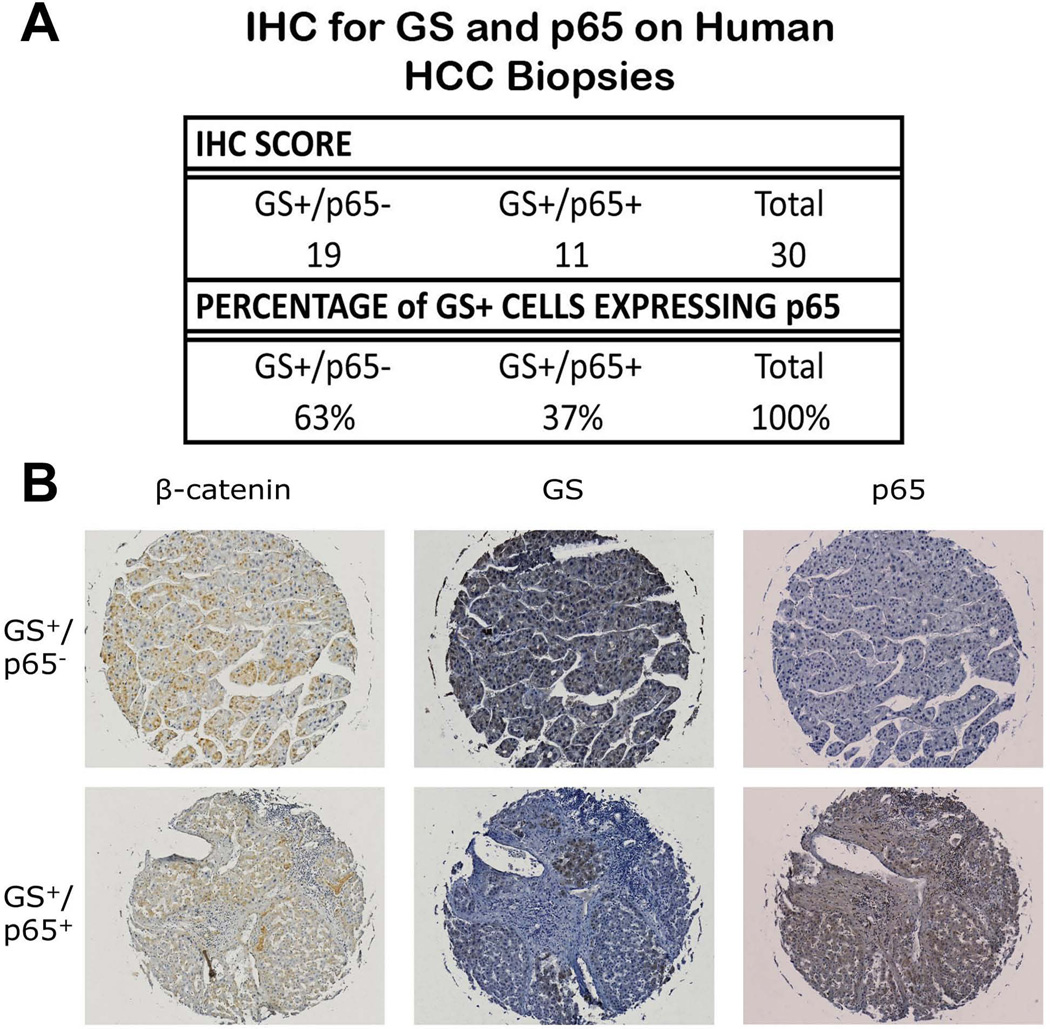
We next examined conditions in which β-catenin was overexpressed both in vitro and in vivo to determine the effect on p65 expression and activity. Hep3B cells were transfected with control plasmid or plasmid expressing constitutively active S33Y-β-catenin or S45Y-β-catenin, simultaneous with p65 or TOPflash reporters. While expression of mutated β-catenin induces TOPflash activity, it also results in significantly repressing p65 activity (Figure 7E). We next treated Hep3B cells with escalating dose of lithium chloride (LiCl), a known inhibitor of GSK-3β that in turn induces β-catenin protein stabilization. This led to a dose-dependent increase in TOPflash reporter activity and a concomitant and significant decrease in p65 reporter activity (Figure 7F). Lastly, we analyzed human HCC tissue array by IHC. Tumor-wide GS staining is a good indicator of β-Catenin mutations (28, 29). Of 93 HCC tumors on Biomax HCC tissue array, 30 were GS+ve, which is consistent with the numbers of HCC with β-catenin gene mutations (reviewed in (9)). Of this subset, majority of GS-positive HCC (63%; 19/30) were negative for p65 (Figure 8A and 8B). Thus, β-catenin activation in HCC affects p65 expression and NF-κB activity.
Figure 8. Overexpression of β-catenin in HCC correlates inversely with p65 expression.
(A) The majority of patient HCC samples that are GS+ are also p65-, as classified by IHC on Biomax HCC tissue array. Samples are grouped by p65 status secondary to GS status.
(B) Representative IHC for β-catenin, GS, and p65 demonstrates the two different GS-dependent p65 classifications.
DISCUSSION
β-Catenin is a crucial component of the Wnt pathway that plays multiple roles in liver homeostasis through its regulation of proliferation, differentiation and regeneration. However its role in hepatic injury remains unexplored. The analysis was initiated to test two common modes of hepatocyte apoptosis - Fas- and TNF-α- mediated cell death. Previously, we have reported that β-catenin and the hepatocyte growth factor (HGF) receptor c-Met associate at the cell surface (15). c-Met sequestration of the Fas receptor that can prevent Fas-mediated apoptosis in hepatocytes has also been reported (13). We also identify Fas-β-catenin complex in the liver. Since HGF mRNA upregulation along with a dramatic reduction in Met protein levels was evident in KO livers, we hypothesized that basal apoptosis may be due to destabilization of c-Met-Fas-β-catenin complexes, which may enhance free-Fas levels available for engagement with Fas ligands like Jo-2. However, the KO mice were as susceptible as WT to Fas-activation.
Since several TNF-α related genes were upregulated basally in KO livers, we next reasoned that the prevalence of basal apoptosis maybe a consequence of TNF-α activation. However, we found that β-catenin KO mice were paradoxically resistant to the effects of GalN/LPS induced hepatocyte apoptosis. The time to morbidity in WT C57BL/6 mice following GalN/LPS treatment is generally 6–8 hours (17). We observed 100% of the WT mice displaying morbidity by 6 hours. In contrast, nearly all (93.3%) KO mice were still alive at this time, with times to morbidity ranging from 7.5 hours to 10.5 hours in a subset of mice while many animals remained healthy until the outer limit of the predicted survival time of 12 hours. All of the hallmarks of apoptotic death which were present in the WT mice including elevated liver enzymes, hepatic caspase activation, and TUNEL-positivity were absent or greatly diminished in KO.
To address the mechanism of relative resistance to TNF-α induced liver injury, we first proceeded to determine if the metabolism of D-galactosamine used as a transcriptional suppressor prior to LPS administration could be a factor. We previously showed a perturbation in vitamin C biosynthesis in β-catenin KO mice (30), which could result in accumulation of D-glucuronate, a precursor of vitamin C (31). D-galactosamine is known to reduce the hepatic content of UDP-glucose (32), which would decrease the amount of glucuronate, as well as inhibit the formation of de-novo glucuronate by depleting UDP-glucuronate. However, pretreatment of KO and WT before LPS with actinomycin-D, an independent transcriptional inhibitor, in lieu of GalN, also recapitulated the observations in GalN/LPS-treated mice (data not shown).
The transcription factor NF-κB plays a key role in both innate and adaptive immunity. It plays a direct role in hepatocyte survival and regeneration (33), and is known to positively regulate the transcription of anti-apoptotic genes such as c-IAPs, Trafs, Bcl-XL, and c-FLIP (34), making it a likely candidate for cytoprotective role in KO in response to TNF-α. Indeed, we found greater activation of NF-κB along with high expression of many of its downstream targets in KO mice after TNF-α. KO livers demonstrated an increase in basal inflammation, which are a prominent source of TNF-α, which in turn may be due to higher total hepatic bile acids at baseline in chow fed KO (35). In addition levels of TLR-4, which has been shown to activate NF-κB, are higher in KO (36). These two factors may be the priming mechanisms for NF-κB activation, especially in the absence of β-catenin-p65 complex in hepatocytes. Interestingly, however, NF-κB was not active in KO under resting conditions, which may be because of negative feedback regulation due to NF-κB-dependent transcription of inhibitory targets such as IκB (37). Similarly, we do observe heterogeneity in NF-κB activation, which explains inter-animal variability in susceptibility of KO to TNF-α in the KO, albeit its basis remains undetermined.
While others have previously described an association between β-catenin and p65 in cancer (22), we report this association under normal physiologic conditions and ascribe to it functionality within the cell and in the context of acute liver injury. We demonstrate β-catenin-p65 complex to change dynamically over time in response to TNF-α stimulation and acute liver injury. While basal p65-β-catenin association prevents NF-κB activation during resting conditions, upon TNF-α signaling, β-catenin-p65 complex undergoes dissociation allowing nuclear p65 translocation that regulates cell survival through expression of specific anti-apoptotic downstream targets.
The relevance of p65-β-catenin association is demonstrated by manipulating β-catenin levels. We observe greater p65 activity upon silencing of β-catenin gene. Intriguingly, however ICG-001, a known blocker of β-catenin’s downstream transcriptional activity through blockade of β-catenin-CBP complex, decreased p65 reporter activity (38). This is not surprising since ICG-001 treatment does not decrease total β-catenin levels, in fact its free pool is increased (27). This result thus shows that not all anti-β-catenin therapies will be effective in stimulating NF-κB signaling and only those agents that decrease total β-catenin levels and not its activity alone may be useful, as it is the physical presence of β-catenin protein that directly affects p65 activity through formation of the inhibitory complex. Nonetheless, β-catenin inhibition to enhance p65 activation may be therapeutically exploitable to treat certain hepatic injuries where TNF-α signaling is the chief perpetrator.
We also demonstrate that stabilizing β-catenin by LiCl treatment represses p65 activity. Although inhibition of GSK-3β may inhibit p65 directly, this effect is likely due to increased β-catenin binding to p65, as shown previously (39). It is possible that APC/Axin/GSK-3β/β-catenin complex may be in close proximity to or in direct association with β-catenin/NF-κB/IκK complex. If true, this association could serve as a mode of crosstalk and integration of two distinct signaling pathways. Whether β-catenin-p65 complex exists as a part of a larger multimeric complex will need additional investigation.
β-Catenin gene mutations leading to its activation and nuclear translocation are frequent in HCC and this led to decreased p65 activity and expression in hepatoma cells and tissues. This is in agreement with previous studies that showed enhanced β-catenin can bind to and inhibit NF-κB transcriptional activation in cancer cells (22, 23). While the functional consequences of this observation will need to be investigated more thoroughly, it is previously reported that β-catenin may act as a negative regulator of inflammation through repression of NF-κB signaling (40, 41). That may explain why in certain β-catenin mutated HCC lesser cirrhosis is evident, since inflammation induces hepatic injury and fibrosis (28, 42). However, the role of NF-κB in liver injury and hepatocyte survival is pleiotropic since its activation can be contextually anti-apoptotic or pro-inflammatory (8). Similarly, β-catenin antagonism that has been touted for cancer therapeutics (9) may have unintended consequences of promoting tumor cell survival depending on NF-κB status. Further pre-clinical work would be necessary to determine the long-term effects of NF-κB activation in the context of β-catenin inhibition.
Supplementary Material
Acknowledgments
Grant Support: This study was funded by NIH grants 1R01DK62277 and 1R01CA124414 to SPSM and by Rango’s Fund for the Enhancement of Pathology Research.
Abbreviations
- LPS
Lipopolysaccharide
- KO
β-catenin conditional knockouts
- WT
littermate controls
- TNF
Tumor necrosis factor
- TNFR
Tumor necrosis factor receptor
- GalN
D-galactosamine
- LPS
Lipopolysaccharide
- HCC
Hepatocellular cancer
- FADD
Fas-associated death domain
- DISC
Death-inducing signaling complex
- TRADD
TNFR-associated death domain
- PH
Partial hepatectomy
- EGFR
Epidermal growth factor receptor
- HGF
Hepatocyte growth factor
- ALT
Alanine aminotransferase
- AST
Aspartate animotransferase
- TLR
Toll like receptor
- GSK
Glycogen synthase kinase
- GS
Glutamine synthetase
- IHC
Immunohistochemistry
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- IP
intraperitoneal
- BW
body weight
- WB
Western blot
Footnotes
Disclosure: None of the authors have any potential conflicts of interest pertaining to the current manuscript.
REFERENCES
- 1.Ding WX, Yin XM. Dissection of the multiple mechanisms of TNF-alpha-induced apoptosis in liver injury. J Cell Mol Med. 2004;8:445–454. doi: 10.1111/j.1582-4934.2004.tb00469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641–1654. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiratori Y, Tanaka M, Hai K, Kawase T, Shirna S, Sugimoto T. Role of endotoxin-responsive macrophages in hepatic injury. Hepatology. 1990;11:183–192. doi: 10.1002/hep.1840110205. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol. 2008;8:837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 5.Guicciardi ME, Gores GJ. Life and death by death receptors. Faseb J. 2009;23:1625–1637. doi: 10.1096/fj.08-111005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 7.Lehmann V, Freudenberg MA, Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and D-galactosamine-treated mice. J Exp Med. 1987;165:657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luedde T, Schwabe RF. NF-kappaB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nejak-Bowen KN, Monga SP. Beta-catenin signaling, liver regeneration and hepatocellular cancer: sorting the good from the bad. Semin Cancer Biol. 2011;21:44–58. doi: 10.1016/j.semcancer.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehwald N, Tao GZ, Jang KY, Sorkin M, Knoefel WT, Sylvester KG. Wnt-beta-catenin signaling protects against hepatic ischemia and reperfusion injury in mice. Gastroenterology. 2011;141:707–718. e701–e705. doi: 10.1053/j.gastro.2011.04.051. 718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131:1561–1572. doi: 10.1053/j.gastro.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 12.Li G, Han C, Xu L, Lim K, Isse K, Wu T. Cyclooxygenase-2 prevents fas-induced liver injury through up-regulation of epidermal growth factor receptor. Hepatology. 2009;50:834–843. doi: 10.1002/hep.23052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, DeFrances MC, Dai Y, Pediaditakis P, Johnson C, Bell A, Michalopoulos GK, et al. A mechanism of cell survival: sequestration of Fas by the HGF receptor Met. Mol Cell. 2002;9:411–421. doi: 10.1016/s1097-2765(02)00439-2. [DOI] [PubMed] [Google Scholar]
- 14.Boon EM, van der Neut R, van de Wetering M, Clevers H, Pals ST. Wnt signaling regulates expression of the receptor tyrosine kinase met in colorectal cancer. Cancer Res. 2002;62:5126–5128. [PubMed] [Google Scholar]
- 15.Monga SP, Mars WM, Pediaditakis P, Bell A, Mule K, Bowen WC, Wang X, et al. Hepatocyte growth factor induces Wnt-independent nuclear translocation of beta-catenin after Met-beta-catenin dissociation in hepatocytes. Cancer Res. 2002;62:2064–2071. [PubMed] [Google Scholar]
- 16.Zeng G, Apte U, Micsenyi A, Bell A, Monga SP. Tyrosine residues 654 and 670 in beta-catenin are crucial in regulation of Met-beta-catenin interactions. Exp Cell Res. 2006;312:3620–3630. doi: 10.1016/j.yexcr.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galanos C, Freudenberg MA, Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979;76:5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morikawa A, Sugiyama T, Kato Y, Koide N, Jiang GZ, Takahashi K, Tamada Y, et al. Apoptotic cell death in the response of D-galactosamine-sensitized mice to lipopolysaccharide as an experimental endotoxic shock model. Infect Immun. 1996;64:734–738. doi: 10.1128/iai.64.3.734-738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Josephs MD, Bahjat FR, Fukuzuka K, Ksontini R, Solorzano CC, Edwards CK, 3rd, Tannahill CL, et al. Lipopolysaccharide and D-galactosamine-induced hepatic injury is mediated by TNF-alpha and not by Fas ligand. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1196–R1201. doi: 10.1152/ajpregu.2000.278.5.R1196. [DOI] [PubMed] [Google Scholar]
- 20.Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274:30353–30356. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- 21.Schwabe RF, Brenner DA. Role of glycogen synthase kinase-3 in TNF-alpha-induced NF-kappaB activation and apoptosis in hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2002;283:G204–G211. doi: 10.1152/ajpgi.00016.2002. [DOI] [PubMed] [Google Scholar]
- 22.Deng J, Miller SA, Wang HY, Xia W, Wen Y, Zhou BP, Li Y, et al. beta-catenin interacts with and inhibits NF-kappa B in human colon and breast cancer. Cancer Cell. 2002;2:323–334. doi: 10.1016/s1535-6108(02)00154-x. [DOI] [PubMed] [Google Scholar]
- 23.Du Q, Zhang X, Cardinal J, Cao Z, Guo Z, Shao L, Geller DA. Wnt/beta-catenin signaling regulates cytokine-induced human inducible nitric oxide synthase expression by inhibiting nuclear factor-kappaB activation in cancer cells. Cancer Res. 2009;69:3764–3771. doi: 10.1158/0008-5472.CAN-09-0014. [DOI] [PubMed] [Google Scholar]
- 24.Blackwell TS, Yull FE, Chen CL, Venkatakrishnan A, Blackwell TR, Hicks DJ, Lancaster LH, et al. Multiorgan nuclear factor kappa B activation in a transgenic mouse model of systemic inflammation. Am J Respir Crit Care Med. 2000;162:1095–1101. doi: 10.1164/ajrccm.162.3.9906129. [DOI] [PubMed] [Google Scholar]
- 25.Tapalaga D, Tiegs G, Angermuller S. NFkappaB and caspase-3 activity in apoptotic hepatocytes of galactosamine-sensitized mice treated with TNFalpha. J Histochem Cytochem. 2002;50:1599–1609. doi: 10.1177/002215540205001204. [DOI] [PubMed] [Google Scholar]
- 26.de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci U S A. 1998;95:8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, Moon RT, et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected] Proc Natl Acad Sci U S A. 2004;101:12682–12687. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cieply B, Zeng G, Proverbs-Singh T, Geller DA, Monga SP. Unique phenotype of hepatocellular cancers with exon-3 mutations in beta-catenin gene. Hepatology. 2009;49:821–831. doi: 10.1002/hep.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zucman-Rossi J, Benhamouche S, Godard C, Boyault S, Grimber G, Balabaud C, Cunha AS, et al. Differential effects of inactivated Axin1 and activated beta-catenin mutations in human hepatocellular carcinomas. Oncogene. 2007;26:774–780. doi: 10.1038/sj.onc.1209824. [DOI] [PubMed] [Google Scholar]
- 30.Nejak-Bowen KN, Zeng G, Tan X, Cieply B, Monga SP. Beta-catenin regulates vitamin C biosynthesis and cell survival in murine liver. J Biol Chem. 2009;284:28115–28127. doi: 10.1074/jbc.M109.047258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linster CL, Van Schaftingen E, Vitamin C. Biosynthesis, recycling and degradation in mammals. Febs J. 2007;274:1–22. doi: 10.1111/j.1742-4658.2006.05607.x. [DOI] [PubMed] [Google Scholar]
- 32.Decker K, Keppler D. Galactosamine hepatitis: key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev Physiol Biochem Pharmacol. 1974:77–106. doi: 10.1007/BFb0027661. [DOI] [PubMed] [Google Scholar]
- 33.Taub R. Liver regeneration 4: transcriptional control of liver regeneration. Faseb J. 1996;10:413–427. [PubMed] [Google Scholar]
- 34.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 35.Behari J, Yeh TH, Krauland L, Otruba W, Cieply B, Hauth B, Apte U, et al. Liver-specific beta-catenin knockout mice exhibit defective bile acid and cholesterol homeostasis and increased susceptibility to diet-induced steatohepatitis. Am J Pathol. 2010;176:744–753. doi: 10.2353/ajpath.2010.090667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akashi-Takamura S, Furuta T, Takahashi K, Tanimura N, Kusumoto Y, Kobayashi T, Saitoh S, et al. Agonistic antibody to TLR4/MD-2 protects mice from acute lethal hepatitis induced by TNF-alpha. J Immunol. 2006;176:4244–4251. doi: 10.4049/jimmunol.176.7.4244. [DOI] [PubMed] [Google Scholar]
- 37.Nelson DE, Ihekwaba AE, Elliott M, Johnson JR, Gibney CA, Foreman BE, Nelson G, et al. Oscillations in NF-kappaB signaling control the dynamics of gene expression. Science. 2004;306:704–708. doi: 10.1126/science.1099962. [DOI] [PubMed] [Google Scholar]
- 38.Eguchi M, Nguyen C, Lee SC, Kahn M. ICG-001, a novel small molecule regulator of TCF/beta-catenin transcription. Med Chem. 2005;1:467–472. doi: 10.2174/1573406054864098. [DOI] [PubMed] [Google Scholar]
- 39.Deng J, Xia W, Miller SA, Wen Y, Wang HY, Hung MC. Crossregulation of NF-kappaB by the APC/GSK-3beta/beta-catenin pathway. Mol Carcinog. 2004;39:139–146. doi: 10.1002/mc.10169. [DOI] [PubMed] [Google Scholar]
- 40.Duan Y, Liao AP, Kuppireddi S, Ye Z, Ciancio MJ, Sun J. beta-Catenin activity negatively regulates bacteria-induced inflammation. Lab Invest. 2007;87:613–624. doi: 10.1038/labinvest.3700545. [DOI] [PubMed] [Google Scholar]
- 41.Sun J, Hobert ME, Duan Y, Rao AS, He TC, Chang EB, Madara JL. Crosstalk between NF-kappaB and beta-catenin pathways in bacterial-colonized intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G129–G137. doi: 10.1152/ajpgi.00515.2004. [DOI] [PubMed] [Google Scholar]
- 42.Dal Bello B, Rosa L, Campanini N, Tinelli C, Torello Viera F, D'Ambrosio G, Rossi S, et al. Glutamine synthetase immunostaining correlates with pathologic features of hepatocellular carcinoma and better survival after radiofrequency thermal ablation. Clin Cancer Res. 2010;16:2157–2166. doi: 10.1158/1078-0432.CCR-09-1978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.